Abstract
Background
The Selenium and Vitamin E Cancer Prevention Trial (SELECT) was a randomized, double blind, placebo-controlled prostate cancer prevention study funded by the National Cancer Institute and conducted by SWOG (Southwest Oncology Group). A total of 35,533 men were assigned randomly to one of four treatment groups (vitamin E + placebo, selenium + placebo, vitamin E + selenium, placebo + placebo. The independent Data and Safety Monitoring Committee recommended the discontinuation of study supplements because of the lack of efficacy for risk reduction and because futility analyses demonstrated no possibility of benefit of the supplements to the anticipated degree (25% reduction in prostate cancer incidence) with additional follow-up. Study leadership agreed that the randomized trial should be terminated but believed that the cohort should be maintained and followed as the additional follow-up would contribute important information to the understanding of the biologic consequences of the intervention. Since the participants no longer needed to be seen in person to assess acute toxicities or to be given study supplements, it was determined that the most efficient and cost-effective way to follow them was via a central coordinated effort.
Purpose
A number of changes were necessary at the local Study Sites and SELECT Statistical Center to transition to following participants via a Central Coordinating Center. We describe the transition process from a randomized clinical trial to the observational Centralized Follow-up (CFU) study.
Methods
The process of transitioning SELECT, implemented at more than 400 Study Sites across the United States, Canada and Puerto Rico, entailed many critical decisions and actions including updates to online documents such as the SELECT Workbench and Study Manual, a protocol amendment, reorganization of the Statistical Center, creation of a Transition Committee, development of materials for SELECT Study Sites, development of procedures to close Study Sites, and revision of data collection procedures and the process by which to contact participants.
Results
At the time of the publication of the primary SELECT results in December 2008, there were 32,569 men alive and currently active in the trial. As of December 31, 2011, 17,761 participants had been registered to the CFU study. This number is less than had been anticipated due to unforeseen difficulties with local Study Site IRBs. However, from this cohort we estimate that an additional 580 prostate cancer cases and 215 Gleason 7 or higher cancers will be identified. Over 109,000 individual items have been mailed to participants. Active SELECT ancillary studies have continued. The substantial SELECT biorepository is available to researchers; requests to use the specimens are reviewed for feasibility and scientific merit. As of April 2012, 12 proposals had been approved.
Limitations
The accrual goal of the follow-up study was not met, limiting our power to address the study objectives satisfactorily. The CFU study is also dependent on a number of factors including continued funding, continued interest of investigators in the biorepository and the continued contribution of the participants. Our experience may be less pertinent to investigators who wish to follow participants in a treatment trial or participants in prevention trials in other medical areas.
Conclusions
Extended follow-up of participants in prevention research is important to study the long-term effects of the interventions, such as those used in SELECT. The approach taken by SELECT investigators was to continue to follow participants centrally via an annual questionnaire and with a web-based option. The participants enrolled in the CFU study represent a large, well-characterized, generally healthy cohort. The CFU has enabled us to collect additional prostate and other cancer endpoints and longer follow-up on the almost 18,000 participants enrolled. The utility of the extensive biorepository that was developed during the course of the SELECT is enhanced by longer follow-up.
Introduction
The Selenium and Vitamin E Cancer Prevention Trial (SELECT) was a randomized, double blind, placebo-controlled prostate cancer prevention study funded by the National Cancer Institute (NCI) and conducted by the Southwest Oncology Group (SWOG) [1]. A total of 35,533 men were assigned randomly to one of four treatment groups, i.e., vitamin E + placebo, selenium + placebo, vitamin E + selenium, placebo + placebo, between August 2001 and June 2004. On September 15, 2008, the independent Data and Safety Monitoring Committee (DSMC) recommended discontinuation of study supplements because of lack of efficacy for risk reduction and because futility analyses demonstrated no possibility of benefit from the supplements to the anticipated degree, i.e., 25% reduction in prostate cancer incidence, with continued supplementation and additional follow-up. SELECT participants were notified of the decision to end study supplementation by an IRB-approved letter prepared by study leadership, including the independent National Participant Advisory Board. Study leadership agreed that the randomized trial should be terminated but decided that the cohort should be maintained to better understand the biologic consequences of the interventions. Since the participants no longer needed to be seen in person to assess acute toxicities or to be given study supplements, it was determined that the most efficient and cost-effective way to follow them was via a central coordinated effort.
Investigators in the Breast Cancer Prevention Trials (BCPT P-1 and P-2 [STAR])[2], conducted by the National Surgical Adjuvant Breast and Bowel Project (NSABP), continued to follow participants after the initial publication of trial results. The continued follow-up provided additional outcomes and long-term safety data which allowed the investigators to evaluate the effect of the agents over prolonged periods of time, before the agent was stopped. Tamoxifen was found to have a beneficial effect even after the participants stopped taking the study drug, a finding that would not have been known had the women not been followed long-term. For BCPT P-1, a protocol amendment and additional participant consent were needed to follow participants longer because the original consent only allowed for seven years of participant follow-up. For BCPT P-2, additional consent was not necessary because the original consent allowed for indefinite follow-up. Participants continued to be followed every 6 months; they were encouraged to continue visits to study sites rather than to be followed by telephone or mail because (1) they were women at high risk of developing breast cancer and, (2) for those who were receiving tamoxifen, evaluation of potential side effects was desired. Follow-up was terminated after 8 additional years in BCPT P1 and after 6 additional years in BCPT P2 because the long-term study objectives had been met.
