Abstract
Aims
To test the hypothesis that the expression of aquaporins (AQPs) 4 and 5 is altered in the lacrimal glands (LG) of rabbits with induced autoimmune dacryoadenitis (IAD).
Materials and Methods
LGs were obtained from adult female rabbits with IAD, and age-matched female control rabbits. LGs were processed for laser capture microdissection (LCM), real time RT-PCR, Western blot, and immunofluorescence for the detection and quantification of protein and mRNAs of AQP4 and AQP5 in whole LGs, and purified acinar cells and duct cells from specific duct segments.
Results
In rabbits with IAD, abundances of mRNAs for AQP4 and AQP5 from whole LGs were significantly lower than controls. Levels of mRNA for AQP4 were lower in most duct segments from rabbits with IAD. However, the mRNA abundance for AQP5 was significantly lower in acini from rabbits with IAD, while its abundance was higher in each duct segment. Western blot showed that the expression of AQP4 in LGs from rabbits with IAD was 36% more abundant than normal controls, whereas AQP5 was 72% less abundant. Immunofluorescence indicated that AQP4 immunoreactivity (AQP4-IR) was present on the basolateral membranes of acinar and ductal cells in control and diseased LGs, with ductal cells showing stronger AQP4-IR than acinar cells. AQP5-IR was found on apical and basolateral membranes of acinar cells, and showed a “mosaic” pattern, i.e., with some acini and/or acinar cells showing stronger AQP5-IR than others. Minimal AQP5-IR was detected in ductal cells from control animals, while its intensity was significantly increased in rabbits with IAD.
Conclusions
These data strongly support our hypothesis that expressions of AQPs are altered in rabbits with IAD, and that specific ductal segment play important roles in lacrimal secretion.
Keywords: Lacrimal gland, Aquaporin, Sjögren’s syndrome, Dry eye
INTRODUCTION
Dry eye syndrome afflicts millions of people worldwide, and is the leading cause of visits to eye care clinicians.1 Sjögren’s syndrome is an autoimmune disease that causes functional impairment of the lacrimal and salivary glands, and is one of the most frequent causes of dry eye. Although many theories have been proposed to explain the etiologies of secretory dysfunction in Sjögren’s syndrome, the exact mechanism is still unknown.2
Rabbits with induced autoimmune dacryoadenitis (IAD) mimic many of the ocular surface symptoms as well as lacrimal gland (LG) pathologic features characteristic of Sjögren’s syndrome, and have been used extensively to study its pathophysiology.3–5
Lacrimal fluid secretion is an osmotic process driven by transepithelial secretion of electrolytes that is mediated by ion transporters and following water movement.6–9 Since water movement is involved in lacrimal secretion, aquaporins (AQPs) have been proposed to be involved in lacrimal fluid production.
AQPs, a group of water channel proteins that are responsible for rapid movement of water across plasma membranes, have recently been identified to be involved in transepithelial water movement in many organisms.10–12 At least 13 subtypes of AQPs have been characterized in various tissue types, and at least two subtypes have been found in LGs. In mice and humans, aquaporin 5 (AQP5) is localized to the apical membranes of lacrimal acinar and ductal cells.13–19 Aquaporin 4 (AQP4) is found on the basolateral membranes of acinar and ductal cells in mouse.13 In rabbit, we recently reported that AQP4 was found in the basolateral sides of acinar and duct cells, and AQP5 in both apical and basolateral sides of acinar cells.20 Interestingly, AQP5 immunoreactivity (AQP5-IR) in the rabbit LG showed a “mosaic” pattern, with some acini and/or acinar cells demonstrating much stronger staining than others.
Available data of AQP5 and AQP4 appear to support their involvement in normal secretion and their contribution to the pathogenesis of Sjögren’s syndrome in the LG16,17,19,21–23 although some knockout studies indicated that AQPs may not be required at physiological conditions.15,18
Like other exocrine secretions, lacrimal fluid is believed to be produced in two stages, formation of primary fluid in the acini and modification into the final fluid during transit through the duct system. Most studies have focused on the roles that acinar cells play in lacrimal secretion, with fewer considering the functional roles of the duct system.7,8,20,24 However, increasing evidence supports the notion that the lacrimal duct system also plays an important role in lacrimal secretion.
The aim of the present study was to study the expression changes of AQP4 and AQP5 in whole LGs, acinar cells and ductal cells from specific duct segments in rabbits with IAD. It is our hypothesis that there are significant changes of these two AQPs in acini and the duct system in rabbits with IAD.
MATERIALS AND METHODS
Animals
Adult female New Zealand White rabbits (Irish Farms, Norco, CA) were used throughout this study. The procedures for the generation of IAD have been previously published.3,4 Rabbits were narcotized with a mixture of ketamine (40 mg/ml) and xylazine (10 mg/ml) and given an overdose of Nembutal (80 mg/kg) for euthanasia. Inferior LGs were removed and embedded in OCT, frozen in liquid nitrogen, and stored at −80°C until use. This study conformed to the standards and procedures for the proper care and use of animals as described in the ARVO Statement for the Use of Animals in Ophthalmic Research.
