Abstract
A forkhead transcription factor gene, fktf-1, which we propose to be orthologous to the Caenorhabditis elegans dauer-regulatory gene daf-16 has been discovered in the parasitic nematode Strongyloides stercoralis. Genomic and cDNA sequences from both species predict alternately spliced a and b message isoforms. In contrast to C. elegans, where two a isoforms, daf-16a1 and daf-16a2, are found, a single fktf-1a isoform is found in S. stercoralis. Five of the 10 introns found in the C. elegans gene are found in the proposed S. stercoralis ortholog. Functional motifs common to DAF-16 and several mammalian forkhead transcription factors are conserved in FKTF-1. These include the forkhead DNA binding domain, four Akt/protein kinase B phosphorylation sites and a C-terminal domain that may associate with factors such as the steroid receptor coactivator and other factors necessary for transcriptional regulation. An N-terminal serine-rich domain found in DAF-16A is greatly expanded in FKTF-1A. This domain is missing in DAF-16B, FKTF-1B and all mammalian orthologs. FKTF-1 shows the closest phylogenetic relationship to DAF-16 among all known mammalian and nematode forkhead transcription factors. Like its proposed Caenorhabditis ortholog, the fktf-1 message is expressed at all stages of the life cycle examined thus far. Discovery of fktf-1 indicates the presence of an insulin-like signalling pathway in S. stercoralis similar to that known to regulate dauer development in C. elegans. This pathway is a likely candidate to control infective larval arrest and reactivation as well as regulation of the switch between parasitic and free-living development in the parasite.
Keywords: fktf-1, Strongyloides stercoralis, daf-16, Forkhead transcription factor, Dauer larva, Infective larva
1. Introduction
Parasitic nematodes of many species, including some of medical or veterinary importance invade their definitive hosts as infective third-stage larvae (L3i). The host may acquire these L3i by various routes and from various sources including direct skin penetration from contaminated soil, ingestion with contaminated herbage, intermediate or paratenic hosts, or transfer during blood or tissue feeding by an arthropod vector (Noble et al., 1989; Bowman, 1995; Despommier et al., 2000). Despite the varied nature of their hosts, habitats and modes of transmission, parasitic nematode L3i share some common biological attributes. Basic among these is their programmed developmental arrest followed by reactivation upon encountering the host or host-like conditions. In vitro culture methods have allowed some organism-scale studies of extrinsic factors governing the reactivation of L3i (Franke and Weinstein, 1984a, b; Farrar and Klei, 1985; Abraham et al., 1987; Wisnewski and Weinstein, 1993) and of the early events following reactivation (Abraham et al., 1987; Lustigman et al., 1995, 1996). Pioneering microsurgical techniques have been used recently to study the neuronal control of arrest and reactivation of Strongyloides stercoralis L3i (Ashton et al., 1998). Recent studies of growth regulating effects of muscarinic agents provide indirect evidence that insulin-like signalling participates in reactivation of Ancylostoma caninum L3i (Tissenbaum et al., 2000). An understanding of the molecular mechanisms underlying programmed arrest and reactivation of L3i has been slow to develop. This is due in large part to the fact that the complex and often protracted life histories of parasitic nematodes have hampered the development of basic tools for genetic, cell and molecular biological investigation.
The free-living nematode Caenorhabditis elegans is, in many respects, an ideal subject for genetic and molecular biological study, and consequently a relative wealth of basic information on the molecular, cellular, and developmental biology of that organism exists (Epstein and Shakes, 1995; Riddle and Albert, 1997). Caenorhabditis elegans has been proposed by numerous authors as a general model for many aspects of basic molecular, cellular and developmental biology in the less tractable parasitic nematodes (Blaxter, 1998; Burglin et al., 1998; Aboobaker and Blaxter, 2000). One of the most frequent of such analogies is that drawn between the arrest and reactivation of parasitic L3i and the dauer developmental pathway in C. elegans (Hotez et al., 1993). In response to adverse environmental conditions, larval C. elegans arrest their development at a specialised third-stage form termed the dauer larva. These dauer larvae re-enter the continuous, reproductive developmental pathway (i.e. reactivate) if optimal growth conditions are restored.
