Key Points
The adaptor molecule SAP is required for T-cell receptor-induced iNKT cell killing of T- and B-cell targets.
SAP-deficient iNKT cells adhere poorly to T-cell lymphoid targets and exhibit reduced polarization of lytic machinery to the immunologic synapse.
Abstract
The adaptor molecule signaling lymphocytic activation molecule–associated protein (SAP) plays critical roles during invariant natural killer T (iNKT) cell ontogeny. As a result, SAP-deficient humans and mice lack iNKT cells. The strict developmental requirement for SAP has made it difficult to discern its possible involvement in mature iNKT cell functions. By using temporal Cre recombinase–mediated gene deletion to ablate SAP expression after completion of iNKT cell development, we demonstrate that SAP is essential for T-cell receptor (TCR)–induced iNKT cell cytotoxicity against T-cell and B-cell leukemia targets in vitro and iNKT-cell–mediated control of T-cell leukemia growth in vivo. These findings are not restricted to the murine system: silencing RNA–mediated suppression of SAP expression in human iNKT cells also significantly impairs TCR-induced cytolysis. Mechanistic studies reveal that iNKT cell killing requires the tyrosine kinase Fyn, a known SAP-binding protein. Furthermore, SAP expression is required within iNKT cells to facilitate their interaction with T-cell targets and induce reorientation of the microtubule-organizing center to the immunologic synapse (IS). Collectively, these studies highlight a novel and essential role for SAP during iNKT cell cytotoxicity and formation of a functional IS.
Introduction
Invariant natural killer T (iNKT) cells comprise a unique lineage of innate-type T lymphocytes with pleiotropic roles in host immunity, including promotion of graft tolerance, prevention of autoimmunity, and protection against specific pathogens and cancers.1 Most iNKT cells express a common or “invariant” T-cell receptor (TCR), which confers reactivity to self and microbial-derived lipids, as well as the potent iNKT cell agonist α-galactosyl ceramide (αGC).1 Following TCR engagement, iNKT cells rapidly upregulate costimulatory molecules, secrete cytokines, and elicit cytotoxic responses.1 As a result, iNKT cells stimulate and direct the development of immune responses. However, the mechanisms that control iNKT cell functions are poorly understood.
In this study, we sought to examine whether the adaptor molecule SAP (signaling lymphocytic activation molecule [SLAM]–associated protein) regulates mature iNKT cell activation. SAP is encoded by SH2D1A, the gene mutated in X-linked lymphoproliferative disease (XLP), a primary immunodeficiency characterized by the development of fulminant Epstein-Barr virus infection, B-cell lymphomas, and humoral immune defects.2-4 Although SAP is abundantly expressed in iNKT cells, it has been difficult to elucidate its functions within the mature iNKT cell compartment because of the crucial role for SAP during iNKT cell ontogeny.5-7 As a consequence, XLP patients and Sap−/− mice lack iNKT cells.5-7 Previously, we introduced a transgene encoding the rearranged α-chain of the invariant TCR (Vα14 Tg) onto the Sap−/− genetic background and observed that this maneuver supported the emergence of iNKT cells.8 These SAP-deficient Vα14 Tg+ iNKT cells secreted significantly less interleukin-4 (IL-4) and interferon gamma (IFN-γ) in response to TCR ligation, yet they exhibited a markedly abnormal surface phenotype. Therefore, it was not possible to discern whether the observed cytokine defects were due to the intrinsic lack of SAP protein or rather from altered ontogeny due to absence of SAP during iNKT cell development.
To definitively elucidate SAP’s role within mature iNKT cells, we used temporal gene deletion and silencing RNA (siRNA)–mediated gene repression to eliminate SAP expression in postthymic murine and human iNKT cells, respectively, and examined the effects of these maneuvers on iNKT cell function. We observe that the loss of SAP significantly impairs TCR-induced cytolysis. SAP-null iNKT cells form fewer conjugates with T-cell targets and exhibit reduced polarization of the microtubule-organizing center (MTOC) to the immunologic synapse (IS). Together, these studies highlight a novel role for SAP in iNKT cell cytotoxicity and formation of a functional IS.
Materials and methods
Mice
Sap−/− (official gene name, Sh2d1a),9 UBC-CreT2,10 R26R-YFP11 and SapR78A12 mice were as described. C57BL/6 (B6), Fyn−/−, and NOD.Cg-PrkdcscidIl2rgtm1Wjl/Szj (NSG) were from Jackson Laboratories (Bar Harbor, ME). Animal studies were approved by the Institutional Animal Care and Use Committee at The Children’s Hospital of Philadelphia.
Generation of Sapfl mice
Homologous recombination was used to flank exon 1 of Sap with loxP sites in an ES cell line of 129 origin. Following germline transmission, the neomycin cassette was excised by using the FLP1 recombinase. All mice were backcrossed onto the B6 genetic background for >9 generations.
Cell lines and reagents
EL4 cells were from American Type Culture Collection (Manassas, VA) and luciferase expressing EL4 cells were from Caliper Life Sciences (Hopkinton, MA). Recombinant human (rh) IL-2 and IL-15 were from Peprotech (Rocky Hill, NJ) and Sigma (St. Louis, MO). PBS57 and PBS44 (Paul Savage, Brigham Young University, Provo, UT) are synthetic αGC analogs that function in a manner comparable to αGC to activate iNKT cells. Initial studies used PBS57; however, once this reagent was depleted, later studies used PBS44.
Antibodies and flow cytometry
Antibodies included anti-CD4, CD8, CD122, NK1.1 CD11b, CD69, IL-4, IFN-γ, Thy1.2, B220, and FasL, (BD Biosciences, San Jose, CA); TCR-β and CD24 (BioLegend, San Diego, CA); CD44, TRAIL, and perforin (eBiosciences, San Diego, CA); Vα24 and Vβ11 (Beckman Coulter, Brea, CA); CD3, CD56, and CD16 (BD PharMingen), and SAP (André Veillette). Data were collected on an LSRII flow cytometer (BD Biosciences) and analyzed by using FlowJo software (Tree Star, Ashland, OR).
Isolation of murine iNKT cells
iNKT cells were obtained by staining liver lymphocytes or CD8-depleted thymocytes with anti-NK1.1 and anti–TCR-β antibodies and by sorting with a BD FACSAria cell sorter (BD Biosciences). Isolated cells were >97% NK1.1+TCR-β+ or >92% PBS57-CD1d tetramer reactive.
In vitro cytotoxicity assay
EL4 or A20-CD1d target cells were labeled with 100 μCi 51Cr (Na2CrO4; Perkin Elmer; Waltham, MA) for 1 to 2 hours at 37°C and washed. 51Cr-labeled targets were loaded with PBS44 (100 ng/mL) or left untreated and were then washed and cultured in triplicate at varying effector:target (E:T) cell ratios. Supernatants were collected, radioactivity measured and percent specific lysis calculated as described.13
Stable knockdown of SAP and Fyn in DN3A4-1.2 iNKT hybridoma cells
Sap (TRCN0000081158 [S1], TRCN0000081162 [S2]), Fyn (TRC0000023379 [F1] and TRC0000023380 [F2]) and control (SHC002) Mission short hairpin RNA (shRNA) lentiviral plasmids were from Sigma. Lentiviruses were generated and iNKT transduction and selection were completed using standard protocols.14 Cells were used within 5 to 7 days following puromycin selection.
