The steroid hormones are synthesized in the adrenal cortex, the gonads, and the placenta; are all derived from cholesterol and many are of clinical importance. Steroid hormones are synthesized in the mitochondria and smooth endoplasmic reticulum. Because they are lipophilic, they cannot be stored in vesicles from which they would diffuse easily and are therefore synthesized when needed as precursors. Upon stimulation of the parent cell, steroid hormone precursors are converted to active hormones and diffuse out of the parent cell by simple diffusion as their intracellular concentration rises.
Because all steroid hormones are derived from cholesterol, they are not soluble in plasma and other body fluids. As a result, steroids are bound to transport proteins that increase their half-life and insure ubiquitous distribution. The protein-bound steroids are in equilibrium with a small fraction of free steroids, which are ‘active.’ Steroids can act quickly, by binding to cell surface receptors, or slowly, by binding to cytoplasmic or nucleic receptors and ultimately activate gene transcription.
The adrenal glands are composed of the adrenal medulla and the adrenal cortex. The adrenal cortex is divided into three major anatomic zones: the zona glomerulosa, which produces aldosterone; and the zonae fasciculata and reticularis, which together produce cortisol and adrenal androgens. The medulla synthesizes catecholamines. More than 30 steroids are produced in the adrenal cortex; they can be divided into three functional categories: mineralocorticoids, glucocorticoids, and androgens.
The steroids that are made almost exclusively in the adrenal glands are cortisol, 11-deoxycortisol, aldosterone, corticosterone, and 11-deoxycorti-costerone. Most other steroid hormones, including the estrogens, are made by the adrenal glands and the gonads [1].
Mineralocorticoids
The mineralocorticoids are formed in the zona glomerulosa. The main function of the mineralocorticoids is to promote tubular reabsorption of sodium and secretion of potassium and hydrogen ions at the collecting tubule, distal tubule, and collecting ducts [2]. When sodium is reabsorbed, water is absorbed simultaneously. The absorption of sodium and water increases fluid volume and arterial pressure.
Aldosterone is the most potent mineralocorticoid and accounts for about 90% of the total mineralocorticoid activity. Mineralocorticoid potency in descending order is: aldosterone, 11-deoxycorticosterone, 18-oxocortisol, corticosterone, and cortisol [1]. Although cortisol has mainly glucocorticoid activity, it also has some mineralocorticoid activity. Cortisol has 1/400 of the potency of aldosterone, but its concentration is about 80 times greater than that of aldosterone [4]. The adrenal production of cortisol is approximately 25 mg/day and that of aldosterone is 100μg/day. Corticosterone has mainly glucocorticoid activity and some mineralocorticoid activity.
Aldosterone secretion is regulated primarily by the renin-angiotensin system; it also is stimulated by increased serum potassium concentrations. Hyperkalemia and angiotensin II cause an increase in aldosterone. To a lesser degree, elevated sodium concentration suppresses aldosterone secretion and corticotropin allows aldosterone secretion.
Glucocorticoids
The glucocorticoids are produced primarily in the zona fasciculata. The glucocorticoids affect metabolism in several ways. Glucocorticoids stimulate gluconeogenesis and decrease the glucose use by cells. Cortisol reduces protein stores in all cells of the body, except the liver, and increases protein synthesis in the liver. Cortisol also increases amino acids in the blood, decreases transport of amino acids into extrahepatic cells, and increases transport of amino acids into hepatic cells. Cortisol mobilizes fatty acids from adipose tissue, increases free fatty acids in the plasma, and increases free fatty acid use for energy. Cortisol, the most clinically important glucocorticoid, accounts for about 95% of all glucocorticoid activity [3]. Corticosterone accounts for a small, but significant, amount of the total glucocorticoid activity. Cortisol secretion is regulated almost entirely by corticotropin, which is secreted by the anterior pituitary gland in response to corticotropin-releasing hormone (CRH) from the hypothalamus. Serum cortisol inhibits secretion of CRH and corticotropin, which prevents excessive secretion of cortisol from the adrenal glands. Corticotropin stimulates cortisol secretion and promotes growth of the adrenal cortex in conjunction with growth factors, such as insulin-like growth factor (IGF)-1 and IGF-2. There is a circadian rhythm to cortisol secretion; the highest cortisol levels occur about 1 hour before arising. Stress, pain, and inflammation cause increased cortisol production.
Androgens
The term “androgen” refers to any steroid hormone that has masculinizing effects. In men, androgens are responsible for the development of secondary sexual characteristics. The androgens play a less important role in women; however, the adrenal androgens are responsible for much of the growth of pubic and axillary hair. Testosterone is the major androgen. Androgens are produced in the adrenal glands and the gonads. In men, about 100μg/day of testosterone is made by the adrenals and about 7000μg/day is made by the testes [1]. In women, 50% to 60% of the testosterone is derived from androstenedione conversion in peripheral tissues, 30% is produced directly by the adrenals, and 20% is produced by the ovary [4].
The adrenal androgens are formed primarily in the zona reticularis. Dehydroepiandrosterone (DHEA) is the principal steroid that is produced by the adrenal glands. Sulfation of DHEA produces DHEA sulfate (DHEA-S). Adrenal androgens are moderately active male sex hormones. Some of the adrenal androgens are converted to testosterone. The mechanism of stimulation of androgen secretion from the adrenals is not well understood. Adrenarche is the maturation of the adrenals, which causes an increase in these androgens and occurs between age 5 and 20 [5]. Adrenarche, therefore, begins well before puberty. Adrenal androgen secretion is regulated partially by corticotropin but also by other unknown factors.