In contrast to parasitic L3i, the developmental biology of C. elegans dauers is well studied at the molecular genetic level. Currently, over 30 genes, designated daf- for their direct effect on dauer formation, and numerous genes not bearing daf designations have been shown to be necessary for normal dauer development (Riddle and Albert, 1997). Epistasis analysis (Vowels and Thomas, 1992; Thomas et al., 1993) coupled with cloning of many daf and related genes in C. elegans has revealed that dauer development is controlled in part by a signal transduction pathway closely resembling mammalian insulin signalling (Gottlieb and Ruvkun, 1994; Morris et al., 1996; Kimura et al., 1997; Ogg et al., 1997; Ogg and Ruvkun, 1998; Paradis and Ruvkun, 1998; Paradis et al., 1999). Under optimal growth conditions, signalling through DAF-2, an insulin/insulin-like growth factor (IGF) receptor ortholog, and the intervening kinase cascade negatively regulate the forkhead transcription factor DAF-16, allowing development through the continuous reproductive pathway. Under dauer inducing conditions, insulin-like signalling is downregulated and DAF-16 is released to promote dauer development. If the development of dauer larvae and parasitic L3i is controlled by similar molecular signalling mechanisms as has been proposed by Beall and Pearce (2002), then we would expect that this latter condition operates constitutively in the preinfective larvae of most parasitic nematodes for which dauer-like arrest of the L3i is the sole developmental alternative. On the other hand, in the case of the thread-worm, S. stercoralis, a subset of postparasitic larvae develop to free-living adults while others arrest as L3i. In some related species, Strongyloides ratti, Strongyloides planiceps and Strongyloides ransomi, the proportions of postparasitic larvae entering the two alternative developmental pathways are governed both by the genetic background of the worms (Viney et al., 1992) and by environmental factors such as pH, temperature and the concentration of organic nutrients (Arizono, 1976a, b; Moncol and Triantaphyllou, 1978; Minematsu et al., 1989). Therefore, if dauer and L3i development are similarly regulated in C. elegans and Strongyloides, then insulin-like signalling, particularly involving a pivotal regulatory molecule like DAF-16, could be critical in switching between these developmental alternatives. For this reason, we are conducting a PCR based search for insulin-like signal pathway intermediates in S. stercoralis. In the present paper we report the discovery of fktf-1 in S. stercoralis, a proposed ortholog of daf-16 in C. elegans, and present information on its developmental expression patterns and its phylogenetic relationship to other forkhead transcription factors.
2. Materials and methods
2.1. Parasite culture
All studies were performed on the University of Pennsylvania dog strain of S. stercoralis (SsUPD), which is maintained as described (Schad et al., 1984). Free-living adults and all larval stages were isolated by Baermannisation of faecal-charcoal cultures established with freshly deposited faeces from an infected dog (Nolan et al., 1993) and maintained at 70% relative humidity under different time and temperature regimens. Postparasitic first-stage larvae were obtained immediately after dispersing fresh faeces on activated charcoal. Mixed male and female free-living adults were obtained from cultures maintained for 48 h at 22 °C. Post-free-living L1 were manually selected from the mixed population of old free-living adults and newly hatched L1 in the Baermann proceeds from cultures incubated for 24 h at 22 °C and then for 24 h at 25 °C. L3i were obtained from cultures incubated for 7 days at 25 °C. Parasitic adult female worms were obtained by allowing them to emerge from slit intestines of experimentally infected jirds hanging in Dulbecco’s minimal essential medium (DMEM) with gentamicin for 1 h at 37 °C (Nolan et al., 1993).
2.2. DNA and RNA preparation from mass L3i cultures
Large scale DNA and RNA preparations were made from L3i of SsUPD grown in faecal-charcoal culture as described (Massey et al., 2001).
2.3. PCR screening and sequencing of S. stercoralis daf-16 sequences
PCR reactions were primed with degenerate guessmers FKHF1 (AAC AAG AMG WAA TGC WTG GGG), FKHR2 (GGA ACR TTT TNW ACC ATC CAT TC), FKHR3 (CAG CWG AWS WAT TWG AAT CTC C), FKHF3 (TTC WAA TWS WTC WGC TGG ATG G), and FKHR4 (SCW TCT GGR TTW ATW ACC CAC C) (where M = A + C; W = A + T; R = A + G; N = A + T+ G + C; and S = G + C) designed using codon frequencies for S. stercoralis (Moore et al., 1996; Massey et al., 2001) and based upon sequences conserved between C. elegans daf-16a, daf-16b and the human forkhead transcription factors AFX and FKHR (Ogg et al., 1997) using conditions modified for the (A + T)-rich genome of this species (Massey et al., 2001). Primer combinations FKHF1 plus FKHR2 and FKHF1 plus FKHR3 yielded 236 and 274 bp fragments, respectively, from the fktf-1a region, and FKHF1 plus FKHR4 and FKHF3 plus FKHR4 yielded 431 and 287 bp fragments, respectively, from the fktf-1b region. These fragments were sequenced directly from PCR as described (Massey et al., 2001). Outward facing gene-specific primers (not shown) were designed and the cDNA sequence of fktf-1b was amplified by modified 5′ and 3′ rapid amplification of cDNA ends (RACE) procedures (Massey et al., 2001) and sequenced. Low extension temperature inverse PCR (Massey et al., 2001) with outward facing gene-specific primers SsFKHF162 (GCA ACA TCA TGT TCA ATT CAC AAT GG) and SsFKHR65 (TGT ATC CTG TCA TTG GTG TAT CTG G) was used to capture 2.6 kb of sequence reading into the large intron between fktf-1a and fktf-1b from a circularised Bgl II digest of SsUPD genomic DNA, and primers SsFKHF4880 (CAT GAA ATT TCT ATA AAT AGT AAT GCT CC) and SsFKHR4822 (CAT TAA TGA CTC AAA ATC CAT TTC CG) were used to capture 1.5 kb of 3′ flanking sequence from a circularised Sau3AI digest. Gene-specific primers SsFKHaFA (GCA TGA GAC TAA TTC ATT TAC TG) and SsFKHaRB (CTC ATG CCT ATT ATTAAGAAGTG) were used to capture most of the coding region of fktf-1a from a circularised Msp I digest, and 600 bp reading into the large intron from a circularised Sau3AI digest. Two kilobases of the fktf-1a 5′ flanking region was captured from a circularised Eco RI digest with primers SsFKHaMF (GTT CTG GTG CCT CTT CAG C) and SsFKHaMR (CTG TTT CAT AAG AAG GAA TAC CG).
