Abstract
Objectives
To determine age and sex-specific pediatric reference intervals for serum alpha fetoprotein, homocysteine, insulin, insulin-like growth factor-I, insulin-like growth factor binding protein-3, C-peptide, immunoglobulin E and parathyroid hormone.
Design and methods
The study was conducted at both Children’s National Medical Center and Georgetown University, Washington D.C. Results for the above analytes were obtained from the Children’s National Medical Center laboratory information system over the period of 1/5/2001–3/8/2007.Patient results using the IMMULITE 2000® were accessed and used to establish reference intervals for the analytes studied. All patient identifiers were removed except age and sex. Analysis of the data was performed at Georgetown University in the Bioanalytical Core Laboratory. The data was analyzed using the Hoffmann approach, and was computer adapted. The number of patient samples studied varied with each analyte and were: Alpha fetoprotein (557), homocysteine (924), insulin-like growth factor-1 (1352), insulin-like growth factor binding protein-3 (711), insulin (3239), C-peptide (267), immunoglobulin E (2691) and parathyroid hormone (513).
Results and conclusions
This study provides pediatric reference intervals for the eight analytes for children from birth to 18 years of age. All the analytes exhibited at least some age dependence. Sex differences between early and late childhood and adolescence were also frequently found.
Keywords: Pediatric, Reference intervals, Alpha fetoprotein, Homocysteine, Insulin, Insulin-like growth factor-I, Insulin-like growth factor binding protein-3, C-peptide, Immunoglobulin E, Parathyroid hormone
Introduction
Pediatric reference intervals for many analytes can vary significantly by both sex and age from those found in adults, and are of great importance in differentiating between the “healthy” and the “diseased” populations [1–7]. Reference intervals for alpha fetoprotein (AFP), homocysteine (HCS), insulin, insulin-like growth factor-1(IGF-1), insulin-like growth factor binding protein-3 (IGFBP-3), C-peptide, immunoglobulin E (IgE) and intact parathyroid hormone (i-PTH) are currently unavailable on the IMMULITE 2000® analyzer (Siemens, Los Angeles, CA). The objective of this study was to determine sex and age-specific pediatric reference intervals for these analytes on the IMMULITE 2000®.
AFP is produced by the liver, and is used in the diagnosis of certain tumors and in children [8]. Circulating HCS concentrations correlate with early atherosclerosis, as early as the second decade of life and are associated with cardiovascular disease [9]. In both adults and children, elevated concentrations of HCS indicate a high level of heart disease risk and suggest decreased folate or cobalamin function in tissues [10].
Insulin is secreted by the pancreas and involved in the regulation of blood sugar [11]. Hypoglycemia and insulin resistance can be detected and managed by testing insulin concentrations [12]. IGF-1, also known as Somatomedin C, is structurally similar to insulin and its production is stimulated by growth hormone (GH). Therefore, the concentrations of serum IGF-1 correlate strongly with GH release. The concentrations of GH fluctuate significantly during the day. Low concentrations of IGF-1 imply GH deficiency, and high concentrations suggest GH excess (acromegaly). Primary IGF-1 deficiency is also associated with short stature [13].
IGFBP-3 is the major binding protein for IGF-1 and IGF-2; it modulates IGF activity, and inhibits cell growth. Endothelial IGFBP-3 concentrations increase in the presence of IGF-1, as well as insulin and other growth-stimulating factors such as GH and epidermal growth factor (EGF). Low IGFBP3 concentrations indicate GH deficiency, while high concentrations reflect GH excess. IGFBP-3 concentrations have been shown to have good predictive value for complete, but not partial, growth hormone deficiency (GHD) [14].
C-peptide, is formed when proinsulin is split into insulin and C-peptide on its release from the pancreas into the circulation in response to a rise in serum glucose. C-peptide and insulin are secreted in equimolar amounts from pancreatic beta-cells; hence, circulating C-peptide concentrations provide a measure of beta-cell secretory activity and are a marker of endogenous insulin production [15].
IgE is an antibody that plays an important role in allergy and is especially associated with type-1 hypersensitivity [16] and immune defense against certain protozoan parasites [17]. i-PTH controls calcium and phosphorus levels in the blood. Measuring PTH concentrations are useful in identifying hyperparathyroidism or to find the cause of abnormal calcium concentrations [18].