Human iNKT cell expansion, purification, and transient siRNA transfections
Human peripheral blood mononuclear cells were cultured in AIM-V medium with 10% fetal calf serum, rhIL-2 (50 U/mL), and αGC (500 ng/mL; Enzo Life Sciences, Farmingdale, NY). After 4 days, cultures were supplemented with rhIL-15 (10 ng/mL) and rhIL-2 (10 U/mL). After 4 more days, iNKT cells were purified by using fluorescein isothiocyanate–conjugated anti-Vα24 antibody and anti-fluorescein isothiocyanate microbeads (Miltenyi Biotec; >98% PBS57-CD1d tetramer reactive). iNKT cells were transfected with 300 pmol of control (Invitrogen), SAP-specific (Stealth Select siRNA, Invitrogen; Mission siRNA, Sigma), or FYN-specific siRNA (Invitrogen) by using Amaxa nucleofection (Lonza, Walkersville, MD).
Confocal microscopy
iNKT and target cells were incubated at 37°C. After 4 or 14 hours, conjugates were fixed and stained with rabbit anti–α-tubulin (Abcam; Cambridge, MA) and anti-rabbit immunoglobulin G Alexa Fluor 405 (Molecular Probes) to detect the MTOC and Alexa Fluor 568–conjugated phalloidin to detect F-actin. Images were acquired with a spinning disk confocal microscope (Zeiss Observer-Z.1 with Hamamatsu cooled-CCD camera) and analyzed by using Volocity software (Perkin Elmer).15
Statistics
Statistical analyses were performed by using GraphPad PRISM software (GraphPad, San Diego, CA).
Results
Conditional deletion of Sap does not impact iNKT cell number, phenotype, or in vivo responsiveness to lipid antigens
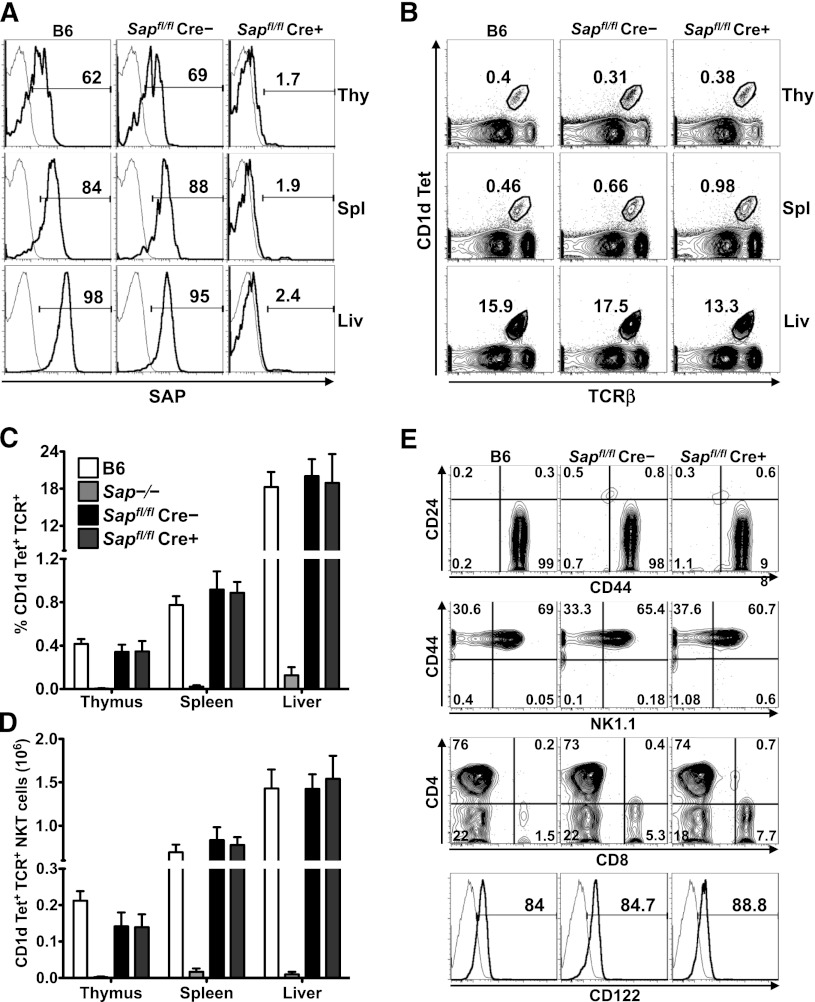
To address whether mature iNKT cells require SAP to carry out their functions, we generated a mouse strain in which exon 1 of the Sap gene was flanked by LoxP sites. Sapfl/fl mice were crossed to animals expressing a Cre recombinase whose activity is inducible by tamoxifen (UBC-CreT2; [Cre])10 as well as a Cre-responsive YFP reporter gene (R26R-YFP; [YFP]).11 Because a burst of iNKT cell ontogeny occurs during the first several weeks of postnatal life,16 Sapfl/flCre–YFP+ and Sapfl/flCre+YFP+ mice were aged for 6 to 8 weeks to allow for iNKT cell development. Subsequently, mice were fed tamoxifen,17 rested for 5 to 7 days to allow for degradation of SAP protein, and analyzed for SAP expression and iNKT cell incidence, number, and phenotype (supplemental Figure 1). By using this approach, ≥95% of lymphocytes, including all YFP+ iNKT cells, were devoid of SAP protein (Figure 1A and not shown). Importantly, iNKT cell incidence, number, and phenotype were preserved in Sapfl/flCre–YFP+ (SAP-sufficient; SAP+) and Sapfl/flCre+YFP+ (SAP-deficient; SAP–) mice (Figure 1B-E), even when examined up to 6 months following tamoxifen treatment (not shown).
Figure 1.
Conditional Sap deletion leads to loss of SAP expression yet retention of mature iNKT cells. (A) PBS57-CD1d tetramer (Tet)+TCR-β+ iNKT cells from the organs of wild-type (B6) and tamoxifen-treated Sapfl/flCre– and Sapfl/flCre+ mice were stained for intracellular SAP by using an anti-SAP (bold histogram) or isotype control (fine histogram) antibody and analyzed by flow cytometry. (B) Representative flow cytometric plots showing the percentages of iNKT cells in the organs of B6 and tamoxifen-treated Sapfl/flCre– or Sapfl/flCre+ mice. Average percentage (C) and absolute number (D) of CD1d-Tet+TCR-β+ iNKT cells in the organs of B6, Sap−/−, and tamoxifen-treated Sapfl/flCre– or Sapfl/flCre+ mice. Data represent the mean ± standard error of the mean (SEM) of 6 animals per genotype. (E) The maturation status of liver iNKT cells was assessed by examining the surface levels of expression of CD24, CD44, NK1.1, CD4, CD8, and CD122. Data in A, B, and E are representative of 6 age-matched mice of each genotype. Liv, liver; Thy, thymus; Spl, spleen.
