Abstract
Aims
Assess whether automatic remote home monitoring (HM) permits same-day evaluation of implantable cardioverter defibrillator (ICD) system dysfunction.
Methods and results
Compromised ICD system integrity (generator/lead) demands prompt evaluation. Home monitoring promises earlier discovery but may be limited by technological differences and follow-up mechanism. We tested whether HM enabled event review within 24 h, and contrasted differing messaging mechanisms. Nine hundred and eight patients in the TRUST prospective multicentre trial were followed by HM for 15 months. ICD system problems automatically triggered notifications: repeatedly (‘redundant’) for impedance deviations and elective replacement indication (ERI), but only a single transmission for ‘30 J ineffective’. Detection time from event onset to physician evaluation was measured. Forty-three system-related alerts occurred; 42% were asymptomatic, 42% were actionable, and 22 of 43 (51%) were viewed within 24 h. Redundant notifications were: 1 ERI, 9 shock impedance, 2 ventricular and 6 atrial pacing impedance. Most (11/18; 61%) were detected in <24 h. Others elicited daily notifications without interruption until resolution. For single transmissions, 11 of 25 (44%) events were detected on the same day. Most (56%, 14/25) were detected between 1 and 39 days (mean 10.0 ± 13.0 days). Ten of 14 events were detected by HM and 4 at the time of office visits. These observations suggest single transmissions were vulnerable to detection failure. Mean detection time of redundant events was 1.1 ± 1.8 vs. single transmission 5.6 ± 10.9 days (P = 0.05). Hence, redundant notification avoided late detection.
Conclusion
Same-day discovery of ICD dysfunction, even if asymptomatic, was achievable. For those events not evaluated within 24 h, repetitive messaging promoted earlier discovery. Reorganization of clinical follow-up methods may maintain early reaction ability.
Clinical Trials registration information
ClinicalTrials.gov; NCT00336284.
Keywords: Defibrillators, Patient monitoring, Pacemaker, Artificial, Follow-up, Remote Monitoring, Early detection
What's new?
Remote monitoring technology promises earlier discovery of ICD system integrity (generator/lead) problems, but clinical application is not well tested.
TRUST results showed that automatic remote monitoring enabled assessment within 24h in 51% of cases
Differing messaging mechanisms influenced early discovery: thus single transmissions were vulnerable to detection failure but late detection was reduced by repeating alerts.
Changes in clinical follow up methods are required to maintain early reaction ability
The trial shows that same day evaluation of lead and generator problems is possible but is affected by engineering differences, transmission frequency, methods of alert notification, and workflow patterns.
Introduction
Implantable cardioverter defibrillator (ICD) system (lead/generator) dysfunction is concerning and ideally demands near-immediate evaluation. Remote monitoring of patients with implantable devices promises prompt problem discovery. This was the underlying rationale for announcements from professional societies for ‘development and utilization of wireless and remote monitoring technologies’, carrying the expectation of early detection and correction of device malfunction.1,2 However, ‘early’—especially important for the detection of ICD system malfunction in this era of multiple lead advisories—remains undefined, although expectations are for as soon as possible. Moreover, the capability of remote monitoring to deliver on this function may extend from months to minutes, according to the system selected.3,4 Even automatic technologies differ, and their ideal operating characteristics (and their handling by follow-up facilities) are relatively unexplored. For example, transmission mechanism may employ a single attempted alert, although this may be vulnerable to transmission and/or detection failure.5 Others may signal repeatedly until resolution.3,6 This may improve detection ability, but potentially creates redundant and distracting data.
We tested the hypothesis that remote monitoring for device function could permit physician evaluation of altered function within 24 h in the TRUST (Lumos-T Safely RedUceS RouTine Office Device Follow-up) trial. In addition, we contrasted the effect of redundant messaging vs. single notification alerts on achieving this demanding goal.
Methods
TRUST was a prospective randomized multicentre clinical trial comparing the safety and utility of automatic remote home monitoring (HM) in ICD recipients compared with standard in-clinic follow-up. The study was an investigator-initiated clinical trial designed by a steering committee consisting of physicians (who also served as investigators) in collaboration with the sponsor. The protocol was written by the principal investigator and sponsor. All hypotheses and data queries were initiated by the principal investigator without sponsor involvement. The primary endpoints of the trial to demonstrate safety and efficacy of HM relative to conventional care to reduce overall clinic burden have been reported previously.7 The objectives of the current analysis were primarily to study whether the promise of same-day notification could be realized by available technology, and secondarily to assess whether this was influenced by different messaging systems, to assess reactions following message delivery.