The percentile approach to the determination of reference intervals is easily applied to adult populations. However, it is almost impossible to obtain ethical and parent permission to perform reference interval studies in healthy children and especially those <5 years of age. We used the IMMULITE® 2000 system which performs chemiluminescent immunometric methods to measure these analytes over several years in a population that was approximately 50% “healthy” outpatient and 50% inpatient. Furthermore, the population serviced by Children’ National Medical Center is approximately 60% African/American. We then employed the Hoffmann approach [19] to determine the normal pediatric (0–18 years) reference intervals for females and males. In this approach outliers and the “sick” population are removed statistically by plotting the analyte value (or log of analyte value) vs. % cumulative frequency. A straight line drawn to the central 50th percentile leaves out the “sick” population at both ends of the graph, the latter deviating from linearity found for the mid 50th percentile. A straight line (over the 25–75th percentile) is only obtained with a Gaussian distribution. If this distribution is not Gaussian, it is made Gaussian by plotting the log of the value. We have employed this approach extensively over the past 25 years and found it to provide reference intervals that compare very closely to those obtained on relatively small numbers of healthy children using the percentile approach [20]. Reference intervals obtained in this manner have been used extensively and for 2 decades at Children’s National Medical Center and have been well accepted by the clinical staff. In addition, our reference interval studies have been published widely in the peer reviewed literature and in the textbook “Pediatric Reference Intervals” now in its 6th edition [21].
Materials and methods
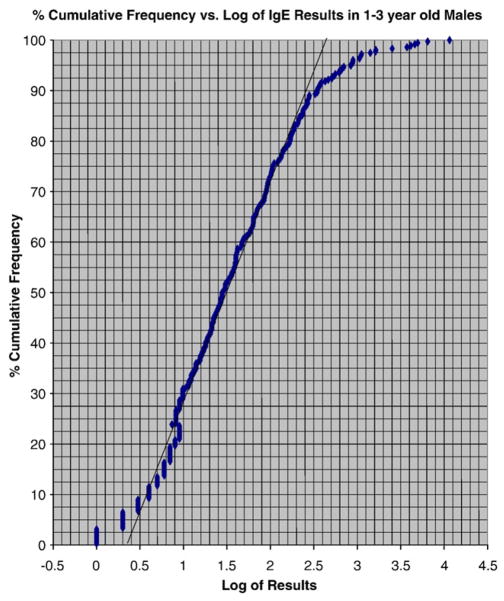
The IMMULITE® 2000 is a continuous random access analyzer with a processing throughput of 200 tests per hour. This study was conducted at Georgetown University Medical Center (GUMC) and Children’s National Medical Center (CNMC). Results on serum or plasma samples from in- and outpatients (approximately 50/50) and with all identifiers removed, except for age and sex, were obtained from the laboratory information system at Children’s over the time frame of January 2001–March 2007 and used to determine the reference intervals for children age 0–18 y. IRB approval to conduct the study was obtained. Prior to performing the assay, the samples were kept refrigerated for no longer than 24 h at 2–4 °C. For quality control, two samples of known analyte concentrations were tested with each batch of analyses. The data base for C-peptide was obtained by running left-over samples. Physician ordered tests were run for all the other analytes. As the Hoffmann approach automatically excludes the sick population there is no need to exclude specific patient populations provided they do not account for more than 50% of the population studied. Our analysis showed that that the “high sick” and “low sick” population for any analyte was never >20% of the population studied thereby validating this approach. Fig. 1 shows results for IgE. As can be seen the Hoffmann approach provides reliable reference intervals under these circumstances.
Fig. 1.
Plot of % cumulative frequency vs log IgE in 1–<3 year old males.
IMMULITE® 2000 assays
AFP and IgE were measured on serum while HCS, insulin, IGF-1, IGFBP-3, C-peptide and parathyroid hormone were all measured on either serum or plasma samples. Assays were performed according to the manufacturer’s instructions. Table 1 documents calibration ranges used, assay sensitivity and in-house between-day precision afforded for each analyte during this study.
Table 1.