Following in vivo administration, lipid antigens such as αGC or its analogs PBS44 or PBS57 are presented by CD1d-expressing dendritic cells (DCs) to iNKT cells, resulting in iNKT cell cytokine production and iNKT cell-dependent activation of bystander cells. To investigate whether the loss of SAP influences these events, tamoxifen-treated mice were injected intraperitoneally with PBS57, and 2 to 6 hours later, they were examined for iNKT- and NK-cell cytokine production, serum cytokine levels, and upregulation of the CD69 activation marker on splenocytes and hepatic lymphocytes. In each of these assays, SAP+ and SAP– animals exhibited comparable responses (supplemental Figure 2). These data concur with a recent report describing an independently generated mouse strain harboring a targeted SAP allele.18 Furthermore, they reveal that following completion of normal iNKT cell ontogeny, the deletion of SAP does not influence iNKT phenotype, persistence, or the ability to respond to DC-mediated presentation of lipid antigens. Additionally, these data suggest that the poor αGC-induced cytokine responses previously observed in Sap−/− Vα14 Tg+ mice8 likely resulted from the perturbed development of iNKT cells in a SAP-deficient thymic environment.
Loss of SAP expression impairs in vitro TCR-induced iNKT cell cytotoxicity
iNKT cells express cytolytic proteins and are capable of cytotoxicity. This function has been most thoroughly examined in the context of cancer, where iNKT cells potently kill a variety of tumor cell lines in vitro.19-24 To address whether SAP regulates iNKT cell cytotoxicity, SAP+ and SAP– iNKT cells were purified from the thymuses or livers of tamoxifen-treated mice and cultured with 51Cr-labeled EL4 T-cell lymphoma cells that were loaded or not with PBS44. Regardless of the organ of origin, SAP+ iNKT cells robustly killed PBS44-loaded, but not unloaded, EL4 cells (Figure 2A-B). In contrast, SAP– iNKT cells exhibited diminished killing that was, on average, 40% to 80% less than that of the SAP+ cells (Figure 2A-B,E-F). SAP– iNKT cells also exhibited poor killing of PBS44-loaded CD1d-transfected B-cell leukemia cells (A20-CD1d; Figure 2C-D,G-H). SAP+ and SAP– iNKT cells expressed equivalent levels of perforin, granzyme B, FasL, and TRAIL (supplemental Figure 3), and they did not express EAT-2 or ERT (supplemental Figure 4), proteins with homology to SAP that inhibit murine NK-cell natural cytotoxicity and cytokine production.25 Thus, the SAP-dependent defects in killing are not due to the altered expression of these death-promoting or inhibitory proteins.
Figure 2.
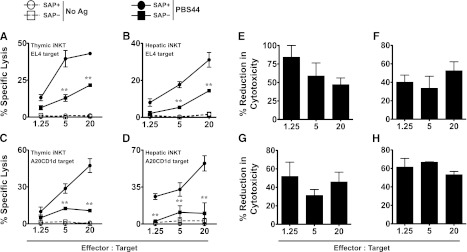
SAP-deficient iNKT cells exhibit defective killing of antigen-pulsed target cells in vitro. (A-B) EL4 target cells were pulsed with 100 ng/mL of PBS44 or left untreated (No Ag) and used as targets in an in vitro cytotoxicity assay. Cytolysis of PBS44-loaded or unloaded EL4 cells by iNKT cells from the thymuses (A) or livers (B) of tamoxifen-treated Sapfl/flCre– (SAP+) or Sapfl/flCre+ (SAP−) mice, respectively. (C-D) In vitro cytotoxicity against PBS44-loaded (100 ng/mL) or unloaded A20-CD1d cells by SAP+ or SAP− iNKT cells. In A-D, representative data from 1 of 3 experiments is shown. (E-H) Mean percent reduction ± SEM in cytolysis of antigen-pulsed EL4 (E-F) and A20-CD1d (G-H) cells by SAP− versus SAP+ iNKT cells. Data are averaged from 3 experiments. Statistical significance in percent specific lysis of PBS44-loaded target cells by SAP+ iNKT cells compared with SAP− iNKT cells was determined by two-way analysis of variance (ANOVA) test. **P < .001.
SAP is required for iNKT-cell–mediated control of tumor growth in vivo
We next sought to address whether SAP was required for iNKT-cell–mediated clearance of T-cell leukemia cells in vivo. For these studies, T-, B- and NK-cell deficient NSG mice were reconstituted or not with SAP+ or SAP– iNKT cells, and 3 days later, challenged with PBS44-loaded EL4 cells that stably expressed luciferase (EL4-LUC). Subsequently, tumor burden was monitored by using serial bioluminescence imaging of live mice, as well as bioluminescence imaging, flow cytometric, and histologic analyses of organs 2 weeks following tumor cell injection. Compared with mice that received no iNKT cells, animals receiving SAP+ iNKT cells exhibited a significantly lower tumor burden, as determined by lower live animal radiance (Figure 3A-B) and reduced radiance (Figure 3C-E) and weights (Figure 3F-G) of tumor-bearing organs. Similarly, flow cytometric analyses of SAP+ iNKT-reconstituted mice demonstrated a lower number of tumor cells in the liver and spleen (Figure 3H-I), and histologic analyses showed very few if any tumor cell aggregates (Figure 3L and not shown). In contrast, SAP– iNKT cells exhibited significantly poorer control of EL4 cell growth in vivo compared with SAP+ iNKT cells (Figure 3A-I,L). Of note, there was no difference in the number of SAP+ or SAP– iNKT cells recovered from the organs of tumor-bearing mice (Figure 3J-K). Therefore, the suboptimal in vivo clearance of tumor is not due to the inability of SAP– iNKT cells to traffic to the tumor site but may reflect their reduced capacity to kill.
Figure 3.
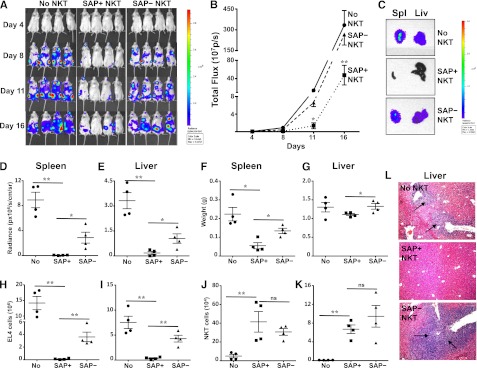
SAP-deficient iNKT cells exhibit defective in vivo control of antigen-pulsed EL4 cells. NSG mice were reconstituted or not (No NKT) with 4 × 105 sort-purified iNKT cells from tamoxifen-treated Sapfl/flCre– (SAP+) or Sapfl/flCre+ (SAP−) mice. Three days later, mice were injected intravenously with 1 × 105 PBS44-loaded EL4-Luc cells. (A) Whole body distribution of EL4-Luc cells over the first 2 weeks was determined by bioluminescence imaging. (B) Quantification of bioluminescence images at serial time points. Data points are the average radiance emitted from 4 mice per group. Statistical significance was determined by two-way ANOVA. *P < .05 and **P < .001 denotes comparisons among cohorts at specific time points. (C) Total bioluminescence in spleen (Spl) and liver (Liv) from a representative mouse in each group is shown. Bioluminescence (D-E) and weights (F-G) of organs were measured and the number of EL4 (H-I) and iNKT cells (J-K) was determined by flow cytometry. (L) Tumor burden in the liver was assessed histologically. Black arrows indicate tumor aggregates. Data are from 1 of 2 experiments in which a total of 7 to 8 mice in each cohort was examined. Error bars represent standard deviation (SD). Statistical significance was determined by unpaired two-tail t test. *P < .05; **P < .001; ns, not significant.