The testes secrete testosterone, dihydrotestosterone (DHT), and androstenedione. Gonadal production of androgens is controlled by hypothalamic secretion of GnRH, which causes the anterior pituitary to release follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Testosterone is secreted by the Leydig cells of the testes in response to LH stimulation. Most of the testosterone is converted to the more active DHT in the target tissues.
Estrogens and progestins
In women, the main function of estrogens is to promote proliferation and growth of specific cells in the body that are responsible for the development of most of the secondary sexual characteristics. The progestins are responsible for the preparation of the uterus for pregnancy and the breasts for lactation. In men, estrogens and progestins usually do not play a clinically significant role in the development of sexual characteristics. In women, estrogens and progestins are derived from the adrenal gland or the gonad. In women who have intact ovaries, the adrenal contribution to circulating estrogens is insignificant. Estrogens and progestins are secreted in differing rates during the different parts of the female menstrual cycle. Estradiol is the prominent ovarian estrogen; estrone and estriol are two other estrogens. Estradiol is 12 times as potent as estrone and 80 times as potent as estriol [3]. Estrone is made in small amounts by the ovaries, but mostly is formed by peripheral conversion from androgens. Estriol is mainly a metabolite of estrone and estradiol in nonpregnant women. In pregnancy, however, estriol is the major estrogen of the placenta. DHEA-S from the fetal adrenal glands is converted to estriol by the placenta.
The major progestin is progesterone; a minor progestin is 17-hydroxy-progesterone. In the first half of the menstrual cycle, small amounts of progesterone are produced—about half from the ovaries and about half from the adrenal cortex. Larger amounts of progesterone are secreted in the latter half of the menstrual cycle by the corpus luteum. Men produce a small amount of estrogens (about 1/5 that of a nonpregnant woman). The Sertoli cells convert a small amount of testosterone to estrogen. Also, estrogens are formed from testosterone and androstenediol peripherally in the liver.
Transport and fate of steroid hormones
Cortisol combines with cortisol-binding globulin (CBG) and albumin. About 3% to 10% of cortisol is free, 80% to 90% is bound to CBG, and 5% to 10% is bound to albumin [6,7]. Only the free portion of cortisol is active. Some clinical situations can cause an increase or decrease in the CBG. For example, an increase in estrogens or in thyroid hormone can cause an increase in the CBG. Alternately, hypothyroidism, increased androgens, acute stress, and nephrotic syndrome can cause a decreased CBG. A change in the amount of CBG affects the total cortisol level, but not the free cortisol level. Also, cortisone, corticosterone, 11-deoxycorticosterone, progesterone, and 17-hydroxyprogesterone bind to CBG [1]. Aldosterone, in contrast, is bound loosely to CBG, albumin, and red blood cells so that about 50% of aldosterone is free. DHEA and androstenedione are bound weakly to albumin; DHEA-S is bound tightly to albumin. Testosterone and estradiol are bound to sex hormone–binding globulin (SHBG), which is sometimes referred to as testosterone binding globulin. About 60% to 75% of serum testosterone is bound to SHBG, about 20% to 40% is bound to albumin, and 1% to 2% is free [4]. The decreasing order of affinity for SHBG is: DHT, testosterone, androstenediol, estradiol, and estrone [1]. Androstenedione and DHEA are bound weakly to albumin. Estrogen, diabetes mellitus, hyperthyroidism, and cirrhosis can cause an increase in SHBG, whereas testosterone and age can cause a decrease in SHBG [8]. Cortisol and aldosterone either become fixed in the target tissue or degraded in the liver. About 25% of the degraded steroid is excreted in the bile and then feces and about 75% is excreted in the urine [3]. The half-life of cortisol is 60 to 100 minutes. The half-lives of aldosterone, DHEA, androstenedione, testosterone, and estradiol are less than 20 minutes. The half-life of aldosterone is less than 15 minutes. Unmetabolized cortisol accounts for about 0.1% of the total urinary cortisol metabolites [1].
Selected clinical abnormalities of steroid hormones
Primary hyperaldosteronism
Primary hyperaldosteronism occurs in about 2% of patients who have hypertension. The most common cause of primary hyperaldosteronism is an aldosterone-producing adenoma that also is known as Conn’s syndrome. Other causes are idiopathic hyperaldosteronism, primary adrenal hyperplasia, dexamethasone suppressible hyperaldosteronism, and adrenal cortical carcinoma. Primary hyperaldosteronism is distinguished from other causes of hyperaldosteronism by a high plasma aldosterone (PA) level and a low plasma renin activity (PRA). A low PRA and low PA are seen in patients who have a real or apparent mineralocorticoid excess that is not caused by aldosterone (caused by 11-β-hydroxylase deficiency, 17-hydroxylase deficiency, Liddle syndrome, 11-β-hydroxysteroid dehydrogenase deficiency, or licorice ingestion). A high PRA and high PA suggest secondary hyper-aldosteronism. Secondary hyperaldosteronism with hypertension can be caused by renal artery stenosis, a renin-secreting tumor, malignant hypertension, or chronic renal disease. Secondary hyperaldosteronism with nor-motension can be caused by renal disorders (eg, renal tubular acidosis or Barters syndrome), diuretic or laxative use, cirrhosis, congestive heart failure, vomiting, and familial chloride diarrhea.