To determine intron/exon boundaries, complete cDNA sequences were compiled from 5′ and 3′ RACE products generated and sequenced directly from PCR using sequence-specific primers and anchor primers directed to the SL1 5′ splice leader or the 3′ polyadenylated tail as described (Massey et al., 2001).
2.4. RT-PCR assays for stage-specific expression of fktf-1a and fktf-1b mRNAs
Five hundred freshly collected worms at selected developmental stages were concentrated by centrifugation to a volume of 50 μl in 1.5 ml polypropylene microcentrifuge tubes immediately after collection. Worms were never stored on ice, since this resulted in loss of certain labile mRNAs (unpublished results). An equal volume of 2 × RNA lysis buffer (100 mM Tris–HCl, pH 8.0, 200 mM NaCl, 2% SDS, 200 mM EDTA) and 7 μl proteinase K (15 mg/ml) were added and the tubes were frozen for at least 5 min at −20 °C followed by incubation at 55 °C for 15 min. One millilitre of TRIZOL reagent (Invitrogen) was added and incubation was continued for 15 min at 55 °C. After cooling to ambient temperature for 3 min, 200 μl of chloroform was added and RNA extraction was continued according to the manufacturer’s protocol. After final resuspension of the product in 50 μl diethyl pyrocarbonate treated water, 1.0 μl aliquots were amplified using Super-Script One-Step RT-PCR with Platinum Taq (Invitrogen) with an annealing temperature of 47 °C and extension at 60 °C. Primer sets FK1aQF (ATA CAT GGC CAT TAA GAA GAG C) plus FK1aQR (TCC AAC CGG CAG AAC TAT) and FK1bQF (CTC CGA GCG CGT TGT TAC) plus FK1bQR (CCC CAT GGA TTA GGT TTT T) were used to amplify fktf-1a- and fktf-1b-specific message, respectively, and the RBPS21QF (ATG CAA AAC GAC AAA GGA GAA) plus RBPS21QR (AGC TAA ACG AAG AAG ACA ATC ATC) pair was used to amplify a fragment from rbps-21, the cDNA for the ribosomal small subunit protein S21 (Genebank BE579329) as an internal standard. In all cases, amplification sites crossed introns to allow message to be distinguished from genomic sequences by size. Cycle numbers (30–35) were empirically chosen to give sub-saturated levels of all products for relative quantitation. Band densities on agarose gels stained with ethidium bromide were compared using the Bio-Rad Gel Doc 2000 Gel Documentation System using Quantity One quantitation software.
2.5. Sequence and phylogenetic analysis
Raw sequence data were edited using SeqEd v1.0.3 (Applied Biosystems) and sequence homologies detected using BLAST servers (Altschul et al., 1990) at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST/), the Sanger Centre (http://www.sanger.ac.uk/Projects/C_elegans/blast_server.shtml), and the European Bioinformatics Institute (http://www.ebi.ac.uk/blast2/parasites.html).
Alignment and analysis of protein sequences were performed using Clustal W algorithm in the Lasergene software suite (DNASTAR) with manual adjustments. Evolutionary trees and Bootstrap analysis were generated using the PAUP 3.1.1 program (Sinauer).
3. Results
3.1. Genomic organisation of fktf-1
The S. stercoralis fktf-1 gene is organised much like C. elegans daf-16, although introns are fewer and often smaller than in daf-16 (Fig. 1). Analysis of cDNA sequences from both species shows alternately spliced isoforms differing in a region including their N-terminal domains and an N-terminal portion of the forkhead domain. In C. elegans, the region specific to the two daf-16a isoforms consists of four exons separated by three introns. Isoforms daf-16a1 and daf-16a2 differ by two amino acid residues due to alternative splice acceptor sites six bases apart at the end of the second intron. In S. stercoralis fktf-1, there is no intron corresponding to intron 2 of daf-16, allowing for only a single fktf-1a isoform. The (fktf-1/daf-16)a region is separated from the rest of the coding region by the large intron 4 in daf-16, or intron 3 in fktf-1, which is 6.2 kb in length in C. elegans and approximately 5 kb in S. stercoralis as judged by the size of PCR fragments spanning this region. A single (fktf-1/daf-16)b-specific exon in both species is located downstream of this large intron. A smaller intron separates this region from a common region consisting of the remaining exons of the gene. The messages for (FKTF-1/DAF-16)A or (FKTF-1/DAF-16)B contain their respective upstream regions spliced to this common region. A single intron corresponding to C. elegans intron 7 is found in this part of the S. stercoralis gene. Promoters upstream of the daf-16a-specific exons and in the large intron upstream of the daf-16b-specific exon drive expression of the respective isoforms of this gene in C. elegans. The region upstream of the fktf-1a-specific exons can drive expression of a green fluorescent protein reporter in C. elegans transformants in a pattern very similar to the daf-16α promoter (not shown), although very little sequence similarity exists between these promoters in the two species. There is likewise very little similarity between the two species in the presumptive (fktf-1/daf-16)b-specific promoter regions, although the large size of the intron in this region is conserved.