A pre-specified TRUST hypothesis was that event notifications would provide the mechanism for early detection of device-related function as expressed in societies' statements.6 Since HM had demonstrated high-fidelity transmission with notification ability within minutes,3 TRUST tested whether device dysfunction could be detected the same day. In an added post hoc analysis, the effects of different messaging systems (in-built and non-programmable) on early detection were evaluated. The trial design has been reported previously.6 Briefly, ICD patients were randomized post-implant in a 2 : 1 scheme to HM or conventional care with remote monitoring disabled and followed by in-clinic follow-up sessions. Both groups received a standard post-implant 3-month clinic follow-up and were followed up for 15 months post-implant. Conventionally managed patients returned for face-to-face scheduled checks every 3 months. The HM patients were followed remotely for 15 months and formed the current study group.
Home monitoring is based on a low-power wireless transmitter within the pulse generator transmitting stored data daily to a bedside communicator for relay telephonically (cellular and/or landline) to a service centre for automatic processing and online review.3 Transmissions for critical events and specified ‘out-of bounds’ conditions were transmitted immediately without patient interaction, and flagged for attention, suiting this remote technology for prompt discovery of silent problems.8,9 Protocol-required system-related event notifications were end of service, elective replacement indication (ERI), atrial impedance <250 or >1500 Ω, ventricular impedance <250 or >1500 Ω, daily shock impedance <30 or >100 Ω), and ineffective ventricular maximum energy (30 J) shock (notified if first shock failed in a given episode sequence). Lead impedance changes triggered event notifications when values deviated from baseline trends (ranges were programmable but not pre-specified and permitted to be individualized by following physician). The mechanisms for signalling within the HM platform differed and were not programmable. Thus, impedance deviations and ERI signaled alerts immediately and then daily (until resolution by the clinic) but ‘30 J ineffective’ events elicited a single immediate transmission only.
Event detection time was measured as time elapsed from event onset (according to its device time stamp) to physician evaluation. Hence, the measured interval incorporated two separate elements—transmission time by technology and reaction time to transmission by the physician. Events without symptoms were classified as silent events. Events were categorized as ‘actionable’ if reprogramming changes, system revision or change in anti-arrhythmic medications were performed in response. However, no interventional algorithm was pre-specified and in-person follow-up was determined by physician discretion. Redundant vs. single mechanisms for alert notifications were contrasted for early detection ability.
An independent Clinical Events Committee comprising three physicians not participating in the trial and blinded to investigational sites, patient identities, and randomization assignment adjudicated disputed classifications.
Analysis and statistics
Only patients completing at least one in-office follow-up in HM were used for analysis. Four patients in the conventional group with the Sprint Fidelis lead crossed over to the remote monitoring arm on receipt of the advisory notice,10 but these patients were analysed as conventional patients (intention-to-treat analysis). Continuous variables were summarized as means and standard deviations, unless otherwise noted. Categorical variables were summarized in frequency distributions. Group differences were compared with Student's t-tests. A P value of 0.05 was considered evidence of statistical significance.
Results
A total of 908 HM patients formed the group for analysis. Demographics at enrolment were: age 63.3 ± 12.8 years; 72% male; New York Heart Association Class II 55.9%; primary prevention indication 72.2%; left ventricular ejection fraction 29.0 ± 10.7%; ischaemic aetiology 64.8%; dual-chamber implants 57.8%; beta-blocker usage 34.3%; and amiodarone usage 13.2%. Systems implanted comprised Biotronik generators capable of HM [Lumax 300 DR-T (1.1%), Lumax 300 VR-T (1.3%), Lumax 340 DR-T (22.7%), Lumax 340 VR-T (12.0%), Lumos DR-T (34.0%), and Lumos VR-T (28.9%)] coupled to the following leads Biotronik (93.5%), Guidant (2.2%), St Jude Medical (2.4%), Medtronic (1.9%), and Oscor (0.08%).