Laser Capture Microdissection
Frozen sections were collected with PEN membrane-coated slides (Leica Microsystems) and stained with cresyl violet in RNase-free conditions with the LCM Staining Kit (Applied Biosystems, Foster City, CA). Areas of interest in tissue sections were laser captured using a PixCell II LCM System (Arcturus Bioscience, Mountain View, CA). Around 100 cells were collected for each acinus and duct segment sample, and six replicate samples of acinar cells and epithelial cells from each duct segment were collected from each animal.20
RNA extraction and reverse transcription
Total cellular RNA was isolated from RNALater-treated samples with RNeasy® midiKit plus on-column DNase digestion to reduce the possibility of DNA contamination (Qiagen, Valencia, CA). RNA quality and quantity was evaluated using a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). Five microgram samples of total RNA samples were then reverse-transcribed to cDNA only if the 260/280 ratio was above 1.9 (High Capacity cDNA Reverse Transcription Kit containing random primers and MultiScribe™ Reverse Transcriptase, Applied Biosystems) according to the manufacturer’s instructions.
Real time RT-PCR Analysis and Pre-amplification
The sequences of primers and probes used in this study were listed in our previous publication.20 Sequences were selected using Primer Express™ software (Applied Biosystems) and synthesized by Applied Biosystems. All probes incorporated the 5′ reporter dye 6-carboxyfluorescein (FAM) and the 3′ quencher dye 6-carboxytetramethylrhodamine (TAMRA).
For LCM samples, pre-amplification was performed using TaqMan® PreAmp Master Mix Kit (Applied Biosystems). The pooled assay mix was prepared by combining up to 50 of 20× TaqMan® Gene Expression Assays into a single tube and using Nuclease-free water to dilute the pooled assays to a final concentration of 0.2×. The 50 μl pre-amplification reaction included 25 μl of 2× TaqMan® PreAmp Master Mix, 12.5 μl of 0.2× pooled assay mix, and 12.5 μl of cDNA sample. The reactions were incubated in the DNA Engine® Thermal Cycler for 10 min at 95°C followed by 14 cycles at 95°C for 15 sec and 4 min at 60°C and then held at 4°C. The pre-amplification product was then diluted 1:20 with 1× TE buffer and analyzed by TaqMan® real time RT-PCR.
Amplification was carried out with an ABI PRISM® 7900HT Sequence Detection System (Applied Biosystems) using TaqMan® Gene Expression Master Mix (Applied Biosystems) containing the internal dye, ROX, as a passive reference. The PCR reaction volume was 10 μl. It contained 1×TaqMan® Gene Expression Master Mix, 300 nM forward and reverse primers, 250 nM probes, and 50 ng of cDNA template. The FAM signal was measured against the ROX signal to normalize for non-PCR-related fluorescence fluctuations. The cycle threshold (CT) value represented the refraction cycle number at which a positive amplification reaction was measured and was set at 10× the standard deviation (SD) of the mean baseline emission calculated for PCR cycles 3–15. Each sample was measured in triplicate. The difference between the CT values for each target mRNA and for the internal housekeeping gene, GAPDH, in each sample was used to calculate the abundance of target mRNA relative to the abundance of GAPDH mRNA in the same sample.
Immunofluorescence and Confocal Microscopy
Primary antibodies used were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The dilution for AQP4 (goat polyclonal, C-19) was 1:50, and 1:40 for AQP5 (goat polyclonal, C-19). Secondary antibodies used were fluorescein isothiocyanate (FITC)-conjugated AffiniPure donkey anti-goat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) at a dilution of 1:200.
The immunofluorescence technique has been described in detail previously.20 The slides were observed with a confocal laser scanning microscope (Zeiss LSM 710, Thornwood, NY). FITC-conjugated secondary antibodies were visualized by excitation at 488 nm using an argon laser. Images were analyzed with LSM image browser software and PhotoShop (Adobe Systems, Mountain View, CA).
Western Blot
LG samples were homogenized in isolation buffer (5% sorbitol, 0.5mM disodium EDTA, 0.2 mM phenylmethylsulfonyl fluoride, protease inhibitor cocktail, 5 mM histidine-imidazole buffer, pH 7.5), and centrifuged at 2000 ×g for 20 min. The supernatants were denatured in SDS-PAGE sample buffer for 20 min at 60°C, resolved on a 4–20% gradient SDS-PAGE gel (Bio-Rad), and then transferred onto PVDM (Millipore Immobilon-P). To assess AQP proteins, a constant amount of protein from each sample was analyzed. Membrane blots were probed with AQP4 at the dilution of 1:250, and AQP5 at 1:1000. All blots were incubated with Alexa 680-labeled donkey anti-goat secondary antibody (Molecular Probes, Eugene, OR) and detected with an Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE). Densitometry analysis of resulting gel was performed by the manufacturer’s software.
Statistics
For mRNA data from whole gland samples, unpaired t-tests were performed. Relative mRNA abundance data from LCM samples and densitometry results of Western blots were subjected to analysis of variance (ANOVA) with Sigmaplot 11.0 (Systat Software, San Jose, CA).