Measurement of aldosterone concentration by itself is not a useful screening test because there is overlap between primary hyperaldosteronism, secondary hyperaldosteronism, and essential hypertension. To evaluate for primary hyperaldosteronism, the ratio of PA:PRA is used. Measurement of the ratio of PA:PRA is performed ideally when the patient is not taking any medications (especially diuretics, ace inhibitors, and β-blockers) for 2 to 4 weeks before testing and after 2 hours of standing. Although there is some disagreement, a ratio of PA (ng/dL) to PRA (ng/mL/hour) of greater than 25 is suggestive primary hyperaldosteronism; a ratio of greater than 50 is diagnostic of primary hyperaldosteronism [9].
After a diagnosis of primary hyperaldosteronism is suspected based on the ratio of PA:PRA, the usual next step is confirmatory testing to demonstrate the autonomy of aldosterone secretion. This can be performed by giving two liters of saline over 4 hours and looking for possible aldosterone suppression. If the aldosterone level suppresses to less than 5 ng/dL this is considered normal. If the aldosterone level remains greater than 10 ng/dL, this confirms the diagnosis of hyperaldosteronism [9].
Adrenal insufficiency
Lack of cortisol can be caused by an inability of the adrenal glands to produce cortisol (primary adrenal insufficiency) or by lack of CRH or corticotropin (secondary or tertiary adrenal insufficiency). In primary adrenal insufficiency (Addison’s disease) there is deficiency of cortisol and aldosterone. In secondary and tertiary adrenal insufficiency, the adrenal glands are able to make aldosterone (because of intact stimulation of the adrenal glands by angiotensin II) but not cortisol. Primary adrenal insufficiency has several causes, including autoimmune disease; adrenal hemorrhage; HIV; or infiltration of the adrenals by tuberculosis, sarcoidosis, or amyloidosis. Causes of secondary or tertiary adrenal insufficiency are infiltration of the anterior pituitary or hypothalamus by craniopharyngioma, pituitary adenoma, metastasis, sarcoidosis, or tuberculosis or by suppression of corticotropin by long-term steroid use.
Chronically, adrenal insufficiency can manifest with weakness, fatigue, anorexia, nausea, abdominal pain, and diarrhea. Hyponatremia can be present in any form of adrenal insufficiency. Hyperkalemia can be present in primary adrenal insufficiency because of the lack of aldosterone. In acute adrenal insufficiency, patients may be hypotensive from decreased vascular tone, decreased cardiac output, and relative hypovolemia. If untreated, adrenal insufficiency can lead to coma and death.
Evaluation of adrenal insufficiency usually is performed by measurement of serum cortisol. Because of the diurnal pattern of cortisol secretion, random cortisol levels are of little value. In a critically ill patient, a serum cortisol level of greater than 18μg/dL usually is considered adequate. In the outpatient setting, the preferred tests are either a morning cortisol or a corticotropin-stimulation test. A morning cortisol level of less than 3μg/dL is diagnostic of adrenal insufficiency and a morning cortisol level of greater than 18μg/dL rules out adrenal insufficiency. Morning serum cortisol levels that are between 3μg/dL and 18μg/dL can be evaluated by a corticotropin-stimulation test [10].
During a corticotropin-stimulation test, 250μg of synthetic cosyntropin is given intravenously (or intramuscularly); cortisol levels are measured at baseline, at 30 minutes, and at 60 minutes [10]. A peak cortisol value of 18 to 20μg/dL or greater usually is used to rule out adrenal insufficiency. If acute secondary adrenal insufficiency is suspected, another type of stimulation test, such as a CRH-stimulation test, must be used to rule out adrenal insufficiency. Complicating factors in the evaluation of serum cortisol levels during acute stress are that the albumin and CBG may decrease. Therefore, the total serum cortisol may be decreased even if the level of free (active) cortisol is unchanged [11] Methods have been developed for measuring serum free cortisol; however, they are technically demanding, expensive, and are not readily available [12]. A ratio of cortisol:CBG has been proposed to determine a free cortisol index that is proportionate to the serum free cortisol [13].
Cushing’s syndrome
The syndrome of persistent and inappropriate excess cortisol is called “Cushing’s syndrome.” Excess cortisol can be caused by exogenous glucocorticoids, excess production of cortisol by the adrenal glands (by an adrenal adenoma, carcinoma, or nodular hyperplasia), or excess production of corticotropin or CRH. Corticotropin can be produced by the anterior pituitary gland or by an ectopic source, such as bronchial carcinoid or small cell lung cancer. Likewise, ectopic production of CRH can be produced by bronchial carcinoid, medullary thyroid cancer, or metastatic prostate cancer. Clinical manifestations of excess cortisol include hypertension, diabetes mellitus, androgen-type hirsutism, irregular menses, weight gain, ecchymoses, myopathy, osteopenia, truncal obesity, and purple striae.