Mean follow-up duration was 407 ± 103 (range 21–617) days. Mean follow-up times were <15 months because of the allowable window around the 15-month visit and subjects who withdrew during the study. Primary and secondary endpoints have been reported separately. Most events resulted from arrhythmias.9
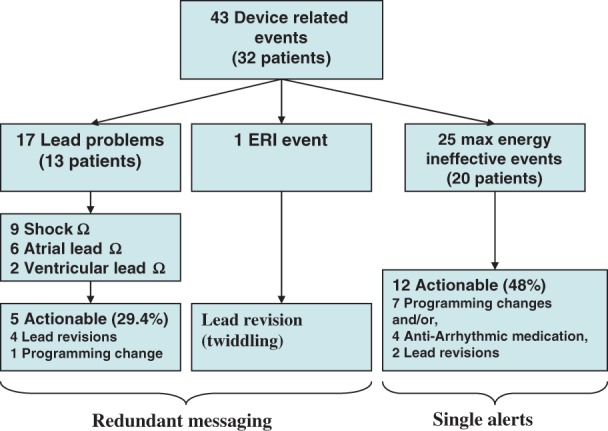
Forty-three device-related events [originating from 32 (3.5%) patients] occurred in HM (Figure 1). This represented a rate of 0.043 per patient-year. Events were captured progressively during follow-up.11 The trial protocol required HM checks to be performed daily and prior to any in-office evaluations. Thirty-five of 43 events (81%) were notified by automatic event triggers. Eight of 43 were detected during in-person evaluations. Two of these eight events resulted from oversight (i.e. event notification not attended to) and one from transmission loss. In the remainder, notifications occurred on the day of travel to the hospital where a face-to-face interrogation was performed. In all, 41.9% of these system-related events were clinically silent. Clinical actions were taken in 18 of 43, representing a rate of 0.016 per patient-year. No deaths were attributable to system malfunction.
Figure 1.
Events.
Twenty-two of 43 (51%) of all system-related alerts were viewed the same day (Figure 2). Overall, average time from onset to physician evaluation of events was 3.7 ± 8.6 days [median 0 (range 0–39, IQ 0–3.5) days].
Figure 2.
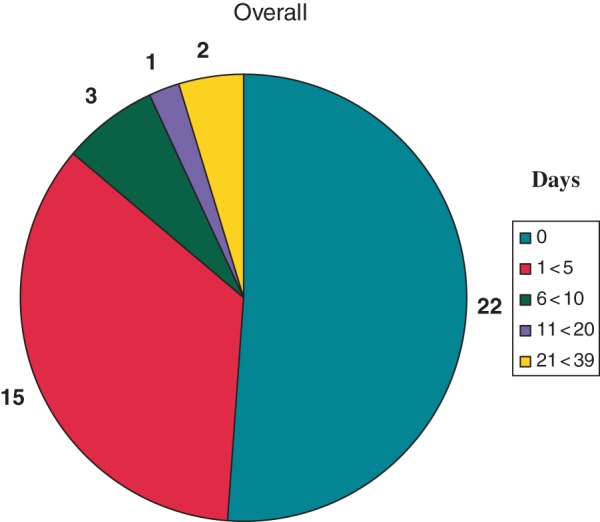
Days to detection of ICD problems in patients assigned to remote HM. Overall, 22 of 43 (51%) were detected within 24 h.
Redundant notifications
A total of 17 lead problem notifications comprising out of range atrial and ventricular lead impedance and out of range shock impedance were observed in 13 (1.43%) HM patients. Home monitoring lead notifications included six atrial impedance out of range and two ventricular pacing impedance out of range (Figure 3). Out-of-range shock impedance values were reported in six (0.7%) patients declaring nine events, and 29.4% were actionable (n = 5). Actionable causes were surgical lead revision (n = 4, 80%), e.g. fracture (Figure 3). In the other case, management entailed reprogramming changes only. One HM patient reached ERI voltage, but this was due to twiddling with retraction of the ventricular lead into the pectoral pocket followed by shocks that caused high-voltage circuitry failure and premature battery depletion. The detection time of these system-related problems were as follows: nine shock impedance, five detected the same day, others at 1,1, 2, and 4 days; two ventricular lead impedance, one detected the same day, the other at 4 days; six atrial lead impedance, four detected the same day, others at 2 and 6 days; and one ERI detected the same day. Thus, 11 of 18 (61%) were detected in <24 h, and others in <3 days. Events not detected the same day all elicited daily notifications without interruption until resolution, indicating robust transmission characteristics. Since these were resolved early, thus interrupting the transmission cycle, the total number of notifications ultimately received were few, i.e. a redundant notification mechanism did not impose significant data overload.