Testing in children
| Assay | Calibration range | Sensitivity | Between-day precision %CV | Clinical applications* |
|---|---|---|---|---|
| AFP | 0.2–300 ng/mL | 0.24 ng/mL | 4.5–7.5 | Diagnose neural tube defects and development abnormalities |
| HCS | 2–50 μmol/L | 0.5 μmol/L | 2.9–8.6 | Diagnose: pediatric atherosclerosis, cardiovascular disease, schizophrenia |
| Insulin | 2–300 μIU/mL | 2 μIU/mL | 3.6–7.6 | Management of insulin-dependent diabetes |
| IGF-1 | 25–1600 ng/mL | 20 ng/mL | 2.9–4.9 | Diagnose growth disorder (GH deficiency or excess); assess nutritional status |
| IGFBP-3 | 0.5–16 μg/mL | 0.1 μg/mL | 3.4–7.7 | Differential diagnosis of growth disorders |
| C-peptide | 0.5–7 ng/mL | 0.3 ng/mL | 2.8–9.1 | A marker for insulin production |
| IgE | 1–2000 IU/mL | 1 IU/mL | 5.1–9.6 | Indicates allergic reaction |
| i-PTH | 1–2500 pg/mL | 1 pg/mL | 6.1–8.5 | Help identify hyperparathyroidism or to find the cause of abnormal calcium levels |
Tietz, Textbook of Clinical Chemistry and Molecular Diagnostics, 4th edition 2006, eds. Burtis, Ashwood, Bruns. Publishers: Elsevier Saunders.
Statistical analysis
After separating the data obtained into male and female, appropriate age ranges were determined so that the numbers, although not always optimal, would have statistical significance and maintain fairly narrow age ranges. Using computers, the Hoffmann approach was used to analyze the data by plotting either the value or the logarithm of the value versus the percent cumulative frequency. Outliers were automatically eliminated as a straight line was drawn to the central 50% (25–75th percentile) of the data. The linear portion of this line was extended and the 2.5th and 97.5th percentiles were calculating using this line. These were used as the reported reference intervals.
Results and discussion
Over the course of this study the CVs for all the analytes in question ranged between 2.8 and 9.6%. Switching from one calibrator lot to another did not significantly affect the results of the quality controls performed daily. The reference intervals for AFP were obtained from 557 children. AFP concentrations ranged between 40 and 19,953 ng/mL for newborn, 3.6 and 13,183 ng/mL for infants three and six months of age and declined rapidly thereafter (Table 2). For infants 3 years and older AFP concentrations ranged between 0.6 ng/mL and 2.0 ng/mL which are similar to values found in adults. These values confirm AFP reference intervals previously published on the ABBOTT platform [22].
Table 2.
Alpha fetoprotein reference intervals
| n | ng/mL
|
ng/mL
|
|
|---|---|---|---|
| 2.5th | 97.5th | ||
| Females | |||
| 0–<3 mo* | 52 | 40 | 19953 |
| 3–<6 mo* | 35 | 3.6 | 13183 |
| 6 mo–<1 y* | 53 | 3.6 | 94 |
| 1–<3 y | 52 | 1.6 | 37 |
| 3–<18 y* | 320 | 0.6 | 2.0 |
| Males | |||
| 0–<3 mo* | 52 | 40 | 19953 |
| 3–<6 mo* | 35 | 3.6 | 13183 |
| 6 mo–<1 y* | 53 | 3.6 | 94 |
| 1–<3 y | 45 | 0.9 | 16 |
| 3–<18 y* | 320 | 0.6 | 2.0 |
n=557;
males and females combined.
Homocysteine reference intervals were calculated for 924 children. Reference intervals are somewhat higher for females 1 month–1 year with 97.5th percentiles greater than those found in age matched males. The reverse is true for the 97.5th percentiles between 15–18 years (see Table 3) with concentrations in males being > than in females.
Table 3.