Diagnostic evaluation for Cushing’s syndrome usually begins with a screening test. Difficulties with screening tests for Cushing’s syndrome include a high false-positive rate. Only patients who have suspected Cushing’s syndrome should be screened (ie, patients who have central obesity, facial plethora, proximal muscle weakness, purple striae, and so forth). A 24-hour urine measurement of free cortisol is a sensitive (95%–100%) and specific (98%) screening test [14]. Alternately, a 1-mg overnight dexamethasone suppression test often is used for screening purposes. In this test, 1 mg of dexamethasone is taken orally at 10 pm or 11 pm and a cortisol level is obtained at 8 am the next morning [14]. Although there is some disagreement in the literature, a serum cortisol level of less than 5μg/dL rules out Cushing’s syndrome. If the diagnosis of Cushing’s syndrome is suggested or ambiguous after screening tests are performed, then further testing should be performed. A newer technique for evaluating Cushing’s syndrome is the measurement of salivary cortisol levels. Because the serum free cortisol diffuses freely into saliva, the salivary cortisol reflects the serum free cortisol level [15]. Saliva can be collected at various times of the day as an outpatient, which allows serial cortisol measurements without performing serial blood draws [16].
Disorders of gonadal function
Tests of gonadal function are most commonly performed in men for hypogonadism and in women for menstrual disorders, hirsutism, and virilization. Usually, LH and FSH are measured along with the androgens or estrogens to help determine the cause of the problem.
There are many causes of male sexual dysfunction. One of the causes is a disorder of the hypothalamic-pituitary-gonadal axis. A good screening test is measurement of serum total testosterone level in the morning. A total serum testosterone measurement of less than 300 ng/dL usually is indicative of hypogonadism [17]. FSH and LH can be measured simultaneously to help determine the cause of the disorder.
About 5% to 10% of women of reproductive age have some symptoms of hyperandrogenism (hirsutism, acne, or menstrual dysfunction). Most symptoms are secondary to polycystic ovarian syndrome or idiopathic hirsutism. A small percentage of cases of hyperandrogenism has a more pathologic cause, such as androgen secreting ovarian or adrenal tumors, pituitary tumors, Cushing’s syndrome, or late-onset congenital adrenal hyperplasia. The best test to rule out an androgen-secreting tumor is a serum total testosterone and a serum DHEA-S. If the total testosterone is less than 150 to 200 ng/dL and the DHEA-S is less than 800 to 900μg/dL there is less likelihood of an androgen secreting tumor [18].
Congenital adrenal hyperplasia
Congenital adrenal hyperplasia (CAH) is another clinical disease that affects the levels of steroid hormones that are produced by the adrenal cortex. Congenital adrenal hyperplasia is a group of inherited diseases that result in defective activity of one of five enzymes in the adrenal cortex. The defective enzyme leads to decreased production of cortisol, and, therefore, an increased production of corticotropin, excess production of hormones proximal to the enzyme defect, and glandular enlargement.
The two most common types of CAH are 21-hydroxylase deficiency (defect in the p450c21enzyme) and 11-β-hydroxylase deficiency (defect in the p450c11 enzyme). More than 90% of cases of CAH are caused by 21-hydroxylase deficiency; there is a classic form and a nonclassic form. In the classic form of salt-wasting 21-hydroxylase deficiency CAH, girls are born with ambiguous genitalia and boys and girls may have Addisonian crisis and hypotension. In simple virilizing 21-hydroxylase CAH, girls express ambiguous genitalia but do not experience Addisonian crisis. Boys who have 21-hydroxylase CAH are not ambiguous. Untreated male nonsalt losers present with precocious puberty. Like girls who have salt-losing 21-hydroxylase CAH, boys present with Addisonian crisis in the first few weeks of life. The frequency of CAH is about 1 in 14,000 [19].
The nonclassic form of 21 hydroxylase deficiency is more common (1 in 100) and presents at puberty in girls who have signs of excess androgen [20]. A deficiency in 21-hydroxylase causes an increase in the hormone 17-hydroxyprogesterone, as well as excess testosterone and DHEA-S. A 17-hydroxyprogesterone level of less than 200 ng/dL at 8 am excludes the diagnosis in nonclassic 21-hydroxylase deficiency [21]. 21-hydroxylase deficiency is diagnosed by looking for an increase in 17-hydroxyprogester-one from baseline to 60 minutes during a corticotropin-stimulation test [21].
The next most common form of CAH is 11-β-hydroxylase deficiency (1 in 100,000) [22]. Like 21 hydroxylase deficiency, 11-β-hydroxylase deficiency presents in newborn girls with ambiguous genitalia. 11-β-hydroxylase deficiency presents with sodium retention, volume expansion, and hypokalemia from increased amounts of the mineralocorticoid 11-deoxycorticosterone.
Methods of measurement
Immunoassays (IAs) are among the most sensitive and precise analytical methods; however, recent studies [23–36] showed that many immunoassays lack specificity as a result of cross-reactivity. Furthermore, results from the College of American Pathologists Proficiency Testing Program for the year 2002 (Y-survey) [36] clearly showed that the antibodies that are used in the commercially available immunoassays for steroids lack specificity. Table 1 gives the mean “low” and “high” values for each steroid using the different immunoassays that are available; the data strongly illustrate their lack of specificity. In the past, steroids usually were analyzed individually, using gas chromatography-mass spectrometry (GC-MS) or immunoassay. GC-MS is sensitive and specific, but requires tedious and time-consuming sample preparation, whereas it is clear that immunoassays lack specificity. Liquid chromatography-mass spectrometry (LC-MS) and liquid chromatography-tandem mass spectrometry are specific and offer simpler approaches to sample preparation without sample derivatization steps. Recently, several LC-MS–based methods that use different ion sources were reported for the determination of the following steroid hormones: testosterone [37–39], cortisol [40–44], 11-deoxycortisol [45], androstenedione [38,39], DHEA [46], DHEA-S [38,46], progesterone [47], 17-hydroxyprogesterone [48], estriol [49], and estradiol [38,49].