iNKT killing is dependent on the presence of Fyn
SAP functions in T and NK cells to transmit signals initiated by one or more of the SLAM family receptors.26 Following receptor engagement, SAP binds to the SLAM receptor tails to which it often recruits the tyrosine kinase Fyn.27,28 Fyn then phosphorylates the SLAM receptor tails and associated signaling proteins, leading to the initiation of a phosphotyrosine signal that is critical for cell functions. To address whether Fyn and/or the SAP-Fyn interaction were involved in iNKT cell lysis, we first examined the killing activity of iNKT cells isolated from the livers of Fyn−/− mice or mice expressing a mutant version of SAP (SapR78A) that cannot bind to Fyn.12 Consistent with the known cooperative roles for SAP and Fyn during iNKT cell selection,29 iNKT cell numbers were very low in each of these mouse strains (not shown). Nonetheless, sufficient cells were obtained for these studies, which to our surprise revealed no defects in target cell lysis (supplemental Figure 5). These results were unanticipated because SAP and Fyn must each be present and physically interact with one another to promote optimal NK cell lysis of target cells.30,31
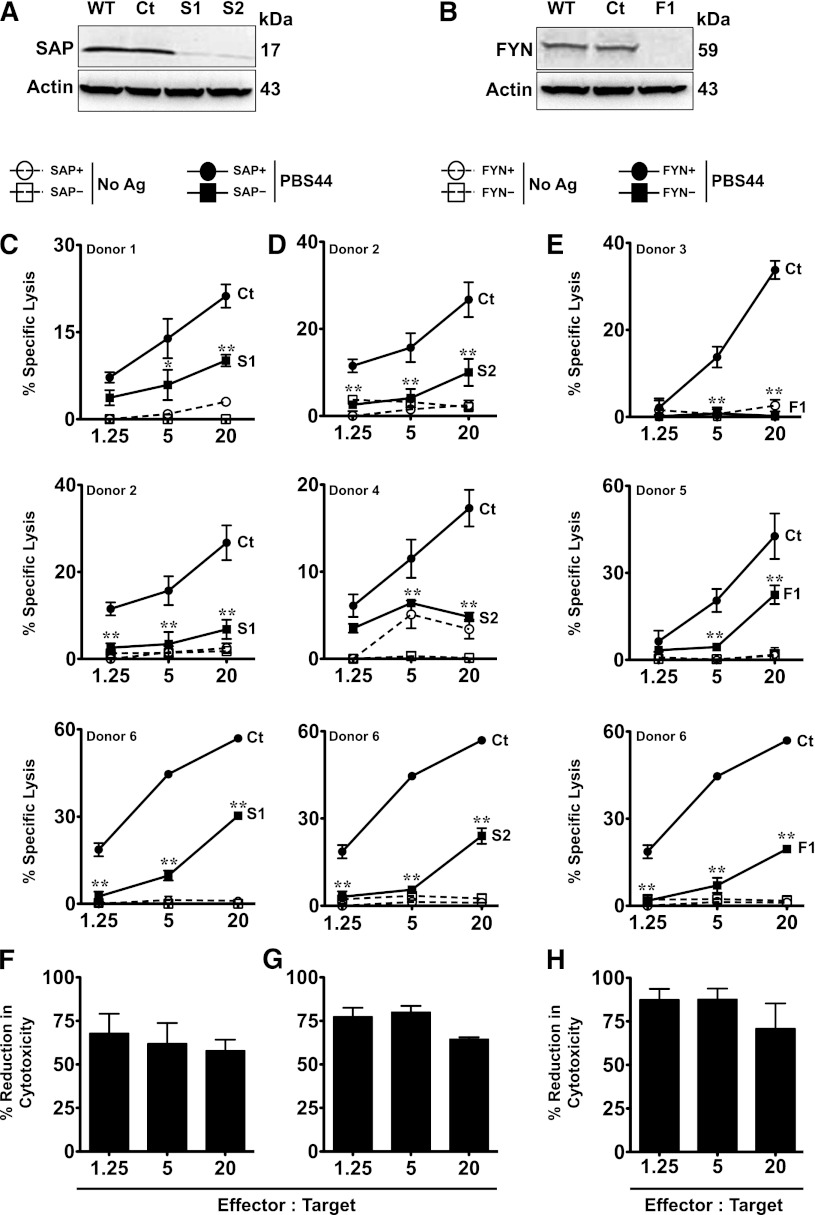
In these experiments, it is possible that Fyn−/− or SapR78A iNKT cells do not reflect the functions of normal iNKT cells because of their altered thymic development based on autonomy from Fyn. Therefore, we also silenced the expression of Fyn in murine DN3A4-1.2 NKT hybridoma cells, which express high levels of SAP and Fyn and exhibit potent killing activity (H.B. et al, manuscript submitted February 2013). As proof-of-principle, we first confirmed that DN3A4-1.2 killing was SAP dependent. DN3A4-1.2 cells were transduced with lentiviruses encoding control or Sap-targeted shRNA sequences. After selection, we examined SAP expression by western blotting and EL4 lysis via the in vitro cytotoxicity assay. Transduction with lentiviruses encoding a control shRNA had no effect on SAP expression or target cell lysis (Figure 4A,C-D). In contrast, transduction with the Sap-specific shRNAs S1 and S2 led to a ≥90% reduction in SAP protein levels (Figure 4A), and similar to SAP– primary iNKT cells, Sap-silenced DN3A4-1.2 cells exhibited a 52% ± 11% (S1) and 46% ± 9% (S2) reduction in the killing of EL4 cells (E:T ratio 1.25:1) (Figure 4C-D,G-H). shRNA-mediated gene silencing also efficiently lowered Fyn protein expression (Figure 4B). Strikingly, Fyn-silenced cells showed even poorer target cell killing that was, on average, 76% ± 19% (F1) and 73% ± 12% (F2) less than control shRNA-transduced cells (E:T ratio 1.25:1) (Figure 4E-F,I-J). Collectively, these latter studies reveal that SAP and Fyn are each essential for iNKT cell cytotoxicity. Furthermore, they highlight critical issues surrounding the study of Fyn−/− and SapR78A iNKT cells, which appear to develop and function in a Fyn-independent manner.
Figure 4.