After the early closure of another SWOG study, the Prostate Cancer Prevention Trial (PCPT) in 2003 because the primary objective had been reached the conclusions were extremely unlikely to change with additional diagnoses of prostate cancers, it took two years for a decision to be made about how to follow the men and to activate a follow-up protocol. Ultimately, the investigators determined that there was sufficient scientific rationale to follow only the men who had developed prostate cancer while participating in the trial. The objective of the follow-up study was to measure the difference in time to metastases between men on the active intervention arm (finasteride) and men on placebo [3]. However, because of the delay, many sites already either had closed or had stopped following participants. As a result, the target follow-up study accrual goal was not met; the follow-up study was closed in 2009 at the recommendation of the DSMC due to lack of feasibility. The PCPT experience showed that early planning is critical and that, there is little hope of obtaining the consent of participants to additional follow-up once participation of study sites has ended.
The intervention in the Carotene and Retinol Efficiency Trial (CARET) [4] was stopped in 1996 and a final in-person contact was attempted by staff at the participant’s local study center. During the next two years (1997 – 1998), telephone contacts were made by the study centers. During the subsequent two years (1999 – 2000) there was a transition to central follow-up by the CARET Coordinating Center via an annual mailed questionnaire. Prior to the last routine telephone calls from the local study center, participants were mailed a letter that explained the change. During the call participants were asked whether they had received the letter and whether they had any questions. Passive consent was assumed as long as the participant acknowledged receiving the letter and did not explicitly decline further contact. In 2005, participant follow-up ended due to lack of funding.
The Women’s Health Initiative (WHI) [5] consisted of three randomized clinical trials and an observational cohort study. During the active follow-up period, the study centers conducted semiannual clinic visits with the participants in the clinical trials and one follow-up visit with observational study participants. After closure of the randomized clinical trials, the women were offered the opportunity to continue follow-up contacts through annual mailings from the WHI Coordinating Center. Women had to sign a new consent form that was administered by the local study center staff. Upon notification of an endpoint, the study center requested a signed medical release, collected the necessary medical records, and forwarded the medical record packet to the WHI Coordinating Center for adjudication.
These experiences with transition of other cancer prevention randomized trials to observational follow-up studies informed the process we developed for transitioning the SELECT randomized clinical trial into the observational cohort study, the SELECT Centralized Follow-up (CFU) study that we describe
Background
SELECT was a highly successful cancer prevention clinical trial. Accrual to the trial was rapid with 35,533 men randomized in 34 months, 2 years less than initially planned; more than 400 Study Sites in the United States, including Puerto Rico, and Canada participated. Overall, men were adherent to the study supplements; more than 70% of them took at least 80% of the expected amount and regularly visited the Study Site. Study Site staff met SWOG performance criteria by submitting data in a timely manner. While the result of the trial was not what had been hoped for, i.e., a proven preventive agent, the trial successfully answered the primary null hypothesis that there is no role for commonly-used vitamin E and selenium supplements in prostate cancer prevention.
Following the release of the trial results, the study leadership considered whether or not to continue following the trial participants, and if so, how. Given the early stopping of the interventions in the trial, there were a number of complex issues to consider including what would be learned by following men from a null trial, staffing and budgeting of local Study Sites, the Statistical Center and other central facilities, and management of the large biorepository. Perhaps most importantly, there was the question of the role of a cooperative group with a primary focus on clinical trials in managing an observational cohort. It was decided that continuing the trial in its current local site-based format would be expensive and that a more efficient method would be to follow the men via central follow-up by a single coordinating center. Additional participant follow-up was expected to characterize the long-term effects of vitamin E and selenium usage and result in a biorepository enriched with additional cancer endpoints and longer participant follow-up that could be utilized to understand the biology of prostate cancer [6].
The initial goals of the CFU study were to monitor the incidence of new prostate cancer, lung cancer, colon cancer and other cancer endpoints during long-term follow-up of surviving SELECT participants and to obtain more precise estimates of incidence. When the intervention initially was stopped, the study sites were given time to close out the study participants; in the process, person-years of follow-up increased by 23%. While it was expected that the difference in the incidence rates between supplement and placebo groups would diminish, instead the difference had increased. An updated analysis [7] in May 2011, which included all data collected prior to the transition of any of the men to the CFU study, revealed that the men who had been taking vitamin E and the selenium placebo had a 17% increased risk of prostate cancer compared to the men who took two placebos (p=.008). Rates of prostate cancer also were higher among the men taking selenium and the vitamin E placebo and among men taking the combination of selenium and vitamin E but the differences between the rates were not statistically significant. The updated findings raised the question of a biologic effect that persisted beyond the intervention period. The goals of the CFU study were modified based on these new findings. With additional follow-up in the CFU study, it would be possible to ascertain and understand better the true long-term risk of the supplements and whether the biologic effects seen in 2011 persisted, increased, or decreased with time.
Methods
The process of transitioning SELECT, implemented at a large number of Study Sites across the United States, Canada and Puerto Rico, entailed many critical decisions and actions which we detailed below. A timeline for the transition is provided in Table 1.
Table 1.