Table 1.
Problems with immunoassays: data acquired from CAP PT Program 2002
| Steroid | Mean (low) | Mean (high) |
|---|---|---|
| Androstenedione (ng/dL) | 17.3 | 23.1 |
| 17-Hydroxyprogesterone (ng/dL) | 16.0 | 82.7 |
| Estradiol (pg/mL) | 25.7 | 220.7 |
| Progesterone (ng/mL) | 1.56 | 3.85 |
| Testosterone (ng/dL) | 20.1 | 51.2 |
| DHEA-S (μg/dL) | 34.9 | 59.9 |
| Estriol (ng/mL) | 5.57 | 20.5 |
Before the advent of the atmospheric pressure photoionization (APPI) ion source [50], the LC-MS–based methods used an atmospheric pressure chemical ionization (APCI) or electrospray ionization (ESI) source. Without multi-step sample preparation procedures, the APCI source usually cannot provide adequate sensitivity for some steroids, such as estradiol and DHEA, in human serum. The ESI source is considered to be more sensitive than the APCI source for polar compounds. For the nonpolar or low polar compounds, such as most steroid molecules, the sensitivity that is provided by the ESI source is less satisfactory [44,48] than the APCI source. The more recently introduced APPI source is significantly more sensitive than APCI for certain compounds [50]. Alary [51] used APPI-tandem mass spectrometry for the detection of steroids in biologic matrices. In selected ion monitoring mode and multiple reaction monitoring (MRM) mode, the signal that was obtained by photoionization was more intense by a factor of 3 to 10 when compared with the APCI source.
Because of the high sensitivity that is provided by the APPI source for steroids, we hypothesized that stable isotope dilution tandem mass spectrometry in the MRM mode would allow for the rapid simultaneous quantitation of numerous steroids in a single sample. We recently measured nine steroids in a 760μL sample of serum or plasma simultaneously, without derivatization and with minimal sample work-up and acetonitrile protein precipitation (unpublished data). The reliability of the method was evaluated by correlation with currently used immunoassays (Table 2) and assessment of within-day and between-day imprecision, recovery, and accuracy. Comparison of results that were obtained by tandem mass spectrometry with the all-method mean [36] in the CAP PT Program also was made (Table 3).
Table 2.
Correlations between tandem MS and immunoassays
| Steroid | Sy,x | Equations | n | Correlation coefficient (r) | Concentration ranges (ng/mL) |
|---|---|---|---|---|---|
| DHEASa | 241.5 | y = 1.15x + 43.18 | 50 | 0.971 | 14–3970 |
| Cortisolb | 17.98 | y = 1.036x + 18.28 | 50 | 0.983 | 9.71–554 |
| Androstenedionec | 0.564 | y = 1.051x + 0.769 | 50 | 0.905 | 0.1–6.8 |
| Estriold,e | 0.271 | y = 1.132x + 0.079 | 13 | 0.959 | 0.8–3.3 |
| Progesteronef | 2.176 | y = 1.236x − 0.502 | 50 | 0.988 | 0.215–52.8 |
| DHEAc | 1.26 | y = 1.973x + 2.063 | 27 | 0.886 | 0.1–5.5 |
| 11-Deoxycortisolg | 0.70 | y = 0.668x + 1.711 | 9 | 0.888 | 1.0–6.0 |
| Testosteroneg | 0.633 | y = 0.919x − 0.064 | 50 | 0.971 | 0.11–17.2 |
| 17-Hydroxyprogesteroneh | 0.232 | y = 1.587x + 0.123 | 46 | 0.988 | 0.11–6.07 |
| Estradiola | 1.392 | y = 1.436x + 0.252 | 43 | 0.969 | 0.15–14.1 |
Immunoassay/DPC Immulite (Diagnostic Products Corporation).
Immunoassay/Bayer ADVIA Centaur.
RIA/Diagnostic Systems Laboratories, RIA indicates radioimmunoassay.
ColorMetric/Bayer.
Samples of estriol were run in negative ion mode.
RIA/DPC Coat-A-Count.
RIA/ICN Pharmaceuticals.
Extracted RIA/Diagnostic Products Corporation.
Table 3.