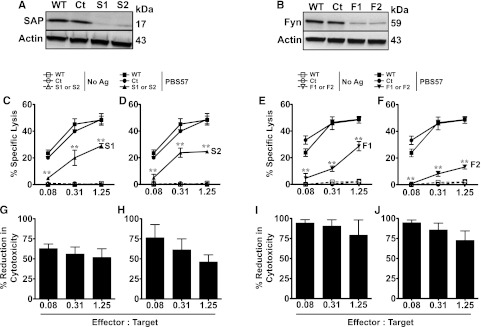
Silencing SAP or Fyn in DN3A4-1.2 iNKT cells results in reduced cytotoxicity. (A-B) DN3A4-1.2 (1.2) cells were stably transduced with lentiviruses encoding control (Ct), SAP- (S1, S2; [A]), or Fyn-specific (F1, F2; [B]) shRNA. After 5 days, cell lysates prepared from untransduced (WT) or shRNA-transduced cells were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotted with anti-SAP or Fyn antibodies to assess knockdown of SAP or Fyn expression, respectively. (C-F) The in vitro cytolytic activity of untransduced 1.2 NKT cells or 1.2 cells transduced with control, SAP-specific (C-D), or Fyn-specific (E-F) shRNA was tested against PBS44-loaded EL4 cells. Data are from 1 of 4 experiments. (G-J) Mean percent reduction ± SEM in cytolysis of EL4 cells by SAP or Fyn-silenced 1.2 iNKT cells compared with WT cells (S1 or S2; [G-H] and F1 or F2; [I-J]). Data are averaged from 4 experiments. Statistical significance in percent-specific lysis of PBS44-loaded EL4 cells by SAP- or Fyn-silenced 1.2 iNKT cells compared with WT cells was determined by two-way ANOVA test. **P < .001.
SAP and FYN are required for human iNKT cell cytotoxic responses
To examine whether SAP or FYN are also required for human iNKT cell cytotoxicity, we expanded and purified iNKT cells from the blood of normal donors (supplemental Figure 6) and examined their ability to kill T- or B-cell targets. Human iNKT cells expressed SAP and FYN (Figure 5A-B) and mounted potent cytotoxic responses against PBS44-loaded, but not unloaded, EL4 cells (Figure 5C-E). Expanded iNKT cells from 3 donors were then independently transfected with control, SAP-, or FYN-specific siRNAs and subjected to the in vitro cytotoxicity assay. SAP- or FYN-silenced cells expressed almost no SAP or FYN protein (Figure 5A-B), and similar to murine iNKT cells, they exhibited a significant reduction in target cell killing (Figure 5C-G; S1 or S2 and E,H; F1). Together, these studies indicate that SAP and FYN are each critical mediators of human iNKT cell lysis.
Figure 5.
Silencing SAP or FYN expression in human iNKT cells (hu-iNKT cells) results in reduced cytotoxicity. (A-B) hu-iNKT cells were transfected with control (Ct), SAP-specific (S1 or S2), FYN-specific siRNA (Fyn), or left untransfected (WT). After 72 hours, knockdown of SAP or FYN expression was assessed by immunoblotting. (C-E) Cytolysis of EL4 cells by hu-iNKT cells from three separate healthy controls following transfection with control (Ct), SAP (S1 [C] or S2 [D]), or FYN-specific siRNA (F1 [E]). (F-H) Mean percent reduction ± SEM in cytolysis of EL4 cells by SAP or FYN-silenced hu-iNKT cells compared with control siRNA-transfected cells (S1 [F], S2 [G], and F1 [H]). Data are averaged from 3 donors per condition. Statistical significance in percent specific lysis of PBS44-loaded EL4 cells by SAP- or FYN-silenced hu-iNKT cells compared with control siRNA-transfected cells was determined by two-way ANOVA test. **P < .001.
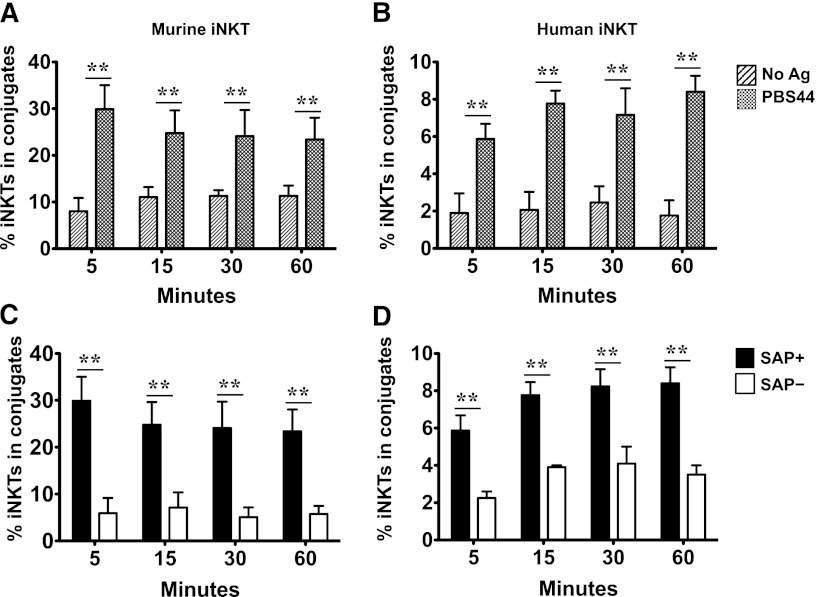
SAP– iNKT cells form fewer conjugates and produce reduced levels of cytokines in response to EL4 targets
In our in vitro assay, iNKT cell cytotoxicity requires 16 to 20 hours to reach its maximal effect, suggesting that multiple or stable interactions may be required for killing and that these interactions could be impaired in the setting of SAP deficiency. To probe this possibility, we used a flow cytometric assay to examine whether SAP+ and SAP– iNKT cells form conjugates with antigen-loaded or unloaded EL4 targets. In these studies, we observed that murine and human iNKT cells did indeed form conjugates following contact with EL4 cells (supplemental Figure 7). Although the percentage of conjugates varied, depending on the source of effectors, they were greater in incidence when EL4 cells were pulsed with PBS44 (Figure 6A-B). Consistent with their lytic defects, both human and murine SAP– iNKT cells formed significantly fewer conjugates with antigen-pulsed EL4 cells (Figure 6C-D).
Figure 6.
SAP-deficient murine and hu-iNKT cells form fewer conjugates with antigen-pulsed targets. iNKT cells from B6, tamoxifen-treated Sapfl/flCre– or Sapfl/flCre+ mice and EL4 cells were stained with 250 nM carboxyfluorescein succinimidyl ester and bodipy red succinimdyl ester, respectively. iNKT cells were mixed at a 1:1 ratio with PBS44-loaded or unloaded targets, spun down at 1200 rpm for 2 minutes, and incubated at 37°C for varying times. Conjugate frequencies were analyzed by flow cytometry for the presence of cellular aggregates staining positive for bodipy red succinimdyl ester and carboxyfluorescein succinimidyl ester. (A-B) Mean percent iNKT cells (of total iNKT cells) in conjugate with PBS44-loaded or unloaded EL4 cells. (C-D) Mean percent of SAP+ or SAP− iNKT cells ± SEM in conjugate with PBS44-loaded EL4 cells. iNKT cells were flow-sorted from B6 mice (A), tamoxifen-treated Sapfl/flCre− (SAP+) or Sapfl/flCre+ (SAP−) mice (C), or control (SAP+) or SAP-specific (SAP−) siRNA transfected hu-iNKT cells (D). Percent iNKT cells in conjugate was calculated by using the formula [% iNKT-containing conjugates (upper right quadrant)]/[% iNKT-containing conjugates (upper right quadrant) + % iNKT cells not in conjugate (lower right quadrant)] × 100. Data presented are from 3 experiments. Statistical significance was determined by unpaired two-tailed t test. **P < .001.