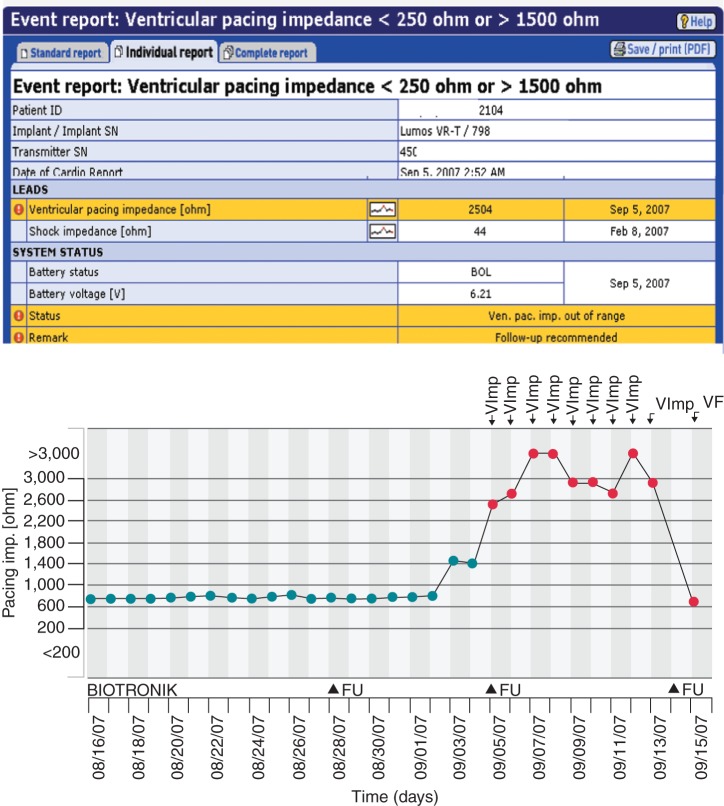
Figure 3.
Example of redundant messaging following ventricular lead fracture in patient with ICD implanted for secondary prevention. Top event notification flagged (red exclamation mark on yellow background) for out of range ventricular lead impedance. The lead impedance trend (below) had been stable, (black dots) but then suddenly increased during sleep (2.52 am) promptly triggering an event notification (red dot on trend). The patient was unaware of this critical device integrity failure which occurred 7 days after a normal conventional check at an office visit (marked FU 8/28/2007). The patient was called immediately the same day (FU 9/5/2007), and lead replacement scheduled. System continued to transmit daily in the interim – a total of 8 redundant messages were sent until problem resolution, i.e. lead replacement and defibrillation threshold testing (marked VF event notification) with return of impedance to normal range (9/15/2007). With a conventional ICD, the issue may have remained unidentified until next device check 1 year later, putting patient at risk, but in this case automatic remote monitoring enabled same-day physician evaluation.
Single transmissions
Twenty patients (2.2%) in HM experienced 25 episodes of ‘ineffective maximum-energy shock’. Although potentially concerning, only five were reported to occur for ventricular fibrillation but these failures were corrected by subsequent therapy. The majority of remaining events related to SVTs or T-wave oversensing. Fifty-two per cent were followed up with continued monitoring. The remainder were actionable events (n = 12, 48%) largely managed conservatively with reprogramming (n = 7, 58.3%) and/or, initiation/change in anti-arrhythmic medications (n = 4, 33.3%). Surgical lead revision was required in only two cases (16.7%): one patient related to twiddling (mentioned above), and one patient requiring lead revision and device upgrade to a high-energy generator. Importantly, 11 of 25 (44%) ‘30 J ineffective’ events were detected the same day and 56% (14/25) of events were detected between 1 and 39 days (mean 10.0 ± 13.0 days.). Ten of these 14 events were detected by HM and 4 in-office.
In summary, approximately one half of all system-related alerts were viewed the same day (Figure 2), but a transmission mechanism of redundant notification improved clinical detection ability [mean time to detect 1.1 ± 1.8 days (range 0–6 days; median 0, i.e. same day)] compared with single notification [mean 5.6 ± 10.9 days, (range 0–39), P = 0.05, Figure 4], principally by avoiding late detection.
Figure 4.