Comparison between the results obtained by tandem MS and the all method mean results from CAP PT Program 2002
| Steroids | Ratio of tandem MS result to all method mean result in percent (%) |
|---|---|
| Androstenedione | 49.9 |
| 17-Hydroxyprogesterone | 79.3 |
| 11-Deoxycortisol | 22.1 |
| Progesterone | 73.7 |
| Testosterone | 85.3 |
| DHEA-S | 88.7 |
| Cortisol | 81.4 |
| Estriol | 75.5 |
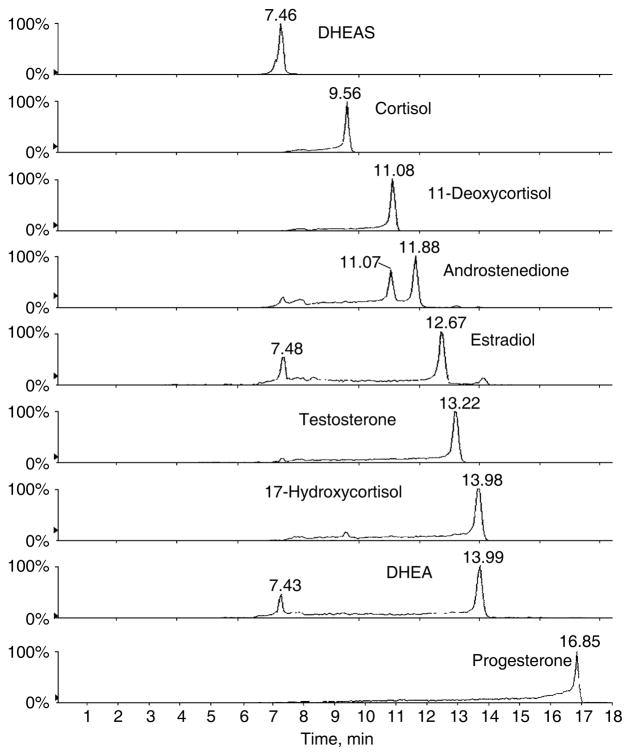
The method allows for the simultaneous quantitation of nine steroids in positive ion mode by tandem mass spectrometry within 18 minutes (Fig. 1). The nine steroids investigated in the positive ion mode and their respective deuterated internal standards were well-separated in 18 minutes. The method is based on isotope dilution, and, unlike immunoassays, is very specific for the analytes of interest. The method possesses adequate sensitivity (because of the use of the APPI source) and precision to be used in the routine clinical laboratory (coefficient of variation [CV] <13% at normal steroid concentrations). The method has been used for the measurement of steroid concentrations in patient samples. Results were compared with immunoassay techniques (see Table 2). Generally, tandem mass spectrometry provides lower values that are due to improved specificity. The correlation coefficients (see Table 2) are satisfactory (r>0.886). The method also has been used to measure urinary free cortisol and the 6-β-hydroxycortisol:cortisol ratio which is a measure of human CYP3A activity [52] and is important because of its role in drug metabolism.
Fig. 1.
Liquid chromatography–tandem mass spectometry (MRM) profiles of the steroids of interest obtained for the injection of a standard mixture.
Unlike immunoassays where each steroid needs to be assayed separately, the current procedure allows for the simultaneous measurement of many steroids, thereby providing a steroid profile on each sample measured. We believe that the greatly improved specificity, accuracy, and simultaneous quantitation features that are afforded by this APPI tandem mass spectroscopy method represent distinct advantages over current IAs.
Acknowledgments
This work was supported by grant M01RR13297-05 from the General Clinical Research Center Program of the National Center for Research Resources, National Institutes of Health, Department of Health and Human Services.
References
- 1.Becker KL, Bilezikian JP, Bremmer WJ, et al. Principles and practice of endocrinology and metabolism. 3. Philadelphia: Lippincott Williams and Wilkins; 2002. CD-ROM. [Google Scholar]
- 2.Rogerson FM, Fuller PJ. Mineralocorticoid action. Steroids. 2000;65:61–73. doi: 10.1016/s0039-128x(99)00087-2. [DOI] [PubMed] [Google Scholar]
- 3.Guyton AC, Hall J. Textbook of medical physiology. 9. W.B. Saunders Co; 1996. [Google Scholar]
- 4.Ravel R. Clinical laboratory medicine. 6. St. Louis: Mosby-Year Book, Inc; 1995. [Google Scholar]
- 5.McKenna TJ, Fearon U, Clarke D, et al. A critical review of the origin and control of adrenal androgens. Baillieres Clin Obstet Gynaecol. 1997;11:229–48. doi: 10.1016/s0950-3552(97)80035-1. [DOI] [PubMed] [Google Scholar]
- 6.Rosner W. The functions of corticosteroid-binding globulin and sex hormone–binding globulin: recent advances. Endocr Rev. 1990;11:80–91. doi: 10.1210/edrv-11-1-80. [DOI] [PubMed] [Google Scholar]
- 7.Hammond GL. Molecular properties of corticosteroid binding globulin and the sex-steroid binding proteins. Endocr Rev. 1990;11:65–79. doi: 10.1210/edrv-11-1-65. [DOI] [PubMed] [Google Scholar]
- 8.Hammond GL. Determinants of steroid hormone bioavailability. Biochem Soc Trans. 1997;25:577–82. doi: 10.1042/bst0250577. [DOI] [PubMed] [Google Scholar]
- 9.Litchfield WR, Dluhy RG. Primary aldosteronism. Endocrinol Metab Clin North Am. 1995;24:593–612. [PubMed] [Google Scholar]
- 10.Grinspoon SK, Biller BM. Clinical review 62: laboratory assessment of adrenal insufficiency. J Clin Endocrinol Metab. 1994;79:923–31. doi: 10.1210/jcem.79.4.7962298. [DOI] [PubMed] [Google Scholar]
- 11.Pugeat M, Bonneton A, Perrot D, et al. Decreased immunoreactivity and binding activity of corticosteroids binding globulin in serum in septic shock. Clin Chem. 1989;35:1675–9. [PubMed] [Google Scholar]
- 12.Robin P, Predine J, Milgrom E. Assay of unbound cortisol in plasma. J Clin Endocrinol Metab. 1978;46:277–83. doi: 10.1210/jcem-46-2-277. [DOI] [PubMed] [Google Scholar]