Fig. 1.
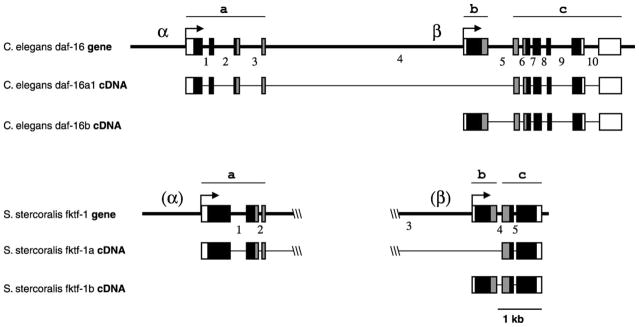
Genomic organisation of fktf-1 compared with its Caenorhabditis elegans ortholog daf-16 (Paradis and Ruvkun, 1998). The genes are arranged similarly in the two nematode species. Narrow bars indicate untranscribed sequences or introns; wide bars indicate exons; white, noncoding; black, N- and C-terminal domains; grey, forkhead domain. Regions specific to a isoforms of the transcription factors are beneath bars marked a. Those specific to b isoforms are beneath bars marked b, and those common to both isoforms are beneath bars marked c. Introns 1, 2, 3, 4 and 5 of Strongyloides stercoralis fktf-1 correspond in position exactly to introns 1, 3, 4, 5 and 7 of C. elegans daf-16, respectively. Arrows indicate direction of transcription and splice acceptor sites for the SL1 splice leader. Promoters for a and b isoforms are marked α and β respectively for C. elegans, and in parentheses at their expected locations for S. stercoralis.
3.2. Three functional domains are conserved between the FKTF-1/DAF-16 isoforms and their mammalian orthologs
Alignment of the conceptual translations of S. stercoralis FKTF-1A and FKTF-1B with the C. elegans DAF-16 isoforms and the orthologous human forkhead transcription factors AFX, FKHR and FKHRL1 shows conservation of the three domain structure common to these transcription factors. This pattern consists of a characteristic N-terminal domain, a central forkhead DNA binding domain, and a characteristic C-terminal domain. The sequences of FKTF-1A, FKTF-1B and other members of this family of proteins are most highly conserved in the DNA binding forkhead domain, with, for example, 79.5% identity between DAF-16A1 and FKTF-1A in the forkhead domain, 51.4% in the N-terminal domain (minus the serine-rich domain described below), and 31.4% in the C-terminal domain. A distinctive feature of the FKTF-1A sequence is a serine-rich region 181 residues long at the N-terminal end of the first domain. The frequency of serine residues in this region is 25.4% compared to 12.1% in the rest of the protein. A similar region consists of only 37 residues in DAF-16A1 and DAF-16A2 and is not found in FKTF-1B, DAF-16B, or any of the human orthologs.
In S. stercoralis FKTF-1 and C. elegans DAF-16, three consensus Akt/protein kinase B (Akt/PKB) phosphorylation sites with the consensus sequence RxxRxR(S/T)Hyd (where R = arginine, (S/T) = serine or threonine, x = any residue, and Hyd = a hydrophobic residue) are found that are highly conserved within the DAF-16/FKHR/AFX family of transcription factors, one in each of the conserved domains. A fourth site overlapping the conserved site in the forkhead domain is also found in both S. stercoralis FKTF-1 and C. elegans DAF-16. The alignment of these sequences and other supplementary information can be found at the following website: http://www.vet.upenn.edu/GraduateGroups/Parasitology/lok.htm.
3.3. Evolutionary relationships of fktf-1
Analysis of an alignment of S. stercoralis FKTF-1A and FKTF-1B with their proposed C. elegans orthologs, the human forkhead proteins FKHR, FKHRL1 and AFX, and other human and nematode forkhead transcription factors shows the S. stercoralis proteins to be most closely related to their proposed C. elegans orthologs (Fig. 2). When sequences from the forkhead domain only are analysed, bootstrapping does not support a clade represented by FKTF-1A and DAF-16A1 separate from that including FKHR, FKHRL1 and AFX. When the sequences from the C-terminal domains of these proteins are added, however, DAF-16A1 and FKTF-1A clearly group together into a distinct clade as shown in Fig. 2.