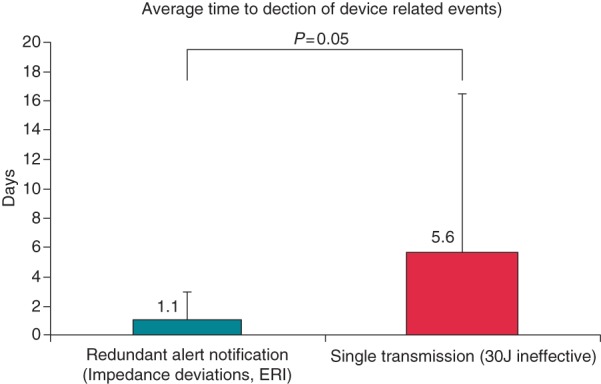
Summary data according to messaging method in patients assigned to remote HM. Days to the detection of ICD problems are contrasted between repeating (redundant) vs. single alerts. The majority (11/18) of redundant events were detected within 24 h (e.g. Figure 3). Single alerts were usually reviewed late.
Discussion
This is the first study to test the ability of remote monitoring to permit physician evaluation of dysfunction in implanted wireless devices within 24 h of occurrence and furthermore compare the effect on early detection of different signalling characteristics. Overall, 51% were detected on the same day. Late detection was reduced when alerts were repeated until problem resolution occurred, compared to a single alert. This result is important for physician selection of and mode of operation of remote monitoring when utilized as an early-warning system for altered device function.
Monitoring of implanted hardware performance is a physician responsibility expressed in recent HRS position statements and often demanded by patients.1,2 The task is daunting in view of increasing volume and device complexity, and added burdens imposed by advisory notices. Intensive monitoring by increasing office visits (e.g. monthly) is impractical, onerous and inefficient (since problem incidence is very low) and is likely to fail to detect potentially catastrophic problems occurring between interrogations.10 Integrated patient-alert mechanisms, for example, beeps for impedance monitoring, are insensitive, prone to false-positive evaluations, and under-detected by elderly patients.12 The development of remote monitoring technologies was called for by professional organizations following the cluster of advisories occurring in 2005, and more have followed since. The promise was that remote monitoring would serve as an instrument for early detection and resolution of system problems, as and when they occurred. However, the details of operation did not receive comment, although many remote monitoring systems, spanning several evolutionary stages, were already available at the time. Importantly, these have different operating characteristics and distinct abilities for problem discovery. For example, inductive (wand-based) systems depend on patient (and hospital) adherence to set schedules (e.g. 3 monthly)13,14 and therefore challenge compliance (especially true for children and the elderly15). Such systems maintain no interim monitoring, and are vulnerable to missing asymptomatic events. Thus, when used to follow-up a pacemaker population, clinically actionable events took several months for discovery (yet described as ‘early detection’) and only 66% of events were detected during remote transmission.4 In contrast, automatic transmission technologies potentially overcome these obstacles but differ in operating characteristics. The system used in the current study has demonstrated proven safety, efficacy, and more rapid problem detection ability (including asymptomatic events and device-related issues) during follow-up of ICD and pacemaker patients, compared with conventional in-person evaluations.7,11,16–18 Diagnostic ability was aided by automatically wirelessly transmitted electrograms.19 However, the strength of this system may be blunted by delays in reaction on the part of clinical follow-up personnel, which is in an important link in the chain of events.
The current results show that 51% remote alerts for device function were viewed on the same day. This indicates that the desired (if ambitious) aim of 24 h detection is achievable in the majority. While representing a considerable advance over conventional in-person evaluations, there remains opportunity for improvement. Interestingly, events that were signalled repeatedly secured earlier review, thereby avoiding late detection. In contrast, physician evaluation of events transmitted only once was more variable. Delays sometimes extended to over a month, diluting the early detection capability offered by remote monitoring. This is important, since event triggers cover an extensive range of potentially lethal system problems (e.g. ERI, lead fracture, high-voltage circuitry failure20), especially since almost one half of these were asymptomatic and associated with high actionability. Any delay in reaction is potentially hazardous and undesirable. An operating model utilizing a single transmission without back-up is particularly concerning since this may be vulnerable to transmission failure. Although transmission failure was observed in only one case in the current study, different proprietary technologies may have different levels of reliability (although not all have published performance characteristics). Thus, in a large prospective study of another wireless system designed to transmit once only for all alerts, 45% of all ICD triggered alerts failed transmission.5 This limits its role as an early-warning mechanism. In this context, repeating alert mechanisms would have ensured added safety, reducing the probability that transmission failure may preclude detection. Hence, from a manufacturer's viewpoint, it would seem prudent to make all alerts repetitive to ensure delivery.