RESULTS
Expression of mRNAs for AQP4 and AQP5
The relative abundance of AQP4 mRNA from whole LGs was 0.068 ± 0.008 in control animals, while its level was decreased to 0.044 ± 0.004 in animals with IAD (p < 0.05), a 34.4% decrease (Figure 1).
FIGURE 1.
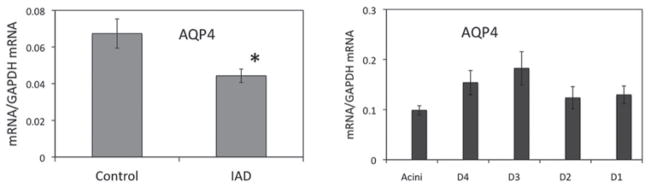
Real-time-PCR of AQP4. The abundance of AQP4 mRNA extracted from whole LGs (left panel) was significantly lower in animals with IAD (p < 0.05, indicated by *, same in all the following figures), represented by a 34.4% decrease. In LCM samples from rabbits with IAD (right panel), mRNA abundance was the lowest in acini, and its abundance was significantly higher in intralobular and interlobular ducts. D4: intralobular duct. D3: interlobular duct. D2: intralobar duct. D1: interlobar duct. Data are presented as mean ± standard error of the mean (SEM) of three animals.
Data from LCM samples from rabbits with IAD indicated that ducts were generally more abundant with AQP4 mRNA than acini, which was consistent with our previous results from normal rabbits.20 In control rabbits, mRNA of AQP4 was significantly more abundant in intralobular, intralobar, and inter-lobar ducts than in interlobular ducts.20 In rabbits with IAD, while mRNA abundance of AQP4 from acini was similar to acini from control animals, the abundance was significantly lower in intralobular, intralobar, and interlobar ducts.
For mRNA of AQP5 extracted from whole LGs, its abundance was reduced from 7.1 ± 0.244 in control rabbits to 3.351 ± 0.087 in IAD animals, a 52.8% decrease (p < 0.05). The relative abundances of mRNAs for AQP4 and AQP5 differed substantially, with mRNA for AQP5 being approximately 100-fold more abundant than AQP4 (Figure 2).
FIGURE 2.
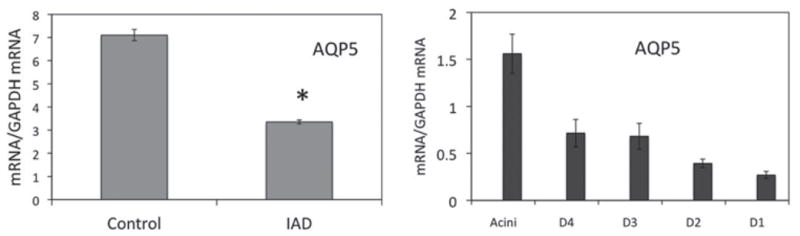
Real-time-PCR of AQP5. The abundance of AQP5 mRNA extracted from whole LG (left panel) was significantly lower than that of control animals (*p < 0.05), represented by a 52.8% decrease. Note the substantial differences of relative abundance of mRNAs between AQP4 and AQP5. In LCM samples from rabbits with IAD (right panel), the abundance of AQP5 mRNA showed a completely different pattern as compared to AQP4. mRNA abundance was the highest in acini and appeared to decrease as the ducts became bigger, with the lowest observed in the interlobar duct. D4: intralobular duct. D3: interlobular duct. D2: intralobar duct. D1: interlobar duct. Data are presented as mean ± SEM of three animals.
In LCM samples from rabbits with IAD, AQP5 mRNA abundance was highest in acini, and its levels decreased gradually as the ducts became bigger, with the lowest found in interlobar ducts, i.e., the largest duct segments sampled. The distribution pattern was roughly similar to that from normal control samples.20 Compared to normal control rabbits, mRNA abundance of AQP5 from rabbits with IAD was significantly lower in acini, whereas its levels were generally higher in all the duct segments, albeit the difference was only significant in interlobular ducts (P < 0.05).20
Western Blot and Densitometry
Immunoblotting of whole LG homogenates demonstrated the expression of AQP4 and AQP5 proteins, as shown in Figure 3. Densitometry analysis showed that the expression of AQP4 in LGs from animals with IAD was 36% more abundant than normal controls, whereas the expression of AQP5 was 72% less abundant in animals with IAD (p < 0.05).
FIGURE 3.
