Abstract
OBJECTIVE
To report the first case of tacrolimus measurement in human milk following maternal dosing in a woman who breast-fed while taking the medication.
CASE REPORT
A 32-year-old white woman who had taken tacrolimus 0.1 mg/kg/d throughout pregnancy contacted the Motherisk Program at 35 weeks’ gestation inquiring about the safety of breast-feeding during maternal tacrolimus therapy. After benefit–risk assessment, the mother decided to breast-feed the baby.
METHODS
Manually expressed milk samples were collected over 12 hours following the first tacrolimus dose of the day; pre-dosing and 1-hour post-dosing blood concentrations were also determined. The samples were analyzed for tacrolimus by tandem-mass spectrometry. Breast milk and blood samples were collected at steady-state.
RESULTS
The highest and mean concentrations of tacrolimus in milk were 0.57 and 0.429 ng/mL, respectively. From these measurements, the exclusively breast-fed infant would ingest, on average, 0.06 μg/kg/d, which corresponds to 0.06% of the mother’s weight-adjusted dose. Given the low oral bioavailability of tacrolimus, the maximum amount the baby would receive is 0.02% of the mother’s weight-adjusted dose. The milk-to-blood ratios of tacrolimus at pre-dosing and 1-hour post-dosing concentrations were calculated to be 0.08 and 0.09, respectively. At 2.5 months of age, the infant was developing well both physically and neurologically.
COMMENT
This report is the first to measure tacrolimus concentrations in established human milk using tandem-mass spectrometry to detect drug while the infant was exclusively breast-fed by the mother, and in which the infant’s growth and development were reported.
CONCLUSIONS
Our results suggest that maternal therapy with tacrolimus for liver transplant may be compatible with breast-feeding.
Keywords: breast-feeding, milk transfer, tacrolimus
Tacrolimus, an immunosuppressant drug used orally for organ transplantation, exhibits variable but usually low absorption from the gastrointestinal tract, and is extensively bound to proteins (99%); the whole-blood-to-plasma ratio is approximately 20.1 Both of these factors would suggest that there is low excretion of the drug into human breast milk and a low risk for absorption by the suckling infant. Presently, however, breast-feeding is generally not recommended during immunotherapy, based on theoretical concerns of affecting the development of immune function in the baby. There has been only 1 published report of the measurement of tacrolimus concentrations in breast milk using immunoassay techniques,2 and the mothers in that study were counseled to abstain from breast-feeding while taking the medication despite very low concentrations of tacrolimus in the milk. We describe the first account, to the best of our knowledge, of tacrolimus measurement in human milk following maternal dosing, in which the mother breast-fed while taking the medication.
Case Report
A 32-year-old white woman (59 kg) had undergone liver transplantation in April 1997. At 35 weeks’ gestation, nearly 5 years after transplantation, after having taken tacrolimus throughout pregnancy, she contacted the Motherisk Program inquiring about the safety of breast-feeding during maternal tacrolimus therapy. The mother had stable graft function throughout her pregnancy, and her tacrolimus dose ranged from 3 to 5 mg twice daily. At the time of the call, she was taking 3 mg twice daily (0.1 mg/kg/d). The immunosuppressive properties of tacrolimus and the existence of only 1 case series reporting its excretion into human milk were explained to the patient, and contrasted with the potential benefits of breast-feeding and the fact that she had taken the medication throughout pregnancy. She decided that, depending on the health of the baby following delivery, she would initiate breast-feeding and asked us to estimate the infant exposure to the drug by measuring tacrolimus concentrations in her milk.
Manually expressed milk samples were collected at 0 (trough), 1, 6, 9, 11, and 12 hours after the morning dose, as well as maternal blood samples at 0 and 1 hour. The samples were stored at −20 °C until analysis by tandem-mass spectrometry (tandem-MS/MS) at the Children’s National Medical Center (Washington, DC) within 8 days. The Sciex API-2000 instrument (MDS Sciex, Toronto, Canada) was used for whole-blood and human milk analysis, as described previously.3 A minor modification to the whole-blood method was required for human milk analysis; following deproteinization, 120 μL (4 times the amount of sample stated in the published whole-blood method) was injected for analysis. This step was necessary to enhance the signal and increase the sensitivity of the method. Other than this modification, the technique for measurement of tacrolimus in human milk is the same as for whole blood. The coefficient of variation at 6 and 0.3 ng/mL is 4% and 20%, respectively. The limit of sensitivity is 0.2 ng/mL, and the injection volume was quadrupled to enhance sensitivity.
The AUC of tacrolimus in milk from time 0 to 12 hours of the dose was calculated by the trapezoidal method. The elimination half-life (t1/2) of tacrolimus in breast milk was calculated using the slope of the elimination phase of the AUC.
By multiplying the AUC of tacrolimus in milk with an assumed infant’s milk intake of 150 mL/kg/d, an infant daily exposure to tacrolimus through breast-feeding was estimated. A hypothetical maximum infant dose was calculated based on the highest measured drug concentration in milk.