SAP-deficient T cells adhere normally to DCs but display poor adhesion to T and B cells. This property is proposed to underlie their normal responses to myeloid cells, yet inadequate response to lymphoid cells.32-34 To explore whether SAP– iNKT cells behave in a similar fashion, murine SAP+ or SAP– iNKT cells were cultured with antigen-pulsed or unpulsed EL4 cells or splenic CD11c+ DCs. After 18 hours, iNKT cell cytokine production was assessed. SAP+ iNKT secreted detectable levels of IL-4 and IFN-γ when cultured with lipid-antigen loaded, but not unloaded, EL4 cells or DCs. Compared with SAP+ iNKT cells, SAP– iNKT cells produced significantly lower levels of cytokines when cultured with antigen-loaded EL4 cells but normal levels of cytokines when cultured with DCs (supplemental Figure 8). Thus, as is the case with T cells, SAP differentially regulates iNKT:lymphoid versus iNKT:myeloid interactions. These findings also help explain the normal in vivo cytokine responses of PBS57-injected SAP– mice (supplemental Figure 2), because these responses depend on DC, not lymphoid, presentation of lipid antigens to iNKT cells.
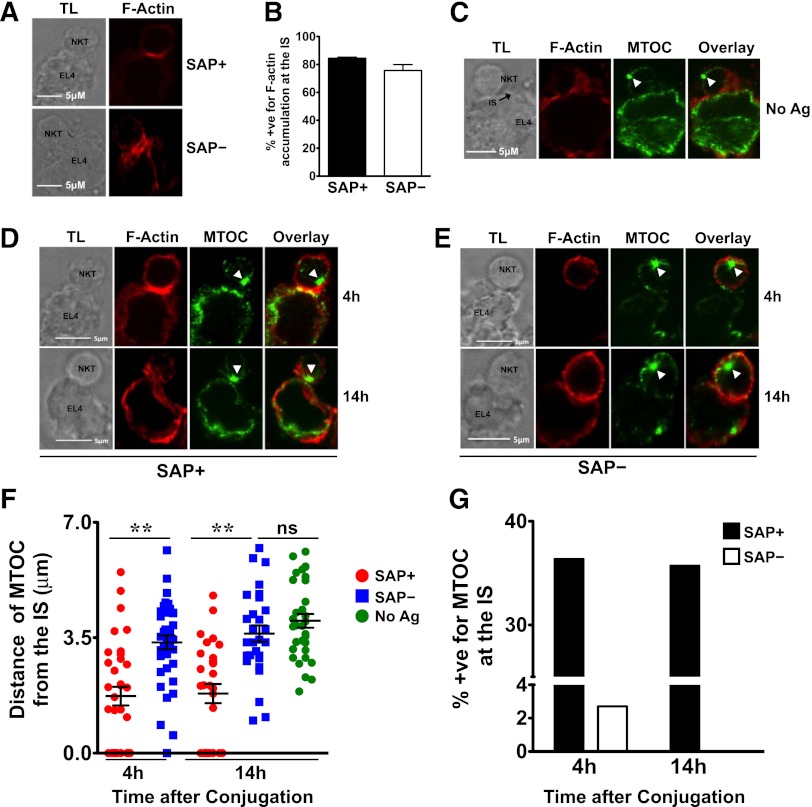
SAP– iNKT cells exhibit defective MTOC polarization to the immunologic synapse (IS)
To understand the mechanism underlying the poor cytotoxic responses of SAP− iNKT cells, we used confocal microscopy to examine the contact site between murine SAP+ and SAP− iNKT cells and EL4 targets. In SAP+ iNKT cells, F-actin accumulated at the IS on exposure to PBS44-loaded as well as unloaded EL4 cells (Figure 7A-B). In contrast, the MTOC polarized toward the IS only in response to antigen-loaded cells (Figure 7C-D), a finding consistent with the known requirement for TCR signals for reorienting the lytic machinery. SAP– cells exhibited no defects in the accumulation of F-actin at the IS (Figure 7A-B); however, they poorly polarized the MTOC to the point of contact with PBS44-loaded targets. This was evident by the longer average distance of the MTOC from the IS (Figure 7D-F) and the reduced percentage of SAP– iNKT-containing conjugates in which the MTOC was completely polarized (Figure 7G). These studies, which are the first to examine the events controlling iNKT cell IS formation, reveal that SAP is a critical signaling molecule required for polarization of the lytic machinery in response to antigen-loaded targets.
Figure 7.
SAP– iNKT cells accumulate actin but demonstrate impaired MTOC polarization at the IS. (A) Conjugates of murine SAP+ or SAP– iNKT cells and antigen-loaded EL4 cells were prepared, fixed at 4 hours, stained, and evaluated by transmitted light (TL) and spinning disk confocal microscopy (Zeiss Observer-Z.1 with Hamamatsu cooled-CCD camera) by using fluorescently labeled phalloidin to detect F-actin. The magnification of the objective lens was ×63 and the numerical aperture was 1.45. Scale bar: 5 μm. (B) Graph represents mean percentage ± SEM of conjugates that were positive (+ve) for F-actin accumulation at the IS. Data are pooled from 3 experiments; scoring was for ≥20 conjugates per condition. Conjugates were evaluated in a blinded fashion and scored positive for F-actin accumulation at the synapse when there was enhanced staining at the site of iNKT-target-cell interface. (C-G) Conjugates were similarly evaluated and scored in a blinded fashion for MTOC polarization at the synapse. (C) Conjugates of SAP+ iNKT cells and unloaded EL4 cells were analyzed for localization of the MTOC at the IS. (D-E) Representative conjugates of SAP+ or SAP– iNKT cells and antigen-loaded EL4 cells analyzed at 4 or 14 hours are shown. White arrowheads indicate the centrosome. (F) MTOC polarization at the IS in SAP+ and SAP– NKT cells was assessed by measuring its distance from the IS at 4 and 14 hours. (G) Average percentage of conjugates positive (+ve) for MTOC at the synapse. Each mean represents data from at least 25 conjugates ± SEM. Statistical significance was determined by unpaired two-tailed t test. **P < .001.
Discussion
It has been recognized for more than a decade that iNKT cells kill activated, infected, and malignant cells. Despite this fact, little is known regarding the signals that promote iNKT cytolysis. In this study, we characterized iNKT cell functions by using a novel mouse strain in which the adaptor molecule SAP can be temporally eliminated in mature iNKT cells following their development in a SAP-sufficient thymic environment. We demonstrate for the first time that SAP is essential for TCR-induced iNKT cell lysis of multiple cellular targets in vitro. Furthermore, we show that the SAP-associated cytolytic defects are also observed in vivo, where mice reconstituted with SAP-null iNKT cells exhibit extremely poor control of T-cell lymphoma growth. TCR-induced iNKT cell lysis is dependent upon Fyn, which often functions in conjunction with SAP to promote cell activation. Finally, we show that siRNA-mediated silencing of SAP or FYN in human iNKT cells significantly impairs TCR-induced target cell lysis. Collectively, these findings provide new insights into iNKT cell biology and highlight the central role for SAP-associated signals in iNKT cell cytotoxicity.