Timeline of transition events: SELECT randomized trial to Central Follow-Up observational study
| Event | Date |
|---|---|
| SELECT accrual opens | August 2001 |
| SELECT accrual completed | June 2004 |
| DSMC recommends discontinuation of study supplements, SELECT leadership concurs | September 2008 |
| Release of primary SELECT results to participating Study Sites | October 2008 |
| Penultimate training Workshop for SELECT Study Site personnel | October 2008 |
| SELECT Workbench update, Study Manual revisions begin for CFU study | October 2008 |
| Primary SELECT results published online | December 2008 |
| SELECT Statistical Center reorganization into the SELECT CFU study Coordinating Center | July 2009 |
| Creation of the Transition Committee | July 2009 |
| Development of materials for Study Sites regarding the transition | July 2009 |
| Protocol amendment creating the CFU study | August 2009 |
| Final Training Workshop for SELECT Study Site personnel | October 2009 |
| Transition Visits begin | November 2009 |
| Data collection via booklets mailed to CFU study participants begins | December 2009 |
| Alternate plans for obtaining CFU study participant consent (Plans B and C) approved | December 2009 |
| SELECT Study Site closures begin | June 2010 |
| Last SELECT Study Site closed | April 2011 |
(a) SELECT Workbench updates and Study Manual revisions (began October 2008)
In SELECT, the trial protocol and other important documents were maintained online on a secure SELECT website, the SELECT Workbench. The transition necessitated the addition of a large amount of new material in an organized way to the SELECT Workbench. The added material included the protocol amendment for establishing the CFU study, revised sections of the Study Manual, a function to register SELECT participants to the CFU study, a new training video, participant materials in both English and Spanish, and downloadable Certificates of Appreciation for all SELECT participants to acknowledge their years of commitment to the trial. Many existing reports and functions had to be updated and new reports had to be created to support the observational study. All transition materials, beginning with announcement of the DSMC’s recommendation through the final participant visits and Study Site closures were made available on the SELECT Workbench.
The Study Manual served as the reference for all SELECT procedures including recruitment, the initial randomization of the participant within SELECT, Study Site visits, adherence and retention, documentation of endpoints, forms and data collection, biospecimen collection procedures and a wide variety of documents to support the trial. Revisions for the CFU study included major changes to the Participant Follow-up and Forms sections with the detailed procedures for implementing the changes outlined in the protocol and the associated new forms, and to the Administration section detailing the closure activities for the SELECT Study Sites. These activities included procedures for closure of the SELECT Study Site Pharmacy and disposition of SELECT-related materials including research records, retention promotion items (e.g., pill dispensers and lapel pins) on hand, SELECT Study Manuals, specimen kits, and regulatory binders.
(b) SELECT Statistical Center Reorganization (July 2009)
The SELECT Statistical Center, which supported the Study Sites throughout the trial, was renamed the SELECT Coordinating Center (SCC) to support follow-up of the participants. The SCC retained its statistical and data management responsibilities for SELECT while its role expanded to disseminate CFU study information to participants, answer participants’ questions, collect data, obtain medical releases and obtain Informed Consent. The earlier duties of many of the SCC staff were modified to accommodate the revised responsibilities. With the transition to the CFU study, the SCC had direct contact with participants, a role that in SELECT was assigned to local Study Sites. SCC staff given primary responsibility for participant contact received additional training; other staff were cross-trained to assure adequate coverage.
With its new role as the SELECT (CFU) Coordinating Center, the role of its IRB also changed. Previously, all materials that were presented to participants had to go through each Study Site’s IRB, even those prepared by the SELECT Statistical Center. With the transition to the CFU study, materials that the SCC disseminates to the CFU participants, including forms, newsletters and website materials are reviewed only by the SCC’s IRB.
(c) Creation and Role of the Transition Committee (July 2009)
A committee was formed to assist the SCC with strategies to inform and retain SELECT participants in the CFU study and to assist with the closeout of SELECT Study Sites. This Transition Committee was composed of Clinical Research Associate (CRA) members of the SELECT Site Coordinators Committee, SCC staff and a Study Site principal investigator who had had a leadership role on the Retention and Adherence Committee throughout the SELECT trial. The Transition Committee met regularly by teleconference and provided recommendations to the SCC regarding strategies and materials for the transition of the SELECT participants to the CFU study and procedures for closure of all SELECT Study Sites.
(d) Development of Materials for SELECT Study Sites (July 2009)
Additional materials to support the transition were developed for SELECT Study Site staff. These included a letter to each site’s IRB that explained the transition and a letter and answers to frequently asked questions (FAQs) for the Study Site personnel to SELECT trial participants to explain what participation in the CFU study would mean to them. A video was created as a training tool for Study Site staff to prepare them to conduct Transition Visits. Dramatization, voice-over narration and text were used throughout the video clips to communicate key messages, procedures and suggested participant communication techniques. Study Site staff were trained to deal effectively with a variety of situations that they might encounter during their interactions with SELECT trial participants as they presented information about the CFU study.
(e) Protocol amendment (August 2009)
Before any other activities could be finalized, the SELECT study protocol had to be amended to reflect a major change in study goals and activities. This amendment established the transition from the clinical trial to the observational cohort, the CFU study. Until the protocol amendment had been approved, SELECT participants continued in-person follow-up visits to their local study sites. The highlights of the transition amendment included:
Modification of the treatment plan and introduction of the Transition Visit
The Transition Visit was the final visit the participant completed at the local Study Site. Final activities included collection of routine clinical data, resolution of outstanding data queries and acknowledgment of the participant’s commitment to the trial with a SELECT lapel pin and a personalized certificate. Importantly, the Transition Visit was the primary avenue for staff familiar with the participant to present and explain the opportunity for the participant to continue his participation and commitment to research through participation in the SELECT CFU study.
Revised Informed Consent
A new consent for the local Study Site staff to present to the SELECT participant allowed the Study Site staff to (a) acknowledge that the participant’s consent was sought on behalf of the principal investigator of the SCC, (b) collect current participant contact information so that the SCC could communicate with the participant, and (c) determine whether the consenting participant was willing to be contacted in future about other research opportunities. The consent form and process were approved by the IRB of the SCC.