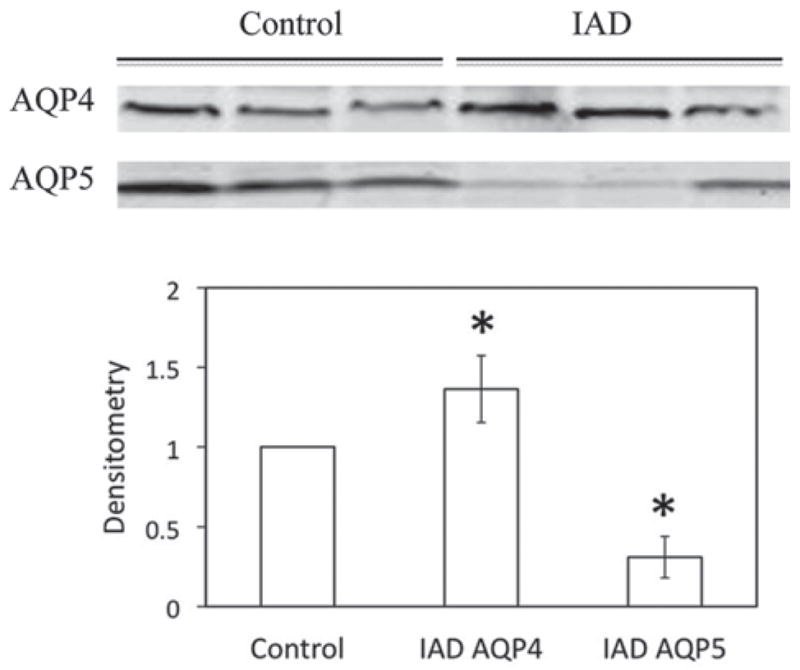
Western blots of AQP4 and AQP5 from whole LG homogenates. AQP4 was significantly increased in LGs from rabbits with IAD, while AQP5 was significantly decreased (*p < 0.05). Data are from three animals each.
Immunofluorescence
AQP4
AQP4 immunoreactivity (AQP4-IR) was observed on the basolateral sides of acinar and ductal cells, with ductal cells showing stronger AQP4-IR than acinar cells (Figure 4). AQP4-IR in acini appears to be membrane associated, in close proximity with or on the basal membrane. No staining of apical membranes was evident in either acinar or ductal cells. AQP4-IR in rabbits with IAD showed a similar distribution pattern to that of control animals.
FIGURE 4.
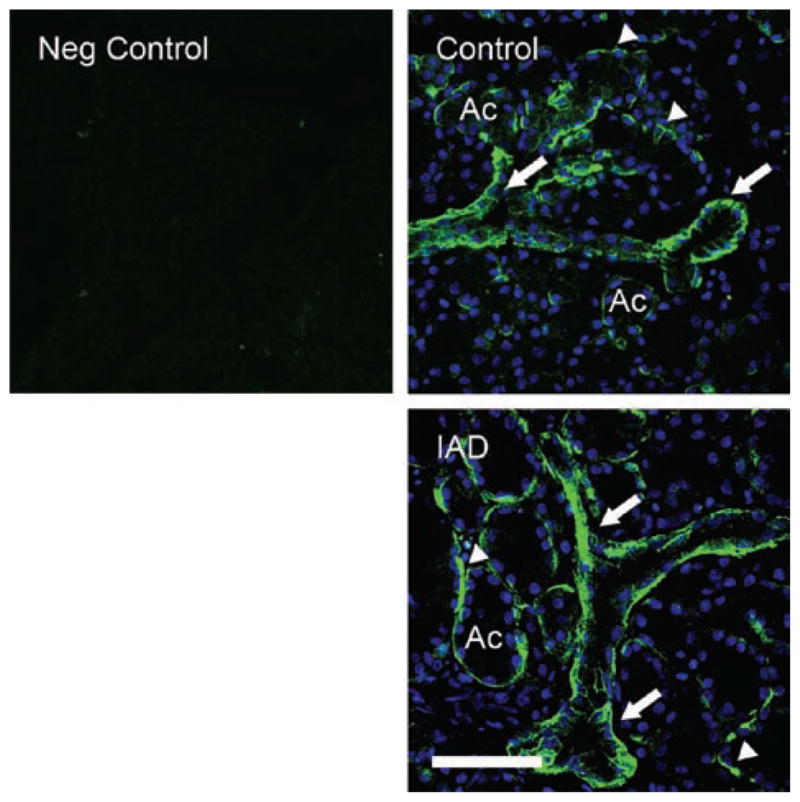
Immunofluorescence of AQP4-IR. Neg Control: negative control without primary antibody, which is for both AQP4 and AQP5. Control: AQP4-IR was observed on the basolateral sides of acinar and duct cells, with ducts (arrows) showing much stronger AQP4-IR than acini (arrowheads). IAD: AQP4-IR in rabbits with IAD showed a similar distribution pattern to that of control animals. In both images, DAPI was used to stain nuclei as bright blue to demonstrate the morphologic profiles of acini and ducts. Ac = acinus. Scale bar = 50 μm.
AQP5
AQP5-IR was found in apical and basolateral membranes of all acinar cells, and distributed among acini in a “mosaic” pattern, with some acini and/or acinar cells in both control rabbits and rabbits with IAD exhibiting stronger AQP5-IR than other acini/acinar cells (Figure 5). Virtually no AQP5-IR was detected in ductal cell membranes of control LGs, in contrast to the significant staining observed in the ducts of rabbits with IAD.
FIGURE 5.