Results
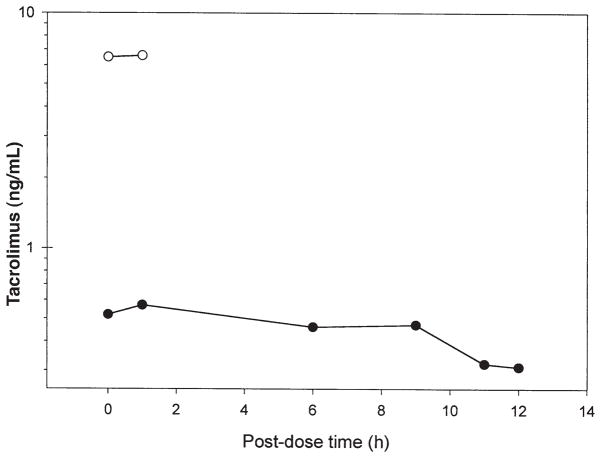
The highest concentration of tacrolimus in milk (0.57 ng/mL) was observed at 1-hour after the dose (Figure 1). This concentration is not necessarily the actual peak concentration of tacrolimus in human milk, as we were limited by the mother’s ability to express milk. The first 2 samples were scheduled, but the remaining samples were drawn based on the feeding times. A more accurate estimate of peak concentration would be obtained if milk samples were taken at 0, 1, 2, 4, 6, and 8 hours after the dose. The AUC and the mean concentration were calculated to be 5.15 h • ng/mL and 0.429 ng/mL, respectively. Therefore, the tacrolimus dose this exclusively breast-fed infant would ingest was 0.06 μg/kg/d (0.429 ng/mL × 150 mL/kg/d), which is 0.06% of the mother’s weight-adjusted dose (0.1 mg/kg/d). A maximum tacrolimus dose the infant would receive through breast-feeding is 0.09 μg/kg/d, which corresponds to about 0.09% of the mother’s weight-adjusted dose. Oral bioavailability of tacrolimus has been reported4 to range from 18% in healthy volunteers to 34% in pediatric liver transplant patients. Using the highest range of bioavailability, the baby could potentially absorb 0.02% of the mother’s weight-adjusted dose.
Figure 1.
Concentration-versus-time profile of tacrolimus in breast milk (●) and maternal whole blood (○). The breast-feeding woman was receiving 0.1 mg/kg/d of oral tacrolimus. Milk samples were collected 2 weeks postnatally. Peak concentration of 0.57 ng/mL was observed 1 hour after the dose. The average concentration, obtained from the AUC, was 0.429 ng/mL; average infant exposure was estimated to be 0.06% of the mother’s weight-adjusted dose.
The maternal whole-blood concentrations of tacrolimus were 6.5 and 6.6 ng/mL at 0 and 1 hour, respectively. Using the human milk concentrations of tacrolimus at the corresponding times, the milk-to-whole-blood ratio of tacrolimus was 0.08 and 0.09, respectively. We are not sure why the difference between the pre-dosing and 1-hour post-dosing maternal whole-blood tacrolimus concentrations is not great. It is likely due to steady-state pharmacokinetics.
The apparent t1/2 of tacrolimus in breast milk was calculated to be 12.85 hours. The t1/2 of tacrolimus in the patient could not be calculated from the available data because only 2 concentrations could be obtained from the patient, but it is approximately 11.7 hours in liver transplant patients and 34.2 hours in healthy volunteers. The t1/2 is usually calculated after single-dose administration or after the last dose in steady-state. We clearly did not have these conditions, and hence could not sample for 3–5 half-lives to characterize the elimination. Therefore, the value we calculated for the t1/2 of tacrolimus in breast milk should be interpreted with caution.
At 2.5 months of age, the infant was developing well both physically and neurologically. His weight and supine length were at the 50th percentile, as was his head circumference. He holds his head up and smiles. Chest ultrasound revealed a normally developed thymus.
Discussion
Tacrolimus concentration in breast milk has been reported previously in 1 other study.2 This group measured tacrolimus concentrations in the colostrum of 6 mothers who had taken the medication throughout pregnancy, but had been advised not to breast-feed their infants. The authors found that the average concentration of tacrolimus in the first breast milk specimen was 0.6 ng/mL. This corresponds with the peak concentration measured in our patient’s breast milk, which is reassuring.
Several elements of this small case series, however, may limit the results.2 First, we disagree with the authors’ method of tacrolimus measurement. Concentrations were measured in the colostrum, the first breast milk specimen, which is known to differ in protein content from mature milk.5,6 The elevated protein levels could theoretically change the way in which tacrolimus is handled by the human milk. Second, an immunoassay was used in the mentioned study for analysis of tacrolimus concentrations in breast milk.2 Immunoassays for tacrolimus have problems with specificity and cross-reactivity with metabolites, which can result in overestimation of the concentration of the parent compound.7–10 Additionally, all concentrations reported from this case series were <9 ng/mL. Ghoshal and Soldin11 recently found that the IMx tacrolimus II assay (which is still used in >99% of laboratories in North America) is not accurate for concentrations of tacrolimus <9 ng/mL. Tandem-MS/MS is a far more sensitive and specific method for tacrolimus measurement because there is no metabolite interference and better sensitivity. Lastly, all of the mothers were counseled to abstain from breast-feeding while taking the medication; in our case, the mother breast-fed while continuing the medication, so we have been able to follow up with the infant’s development.