The initial transition plan was for the Study Sites to present the CFU study to the participants at the Transition Visit, the final visit for the SELECT trial. For those participants who agreed to be followed by the SCC, the local site would collect detailed participant contact information, obtain the participant’s signed consent and register the participant in the CFU study. This preferred method was known as Plan A. Some local IRBs did not approve Plan A, primarily because they would not allow their institution’s staff to elicit informed consent for a study that would not be conducted at their institution. As a result, two alternate plans (known as Plans B and C) were developed.
In Plan B, the site was responsible for all activities as originally described, stopping short of requesting the participant to consent to the CFU study. Instead, the site obtained a Release of Information (ROI) signed by the SELECT participant that authorized the site staff to provide the SCC with the participant’s contact information. The SCC then sent these participants a packet of information that included a description of the CFU study, the consent form and encouragement to contact the SCC with questions via a toll-free telephone number. Upon receipt of the signed consent form, the SCC registered the SELECT participant to the CFU study. While this plan was not ideal because the burden of returning the necessary paperwork to the SCC was on the participant, it was judged to be a reasonable alternative to Plan A.
In Plan C, the site provided the participant with contact information for the SCC and participants interested in learning more about the follow-up study contacted the SCC. The same packet of information, with the addition of a form to collect detailed current contact information, was sent to interested SELECT participants who contacted the SCC. The same registration procedure was used for Plan C as for Plan B. This plan was highly discouraged because it was believed to have the least chance of success for recruiting SELECT participants to the CFU study because the SELECT participant had to take the initiative to contact the SCC.
(f) Training Workshops (October 2008 and October 2009)
Throughout the course of the SELECT trial, semiannual training workshops with scientific and study management were held with Study Site personnel. The initial workshop in August 2000 focused on initiating the trial at more than 400 Study Sites. Subsequent workshops had different emphases which were chosen based on specific problems or external study events that could have affected participant adherence and retention, such as publications about either vitamin E or selenium. The penultimate workshop (October 2008) focused on the early cessation of study supplementation and study results. The final workshop for Study Site staff (October 2009) had a combined focus of training for the Transition Visit along with acknowledgment of the contributions of the site staff, committee members and our National Participant Advisory Board. This workshop included didactic presentations and a preview of the video for training staff on how to conduct the Transition Visit.
(g) Data collection (December 2009)
There were two different data collection issues to consider for the transition of SELECT participants to the CFU study. The first goal was to collect direct contact information for each CFU study participant. During SELECT, the Statistical Center did not have personal contact information for the participants; contact with the participant was only through the Study Site. A form to be administered by Study Site staff was developed to capture the participant’s primary and secondary addresses, phone numbers, e-mail addresses, etc., as well as alternate contacts in case the SCC was unable to locate the participant.
Secondly, we had to consider how to collect the follow-up data for the CFU study. The majority of the data forms used in the SELECT trial were completed by personnel at the Study Sites, usually Clinical Research Associates (CRAs). Some of the questions required the CRA to query the participant but the form and instructions were directed to the CRA. There were a few exceptions in which a form was completed by the participant: a 17-page baseline Food Frequency Questionnaire, family history of cancer form and participant characteristics form, an annual update on current dietary supplement use and a Quality of Life survey given at baseline and selected follow-up visits to a subset of participants.
Data for the CFU study were to be collected annually from the participant via a questionnaire in booklet format mailed directly to the participant. The development of a participant-completed booklet required consideration of a number of factors in order to maximize the number of booklets returned as well as to provide high quality data. In addition to the SELECT participant-completed forms, participant-completed booklets from other cohort studies were obtained and referenced as examples to aid development of the current booklet. Our general approach was to use closed-ended questions with check boxes and to minimize the number of text and numeric answers.
There currently are four versions of the booklet: one for participants not currently diagnosed with prostate cancer and another for those diagnosed with prostate cancer, each available in Spanish or English. The questions in the booklet were kept to a small number and focused on the study endpoints of prostate and other cancers and the participant’s general health. The questions were worded to be understandable to the population; examples were provided when applicable. For example, the questions about prostate cancer treatments used the scientific name(s) as well as the commonly used name of each treatment. While there was some interest in collecting additional covariate data, such as physical activity measures, detailed comorbid conditions and additional body measurements, collection of such data was deemed not to be feasible due to a lack of funding for the additional work and quality control issues.
Producing the booklets involves merging the individual participant data (address, date of last data submission, participant ID) with the correct version based on prostate cancer status and preferred language. To minimize the number of booklets returned for bad addresses, the participant’s address of record is checked against a National Change of Address database at a nominal cost; addresses are updated for a final data merge.
The booklet is mailed during the month of the participant’s birth. Booklets not returned within two months are remailed, for up to three mailings total. An option for the participant to complete the questions online, via a secure website linked to the SELECT public website, was implemented in June 2011, approximately one year after initiation of the CFU study. Participants who report diagnoses of prostate or other cancers are asked to sign a medical release of information that allows the SCC to obtain medical records or specimens. Currently there is no plan to collect medical releases via the web application as the local hospitals have varying requirements and, at a minimum, want an original patient signature.
(h) Study Site closures (June 2010 – April 2011)
After all SELECT participants who were being followed at a Study Site had been contacted to discuss the CFU study, the process of closing the Study Site began. Prior to closure, it was expected that all data would be submitted and all data queries resolved and that the Study Site staff had worked with the SELECT Statistical Center to resolve any participant-specific problems. Transition Visits began in November 2009 with the goal of completion by December 2010. Final closure of the Study Sites took additional time as final paperwork was completed.