Homocysteine reference intervals
| n | μmol/L
|
μmol/L
|
|
|---|---|---|---|
| 2.5th | 97.5th | ||
| Females | |||
| 0–<1 mo* | 66 | 3.0 | 8.5 |
| 1–<6 mo | 59 | 4.4 | 15.1 |
| 6 mo–<1 y | 61 | 3.0 | 13.2 |
| 1–<3 y | 68 | 3.6 | 10.7 |
| 3–<10 y | 71 | 3.0 | 9.8 |
| 10–<13 y | 37 | 3.6 | 8.9 |
| 13–<15 y* | 76 | 4.7 | 10.6 |
| 15–<18 y | 91 | 4.7 | 13.2 |
| Males | |||
| 0–<1 mo* | 66 | 3.0 | 8.5 |
| 1 mo–<1 y | 88 | 3.5 | 8.5 |
| 1–<3 y | 49 | 2.6 | 11.0 |
| 3–<10 y | 103 | 3.6 | 11.0 |
| 10–<13 y | 54 | 4.3 | 9.3 |
| 13–<15 y* | 76 | 4.7 | 10.6 |
| 15–18 y | 60 | 4.7 | 14.5 |
| >18 y | 41 | 5.1 | 19.0 |
n=924
males and females combined.
For children <18 years of age the reference intervals for IGF-1 are somewhat higher for females (n=585) than for males (n=767) (Table 4). For example, the reference interval for females 13 to 15 years old is 148 ng/mL to 912 ng/mL, while for males of the same age the range is 132 ng/mL to 692 ng/mL. The interval increases in value with age for both males and females.
Table 4.
IGF-1 reference intervals
| n | ng/mL
|
ng/mL
|
|
|---|---|---|---|
| 2.5th | 97.5th | ||
| Females | |||
| 3 mo–<1 yr | 30 | 30 | 98 |
| 1–<3 yr | 42 | 44 | 174 |
| 3–<10 yr | 200 | 65 | 457 |
| 10–<13 yr | 127 | 100 | 692 |
| 13–<15 yr | 87 | 148 | 912 |
| 15–18 yr | 58 | 162 | 708 |
| >18 yr | 41 | 69 | 490 |
| Males | |||
| 3 mo–<1 yr | 30 | 30 | 98 |
| 1–<3 yr | 45 | 32 | 91 |
| 3–<10 yr | 227 | 45 | 295 |
| 10–<13 yr | 143 | 100 | 437 |
| 13–<15 yr | 136 | 132 | 692 |
| 15–18 yr | 126 | 129 | 741 |
| >18 yr | 60 | 87 | 562 |
n=1352.
IGFBP3 (Table 5) (n=711) reference intervals increase similarly with age in females and males ranging between 1.3 and 3.5 μg/mL in the first three years of life increasing to 3.0 to 6.0 μg/mL for age 10–<13 y in females.
Table 5.
IGFBP-3 reference intervals
| n | μg/mL
|
μg/mL
|
|
|---|---|---|---|
| 2.5th | 97.5th | ||
| Females | |||
| 1–<3 y | 60 | 1.3 | 3.5 |
| 3–<10 y | 102 | 2.9 | 5.3 |
| 10–<13 y | 49 | 3.2 | 6.0 |
| 13–15 y | 35 | 3.3 | 6.3 |
| >15 y | 46 | 3.3 | 6.6 |
| Males | |||
| 1–<3 y | 60 | 1.3 | 3.5 |
| 3–<10 y | 117 | 2.0 | 5.0 |
| 10–<13 y | 68 | 3.0 | 5.8 |
| 13–<15 y | 80 | 3.6 | 6.0 |
| 15–18 y | 52 | 3.5 | 6.8 |
| >18 y | 42 | 3.3 | 6.6 |
n=711.
Insulin (n=3239) reference intervals for infants until one year of age ranged between 2.3 and 27.5 μIU/mL, and 1.6 and 27.5 μIU/mL in children 1–3 years of age (Table 6). In 3–10 year olds these intervals increase to 4.0–40.7 μIU/mL for females, and 2.9–30.9 μIU/mL for males. Insulin reference intervals increase further with age to 7.9–56.2 μIU/mL in females older than 10 years old, which are similar to those found in age matched males.
Table 6.