In our study, both signalling mechanisms tested used the same remote monitoring platform, which has excellent transmission reliability, delivering >90% of event notifications in <4 min 3,21 and is approved specifically for early detection.22 In this regard, the difference between the two groups studied here (Figure 4) arises from the predilection of a problem signaled by a single notification for late detection. The implication is that delays incurred in evaluation are most probably generated at point of retrieval, i.e. during handling by receiving facilities. This is supported by the current data: a few notifications were overlooked entirely, others discovered late, but those generating repeating messages were more promptly evaluated. (More exaggerated losses were observed in another study using single notifications in which 29% of all delivered alerts failed to be viewed at all, and 50% of the remainder were evaluated >4.6 days after the event with many exceeding a month5). These observations expose current constraints in follow-up clinics as a barrier to maintaining early detection consistently. In this regard, ability for prompt handling by follow-up clinics can range widely according to remote monitoring system utilized.23 The current results indicate that ‘reminders’ with automatic systems may avoid late detection, even though the device system alerts studied were potentially serious and should have commanded immediate review. Operational characteristics of the system used may be important. Longitudinal parameter trends that are updated daily have high temporal definition (Figure 3) permitting adjudication of significant departures from baseline. For example, in a previous report, a sudden change in lead impedance8 of an ICD lead under Class 1 recall remained within specifications, but deviation from historical trend triggered event notification. Alert settings may be individualized in each patient. These processes improve the value of alerts received and likely underlies the observation that, overall, only a median of two event notifications (including arrhythmias) per patient year are received with this system (despite its capacity for redundant messaging) when problems need notification.9,24
The clinical advantages of early detection with automatic remote HM for patient and device care are significant. These included prompt decision making and intervention e.g. surgically for lead failure (Figure 3), or conservatively with reprogramming to prevent potential inappropriate therapies.11 The non-sustained ventricular arrhythmia notification may be triggered by system issues such as lead electrical noise artefacts caused by fracture or non-physiological electrical signals, and direct intervention to preempt shock delivery and reduce patient morbidity.11,25 Interruption of repeated charge cycles which occur in a significant minority of ICD patients26 is important to prevent early battery exhaustion. In the multicenter ECOST trial, clinical reactions enabled by early detection resulted in a large reduction in the number of actually delivered shocks (−72%), the number of charged shocks (−76%), the rate of inappropriate shocks (−52%), and at the same time exerting a favourable impact on battery longevity.27 These results potentially may be improved further by refining current early detection mechanisms.
Limitations
A relatively small number of system-related events occurred in this short-term post-implant study and most device-related problems are anticipated to manifest several years post-implant.28 However, the current findings demonstrate that the range of types of such events (which also cover dangerous advisories) may be appropriately captured and rapidly notified. Programmability of redundant vs. single transmissions was not available, though potentially useful for prioritizing notifications e.g. system-related alerts demanding rapid evaluation vs. less serious conditions e.g. some recurrent arrhythmias.
In summary, merely committing patients to ‘remote monitoring’ is insufficient to guarantee ‘early’ detection of system problems, although the relevance of prompt problem discovery continues to increase in the light of recent lead and device advisories. Although HM may be programmed to simply deliver 3–6 monthly data without interim monitoring, continuous monitoring with use of alerts is valuable, potentially providing almost immediate notification. This study shows that it is possible to realize the ambitious aim of same day evaluation of lead and generator problems, but this intent may be influenced by engineering differences, transmission frequency and methods of alert notification. Also, it is important to recognize that changes in workflow patterns are necessary to capitalize on the rapid detection power afforded by current generation technology. An offer of a remote monitoring service carries an implicit agreement that actual review of transmitted information occurs in a timely manner. Just receiving an alert is insufficient—there has to be a robust response mechanism in place to realize the technological promise. This was illustrated by the current results: even though multiple alerts improved time to detection in many cases, this was still prolonged in others, i.e. clinical practice has lagged behind this innovative technology. Preliminary initiatives with new organizational models to systematically implement remote monitoring into daily clinical practice and maintain early reaction ability are promising.29 Such enhancements may advance the efficacy of automatic continuous remote monitoring as an early warning system and provide assurance to both patients and their physicians.
Conflict of interest: N.V. is the Trial principal investigator; B.B.P. and B.S. are members of the clinical events committee. J.M. is a Biotronik employee.
Trial sponsor: Biotronik.