Participant contact
Participant follow-up from a local site-based model to one in which they are followed centrally resulted in a loss of personal contact with SELECT personnel. Over the eight years of follow-up in SELECT, participants had become accustomed to interacting with staff at the Study Site. Although Study Site staff was trained to bond the participant to the trial, there was also a bond that developed between the participant and individual staff members. In the CFU study, frequent communication with the participants is necessary to ensure that the participants continue to feel that they are part of SELECT through their participation in the CFU study. In addition to the yearly questionnaires, a semiannual newsletter, a public website and access to the SCC through e-mail or a toll-free telephone call are provided.
The first step in introducing the SCC to the CFU study participant was an official “Welcome letter” that was sent within one month of the participant’s registration to the CFU study. In addition to providing the first contact, it was an opportunity to correct any errors in the participant’s primary address before the participant’s local Study Site closed. This letter also included a wallet-sized CFU study participant ID card with the study ID number and the SCC contact information.
The telephone and e-mail contact information for the SCC is included in the annual booklet, in all correspondence, and in each issue of the newsletter. During a typical month, fewer than 10 telephone calls from study participants are received at the SCC; the majority is either to report an endpoint or to provide a change of address or other contact information. A total of approximately 250 contacts, primarily by telephone, were generated by the release of the updated negative results in October 2011.
Effect of Transition on ongoing SELECT ancillary studies
During SELECT, there were four ancillary studies that were independently funded but relied on the infrastructure of SELECT for participant accrual and data collection. By the time of the transition to the CFU study, only two were active. With the transition the process for the continuation of these studies had to be addressed.
The Prevention of Alzheimer’s Disease with Vitamin E and Selenium (PREADVISE) [8] collected neurocognitive functioning data from a short memory screen administered by Study Site staff. Accrual to PREADVISE stopped in September, 2009. A telephone version of the screen had been approved and implemented prior to the closure of SELECT but was used only when the participant was not able to go in to the Study Site. With the transition to the CFU study, the PREADVISE participants were reconsented for extended follow-up and the memory screen is administered exclusively by telephone by staff at the pre-existing PREADVISE Coordinating Center.
The Adenomatous Colorectal Polyps (ACP) study relied on the collection of medical records from participants who reported that they had had specific colorectal screening procedures. The study was implemented in a subset of the SELECT Study Sites. Data collection required a medical release administered by the Study Site staff that subsequently was faxed to the ACP Coordinating Center to facilitate retrieval of the relevant medical records. This study was recruiting men at the time SELECT supplements were stopped. Currently, a consent form and medical release are mailed by the SCC directly to any participant who reports the events of interest. Medical releases signed by consenting CFU participants are forwarded to the ACP Coordinating Center for retrieval of the appropriate material. This ancillary study has benefitted from the transition of SELECT to the CFU study as identification of potentially eligible participants no longer is the responsibility of the Study Sites.
The SELECT Data and Safety Monitoring Committee continues to meet annually to review data relevant to the SELECT trial, the ancillary studies and the additional data collected during the CFU study.
Results
The primary SELECT results were published online in December 2008 and in print in January 2009 [1]. At that time, there were 32,569 men alive and currently active in SELECT. The planned follow-up had been for 7 to 12 years, depending on when the participant enrolled and was randomized. Median follow-up was 5.5 years (range 4.2 – 7.3 years); 89% of the men had been contacted within the prior 7 months; fewer than 4% had their most recent contact more than two years earlier. It was anticipated that up to 75% of the SELECT participants, approximately 24,000 men, who were alive and being followed would be interested in continuing to be followed in the CFU study. At the time of the release of the primary study results, 410 of the 427 Study Sites that enrolled participants in the trial actively were following participants (Table 2).
Table 2.
Sites following participants at the time study supplements were stopped in SELECT (October 2008)
| Participants per site | Number of sites | Number of participantsa at these sites |
|---|---|---|
| 1 – 10 | 77 | 401 |
| 11 – 25 | 71 | 1,198 |
| 26 – 50 | 103 | 3,803 |
| 51 – 100 | 79 | 5,689 |
| 101 – 250 | 56 | 8,239 |
| 251 – 500 | 14 | 4,905 |
| 501 – 1000 | 8 | 5,402 |
| > 1000 | 2 | 2,930 |
| TOTAL | 410 | 32,569 |
Participants alive and not previously having refused further contact.
The timeline for implementation of the Transition Visits at the Study Sites varied greatly. Personnel at some sites were eager to close the trial as soon as possible to allow the staff to focus on other responsibilities. Other sites needed a lot of time to plan and schedule Transition Visits with sufficient time to allow for longer than normal visits. There were also individual staff issues regarding potential loss of employment which may have slowed the process. The initial goal of closing sites by December 2010 was extended to April 2011 because of unanticipated issues with local IRBs and other implementation issues. The final closure of sites was dependent on Statistical Center processing which became backlogged due to the volume of sites that completed transition activities during the same time period.
As of December 31, 2011, there were 17,761 men registered in the CFU study (Table 3), i.e., fewer than the initial goal of 24,000. The majority of sites (n=320) implemented Plan A, 48 sites implemented Plan B, and 14 sites implemented Plan C. Despite the three options for enrolling participants in the CFU study, study personnel at seven sites were either unwilling or unable to implement any of these plans, resulting in a loss of 1,959 potential participants in the CFU study. Finally, 23 sites had closed or were inactive prior to the publication of the primary SELECT results and their participants had not been transferred to an active site.
Table 3.