Insulin reference intervals
| n | μIU/mL
|
μIU/mL
|
|
|---|---|---|---|
| 2.5th | 97.5th | ||
| Females | |||
| 0–<1 y* | 35 | 2.3 | 27.5 |
| 1–<3 y* | 34 | 1.6 | 27.5 |
| 3–<10 y | 323 | 4.0 | 40.7 |
| 10–<13 y | 465 | 7.9 | 56.2 |
| 13–<15 y | 402 | 7.9 | 56.2 |
| 15–18 y | 527 | 7.4 | 51.3 |
| Males | |||
| 0–<1 y* | 35 | 2.3 | 27.5 |
| 1–<3 y* | 34 | 1.6 | 27.5 |
| 3–<10 y | 297 | 2.9 | 30.9 |
| 10–<13 y | 465 | 6.3 | 50.1 |
| 13–<15 y | 288 | 7.9 | 50.1 |
| 15–18 y | 334 | 7.1 | 66.1 |
n=3239;
males and females combined.
Results in females and males were combined for C-peptide reference intervals (n=267; Table 7). For children under the age of 13 y the interval ranged between 0.6 and 7.8 ng/mL, while for females and males older than 13 y the range was 1.3 to 7.9 ng/mL.
Table 7.
C-peptide reference intervals
| Females and males | n | ng/mL
|
ng/mL
|
|---|---|---|---|
| 2.5th | 97.5th | ||
| 0–<1 y* | 73 | 0.6 | 7.8 |
| 1–<5 y* | 43 | 0.6 | 7.4 |
| 5–<13 y* | 75 | 0.8 | 8.5 |
| 13–<18 y* | 76 | 1.3 | 7.9 |
n=267;
males and females combined.
The reference intervals for IgE (n=2691) (Table 8) were different for females and males. This was especially true for the upper limit. For example, the reference intervals for females age 3 to 10 y were 3 to 398 IU/mL while for males of the same age it was 4 to 999 IU/mL. Fig. 1 illustrates how the Hoffmann approach eliminates both outliers and the “sick” populations, which deviate from linearity and are removed from the calculation. Parathyroid hormone (n=573; Table 9) reference intervals did not show sex differences. Ranges under 1 year were 10–112 pg/mL, while those over 15 years were 13–100 pg/mL.
Table 8.
IgE reference intervals
| n | IU/mL
|
IU/mL
|
|
|---|---|---|---|
| 2.5th | 97.5th | ||
| Females | |||
| 0–<4 mo | 31 | 1 | 24 |
| 4 mo–<1 y | 98 | 2 | 63 |
| 1–<3 y | 250 | 3 | 141 |
| 3–<10 y | 396 | 3 | 398 |
| 10–<13 y | 152 | 9 | 407 |
| 13–<15 y | 112 | 23 | 355 |
| 15–18 y | 181 | 5 | 355 |
| Males | |||
| 0–<4 mo | 63 | 1 | 40 |
| 4 mo–<1 y | 125 | 1 | 126 |
| 1–<3 y | 356 | 3 | 398 |
| 3–<10 y | 556 | 4 | 999 |
| 10–<13 y | 169 | 8 | 631 |
| 13–<15 y | 94 | 10 | 562 |
| 15–18 y | 108 | 11 | 447 |
n=2691.
Table 9.
Intact parathyroid hormone reference intervals
| Females and males | n | pg/mL
|
pg/mL
|
|---|---|---|---|
| 2.5th | 97.5th | ||
| 0–<1 y* | 67 | 10 | 112 |
| 1–<3 y* | 62 | 10 | 71 |
| 3–<10 y* | 150 | 13 | 120 |
| 10–<13 y* | 82 | 13 | 85 |
| 13–<15 y* | 76 | 10 | 117 |
| 15–18 y* | 136 | 13 | 100 |
n=573;
males and females combined.
The Hoffmann approach permits use of in- and outpatient samples and is valid provided more than 50% of the population is “healthy” for the analyte under study. As less than 50% of the population have diabetes, allergies, hepatic malignancies etc. this approach works well for the analytes studied in this paper.
Conclusion
We used the IMMULITE 2000® system to measure 8 analytes and determined the normal pediatric (0–18 years) reference intervals for females and males. We studied AFP, HCS, insulin, IGF-1, IGFBP3, IgE, intact-PTH and C-protein concentrations in a pediatric population of 267–3239 children. Where possible the central 95% reference intervals were determined separately for females and males for different age groups to assess age- and sex-related differences. The Hoffmann approach has been used widely to evaluate reference intervals in the sick/hospitalized population. Since we employed the Hoffmann approach all the analyte values from both in- and outpatient samples were used. The central 50% of the values are taken and the value or log of the value plotted (depending on which gave a straight line) against the percent cumulative frequency. The straight line is extended to give the 2.5th and 97.5th percentiles (see Fig. 1).