We observe that SAP-deficient iNKT cells exhibit reduced competency to form conjugates with targets and to assemble a functional lytic synapse. These studies are among the first to examine the nature of iNKT cell-target cell interactions and are concordant with a recent report in which SAP expression within iNKT cells was required to support cognate iNKT cell help to B cells during the generation of lipid antigen-specific B-cell responses.18 SAP-deficient CD8+ cells also exhibit poor formation of conjugates and reduced killing of EL4 cells.35 Interestingly, CD8+ killing varies, depending on the dose and nature of peptides with which the EL4 cells were pulsed. Specifically, Sap−/− CD8+ T cells efficiently formed conjugates and killed when the EL4 cells were loaded with high doses of peptide. In contrast, they killed poorly and formed fewer conjugates when EL4 cells were loaded with reduced doses of peptide or low-affinity ligands. In our hands, iNKT cell killing occurs only when target cells are loaded with high-affinity ligands of the invariant TCR. Nonetheless, SAP– iNKT cells exhibit a significant defect in target-cell killing. On the basis of these observations, we hypothesize that the nature of interactions required for iNKT-cell killing are distinct from those needed by CD8+ T cells. Indeed, iNKT-cell killing is slow and requires up to 20 hours to reach its maximal effect (not shown). Thus, iNKT-cell cytotoxicity may require strong TCR signals and durable contacts with targets, processes that are especially dependent on the presence of SAP.
Many but not all of SAP’s functions rely on its interaction with Fyn and the induction of Fyn kinase activity. To probe the requirement for Fyn or the SAP-Fyn interaction in iNKT cell lysis, we examined the ability of Fyn−/− and SapR78A iNKT cells to kill T- or B-cell targets. To our surprise, iNKT cell killing was indistinguishable from that of SAP+ iNKT cells. This result was unexpected because Fyn−/− and SapR78A NK cells exhibit significantly reduced cytolytic activity. However, NK cells do not rely on SAP or Fyn for development. Thus, it is possible that the few iNKT cells that remain in Fyn−/− and SapR78A mice have bypassed the need for Fyn during ontogeny by interacting with an alterniative Src family kinase. Indeed, the SH2 domain of SAP is reported to bind to Lck in vitro and in yeast36; however, to the best of our knowledge, this interaction has never been recapitulated in vivo. To further probe the role for Fyn during iNKT activation, we transiently silenced Fyn expression in murine and human iNKT cells. Contrary to the prior studies, this procedure significantly dampened iNKT cell killing. Indeed, the effects of Fyn silencing were even greater than those occurring following the silencing of SAP.
These results demonstrate several important points. First, they highlight the potential difficulties that can be encountered when examining the effects of genetic maneuvers that eliminate or alter the expression of proteins essential for T- or iNKT-cell development. Indeed, such maneuvers can significantly perturb TCR expression as well as peripheral T-cell responses.17 By using a temporal deletion strategy, we circumvented the developmental abnormalities and examined the effects of SAP deletion in peripheral iNKT cells that were phenotypically similar to normal iNKT cells. Second, these results highlight the importance of Fyn during regulation of immune cell functions. Our data mirror published reports of Fyn−/− T and NK cells, which are severely impaired in proliferation, cytokine production, and cytotoxicity.12,30 Compared with SAP, Fyn participates in multiple signaling pathways, including those induced by the SLAM receptors as well as the TCR.37 Following TCR engagement, Fyn phosphorylates and activates the actin regulatory protein WASp38-40 and the guanine nucleotide exchange factor Vav,41-43 which are both required for IS formation and MTOC reorientation.44 Thus, by silencing Fyn expression, we are likely impeding multiple aspects of signaling and causing a greater effect on iNKT cell lysis.
SAP exerts dual signaling functions downstream of the SLAM receptors, where it promotes Fyn-dependent signaling and at the same time prevents the recruitment of inhibitory phosphatases to the SLAM receptor cytoplasmic tails.31,35 Consequently, in Sap−/− CD8+ T and NK cells, SLAM receptors bind more readily to SHP-1 and SHIP-1, respectively, leading to dampened cytotoxic responses due to dephosphorylation of signaling proteins such as Lck and PLC-γ. We are currently examining whether SAP uses a similar mode of action to regulate iNKT cytolysis.
Efforts are underway to manipulate iNKT cell functions as a treatment for human diseases such as cancer. Before these efforts can be fully realized, it is necessary to define how iNKT cells recognize and respond to targets, including malignant cells. By using complementary temporal approaches to silence the expression of SAP and Fyn in mature iNKT cells, these studies have defined SAP and Fyn as critical signaling molecules required for TCR-induced cytotoxicity. These studies thus increase our understanding of this poorly understood iNKT cell function and provide a platform for further enhancing iNKT cell lytic activity for therapeutic purposes. Furthermore, these experiments provide insights into the pathogenesis of Epstein-Barr virus–induced hyperinflammatory responses and increased development of lymphoma in XLP patients. Given their ability to activate cells of the innate and adaptive immune systems and exert potent cytotoxicity, iNKT cells may indeed serve to shape effective antiviral and antitumor immune responses. Although their contribution may be less apparent in healthy individuals, iNKT cells could play a prominent role in the setting of NK- and T-cell dysfunction, as occurs in XLP.
Supplementary Material
Acknowledgments
The authors thank Gary Koretzky, Martha Jordan, Taku Kambayashi, and Ed Behrens for comments on this manuscript; Mitch Kronenberg for supplying DN3A4-1.2 and A20-CD1d cells; and the National Institutes of Health Tetramer Core Facility for generating PBS57-loaded CD1d tetramers.
This work was supported by the National Institutes of Health (R01HL089745 [K.E.N.]), XLP Research Trust (K.E.N.), Alex’s Lemonade Stand Foundation (R.D.), and grants from the Canadian Institutes of Health Research (A.V.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.D. designed and performed experiments, analyzed data, and wrote the paper; H.B., P.G., and S.W. assisted with experiments and reviewed the manuscript; P.P.B. and J.S.O. helped with confocal microscopy studies; M.-C.Z. and A.V. provided vital reagents and reviewed the manuscript; and K.E.N. designed and supervised the research and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kim E. Nichols, The Children’s Hospital of Philadelphia, Colket Translational Research Building, Room 3012, 3501 Civic Center Blvd, Philadelphia, PA 19104; e-mail: nicholsk@e-mail.chop.edu.