Registrations to the Centralized Follow-up Study as of December 31, 2011
| IRB Status | # Sites | # Unique IRBs | # Participants Availablea | # Participants Registered | % Registered |
|---|---|---|---|---|---|
| Approved Plan A | 320 | 270 | 22,327 | 15,060 | 67% |
| Approved Plan B | 48 | 44 | 4,690 | 2,591 | 55% |
| Approved Plan C | 14 | 12 | 1,488 | 110 | 7% |
| Not participating | 7 | 2 | 1,959 | 0 | 0% |
| Closed/inactive prior to CFU | 23 | 0 | 167 | 0 | 0% |
| TOTAL – participating sites | 382 | 326 | 28,505 | 17,761 | 62% |
| TOTAL - overall | 412 | 328 | 30,631 | 17,761 | 58% |
Participants alive and not previously having refused further contact
NCI funding for SELECT was provided to conduct the CFU study via funding for SWOG. Costs for following the cohort via central follow-up compared to follow-up in the SELECT clinical trial are substantially lower due to the reduced volume of data collected, the termination of the per capita reimbursement to study sites, and cessation of collection of yearly blood samples from a subsample of men to assess adherence, and collection of pathology materials from biopsied or resected prostate cancers. Furthermore, the SCC budget has been reduced by approximately one-third although SCC staff has assumed additional responsibilities.
Figure 1 presents cumulative CFU registrations by time. Enrollment of SELECT participants in the CFU study by Plan A sites was completed by April 2011; a modest number of additional registrations from Plan B and Plan C sites have occurred as these participants responded to mailings. Additional registrations may happen at any time as participants are made aware of the CFU study.
Figure 1.
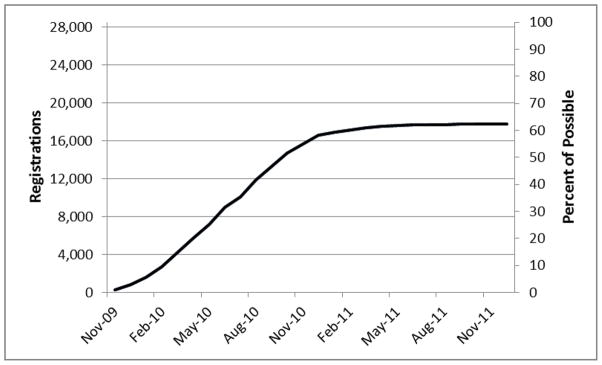
Registrations to the Centralized Follow-Up Study by month – Cumulative and Percent of possible registrations
Baseline characteristics of the men randomized to SELECT and those who have agreed to CFU are presented in Table 4. The CFU population is very similar to the overall SELECT population. During follow-up in SELECT, prostate cancer was diagnosed in 2,279 men (6.5% of the SELECT population); of these men, 1,188 were later enrolled in the CFU study (6.7% of CFU study participants).
Table 4.
Participant Characteristics
| Characteristic | Men Randomized to SELECT N=34,887 | SELECT participants enrolled in CFUa N=17,761 |
|---|---|---|
| BASELINEb | ||
| Age (years) | ||
| Median (IQ range) | 62.0 (58.0 – 67.0) | 62.0 (58.0 – 67.0) |
| 50 – 54 | 1480 (4.2) | 635 (3.6) |
| 55 – 64 | 20,346 (58.3) | 10,800 (60.8) |
| 65 – 74 | 10,809 (31.0) | 5,451 (30.7) |
| ≥ 75 | 2,252 (6.5) | 869 (4.9) |
| Race/ethnicity | ||
| White | 27,571 (79.0) | 14,762 (83.1) |
| African American | 4,318 (12.4) | 1,199 (11.3) |
| Hispanic (non-African-American) | 1,940 (5.6) | 553 (3.1) |
| Hispanic (African American | 361 (1.0) | 73 (0.4) |
| Other | 697 (2.0) | 368 (2.3) |
| Education (highest level) | ||
| ≤ High school graduate or GED | 7,682 (22.0) | 2,855 (16.1) |
| Some college/vocational school | 9,355 (26.8) | 4,642 (26.1) |
| ≥ College graduate | 17,515 (50.2) | 10,129 (57.0) |
| Unknown/missing | 335 (1.0) | 129 (0.7) |
| PSA (ng/ml) | ||
| Median (IQ range) | 1.1 (0.6 – 1.9) | 1.1 (0.7 – 1.9) |
| 0.1 – 1.0 | 16,762 (48.0) | 8,430 (47.5) |
| 1.1 – 2.0 | 10,708 (30.7) | 5,579 (31.4) |
| 2.1 – 3.0 | 4,756 (13.6) | 2,414 (13.6) |
| 3.1 – 4.0 | 2,611 (7.5) | 1,318 (7.4) |
| > 4.0 | 11 (< 0.1) | 5 (< 0.1) |
| Unknown/missing | 39 (0.1) | 9 (< 0.1) |
| Smoking status | ||
| Never | 14,882 (42.7) | 8,159 (46.0) |
| Current | 2,682 (7.7) | 1,010 (5.7) |
| Former | 16,965 (48.6) | 8,456 (47.6) |
| Ever (unknown status) | 191 (0.5) | 75 (0.4) |
| Unknown | 167 (0.5) | 55 (0.3) |
As of December 31, 2011.
At the time of randomization to SELECT.
Over the first five years of the CFU study, accounting for actual enrollment, age and race distribution and the competing risk of death, it is estimated that an additional 580 prostate cancer cases and 215 Gleason 7 or higher cancers will be identified. These additional cases increase the total number of cases by 25% (high grade cases by 35%) and will allow more precise estimation of the difference in the prostate cancer rates and high grade prostate cancer rates between the SELECT treatment arms.