The identification of pediatric AFP, HCS blood concentrations in the higher range may help to identify those children with a higher disease risk, so that preventive strategies can be started as soon as possible. An abnormal result obtained for any of the analytes studied would lead to physician concern and intervention. As with other reference intervals published previously by our group [1,4,6,7,22–24] these new ranges have been implemented at Children’s National Medical Center since August 07 and have been well accepted by the medical staff. They are applied to results obtained for both in- and outpatients. While the IFCC has published recommendations on the estimation of reference intervals [25], obtaining samples from a normal population of children is very difficult particularly in the 0–5 year range. Over the last two and a half decades we have used the Hoffmann approach widely and found that the reference intervals when applied to the population of in- and outpatients at Children’s National Medical Center were useful and appropriate and well accepted by the clinical staff. Furthermore, for some analytes we were able to compare our Hoffmann derived ranges with those obtained on small cohorts of normal children [20] and found agreement to be excellent.
Acknowledgments
The authors have not received research grants or consulting and speaking honoraria from any diagnostic companies, and do not own stock in these companies and the authors’ time on this project was supported by their respective employers. Dr. O.P. Soldin is partially supported by 5U10HD047890-03 NIH/NICHD Obstetrics Pharmacology Research Unit (OPRU) and by the Office of Research on Women’s Health. Dr SJ Soldin was partially supported by the NIH GCRC grant M01RR-023942 and by grant 1 U10HD45993-02 of the National Institute of Child Health and Development, Bethesda, MD. J. Dahlin, E. Gresham and J. King are Colaco Summer Students with Drs. Soldin and contributed to this manuscript.
References
- 1.Soldin OP, Bierbower LH, Choi JJ, Thompson-Hoffman S, Soldin SJ. Serum iron, ferritin, transferrin, total iron binding capacity, HS-CRP, LDL cholesterol and magnesium in children; new reference intervals using the Dade Dimension Clinical Chemistry System. Clin Chim Acta. 2004;342:211–7. doi: 10.1016/j.cccn.2004.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soldin OP, Hilakivi-Clarke, Weiderpass E, Soldin SJ. Trimester-specific reference intervals for thyroxine and triiodothyronine in pregnancy in iodine-sufficient women using isotope dilution tandem mass spectrometry and immunoassays. Clin Chim Acta. 2004;349:181–9. doi: 10.1016/j.cccn.2004.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soldin OP, Tractenberg RE, Hollowell JG, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid. 2004;14:1084–90. doi: 10.1089/thy.2004.14.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soldin OP, Hoffman EG, Waring MA, Soldin SJ. Pediatric reference intervals for FSH,LH, estradiol,T3,free T3, cortisol, and growth hormone on the DPC IMMULITE 1000. Clin Chim Acta. 2005;355:205–10. doi: 10.1016/j.cccn.2005.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soldin OP, Guo T, Weiderpass E, Tractenberg RE, Hilakivi-Clarke L, Soldin SJ. Steroid hormone levels in pregnancy and 1year postpartum using isotope dilution tandem mass spectrometry. Fertil Steril. 2005;84:701–10. doi: 10.1016/j.fertnstert.2005.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soldin SJ, Soldin OP, Boyajian AJ, Taskier MS. Pediatric brain natriuretic peptide and N-terminal pro-brain natriuretic peptide reference intervals. Clin Chim Acta. 2006;366:304–8. doi: 10.1016/j.cca.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kratovil T, Deberardinis J, Gallagher N, Luban NL, Soldin SJ, Wong EC. Age specific reference intervals for soluble transferrin receptor (sTfR) Clin Chim Acta. 2007;380:222–4. doi: 10.1016/j.cca.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Bader D, Riskin A, Vafsi O, et al. Alpha-fetoprotein in the early neonatal period—a large study and review of the literature. Clin Chim Acta. 2004;349:15–23. doi: 10.1016/j.cccn.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 9.Temple ME, Luzier AB, Kazierad DJ. Homocysteine as a risk factor for atherosclerosis. Ann Pharmacother. 2000;34:57–65. doi: 10.1345/aph.18457. [DOI] [PubMed] [Google Scholar]