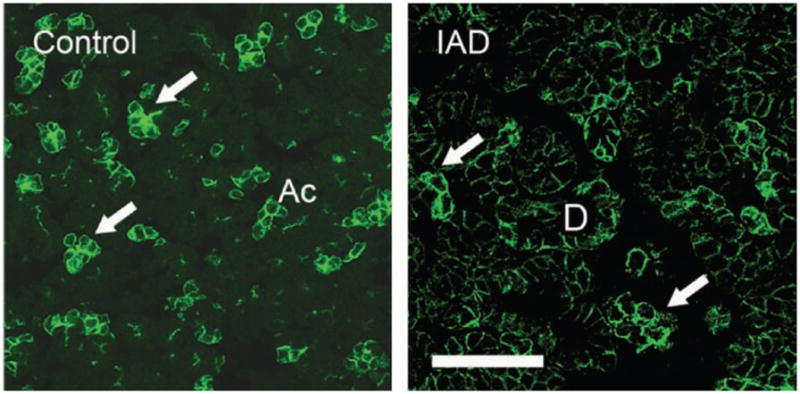
Immunofluorescence of AQP5-IR. Control: AQP5-IR was present in both basolateral and apical membranes of all acinar cells, and distributed among acini in a “mosaic” pattern, with some acini and/or acinar cells demonstrating stronger AQP5-IR (arrows) than the rest of the acini/acinar cells. However, minimal or no AQP5-IR was detected in duct cells. IAD: Like control LGs, AQP5-IR was also present in a “mosaic” pattern (arrows). In contrast to control animals, ductal cells also exhibited significant amounts of AQP5-IR (D = interlobular duct). Ac = acinus. Scale bar = 50 μm.
DISCUSSION
AQPs are the membrane proteins that are responsible for fast water transport across plasma membranes, and the direction water flows is determined by osmotic gradients that are generated by ion transport proteins. Our data confirmed our previous report of the presence of AQP4 and AQP5 in the rabbit LG, which was the first report of the presence of these two subtypes of AQP in the rabbit LG.20 The detection of these two AQPs in the LG suggests that they may play a role in lacrimal secretion.
Our findings indicate that AQP4 is primarily located in the basolateral membranes of both acinar and ductal cells, which is in agreement with previous reports in mouse13 and rat.25 AQP5 was located in both apical and basolateral membranes, consistent with previous reports in mouse13,26,27 and humans.16 Regarding the acinar and ductal cells, it’s evident that AQP4 is predominantly in the duct system, while AQP5 is predominantly in the acini, as illustrated in Figure 6. It is worth noting that the relative abundance of mRNA of AQP5 was about 40 fold that of AQP4 in the acini, while the difference in ducts was much less.
FIGURE 6.
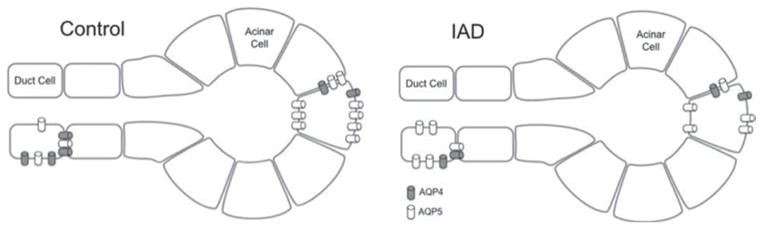
Cellular models of the changes of AQPs in LGs from control rabbits and rabbits with IAD. In acinar cells, AQP4 amounts remained the same during IAD, whereas AQP5 was significantly decreased. In ductal cells, AQP4 amounts decreased during IAD whereas AQP5 increased.
Our results demonstrate that the expression patterns of AQPs in the LGs of IAD animals undergo significant changes, at both gene and protein levels, in acini and specific duct segments. The biggest change happened in acini, where the mRNA abundance of AQP5 in LGs from rabbits with IAD was only 1/3 that of normal rabbits. Similar changes of AQPs have been reported previously.16,17
Although it has been shown that in AQP knockout mice, the ability to secrete lacrimal fluid15,18 and sweat28 was not significantly reduced, in contrast to the significant loss of salivary secretion,29 we believe data from these knockout experiments do not in themselves preclude AQPs’ potential involvement in lacrimal secretion. While knockout mice technology represents a valuable research tool, some important limitations exist, as detailed by the NIH. Therefore, although these studies provide evidence that AQPs may not play a major role in LG secretion, care should be taken in interpreting these results and additional studies are warranted to elucidate AQPs’ role in LG function.
It has been suggested that AQPs are not required at physiological conditions when the secretory rate is low.15,30 Salivary gland secretion rate is very large, with an average daily output of ~75–1,000 ml of saliva in humans,31 therefore AQPs are heavily involved. However, the normal rate of tear production is only ~5 ml per day in humans31, and water secretion, following ionic gradient, can be sufficiently achieved by AQP-independent water permeability by glandular epithelial cells which means AQPs are not required at physiological conditions.30
However, our data shows that the LG is rich in both AQP4 and AQP5, in support of the notion that AQPs may play critical role in LG secretion. Of special interest to us, as the current study is part of a larger project focused on the lacrimal duct system, is the finding that lacrimal ducts are also rich in AQP4 and AQP5, which strongly supports the notion that the duct system also plays an important role in lacrimal secretion. These data are in accordance with previous reports regarding lacrimal ducts’ active role in LG secretion.7,8,20,24
Our recent report showed that the rabbit lacrimal duct system can be divided into four distinctive segments, based on their anatomical and morphological characteristics.20 Real time-PCR data also demonstrated that the ducts are rich in ion transporters and AQPs and there were significant variations among these duct segments, suggesting that lacrimal ducts play critical role in lacrimal secretion and each segment may also have its own distinctive contributions.