To date, over 109,000 items have been mailed directly to the participants. These include Informed Consent packets, Welcome letters, newsletters and Annual Booklets for data collection. In the second half of 2011, the average number of Annual Booklets mailed to participants monthly, including remailings, was 2,026 (range 2,002 – 2,100). For the first mailing of booklets, 76% were completed and returned, 42% of the second mailing were completed and returned, and 24% of the third mailing, for an overall return rate of 85%. Less than 1% of the booklets were returned as undeliverable. At the onset, it was expected that approximately 10% of the participants would have changes to their personal contact information (address and/or telephone number) annually. The current actual rate of changes to the participant’s primary address is close to 5%. The use of the web-based application for participants to enter their data directly into the database is less than was expected; as of February 28, 2012, only 294 “booklets” (< 2%) had been submitted on-line since implementation in June 2011. Discussions are ongoing regarding how to increase annual data collection online in order to lower costs of data collection and data entry.
The number of additional registrations to the ACP ancillary study is currently in excess of 2000, suggesting that this study will be able to meet its recruitment goal.
Over the ten years of the SELECT trial, a substantial biorepository had been developed. The specimen collection included baseline peripheral blood specimens and toenail clippings from all SELECT participants and a peripheral blood specimen from a subset of 2300 men from 24 sites collected at six months and years 1, 2, 4, and 6 post-baseline. This subset was an “adherence cohort” for whom serum levels of selenium and alpha- and gamma-tocopherol were measured. Additionally, a single 5-year post-baseline sample was requested from all participants as well as a Transition Visit blood specimen from prostate cancer cases. A useable baseline plasma sample is available from 29,381 men and a Year 5 sample is available from 20,972 men. An additional post-diagnostic sample from the prostate cancer cases is available for 426 men. The tissue repository has tissue, either unstained slides or paraffin blocks, from about 1000 men with a prostate cancer diagnosis and 100 men without a diagnosis. Baseline toenail samples from 89% of the men are available; these samples are used to measure levels of selenium, other trace minerals, and potential biomarkers (e.g., nicotine) which reflect long term exposure.
Access to these biologic specimens has been made available through a nationally advertised announcement. The scientific merit and feasibility of the proposals to use specimens in the biorepository are evaluated via a review process. To date, there have been two review cycles from which 12 investigators have been awarded access to samples. Approved uses are wide-ranging: genome-wide association studies (GWAS) in prostate cancer and other cancers, studies of inflammation, methionine metabolism, steroid transport and the risk of prostate cancer as well as non-cancer endpoints including the risk of diabetes with selenium supplementation and the association of arsenic with cancer, cardiovascular disease and diabetes incidences. Independent research funding is needed for the approved studies. Additional approved proposals are anticipated.
There is the potential for researchers with approved funding to add questions to the annual SELECT CFU study questionnaire after review by the SWOG Executive Committee for scientific merit and approval. Any additional questions will be asked only of participants who have agreed to participate in additional research as indicated on their signed consent form. The cohort is also a potential resource for investigators who are interested in conducting clinical trials via the mail, telephone or internet.
Limitations
There are limitations of the follow-up study of this newly formed cohort. The accrual goal was not met which limits our power to address the study objectives. The CFU study is also dependent on a number of factors: continued funding from the NCI through an oncologic cooperative group that is focused on clinical trials, outside investigators interested in the biorepository and participants who are willing to continue to be contacted and to provide the necessary medical releases to obtain the documentation to support the cancer endpoints. There also are limitations to the generalizability of our experience. Our experience is with transitioning to follow a cohort of men who initially enrolled in a prostate cancer prevention trial. Different issues may arise when attempts are made to transition from a treatment trial to an observational study or from prevention trials conducted in medical areas other than cancer.
Discussion
Extended follow-up of participants in prevention research is important to study the long-term effects of the intervention [9]. Despite many challenges, the SELECT leadership was successful in transitioning participants and methods from a randomized trial that stopped early to an observational cohort study in which about half the surviving SELECT participants enrolled and are being followed centrally. In SELECT, a negative effect, i.e., an increased rate of prostate cancer among men in the vitamin E arms, which was suggested by the data at the time of the original primary publication, was maintained despite cessation of the supplements and was confirmed by additional diagnoses during longer follow-up. However, another potential negative effect, i.e., an apparent increase in diabetes diagnoses among men in the selenium arm, became of less concern with time. With the CFU study in place, these important new findings were communicated to the SELECT population in an efficient manner. With direct access to many SELECT participants, a communication plan was developed and appropriate support materials were provided in addition to what was available in the lay press. For those participants not registered in the CFU study, dissemination of this information to them was limited.
Little is known about the long-term safety of supplements. During the course of SELECT, a meta-analysis [10] of vitamin E trials hinted at an increased mortality risk for dosages > 500 IU. SELECT provided another example of possible harm from the use of a supplement; the CFU study provides the opportunity to better understand how these supplements work and what the true risks are.
The question of how best to follow participants in large prevention trials after the trial is stopped is not unique to SELECT. There have been several other large trials in the same situation and the approach taken by each differed because of funding, timing and scientific rationale. Extending the follow-up on randomized controlled clinical trials can produce important updated findings which are informative regarding the long term effects of the trial agents. Publications from BCPT [11], STAR [12], CARET [13] and WHI [14, 15] have included such findings. In addition, publication of other findings from WHI [16, 17] on other endpoints was feasible because of continued follow-up. The approach taken by SELECT investigators to continue to follow participants centrally was most similar to CARET and WHI. SELECT investigators benefited from those experiences and developed the plan to obtain consent to extended follow-up, gather annual data using a mailed or online booklet, and obtain the necessary medical records for future endpoint adjudication based on their procedures. The biggest hurdle in the centralization of follow-up of the cohort was the resistance of some of the local IRBs, which we overcame partially by providing alternative recruitment processes.