Summary
Our report is the first to measure tacrolimus concentrations in established human milk (rather than colostrum) using tandem-MS/MS to detect the drug while the mother was exclusively breast-feeding the infant, and in which the infant’s growth and development were reported. Our study may be somewhat limited by lack of a milk sample between 1 and 6 hours after the dose, due to the schedule of feeding the baby. We determined that the exclusively breast-fed infant would ingest only 0.06% of the mother’s weight-adjusted dose of tacrolimus. While more data are needed, our results along with those in a previous study suggest that maternal therapy with tacrolimus for liver transplant may be compatible with breast-feeding.
Contributor Information
Amy E. French, The Motherisk Program, Division of Clinical Pharmacology and Toxicology, The Hospital for Sick Children and The University of Toronto, Toronto, Ontario, Canada.
Steven J. Soldin, Clinical Chemistry and Point-of-Care Testing, Department of Laboratory Medicine, Children’s National Medical Center, Washington, DC.
Offie P. Soldin, Soldin Research and Consultants Inc., Washington, DC; Consultant, The Motherisk Program, Division of Clinical Pharmacology and Toxicology, The Hospital for Sick Children.
Gideon Koren, The Motherisk Program, Division of Clinical Pharmacology and Toxicology, The Hospital for Sick Children and the University of Toronto; Senior Scientist of the Canadian Institutes for Health Research, and holds the Research Leadership for Better Pharmacotherapy During Pregnancy and Lactation.
References
- 1.Jusko WJ, Thomson AW, Fung J, McMaster P, Wong SH, Zylber-Katz E, et al. Consensus document: therapeutic monitoring of tacrolimus (FK-506) Ther Drug Monit. 1995;17:606–14. doi: 10.1097/00007691-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Jain A, Venkataramanan R, Fung JJ, Gartner JC, Lever J, Balan V, et al. Pregnancy after liver transplantation under tacrolimus. Transplantation. 1997;64:559–65. doi: 10.1097/00007890-199708270-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Volosov A, Napoli KL, Soldin SJ. Simultaneous simple and fast quantification of three major immunosuppressants by liquid chromatography—tandem mass-spectrometry. Clin Biochem. 2001;34:285–90. doi: 10.1016/s0009-9120(01)00235-1. [DOI] [PubMed] [Google Scholar]
- 4.Product monograph: Prograf (tacrolimus) Markham, ON: Fujisawa Canada; 2001. [Google Scholar]
- 5.Hytten FE. Clinical and chemical studies in human lactation. Br Med J. 1954;1:175–82. doi: 10.1136/bmj.1.4855.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lonnerdal B, Forsum E, Hambraeus L. A longitudinal study of the protein content of human milk from well-nourished Swedish mothers. Nutr Metab. 1977;21(suppl 1):106–9. doi: 10.1159/000176127. [DOI] [PubMed] [Google Scholar]
- 7.Braun F, Lorf T, Schutz E, Christians U, Grupp C, Sattler B, et al. Clinical relevance of monitoring tacrolimus: comparison of microparticle enzyme immunoassay, enzyme-linked immunosorbent assay, and liquid chromatography mass spectrometry in renal transplant recipients converted from cyclosporine to tacrolimus. Transplant Proc. 1996;28:3175–6. [PubMed] [Google Scholar]
- 8.Braun F, Schutz E, Christians U, Lorf T, Schiffmann JH, Armstrong VW, et al. Pitfalls in monitoring tacrolimus (FK 506) Ther Drug Monit. 1997;19:628–31. doi: 10.1097/00007691-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Staatz CE, Taylor PJ, Tett SE. Comparison of an ELISA and an LC/MS/MS method for measuring tacrolimus concentrations and making dosage decisions in transplant recipients. Ther Drug Monit. 2002;24:607–15. doi: 10.1097/00007691-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Murthy JN, Davis DL, Yatscoff RW, Soldin SJ. Tacrolimus metabolite cross-reactivity in different tacrolimus assays. Clin Biochem. 1998;31:613–7. doi: 10.1016/s0009-9120(98)00086-1. [DOI] [PubMed] [Google Scholar]
- 11.Ghoshal AK, Soldin SJ. IMx tacrolimus II assay: is it reliable at low blood concentrations? A comparison with tandem. MS/MS Clin Biochem. 2002;35:389–92. doi: 10.1016/s0009-9120(02)00338-7. [DOI] [PubMed] [Google Scholar]