- 13.Le Roux CW, Sivakumaran S, Alaghband-Zadeh J, et al. Free cortisol index as a surrogate marker for serum free cortisol. Ann Clin Biochem. 2002;39:406–8. doi: 10.1258/000456302760042182. [DOI] [PubMed] [Google Scholar]
- 14.Meier CA, Biller BM. Clinical and biochemical evaluation of Cushing’s syndrome. Endocrinol Metab Clin North Am. 1997;26:741–62. doi: 10.1016/s0889-8529(05)70280-2. [DOI] [PubMed] [Google Scholar]
- 15.Umeda T, Hiramatsu R, Iwaoka T, et al. Use of saliva for monitoring unbound free cortisol levels in serum. Clin Chim Acta. 1981;110:245–53. doi: 10.1016/0009-8981(81)90353-3. [DOI] [PubMed] [Google Scholar]
- 16.Papanicolaou DA, Mullen N, Kyrou I, et al. Nighttime salivary cortisol: a useful test for the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 2002;87:4515–21. doi: 10.1210/jc.2002-020534. [DOI] [PubMed] [Google Scholar]
- 17.Spark RF, White RA, Connolly PB. Impotence is not always psychogenic: newer insights into hypothalamic-pituitary-gonadal dysfunction. JAMA. 1980;243:750–5. [PubMed] [Google Scholar]
- 18.Derksen J, Nagesser SK, Meinders AE, et al. Identification of virilizing adrenal tumors in hirsute women. N Engl J Med. 1994;331:968–73. doi: 10.1056/NEJM199410133311502. [DOI] [PubMed] [Google Scholar]
- 19.Pang SP, Wallace MA, Hofman L, et al. Worldwide experience in newborn screening for classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics. 1988;81:866–74. [PubMed] [Google Scholar]
- 20.Speiser PW, Dupont B, Rubinstein P, et al. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650–67. [PMC free article] [PubMed] [Google Scholar]
- 21.New MI, Lorenzen F, Lerner AJ, et al. Genotyping steroid 21-hydroxylase deficiency: hormonal reference data. J Clin Endocrinol Metab. 1983;57:320–6. doi: 10.1210/jcem-57-2-320. [DOI] [PubMed] [Google Scholar]
- 22.Rosler A, Leiberman E, Cohen T. High frequency of congenital adrenal hyperplasia (classic 11 beta-hydroxylase deficiency) among Jews from Morocco. Am J Med Genet. 1992;42:827–934. doi: 10.1002/ajmg.1320420617. [DOI] [PubMed] [Google Scholar]
- 23.Soldin SJ, Papanastasiou-Diamandis A, Heyes J, et al. Are immunoassays for digoxin reliable? Clin Biochem. 1984;17:317–20. doi: 10.1016/s0009-9120(84)90637-4. [DOI] [PubMed] [Google Scholar]
- 24.Thong B, Soldin SJ, Lingwood CA. Lack of specificity of current anti-digoxin antibodies, and preparation of a new, specific polyclonal antibody that recognizes the carbohydrate moiety of digoxin. Clin Chem. 1985;31:1625–31. [PubMed] [Google Scholar]
- 25.Soldin SJ. Digoxin—issues and controversies. Clin Chem. 1986;32:5–12. [PubMed] [Google Scholar]
- 26.Soldin SJ, Stephey C, Giesbrecht E, et al. Further problems with digoxin measurement. Clin Chem. 1986;32:1591. [PubMed] [Google Scholar]
- 27.Koren G, Farine D, Grundmann H, et al. Endogenous digoxin-like substance(s) associated with uneventful and high-risk pregnancies. Dev Pharmacol Ther. 1988;11:82–7. doi: 10.1159/000457670. [DOI] [PubMed] [Google Scholar]
- 28.Stone JA, Soldin SJ. An update on digoxin. Clin Chem. 1989;35:1326–31. [PubMed] [Google Scholar]
- 29.Stone J, Bentur Y, Zalstein E, et al. Effect of endogenous digoxin-like substances on the interpretation of high concentrations of digoxin in children. J Pediatr. 1990;117:321–5. doi: 10.1016/s0022-3476(05)80555-4. [DOI] [PubMed] [Google Scholar]
- 30.Murthy JN, Yatscoff RW, Soldin SJ. Cyclosporine metabolite cross-reactivity in different cyclosporine assays. Clin Biochem. 1998;31:159–63. doi: 10.1016/s0009-9120(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 31.Murthy JN, Davis DL, Yatscoff RW, et al. Tacrolimus metabolite cross-reactivity in different tacrolimus assays. Clin Biochem. 1998;31:613–7. doi: 10.1016/s0009-9120(98)00086-1. [DOI] [PubMed] [Google Scholar]
- 32.Shen S, Elin RJ, Soldin SJ. Characterization of cross-reactivity by carbamaz-epine 10, 11-epoxide with carbamazepine assays. Clin Biochem. 2001;34:157–8. doi: 10.1016/s0009-9120(01)00186-2. [DOI] [PubMed] [Google Scholar]
- 33.Ghoshal AK, Soldin SJ. IMx tacrolimus II assay: is it reliable at low blood concentrations? A comparison with tandem. MS/MS Clin Biochem. 2002;35:389–92. doi: 10.1016/s0009-9120(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 34.Soldin SJ, Steele BW, Witte DL, Wang E, Elin RJ. College of American Pathologists Study. Lack of specificity of cyclosporine immunoassays. Results of a College of American Pathologists Study. Arch Pathol Lab Med. 2003;127:19–22. doi: 10.5858/2003-127-19-LOSOC. [DOI] [PubMed] [Google Scholar]