- 10.Ueland PM, Bjørke Monsen AL. Total homocysteine is making its way into pediatric laboratory diagnostics. Eur J Clin Invest. 2001;31:928–30. doi: 10.1046/j.1365-2362.2001.00918.x. [DOI] [PubMed] [Google Scholar]
- 11.Sinaiko AR, Steinberger J, Moran A, Prineas RJ, Jacobs DR. Relation of insulin resistance to blood pressure in childhood. J Hypertens. 2002;20:509–17. doi: 10.1097/00004872-200203000-00027. [DOI] [PubMed] [Google Scholar]
- 12.Moran A, Jacobs DR, Steinberger J, et al. Insulin resistance during puberty: results from clamp studies in 357 children. Diabetes. 1999;48:2039–44. doi: 10.2337/diabetes.48.10.2039. [DOI] [PubMed] [Google Scholar]
- 13.Park P, Cohen P. Insulin-like growth factor I (IGF-I) measurements in growth hormone (GH) therapy of idiopathic short stature (ISS) Growth Horm IGF Res. 2005 Jul;15(Suppl A):S13–20. doi: 10.1016/j.ghir.2005.06.011. Review. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa Y, Hasegawa T, Aso T, et al. Clinical utility of insulin-like growth factor binding protein-3 in the evaluation and treatment of short children with suspected growth hormone deficiency. Eur J Endocrinol. 1994;131:27–32. doi: 10.1530/eje.0.1310027. [DOI] [PubMed] [Google Scholar]
- 15.Bonser AM, Garcia-Webb P. C-peptide measurement: methods and clinical utility. Crit Rev Clin Lab Sci. 1984;19:297–352. doi: 10.3109/10408368409165766. [DOI] [PubMed] [Google Scholar]
- 16.Gould HJ, Sutton BJ, Beavil AJ, et al. The biology of IGE and the basis of allergic disease. Annu Rev Immunol. 2003;21:579–628. doi: 10.1146/annurev.immunol.21.120601.141103. [DOI] [PubMed] [Google Scholar]
- 17.Duarte J, Deshpande P, Guiyedi V, et al. Total and functional parasite specific IgE responses in Plasmodium falciparum-infected patients exhibiting different clinical status. Malar J. 2007;6:1. doi: 10.1186/1475-2875-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hage DS, Taylor B, Kao PC. Intact parathyroid hormone: performance and clinical utility of an automated assay based on high-performance immunoaffinity chromatography and chemiluminescence detection. Clin Chem. 1992;38:1494–500. [PubMed] [Google Scholar]
- 19.Hoffmann RG. Statistics in the practice of medicine. JAMA. 1963;185:864–73. doi: 10.1001/jama.1963.03060110068020. [DOI] [PubMed] [Google Scholar]
- 20.Lockitch G, Halstead AC, Albersheim S, MacCallum C, Quigley G. Age-and sex-specific pediatric reference intervals for biochemistry analytes as measured with the Ektachem-700 analyzer. Clin Chem. 1988;34:1622–5. [PubMed] [Google Scholar]
- 21.Soldin SJ, Brugnara C, Wong EC, editors. Pediatric reference intervals. 6. Washington DC: AACC Press; 2007. [Google Scholar]
- 22.Soldin SJ, Hicks JM, Godwin ID, et al. Pediatric reference ranges for alpha-fetoprotein. Clin Chem. 1992;38:959. (abstract) [Google Scholar]
- 23.Soldin OP, Miller M, Soldin SJ. Pediatric reference ranges for zinc protoporphyrin. Clin Biochem. 2003;36(1):21–5. doi: 10.1016/s0009-9120(02)00405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soldin SJ, Murthy JN, Agarwalla PK, Ojeifo O, Chea J. Pediatric reference ranges for creatine kinase, CKMB, Troponin I, iron, and cortisol. Clin Biochem. 1999;32(1):77–80. doi: 10.1016/s0009-9120(98)00084-8. [DOI] [PubMed] [Google Scholar]
- 25.Solberg HE. The IFCC recommendation on estimation of reference intervals. The RefVal Program. Clin Chem Lab Med. 2004;42:710–4. doi: 10.1515/CCLM.2004.121. [DOI] [PubMed] [Google Scholar]