References
- 1.Carlson MD, Wilkoff BL, Maisel WH, Carlson MD, Ellenbogen KA, Saxon LA, et al. Recommendations from the Heart Rhythm Society Task Force on Device Performance Policies and Guidelines Endorsed by the American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) and the International Coalition of Pacing and Electrophysiology Organizations (COPE) Heart Rhythm. 2006;3:1250–73. doi: 10.1016/j.hrthm.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 2.Maisel WH, Hauser RG, Hammill SC, Hauser RG, Ellenbogen KA, Epstein AE, et al. Recommendations from the Heart Rhythm Society Task Force on Lead Performance Policies and Guidelines: developed in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA) Heart Rhythm. 2009;6:869–85. doi: 10.1016/j.hrthm.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 3.Varma N, Stambler B, Chun S. Detection of atrial fibrillation by implanted devices with wireless data transmission capability. Pacing Clin Electrophysiol. 2005;28(Suppl. 1):S133–6. doi: 10.1111/j.1540-8159.2005.00083.x. [DOI] [PubMed] [Google Scholar]
- 4.Crossley GH, Chen J, Choucair W, Cohen TJ, Gohn DC, Johnson WB, et al. Clinical benefits of remote versus transtelephonic monitoring of implanted pacemakers. J Am Coll Cardiol. 2009;54:2012–9. doi: 10.1016/j.jacc.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Crossley G, Boyle A, Vitense H, Chang Y, Mead RH. The clinical evaluation of remote notification to reduce time to clinical decision (CONNECT) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57:1181–9. doi: 10.1016/j.jacc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Varma N. Rationale and design of a prospective study of the efficacy of a remote monitoring system used in implantable cardioverter defibrillator follow-up: the Lumos-T Reduces Routine Office Device Follow-Up Study (TRUST) study. Am Heart J. 2007;154:1029–34. doi: 10.1016/j.ahj.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 7.Varma N, Epstein A, Irimpen A, Schweikert R, Shah J, Love CJ Investigators. TRUST Investigators . Efficacy and safety of automatic remote monitoring for ICD Follow-Up: the TRUST trial. Circulation. 2010;122:325–32. doi: 10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 8.Varma N. Remote monitoring for advisories: automatic early detection of silent lead failure. Pacing Clin Electrophysiol. 2009;32:525–7. doi: 10.1111/j.1540-8159.2009.02314.x. [DOI] [PubMed] [Google Scholar]
- 9.Varma N, Epstein A, Irimpen A, Gibson L, Love CJ, Investigators T. Event notifications by remote monitoring systems performing automatic daily checks: load, characteristics and clinical utility. Eur Heart J. 2009;30:1909. [Google Scholar]
- 10.Medtronic. 2007. Physician Advisory Letter: Urgent Medical Device Information Sprint Fidelis® Lead Patient Management Recommendations http://www.medtronic.com/product-advisories/physician/sprint-fidelis/PROD-ADV-PHYS-OCT.htm .
- 11.Varma N, Michalski J, Epstein AE, Schweikert R. Automatic remote monitoring of implantable cardioverter-defibrillator lead and generator performance: the Lumos-T Safely RedUceS RouTine Office Device Follow-Up (TRUST) trial. Circ Arrhythm Electrophysiol. 2010;3:428–36. doi: 10.1161/CIRCEP.110.951962. [DOI] [PubMed] [Google Scholar]
- 12.Kallinen LM, Hauser RG, Lee KW, Almquist AK, Katsiyiannis WT, Tang CY, et al. Failure of impedance monitoring to prevent adverse clinical events caused by fracture of a recalled high-voltage implantable cardioverter-defibrillator lead. Heart Rhythm. 2008;5:775–9. doi: 10.1016/j.hrthm.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 13.Schoenfeld MH, Compton SJ, Mead RH, Weiss DN, Sherfesee L, Englund J, et al. Remote monitoring of implantable cardioverter defibrillators: a prospective analysis. Pacing Clin Electrophysiol. 2004;27:757–63. doi: 10.1111/j.1540-8159.2004.00524.x. [DOI] [PubMed] [Google Scholar]
- 14.Joseph GK, Wilkoff BL, Dresing T, Burkhardt J, Khaykin Y. Remote interrogation and monitoring of implantable cardioverter defibrillators. J Interv Card Electrophysiol. 2004;11:161–6. doi: 10.1023/B:JICE.0000042356.52369.89. [DOI] [PubMed] [Google Scholar]