In the absence of approval or resources to contact potential participants directly, registries can be used to capture additional endpoints [18] as an alternative to long-term scheduled follow-up data collection.
The participants enrolled in the SELECT CFU study represent a large, well-characterized, generally healthy cohort. The benefit of their continued follow-up is multifold. The CFU has enabled us to collect additional prostate and other cancer endpoints and longer follow-up on the almost 18,000 participants enrolled. The utility of the extensive biorepository that was developed during the course of the SELECT is enhanced by longer follow-up. The biologic samples from these additional cancer cases from this cohort may be used for either discovery or validation work in biomarker studies. Additional questionnaire-based research also is possible.
Rare event data from the SELECT CFU study, such as pancreatic cancer, pooled with those from other consortia, permit analyses that would not be possible in any individual cohort. The CFU study also provides a framework to allow continued follow-up of the men registered in the ancillary studies and to facilitate study completion. Ultimately, the ability to maintain funding will determine future activities; it is challenging to obtain funding for long-term follow-up studies once a randomized clinical trial is completed. Thus, it is critical that the results of such follow-up, especially the long term risks and benefits of over-the-counter supplements, be disseminated to the medical and lay community.
Acknowledgments
The authors appreciate the information provided by Matt Barnett regarding CARET, Walter Cronin regarding BCPT and STAR, and Bernedine Lund and Garnet Anderson regarding WHI.
Funding/Support: This work was supported in part by Public Health Service Cooperative Agreement grant CA37429 awarded by the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, and in part by the National Center for Complementary and Alternative Medicine (National Institutes of Health). Study agents and packaging were provided by Perrigo Company (Allegan, Michigan), Sabinsa Corporation (Piscataway, New Jersey), Tishcon Corporation (Westbury, New York), and DSM Nutritional Products Inc. (Parsipanny, New Jersey).
Footnotes
Trial registration: clinicaltrials.gov identifier: NCT00006392
References
- 1.Lippman SM, Klein EA, Goodman PG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other Cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogel VG. Follow-up of breast cancer prevention trial and the future of breast cancer prevention efforts. Clinical Cancer Research. 2001;7(Supplement):4413s–4418s. [PubMed] [Google Scholar]
- 3.ClinicalTrials.gov. [accessed 17 July 2012];A service of the US National Institutes of Health. http://clinicaltrials.gov/ct2/show/NCT00288106.
- 4.Omenn GS. Chemoprevention of lung cancers: lessons from CARET, the beta-carotene and retinol efficacy trial, and prospects for the future. European Journal of Cancer Prevention. 2001;16:184–191. doi: 10.1097/01.cej.0000215612.98132.18. [DOI] [PubMed] [Google Scholar]
- 5.Prentice RL, Anderson GL. The Women’s Health initiative: Lessons learned. Annu Rev Public Health. 2007:131–50. doi: 10.1146/annurev.publhealth.29.020907.090947. [DOI] [PubMed] [Google Scholar]
- 6.Goodman GE, Thornquist MD, Edelstein C, et al. Biorepositories: Let’s not lose what we’ve so carefully gathered! Cancer Epidemiol Biomarkers Prev. 2006;15(4):599–601. doi: 10.1158/1055-9965.EPI-05-0873. [DOI] [PubMed] [Google Scholar]
- 7.Klein EA, Thompson IM, Tangen CM, et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306 (14):1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caban-Holt A, Schmitt FA, Runyons CR, et al. Studying the effects of Vitamin E and Selenium for Alzheimer’s disease prevention. In: Vellas B, Fitten LJ, Feldman H, Giacobini E, Grundman M, Winblad B, Kurz A, editors. Research and Practice in Alzheimer’s Disease and Cognitive Decline. Vol. 1.1. Paris: Serdi; 2006. pp. 124–130. [Google Scholar]
- 9.Cuzick J. Long-term follow-up in cancer prevention trials (It ain’t over ‘til it’s over) Cancer Prev Res. 2010;3(6):689–91. doi: 10.1158/1940-6207.CAPR-10-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller ER, 3rd, Patsor-Barriuso R, Dalal D, et al. Meta-analysis: high dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005;142:1–11. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 11.Fisher B, Cosantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: Current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. Journal of the National Cancer Institute. 2005;97(22):1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 12.Vogel VG, Cosantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res. 2010;2(6):696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: Incidence of lung cancer and cardiovascular disease mortality during 6-Year follow-up after stopping β-carotene and retinol supplements. Journal of the National Cancer Institute. 2004;96(23):1743–1750. doi: 10.1093/jnci/djh320. [DOI] [PubMed] [Google Scholar]
- 14.Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13:476–86. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305(13):1305–14. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang JY, Spaunhurst KM, Chlebowski RT, et al. Menopausal hormone therapy and risks of melanoma and nonmelanoma skin cancers: Women’s Health Initiative randomized trials. J Natl Cancer Inst. 2011;103:1469–1475. doi: 10.1093/jnci/djr333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carty CL, Kooperberg C, Neuhouser ML, et al. Low-fat dietary pattern and change in body-composition traits in the Women’s Health Initiative Dietary Modification Trial. Am J Clin Nutr. 2011;93:516–24. doi: 10.3945/ajcn.110.006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Q, You N, Nelson H, et al. Cancer registries: A novel alternative to long-term clinical trial follow-up based on results of a comparative study. Clinical Trials. 2010;7:686–95. doi: 10.1177/1740774510380953. [DOI] [PMC free article] [PubMed] [Google Scholar]