Fig. 2.
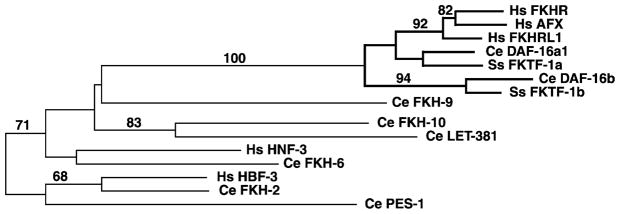
An unrooted phylogenetic tree shows the relationships of Strongyloides stercoralis FKTF-1A and FKTF-1B to other Caenorhabditis elegans and human forkhead transcription factors. Only residues located in the forkhead domains of these proteins were used in the analysis because residues in outlying domains of many of these proteins were too divergent to be aligned. Branches of the tree including FKTF-1A, FKTF-1B, DAF-16A1, DAF-16B, and the FKHR-like group of human forkhead transcription factors are highlighted in boldface. The tree was generated by PAUP 3.1.1 analysis of a Clustal W alignment (DNASTAR software suite) with manual editing. Bootstrapping scores (100 iterations) are shown for robust clades (>50% cutoff). Homo sapiens, Hs; Caenorhabditis elegans, Ce; Strongyloides stercoralis, Ss. GenBank™ accession numbers: Hs FKHR, S40521; Hs AFX, P98177; Hs FKHRL1, CAC26821; Ce DAF-16A1, AAB84390; Ss FKTF-1A, AY281749; Ce DAF-16B, AAB84392; Ss FKTF-1B, AY281750; Ce FKH-9, AAA81139; Ce FKH-10, Z81038; Ce LET-381, NP_491826; Hs HNF-3, AAD51979; Ce FKH-6, AAA80692; Hs HBF-3, X74144; Ce FKH-2, AAL02521; Ce PES-1, CAA82224.
3.4. Developmental expression patterns of the fktf-1 message
Expression of the fktf-1a and fktf-1b messages was found at all developmental stages of S. stercoralis examined. Signal ratios to control Ss rbps-21-specific mRNA, which encodes the ribosomal small subunit protein S21, showed some variation but no consistent developmental pattern at different stages of the S. stercoralis life cycle (Fig. 3).
Fig. 3.
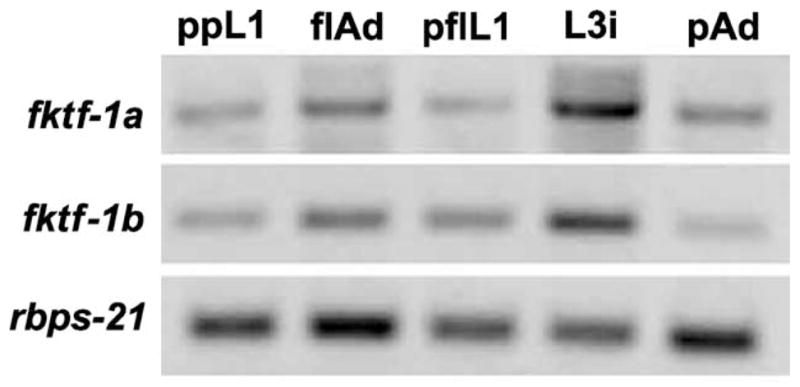
Expression of fktf-1a and fktf-1b messages in larval and adult stages of Strongyloides stercoralis compared with internal standard rbps-21 (see text). Bands in agarose gels of RT-PCR products from postparasitic L1 larvae (ppL1), free-living mixed male and female adults (flAd), post-free-living L1 (pflL1), infective L3 (L3i), and parasitic adult females (pAd).
4. Discussion
It has been shown that the insulin/IGF receptor-like kinase DAF-2 in C. elegans mediates a shift between lipid storage metabolism in dauer larvae and carbohydrate catabolism in actively developing larvae and adults (Kimura et al., 1997). This activity is mediated through a phosphoinositol-3-kinase pathway including orthologs of many of the known intermediates of mammalian insulin and IGF pathways involved in the regulation of gene activity in response to insulin and glucocorticoid signalling (Morris et al., 1996; Kimura et al., 1997; Paradis and Ruvkun, 1998; Paradis et al., 1999; Nasrin et al., 2000). The family of forkhead transcription factors that includes DAF-16 in C. elegans and the human factors FKHR, FKHRL1 and AFX are the terminal effectors in these pathways. DAF-16A1 can functionally replace FKHR in mammalian reporter systems where transcriptional regulation was mediated through binding to insulin response sequences in the promoters of the insulin-like growth factor binding protein 1 and phosphoenolpyruvate carboxykinase genes (Nasrin et al., 2000). Likewise, transgenic FKHRL1 can partially replace DAF-16 function in C. elegans (Lee et al., 2001). The presence of FKTF-1, a close ortholog of DAF-16, in S. stercoralis argues for conservation of this signalling pathway in the parasite.