- 15.Zartner P, Handke R, Photiadis J, Brecher AM, Schneider MB. Performance of an autonomous telemonitoring system in children and young adults with congenital heart diseases. Pacing Clin Electrophysiol. 2008;31:1291–9. doi: 10.1111/j.1540-8159.2008.01180.x. [DOI] [PubMed] [Google Scholar]
- 16.Neuzil P, Taborsky M, Holy F, Wallbrueck K. Early automatic remote detection of combined lead insulation defect and ICD damage. Europace. 2008;10:556–7. doi: 10.1093/europace/eun009. [DOI] [PubMed] [Google Scholar]
- 17.Spencker S, Coban N, Koch L, Schirdewan A, Muller D. Potential role of home monitoring to reduce inappropriate shocks in implantable cardioverter-defibrillator patients due to lead failure. Europace. 2009;11:483–8. doi: 10.1093/europace/eun350. [DOI] [PubMed] [Google Scholar]
- 18.Mabo P, Victor F, Bazin P, Ahres S, Babuty D, Da Costa A, et al. A randomized trial of long-term remote monitoring of pacemaker recipients (The COMPAS trial) Eur Heart J. 2012;33:1105–1011. doi: 10.1093/eurheartj/ehr419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perings C, Bauer WR, Bondke HJ, Mewis C, James M, Bocker D, et al. Remote monitoring of implantable-cardioverter defibrillators: results from the Reliability of IEGM Online Interpretation (RIONI) study. Europace. 2011;13:221–9. doi: 10.1093/europace/euq447. [DOI] [PubMed] [Google Scholar]
- 20.Hauser RG, Kallinen L. Deaths associated with implantable cardioverter defibrillator failure and deactivation reported in the United States Food and Drug Administration Manufacturer and User Facility Device Experience Database. Heart Rhythm. 2004;1:399–405. doi: 10.1016/j.hrthm.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Varma N, Michalski J, Investigators T. Effect of transmission reliability on remote follow-up in ICD patients: automatic home monitoring in the TRUST trial. Heart Rhythm. 2012 9; issue 55, S164 (abstract) [Google Scholar]
- 22.2009. FDA A. Home Monitoring http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/PMAApprovals/ucm166550.htm. P050023/S020.
- 23.Cronin E, Ching EA, Varma N, Martin DO, Wilkoff B, Lindsay BD. Remote monitoring of cardiovascular devices- a time and activity analysis. Heart Rhythm. 2012 doi: 10.1016/j.hrthm.2012.08.002. doi:pii: S1547-5271(12)00860-0. 10.1016/j.hrthm.2012.08.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Theuns DA, Rivero-Ayerza M, Knops P, Res JC, Jordaens L. Analysis of 57,148 transmissions by remote monitoring of implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2009;32(Suppl. 1):S63–5. doi: 10.1111/j.1540-8159.2008.02230.x. [DOI] [PubMed] [Google Scholar]
- 25.Mabo P, Defaye P, Sadoul N, Davy J, Deharo J, Kacet S. 2011. Remote follow-up of patients implanted with an ICD. The prospective randomized EVATEL study http://spo.escardio.org/eslides/view.aspx?eevtid=48&fp=2173 .
- 26.Varma N, Johnson MA. Prevalence of cancelled shock therapy and relationship to shock delivery in recipients of implantable cardioverter-defibrillators assessed by remote monitoring. Pacing Clin Electrophysiol. 2009;32((Suppl. 1):S42–6. doi: 10.1111/j.1540-8159.2008.02288.x. [DOI] [PubMed] [Google Scholar]
- 27.Kacet S. 2011. Safety and Effectiveness of ICD Follow-up using Remote Monitoring: ECOST Study. Presented in Hot Line Session at ESC 2011 Congress http://spo.escardio.org/eslides/view.aspx?eevtid=48&fp=2175 .
- 28.Borleffs CJ, van Erven L, van Bommel RJ, van der Velde ET, van der Wall EE, Bax JJ, et al. Risk of failure of transvenous implantable cardioverter-defibrillator leads. Circ Arrhythm Electrophysiol. 2009;2:411–6. doi: 10.1161/CIRCEP.108.834093. [DOI] [PubMed] [Google Scholar]
- 29.Ricci R, Morichelli L, D'Onofrio A, Zanotto G, Vaccari D, Calò L, et al. Home monitoring manpower, sensitivity and positive predictive value of adverse event detection. Preliminary results from the Home Guide registry. Eur Heart J. 2011;32(Suppl. 1):54. [Google Scholar]