References
- 1.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11(3):197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 2.Coffey AJ, Brooksbank RA, Brandau O, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20(2):129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- 3.Nichols KE, Harkin DP, Levitz S, et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci USA. 1998;95(23):13765–13770. doi: 10.1073/pnas.95.23.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sayos J, Wu C, Morra M, et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395(6701):462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 5.Nichols KE, Hom J, Gong SY, et al. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005;11(3):340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pasquier B, Yin L, Fondanèche MC, et al. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201(5):695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174(6):3153–3157. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- 8.Cen O, Ueda A, Guzman L, Jain J, Bassiri H, Nichols KE, Stein PL. The adaptor molecule signaling lymphocytic activation molecule-associated protein (SAP) regulates IFN-gamma and IL-4 production in V alpha 14 transgenic NKT cells via effects on GATA-3 and T-bet expression. J Immunol. 2009;182(3):1370–1378. doi: 10.4049/jimmunol.182.3.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Czar MJ, Kersh EN, Mijares LA, et al. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci USA. 2001;98(13):7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feil R, Wagner J, Metzger D, Chambon P. Regulation of Cre recombinase activity by mutated estrogen receptor ligand-binding domains. Biochem Biophys Res Commun. 1997;237(3):752–757. doi: 10.1006/bbrc.1997.7124. [DOI] [PubMed] [Google Scholar]
- 11.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson D, Shi X, Zhang S, et al. Genetic evidence linking SAP, the X-linked lymphoproliferative gene product, to Src-related kinase FynT in T(H)2 cytokine regulation. Immunity. 2004;21(5):707–717. doi: 10.1016/j.immuni.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Orange JS, Ramesh N, Remold-O’Donnell E, et al. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci USA. 2002;99(17):11351–11356. doi: 10.1073/pnas.162376099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramanian H, Gupta K, Guo Q, Price R, Ali H. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: resistance to receptor phosphorylation, desensitization, and internalization. J Biol Chem. 2011;286(52):44739–44749. doi: 10.1074/jbc.M111.277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee PP, Orange JS. Quantitative measurement of F-actin accumulation at the NK cell immunological synapse. J Immunol Methods. 2010;355(1-2):1–13. doi: 10.1016/j.jim.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bendelac A, Killeen N, Littman DR, Schwartz RH. A subset of CD4+ thymocytes selected by MHC class I molecules. Science. 1994;263(5154):1774–1778. doi: 10.1126/science.7907820. [DOI] [PubMed] [Google Scholar]
- 17.Wu GF, Corbo E, Schmidt M, et al. Conditional deletion of SLP-76 in mature T cells abrogates peripheral immune responses. Eur J Immunol. 2011;41(7):2064–2073. doi: 10.1002/eji.201040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Detre C, Keszei M, Garrido-Mesa N, et al. SAP expression in invariant NKT cells is required for cognate help to support B-cell responses. Blood. 2012;120(1):122–129. doi: 10.1182/blood-2011-11-395913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metelitsa LS. Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1d-negative tumors in humans. Clin Immunol. 2011;140(2):119–129. doi: 10.1016/j.clim.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Metelitsa LS, Naidenko OV, Kant A, et al. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167(6):3114–3122. doi: 10.4049/jimmunol.167.6.3114. [DOI] [PubMed] [Google Scholar]
- 21.Metelitsa LS, Weinberg KI, Emanuel PD, Seeger RC. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia. 2003;17(6):1068–1077. doi: 10.1038/sj.leu.2402943. [DOI] [PubMed] [Google Scholar]
- 22.Nieda M, Nicol A, Koezuka Y, et al. TRAIL expression by activated human CD4(+)V alpha 24NKT cells induces in vitro and in vivo apoptosis of human acute myeloid leukemia cells. Blood. 2001;97(7):2067–2074. doi: 10.1182/blood.v97.7.2067. [DOI] [PubMed] [Google Scholar]
- 23.Song L, Asgharzadeh S, Salo J, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119(6):1524–1536. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roncagalli R, Taylor JE, Zhang S, et al. Negative regulation of natural killer cell function by EAT-2, a SAP-related adaptor. Nat Immunol. 2005;6(10):1002–1010. doi: 10.1038/ni1242. [DOI] [PubMed] [Google Scholar]
- 26.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 27.Chan B, Lanyi A, Song HK, et al. SAP couples Fyn to SLAM immune receptors. Nat Cell Biol. 2003;5(2):155–160. doi: 10.1038/ncb920. [DOI] [PubMed] [Google Scholar]
- 28.Latour S, Roncagalli R, Chen R, et al. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nat Cell Biol. 2003;5(2):149–154. doi: 10.1038/ncb919. [DOI] [PubMed] [Google Scholar]
- 29.Nunez-Cruz S, Yeo WC, Rothman J, et al. Differential requirement for the SAP-Fyn interaction during NK T cell development and function. J Immunol. 2008;181(4):2311–2320. doi: 10.4049/jimmunol.181.4.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bloch-Queyrat C, Fondanèche MC, Chen R, et al. Regulation of natural cytotoxicity by the adaptor SAP and the Src-related kinase Fyn. J Exp Med. 2005;202(1):181–192. doi: 10.1084/jem.20050449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong Z, Davidson D, Pérez-Quintero LA, Kurosaki T, Swat W, Veillette A. The adaptor SAP controls NK cell activation by regulating the enzymes Vav-1 and SHIP-1 and by enhancing conjugates with target cells. Immunity. 2012;36(6):974–985. doi: 10.1016/j.immuni.2012.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Cannons JL, Qi H, Lu KT, et al. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity. 2010;32(2):253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palendira U, Low C, Chan A, et al. Molecular pathogenesis of EBV susceptibility in XLP as revealed by analysis of female carriers with heterozygous expression of SAP. PLoS Biol. 2011;9(11):e1001187. doi: 10.1371/journal.pbio.1001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature. 2008;455(7214):764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao F, Cannons JL, Dutta M, Griffiths GM, Schwartzberg PL. Positive and negative signaling through SLAM receptors regulate synapse organization and thresholds of cytolysis. Immunity. 2012;36(6):1003–1016. doi: 10.1016/j.immuni.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simarro M, Lanyi A, Howie D, et al. SAP increases FynT kinase activity and is required for phosphorylation of SLAM and Ly9. Int Immunol. 2004;16(5):727–736. doi: 10.1093/intimm/dxh074. [DOI] [PubMed] [Google Scholar]
- 37.Stein PL, Lee HM, Rich S, Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70(5):741–750. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- 38.Badour K, Zhang J, Shi F, Leng Y, Collins M, Siminovitch KA. Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J Exp Med. 2004;199(1):99–112. doi: 10.1084/jem.20030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres E, Rosen MK. Contingent phosphorylation/dephosphorylation provides a mechanism of molecular memory in WASP. Mol Cell. 2003;11(5):1215–1227. doi: 10.1016/s1097-2765(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 40.Torres E, Rosen MK. Protein-tyrosine kinase and GTPase signals cooperate to phosphorylate and activate Wiskott-Aldrich syndrome protein (WASP)/neuronal WASP. J Biol Chem. 2006;281(6):3513–3520. doi: 10.1074/jbc.M509416200. [DOI] [PubMed] [Google Scholar]
- 41.Huang J, Tilly D, Altman A, Sugie K, Grey HM. T-cell receptor antagonists induce Vav phosphorylation by selective activation of Fyn kinase. Proc Natl Acad Sci USA. 2000;97(20):10923–10929. doi: 10.1073/pnas.97.20.10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martín-Cófreces NB, Sancho D, Fernández E, et al. Role of Fyn in the rearrangement of tubulin cytoskeleton induced through TCR. J Immunol. 2006;176(7):4201–4207. doi: 10.4049/jimmunol.176.7.4201. [DOI] [PubMed] [Google Scholar]
- 43.Michel F, Grimaud L, Tuosto L, Acuto O. Fyn and ZAP-70 are required for Vav phosphorylation in T cells stimulated by antigen-presenting cells. J Biol Chem. 1998;273(48):31932–31938. doi: 10.1074/jbc.273.48.31932. [DOI] [PubMed] [Google Scholar]
- 44.Orange JS, Harris KE, Andzelm MM, Valter MM, Geha RS, Strominger JL. The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc Natl Acad Sci USA. 2003;100(24):14151–14156. doi: 10.1073/pnas.1835830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.