The fktf-1 gene in S. stercoralis closely resembles daf-16 in C. elegans in its overall organisation (Fig. 1), with an upstream region specific to the fktf-1a isoform separated from the region specific to the fktf-1b isoform by a large intron (Ogg et al., 1997). Transcription of the daf-16a isoforms is driven by an upstream promoter (α) in C. elegans (Ogg et al., 1997; Lee et al., 2001), and presumably also in the parasite. The large intron separating daf-16a-specific and daf-16b-specific portions of the daf-16 gene is conserved in the fktf-1 gene, suggesting functional conservation in this region, which contains the promoter (β) for the daf-16b message in C. elegans (Lee et al., 2001). The two promoters showed a complementary pattern of expression in tissues, with the green fluorescent protein (GFP) reporter construct daf-16α::gfp driving GFP expression in most somatic tissues excluding the pharynx and somatic gonad, and the daf-16β driven construct showing expression in the pharynx, somatic gonad and a subset of neurons (Ogg et al., 1997; Lee et al., 2001). The similar architecture of the C. elegans daf-16 gene and the S. stercoralis fktf-1 gene region suggests two promoters may function in a similar fashion in both species.
Strongyloides stercoralis fktr-1 has fewer and generally smaller introns than C. elegans daf-16, a feature that has been noted in other genes sequenced in this parasite (Massey et al., 2001). In C. elegans, the number of introns can have a strong positive effect on the efficiency of expression of transgenes (Okkema et al., 1993), suggesting that certain features of message processing in the two species may differ or vary in their degree of influence. Early transformation experiments in this laboratory with S. stercoralis showed high, potentially toxic levels of GFP production during embryogenesis using gfp constructs containing multiple artificial introns designed for efficient expression in C. elegans (Lok and Massey, 2002). The consideration that introns may have greater efficacy in stimulating transcriptional processing in S. stercoralis is being investigated in the context of designing appropriate transformation reporters for the S. stercoralis system.
The DAF-16A1 and DAF-16B proteins were found to be functionally interchangeable in transgenic arrays driven by either of the two promoters in C. elegans (Lee et al., 2001), suggesting that the differential effects of these two factors on growth and longevity were more the product of differential expression from the two promoters than intrinsic differences between the proteins. However, FKTF-1A and DAF-16A share an N-terminal serine-rich sequence, greatly expanded in FKTF-1A, that is not found in FKTF-1B, DAF-16B or their mammalian orthologs. The function of this region is not known, but it might contain regulatory features such as phosphorylation sites or signals for turnover allowing for regulatory pathways unique to these isoforms. It is possible that consequent differences in the regulation of these proteins would not have been evident in transgenic studies where they were over expressed in extrachromosomal arrays (Lee et al., 2001).
FKTF-1A and FKTF-1B show strongest identity with DAF-16A1 and DAF-16B in the central forkhead DNA binding domain (79.5 and 81.1%, respectively), suggesting possible conservation of sequence binding specificity. This domain binds specific DNA sites, which include the insulin response element of the insulin-like growth factor binding protein 1 promoter and the insulin response sequence of the phosphoenolpyruvate carboxykinase gene in mammalian cells (Nasrin et al., 2000), and the DAF-16 family member binding element of sod-3 in C. elegans (Furuyama et al., 2000). DAF-16A1 was shown to promote insulin responsive gene transcription in a manner similar to its human orthologs when transfected into HepG2 cells, with a sensitivity to insulin inhibition being most similar to FKHR (Nasrin et al., 2000). All these proteins bound artificial oligonucleotides with the same eight base core consensus sequence in vitro (Furuyama et al., 2000).
All of these transcription factors share three conserved regulatory Akt/PKB phosphorylation sites, one in each of the conserved domains. In addition to these sites, FKTF-1 and DAF-16 share an additional overlapping Akt/PKB phosphorylation site in the forkhead domain (Ogg et al., 1997). These sites in the C. elegans and human proteins form binding sites for 14-3-3 proteins when phosphorylated that mediate transport out of the nucleus and consequent downregulation of transcription directed by these factors (Cahill et al., 2001). Mutation of the phosphorylated serine or threonine residues in these sites to alanine prevents phosphorylation, resulting in constitutive nuclear retention and upregulation of transcription (Cahill et al., 2001). Ratios of fktf-1 message to a control mRNA indicate that this transcription factor is expressed at similar levels in all life stages. These findings are consistent with the developmental patterns of C. elegans daf-16 expression where daf-16-linked GFP expression occurs at all stages (Ogg et al., 1997; Lee et al., 2001). Since no consistent pattern of stage-specific regulation of message level is apparent, regulation of the activity of these proteins appears to occur primarily by phosphorylation and transport to and from the nucleus rather than at the level of transcription.