In LGs from rabbits with IAD, the present data showed that AQP5 mRNA was increased in ducts, while decreased in acini, which suggests that acini and ducts may play different roles in lacrimal function. In mouse LG, it was reported that in response to pilocarpine-induced lacrimal secretion, AQP5 was increased at the apical membrane of acinar cells.13 In mouse LG with dacryoadenitis, AQPs were found in the tears, suggesting leakage from LG epithelial cells.14 Tsubota and colleagues found that AQP5 processing was disrupted in the LG of Sjögren’s syndrome patients.16 AQP5 was found to congregate within the cytoplasm rather than being transported to the membranes, suggesting AQP5 misprocessing might be involved in the pathogenesis of Sjögren’s syndrome. However, other researchers reported findings that were in contrast to theirs.21
In ductal cells, the significant increase of AQP5-IR in rabbits with IAD, at both protein and mRNA levels, suggests increased transepithelial water transport across the apical membranes. Although water flow could be bidirectional, i.e., either increased secretion or increased reabsorption, the reduced final lacrimal fluid secretion from rabbits with IAD,4 is indicative of increased reabsorption by ductal cells. At the same time, because the AQP4 amount was decreased in duct cells and AQP5 decreased in acinar cells during IAD, there are many potential combinational changes of altered water transport in acinar and duct cells, as documented by previous reports.13,14,16,21 This topic is beyond the scope of the present study and warrants further investigations.
We recognize that there are several other animal models that have been used widely for Sjögren’s syndrome-related dry eye studies, especially murine strains with genetic mutations.32–34 While studies using these models provide valuable information regarding the etiology of Sjögren’s syndrome, the rabbit IAD model has also been demonstrated to be an excellent model and has been used extensively.3–5 We believe studies using this rabbit model, one without genetic defect in contrast to the murine models, will also help us to understand the etiology of lacrimal dysfunction in Sjögren’s syndrome.
We recognize that there were some discrepancies in mRNA and protein expressions of the data present here, which have also been reported in previous studies that many mRNA expression differences are not reflected at the protein levels.35,36 Changes in protein redistribution and recycling during inflammation, such as IAD, may be possible reasons.9,37 Furthermore, differences in protein expressions may also not always reflect functional status in the phenotype. This topic is beyond the scope of the present study but highlights the need and importance of functional studies to investigate the functional changes of LG in rabbits with IAD.
In conclusion, our findings showed that expressions of AQP4 and AQP5 are altered in the LG in rabbits with IAD. These data also support our previous findings that acini and ducts play different roles in LG secretion and that both acinar and ductal cells play important roles in lacrimal secretion. However, further studies are warranted to elucidate the functional implications of these changes.
Acknowledgments
This work was supported by NIH grants EY017731 (CD), EY012689 (MDT), EY005801, EY010550, EY03040 (Doheny Eye Institute Core), and DK048522. The authors thank Drs Austin Mircheff and Joel Schechter for many helpful comments and discussions in the experimental design and manuscript preparation; and Leili Parsa, Tamako Nakamura, Ernesto Barron, and Michele MacVeigh Aloni for excellent technical support.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Pflugfelder S, Tseng S, Sanabria O, Kell H, Garcia C, Felix C, Feuer W, Reis B. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998;17:38–56. doi: 10.1097/00003226-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Zoukhri D. Effect of inflammation on lacrimal gland function. Exp Eye Res. 2006;82:885–898. doi: 10.1016/j.exer.2005.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo Z, Song D, Azzarolo A. Autologous lacrimal lymphoid mixed-cell reactions induce dacryoadenitis in rabbits. Exp Eye Res. 2000;71:23–31. doi: 10.1006/exer.2000.0855. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Z, Stevenson D, Schechter J. Lacrimal histopathology and ocular surface disease in a rabbit model of autoimmune dacryoadenitis. Cornea. 2003;22:25–32. doi: 10.1097/00003226-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Thomas P, Zhu Z, Selvam S, Stevenson D, Mircheff AK, Schechter JE, Trousdale MD. Autoimmune dacryoadenitis and keratoconjunctivitis induced in rabbits by subcutaneous injection of autologous lymphocytes activated ex vivo against lacrimal antigens. J Autoimmun. 2008;31:116–122. doi: 10.1016/j.jaut.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dartt D, Moller M, Poulsen J. Lacrimal gland electrolyte and water secretion in the rabbit: Localization and role of Na+/K+-activated ATPase. J Physiol. 1981;321:557–569. doi: 10.1113/jphysiol.1981.sp014002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mircheff A. Lacrimal fluid and electrolyte secretion: A review. Curr Eye Res. 1989;8:607–617. doi: 10.3109/02713688908995761. [DOI] [PubMed] [Google Scholar]
- 8.Ubels J, Hoffman H, Srikanth S, Resau J, Webb C. Gene expression in rat lacrimal gland duct cells collected using laser capture microdissection: Evidence for K+ secretion by duct cells. Invest Ophthalmol Vis Sci. 2006;47:1876–1885. doi: 10.1167/iovs.05-0363. [DOI] [PubMed] [Google Scholar]
- 9.Selvam S, Thomas P, Gukasyan H, Yu A, Stevenson D, Trousdale M, Mircheff AK, Schechter J, Smith R, Yiu S. Transepithelial bioelectrical properties of rabbit acinar cell monolayers on polyester membrane scaffolds. Am J Physiol Cell Physiol. 2007;293:C1412–1419. doi: 10.1152/ajpcell.00200.2007. [DOI] [PubMed] [Google Scholar]
- 10.Raina S, Preston GM, Guggino WB, Agre P. Molecular cloning and characterization of an aquaporin cDNA from salivary, lacrimal, and respiratory tissues. J Biol Chem. 1995;270:1908–1912. doi: 10.1074/jbc.270.4.1908. [DOI] [PubMed] [Google Scholar]
- 11.Lee MD, Bhakta KY, Raina S, Yonescu R, Griffin CA, Copeland NG, Gilbert DJ, Jenkins NA, Preston GM, Agre P. The human Aquaporin-5 gene. Molecular characterization and chromosomal localization. J Biol Chem. 1996;271:8599–8604. doi: 10.1074/jbc.271.15.8599. [DOI] [PubMed] [Google Scholar]
- 12.Ishida N, Maruo J, Mita S. Expression and characterization of lacrimal gland water channels in Xenopus oocytes. Biochem Biophys Res Commun. 1996;224:1–4. doi: 10.1006/bbrc.1996.0974. [DOI] [PubMed] [Google Scholar]
- 13.Ishida N, Hirai SI, Mita S. Immunolocalization of aquaporin homologs in mouse lacrimal glands. Biochem Biophys Res Commun. 1997;238:891–895. doi: 10.1006/bbrc.1997.7396. [DOI] [PubMed] [Google Scholar]
- 14.Hirai S, Ishida N, Watanabe K, et al. Leakage of aquaporin 5 in the tear of dacryoadenitis mice. Invest Ophthalmol Vis Sci. 2000;41:2432–2437. [PubMed] [Google Scholar]
- 15.Moore M, Ma T, Yang B, Verkman AS. Tear secretion by lacrimal glands in transgenic mice lacking water channels AQP1, AQP3, AQP4 and AQP5. Exp Eye Res. 2000;70:557–62. doi: 10.1006/exer.1999.0814. [DOI] [PubMed] [Google Scholar]
- 16.Tsubota K, Hirai S, King LS, Agre P, Ishida N. Defective cellular trafficking of lacrimal gland aquaporin-5 in Sjogren’s syndrome. Lancet. 2001;357:688–689. doi: 10.1016/S0140-6736(00)04140-4. [DOI] [PubMed] [Google Scholar]
- 17.Konttinen YT, Tensing EK, Laine M, et al. Abnormal distribution of aquaporin-5 in salivary glands in the NOD mouse model for Sjögren’s syndrome. J Rheumatol. 2005;32:1071–1075. [PubMed] [Google Scholar]
- 18.Sasaki Y, Tsubota K, Kawedia JD, Menon AG, Yasui M. The difference of aquaporin 5 distribution in acinar and ductal cells in lacrimal and parotid glands. Curr Eye Res. 2007;32:923–929. doi: 10.1080/02713680701733076. [DOI] [PubMed] [Google Scholar]
- 19.Ohashi Y, Tsuzaka K, Takeuchi T, Sasaki Y, Tsubota K. Altered distribution of aquaporin 5 and its C-terminal binding protein in the lacrimal glands of a mouse model for Sjogren’s syndrome. Curr Eye Res. 2008;33:621–629. doi: 10.1080/02713680802262819. [DOI] [PubMed] [Google Scholar]
- 20.Ding C, Parsa L, Nandoskar P, et al. Duct system of the rabbit lacrimal gland: Structural characteristics and its role in lacrimal secretions. Invest Ophthalmol Vis Sci. 2010;51:2960–2967. doi: 10.1167/iovs.09-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinfeld S, Cogan E, King LS, Agre P, Kiss R, Delporte C. Abnormal distribution of aquaporin-5 water channel protein in salivary glands from Sjogren’s syndrome patients. Lab Invest. 2001;81:143–148. doi: 10.1038/labinvest.3780221. [DOI] [PubMed] [Google Scholar]