- 35.Steele BW, Wang E, Soldin SJ, Klee G, Elin RJ, Witte DL. College of American Pathologists Study. A longitudinal replicate study of immunosuppressive drugs: a College of American Pathologists study. Arch Pathol Lab Med. 2003;127:283–8. doi: 10.5858/2003-127-0283-ALRSOI. [DOI] [PubMed] [Google Scholar]
- 36.College of American Pathologists Proficiency Testing Program. Surveys 2002 Y-A Ligands (Special) 2002. [Google Scholar]
- 37.Joos PE, Ryckeghem MV. Liquid chromatography-tandem mass spectrometry of some anabolic steroids. Anal Chem. 1999;71:4701–10. doi: 10.1021/ac981073s. [DOI] [PubMed] [Google Scholar]
- 38.Dorgan JF, Fears TR, McMahon RP, et al. Measurement of steroid sex hormones in serum: a comparison of radioimmunoassay and mass spectrometry. Steroids. 2002;67:151–8. doi: 10.1016/s0039-128x(01)00147-7. [DOI] [PubMed] [Google Scholar]
- 39.Chang YC, Li CM, Li LA, et al. Quantitative measurement of male steroid hormones using automated on-line solid phase extraction-liquid chromatography-tandem mass spectrometry and comparison with radioimmunoassay. Analyst. 2003;128:363–8. doi: 10.1039/b210111b. [DOI] [PubMed] [Google Scholar]
- 40.Ohno M, Yamaguchi I, Saiki K, et al. Specific determination of urinary 6b-hydroxycortisol and cortisol by liquid chromatography—atmospheric pressure chemical ionization mass spectrometry. J Chromatogr B. 2000;746:95–101. doi: 10.1016/s0378-4347(00)00122-5. [DOI] [PubMed] [Google Scholar]
- 41.Tang PW, Law WC, Wan TSM. Analysis of corticosteroids in equine urine by liquid chromatography–mass spectrometry. J Chromatogr B. 2001;754:229–44. doi: 10.1016/s0378-4347(00)00613-7. [DOI] [PubMed] [Google Scholar]
- 42.Taylor RL, Machacek D, Singh RJ. Validation of a high-throughput liquid chromatography–tandem mass spectrometry method for urinary cortisol and cortisone. Clin Chem. 2002;48:1511–9. [PubMed] [Google Scholar]
- 43.Jönsson BAG, Malmberg B, Amilon Å, et al. Determination of cortisol in human saliva using liquid chromatography–electrospray tandem mass spectrometry. J Chromatogr B. 2003;784:63–8. doi: 10.1016/s1570-0232(02)00753-5. [DOI] [PubMed] [Google Scholar]
- 44.Kushnir MM, Rockwood AL, Nelson GJ, et al. Liquid chromatography–tandem mass spectrometry analysis of urinary free cortisol. Clin Chem. 2003;49:965–7. doi: 10.1373/49.6.965. [DOI] [PubMed] [Google Scholar]
- 45.Kao PC, Machacek DA, Magera MJ, et al. Diagnosis of adrenal cortical dysfunction by liquid chromatography-tandem mass spectrometry. Ann Clin Lab Sci. 2001;31:199–204. [PubMed] [Google Scholar]
- 46.Chatman K, Hollenbeck T, Hagey L, et al. Nanoelectrospray mass spectrometry and precursor ion monitoring for quantitative steroid analysis and attomole sensitivity. Anal Chem. 1999;71:2358–63. doi: 10.1021/ac9806411. [DOI] [PubMed] [Google Scholar]
- 47.Wu ZP, Zhang C, Yang CD, et al. Simultaneous quantitative determination of norgestrel and progesterone in human serum by high-performance liquid chromatography-tandem mass spectrometry with atmospheric pressure chemical ionization. Analyst. 2000;125:2201–5. doi: 10.1039/b005631f. [DOI] [PubMed] [Google Scholar]
- 48.Wudy SA, Hartmann M, Svoboda M. Determination of 17-hydroxyprogesterone in plasma by stable isotope dilution/benchtop liquid chromatography-tandem mass spectrometry. Horm Res. 2000;53:68–71. doi: 10.1159/000023516. [DOI] [PubMed] [Google Scholar]
- 49.Isobe T, Shiraishi H, Yasuda M, et al. Determination of estrogens and their conjugates in water using solid-phase extraction followed by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2003;984:195–202. doi: 10.1016/s0021-9673(02)01851-4. [DOI] [PubMed] [Google Scholar]
- 50.Robb DB, Covey TR, Bruins AP. Atmospheric pressure photoionization: an ionization method for liquid chromatography-mass spectrometry. Anal Chem. 2000;72:3563–659. doi: 10.1021/ac0001636. [DOI] [PubMed] [Google Scholar]
- 51.Alary JF. A010942. Comparative Study: LC-MS/MS analysis of four steroid compounds using a new photoionization source and a conventional APC1 source. Proceedings of the 49th ASMS Conference on Mass Spectrometry and Allied Topics (CD-ROM); Chicago. May 27–31, 2001. [Google Scholar]
- 52.Lykkesfeldt J, Loft S, Poulsen HE. Simultaneous determination of urinary free cortisol and 6 beta-hydroxy cortisol by high performance liquid chromatography to measure human CYP 3A activity. J Chromatogr B Biomed Appl. 1994;660:23–9. doi: 10.1016/0378-4347(94)00265-7. [DOI] [PubMed] [Google Scholar]