In mammalian systems, the C-terminal domain is responsible for interactions with the steroid receptor coactivator and other transcription factors such as p300/CREB binding proteins, indicating a role in integrating glucocorticoid and insulin-like transcriptional responses. Despite limited sequence similarity in this domain, C. elegans DAF-16 is able to mediate recruitment of these factors to promoters and regulate transcription of mammalian insulin responsive genes in vitro and in vivo (Nasrin et al., 2000). The FKTF-1 sequence is 34.1% identical to DAF-16 in this region, suggesting conservation of function in the parasite. The C-terminal domains of the human proteins in this family and DAF-16 have been shown to have similar functional properties despite very limited sequence identity (Nasrin et al., 2000; Lee et al., 2001), suggesting that such properties as conformation and hydropathy may play more important roles in the function of this domain than sequence identity. In C. elegans, daf-12 encodes a member of the steroid nuclear receptor family of transcription factors, and daf-12 interacts with the insulin-like branch of the dauer pathway to regulate dauer development (Riddle and Albert, 1997; Snow and Larsen, 2000). A proposed S. stercoralis ortholog of daf-12 has been identified (Siddiqui et al., 2000). It is possible that cross-talk between insulin-like and glucocorticoid-like regulatory pathways has a regulatory role in dauer arrest in C. elegans and the arrest of L3i in S. stercoralis and other parasitic nematodes, and that the C-terminal domains of forkhead transcription factors like DAF-16 and FKTF-1 may function in this cross-talk.
The discovery of FKTF-1, a proposed DAF-16 ortholog in the parasitic nematode S. stercoralis supports the hypothesis that a dauer-like pathway is functional in this parasite and may function in determining any or all of the following: (1) the choice between parasitic and free-living life cycles, (2) developmental arrest and/or extended life span of infective L3i, and (3) reactivation of development after infection of the host (Hotez et al., 1993; Blaxter, 1998; Burglin et al., 1998; Aboobaker and Blaxter, 2000). The C. elegans system has proven an invaluable tool in investigating many aspects of developmental biology that go far beyond the biology of this species. No such facile system has been found among parasitic nematodes, but the discovery of key intermediates in the dauer pathway in S. stercoralis may allow complementation of relevant mutations in C. elegans with parasite transgenes, as has been done in a study of the function of the cathepsin L protease CPL-1 in parasitic nematodes (Britton and Murray, 2002). The ability of the C. elegans dauer-regulatory genes to function in systems as divergent as mammalian cells, and vice versa, argues for the success of this strategy. The early success in this laboratory of experiments in which S. stercoralis has transiently expressed reporter transgenes (Lok and Massey, 2002) encourages us that it may soon be possible to test the function of these genes in the parasite as well.
Acknowledgments
The authors are grateful to G.A. Schad for critical discussions and for starting material for S. stercoralis cultures. We also thank C.C. Ball, M. Brenes, M. Castelleto, W. Forbes and A. Freeman for technical assistance. This work was supported by grants from the National Institutes of Health (AI-43616 and AI-50668 to J.B. Lok, AI-22662 to G.A. Schad, and RR02512 to M. Haskins), the Ellison Medical Foundation (ID-IA0037-02) and the University of Pennsylvania Research Foundation to J.B. Lok.
Footnotes
Nucleotide sequence data reported in this paper are available in the EMBL, GenBank™ and DDJB databases under the following accession numbers: Strongyloides stercoralis fktf-1a genomic fragment AY281752, and cDNA sequence AY281749, S. stercoralis fktf-1b genomic sequence, AY281750, and cDNA sequence, AY28175.
References
- Aboobaker AA, Blaxter ML. Medical significance of Caenorhabditis elegans. Ann Med. 2000;32:23–30. doi: 10.3109/07853890008995906. [DOI] [PubMed] [Google Scholar]
- Abraham D, Mok M, Mika-Grieve M, Grieve RB. In vitro culture of Dirofilaria immitis third- and fourth-stage larvae under defined conditions. J Parasitol. 1987;73:377–383. [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arizono N. Studies on the free-living generations of Strongyloides planiceps Rogers, 1943 I. Effects of quantity of food and population density on the developmental types. Jpn J Parasitol. 1976a;25:274–282. [Google Scholar]
- Arizono N. Studies on the free-living generations of Strongyloides planiceps Rogers, 1943 II. Effect of temperature on the developmental types. Jpn J Parasitol. 1976b;25:328–335. [Google Scholar]