- 22.Beroukas D, Hiscock J, Jonsson R, Waterman SA, Gordon TP. Subcellular distribution of aquaporin 5 in salivary glands in primary Sjogren’s syndrome. Lancet. 2001;358(9296):1875–1876. doi: 10.1016/S0140-6736(01)06900-8. [DOI] [PubMed] [Google Scholar]
- 23.Beroukas D, Hiscock J, Gannon BJ, Jonsson R, Gordon TP, Waterman SA. Selective down-regulation of aquaporin-1 in salivary glands in primary Sjogren’s syndrome. Lab Invest. 2002;82:1547–1552. doi: 10.1097/01.lab.0000038502.42845.9e. [DOI] [PubMed] [Google Scholar]
- 24.Tóth-Molnár E, Venglovecz V, Ozsvari B, Rakonczay Z, Jr, Varro A, Papp J, Toth A, Lonovics J, Takacs T, Ignath I, Ivanyi B, Hegyi P. New experimental method to study acid/base transporters and their regulation in lacrimal gland ductal epithelia. Invest Ophthalmol Vis Sci. 2007;48:3746–3755. doi: 10.1167/iovs.06-1291. [DOI] [PubMed] [Google Scholar]
- 25.Hamann S, Zeuthen T, La Cour M, Nagelhus EA, Ottersen OP, Agre P, Nielsen S. Aquaporins in complex tissues: Distribution of aquaporins 1–5 in human and rat eye. Am J Physiol. 1998;274(5 Pt 1):C1332–1345. doi: 10.1152/ajpcell.1998.274.5.C1332. [DOI] [PubMed] [Google Scholar]
- 26.Funaki H, Yamamoto T, Koyama Y, et al. Localization and expression of AQP5 in cornea, serous salivary glands, and pulmonary epithelial cells. Am J Physiol. 1998;275:C1151–1157. doi: 10.1152/ajpcell.1998.275.4.C1151. [DOI] [PubMed] [Google Scholar]
- 27.Matsuzaki T, Suzuki T, Koyama H, Tanaka S, Takata K. Aquaporin-5 (AQP5), a water channel protein, in the rat salivary and lacrimal glands: Immunolocalization and effect of secretory stimulation. Cell Tissue Res. 1999;295:513–521. doi: 10.1007/s004410051257. [DOI] [PubMed] [Google Scholar]
- 28.Song Y, Sonawane N, Verkman AS. Localization of aqua-porin-5 in sweat glands and functional analysis using knockout mice. J Physiol. 2002;541:561–568. doi: 10.1113/jphysiol.2001.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krane CM, Melvin JE, Nguyen HV, et al. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem. 2001;29:23413–23420. doi: 10.1074/jbc.M008760200. [DOI] [PubMed] [Google Scholar]
- 30.Tradtrantip L, Tajima M, Li L, Verkman AS. Aquaporin water channels in transepithelial fluid transport. J Med Invest. 2009;56:179–184. doi: 10.2152/jmi.56.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delporte C. Aquaporins in secretory glands and their role in Sjögren’s syndrome. Review. Handb Exp Pharmacol. 2009;190:185–201. doi: 10.1007/978-3-540-79885-9_9. [DOI] [PubMed] [Google Scholar]
- 32.Zoukhri D, Hodges RR, Dartt DA. Lacrimal gland innervation is not altered with the onset and progression of disease in a murine model of Sjogren’s syndrome. Clin Immunol Immunopathol. 1998;89:126–133. doi: 10.1006/clin.1998.4597. [DOI] [PubMed] [Google Scholar]
- 33.Walcott B, Matthews G, Brink P. Differences in stimulus induced calcium increases in lacrimal gland acinar cells from normal and NZB/NZW F1 female mice. Curr Eye Res. 2002;25:253–260. doi: 10.1076/ceyr.25.4.253.13489. [DOI] [PubMed] [Google Scholar]
- 34.Ding C, MacVeigh M, Pidgeon M, et al. Unique ultrastructure of exorbital lacrimal glands in male NOD and BALB/c mice. Curr Eye Res. 2006;31:13–22. doi: 10.1080/02713680500428613. [DOI] [PubMed] [Google Scholar]
- 35.Ebrahimi M, Roudkenar MH, Imani Fooladi AA, Halabian R, Ghanei M, Kondo H, Nourani MR. Discrepancy between mRNA and protein expression of neutrophil gelatinase-associated lipocalin in bronchial epithelium induced by sulfur mustard. J Biomed Biotechnol. 2010:823131. doi: 10.1155/2010/823131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu N, Drinnenberg I, Kelso J, Wu JR, Paabo S, Zeng R, Khaitovich P. Comparison of protein and mRNA expression evolution in humans and chimpanzees. PLoS One. 2007;14(2):e216. doi: 10.1371/journal.pone.0000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rose CM, Qian L, Hakim L, Wang Y, Jerdeva GY, Marchelletta R, Nakamura T, Hamm-Alvarez SF, Mircheff AK. Accumulation of catalytically active proteases in lacrimal gland acinar cell endosomes during chronic ex vivo muscarinic receptor stimulation. Scand J Immunol. 2005;61:36–50. doi: 10.1111/j.0300-9475.2005.01527.x. [DOI] [PubMed] [Google Scholar]