- Ashton FT, Bhopale VM, Holt D, Smith G, Schad GA. Developmental switching in the parasitic nematode Strongyloides stercoralis is controlled by the ASF and ASI amphidial neurons. J Parasitol. 1998;84:691–695. [PubMed] [Google Scholar]
- Beall MJ, Pearce EJ. Transforming growth factor-beta and insulin-like signalling pathways in parasitic helminths. Int J Parasitol. 2002;32:399–404. doi: 10.1016/s0020-7519(01)00348-4. [DOI] [PubMed] [Google Scholar]
- Blaxter M. Caenorhabditis elegans is a nematode. Science. 1998;282:2041–2046. doi: 10.1126/science.282.5396.2041. [DOI] [PubMed] [Google Scholar]
- Bowman DD. Georgi’s Parasitology for Veterinarians. W.B. Saunders; Philadelphia, PA: 1995. pp. 150–245. [Google Scholar]
- Britton C, Murray L. A cathepsin L protease essential for Caenorhabditis elegans embryogenesis is functionally conserved in parasitic nematodes. Mol Biochem Parasitol. 2002;122:21–33. doi: 10.1016/s0166-6851(02)00066-x. [DOI] [PubMed] [Google Scholar]
- Burglin TR, Lobos E, Blaxter ML. Caenorhabditis elegans as a model for parasitic nematodes. Int J Parasitol. 1998;28:395–411. doi: 10.1016/s0020-7519(97)00208-7. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Tzivion G, Nasrin N, Ogg S, Dore J, Ruvkun G, Alexander-Bridges M. Phosphaditylinositol 3-kinase signalling inhibits DAF-16 DNA binding and function via 14-3-3 dependent and 14-3-3 independent pathways. J Biol Chem. 2001;276:13402–13410. doi: 10.1074/jbc.M010042200. [DOI] [PubMed] [Google Scholar]
- Despommier DD, Gwadz RW, Hotez PJ, Knirsch CA. Parasitic Diseases. 4. Chapter V. Apple Trees Productions; New York, NY: 2000. The Nematodes; pp. 97–160. [Google Scholar]
- Epstein HF, Shakes DC, editors. Caenorhabditis elegans: Modern Biological Analysis of an Organism. Academic Press; New York, NY: 1995. [Google Scholar]
- Farrar RG, Klei TR. In vitro development of Strongylus edentatus to the fourth larval stage with notes on Strongylus vulgaris and Strongylus equinus. J Parasitol. 1985;71:489–499. [PubMed] [Google Scholar]
- Franke ED, Weinstein PP. In vitro cultivation of Dipetalonema viteae third-stage larvae: effect of the gas phase. J Parasitol. 1984a;70:493–498. [PubMed] [Google Scholar]
- Franke ED, Weinstein PP. In vitro cultivation of Dipetalonema viteae third-stage larvae: evaluation of culture media, serum, and other supplements. J Parasitol. 1984b;70:618–628. [PubMed] [Google Scholar]
- Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J. 2000;349:629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb S, Ruvkun G. daf-2, daf-16 and daf-23: genetically interacting genes controlling dauer formation in Caenorhabditis elegans. Genetics. 1994;137:107–120. doi: 10.1093/genetics/137.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P, Hawdon JM, Schad GA. Hookworm larval infectivity, arrest and amphiparatenesis: the Caenorhabditis elegans daf-c paradigm. Parasitol Today. 1993;9:23–26. doi: 10.1016/0169-4758(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, An insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Lee RY, Hench J, Ruvkun G. Regulation of C. elegans DAF-16 and its human ortholog FKHRL1 by the daf-2 insulin-like signaling pathway. Curr Biol. 2001;11:1950–1957. doi: 10.1016/s0960-9822(01)00595-4. [DOI] [PubMed] [Google Scholar]
- Lok JB, Massey HC., Jr Transgene expression in Strongyloides stercoralis following gonadal microinjection of DNA constructs. Mol Biochem Parasitol. 2002;119:279–284. doi: 10.1016/s0166-6851(01)00414-5. [DOI] [PubMed] [Google Scholar]
- Lustigman S, Brotman B, Huima T, Castelhano AL, Singh RN, Mehta K, Prince AM. Transglutaminase-catalyzed reaction is important for molting of Onchocerca volvulus third-stage larvae. Antimicrob Agents Chemother. 1995;39:1913–1919. doi: 10.1128/aac.39.9.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustigman S, McKerrow JH, Shah K, Lui J, Huima T, Hough M, Brotman B. Cloning of a cysteine protease required for the molting of Onchocerca volvulus third stage larvae. J Biol Chem. 1996;271:30181–30189. doi: 10.1074/jbc.271.47.30181. [DOI] [PubMed] [Google Scholar]
- Massey HC, Jr, Ball CC, Lok JB. PCR amplification of putative gpa-2 and gpa-3 orthologs from the (A + T)-rich genome of Strongyloides stercoralis. Int J Parasitol. 2001;31:377–383. doi: 10.1016/s0020-7519(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Minematsu T, Mimori T, Tanaka M, Tada I. The effect of fatty acids on the developmental direction of Strongyloides ratti first-stage larvae. J Helminthol. 1989;63:102–106. doi: 10.1017/s0022149x00008841. [DOI] [PubMed] [Google Scholar]
- Moncol DJ, Triantaphyllou AC. Stronglyoides ransomi: factors influencing the in vitro development of the free-living generation. J Parasitol. 1978;64:220–225. [PubMed] [Google Scholar]
- Moore TA, Ramachandran S, Gam AA, Neva FA, Lu W, Saunders L, Williams SA, Nutman TB. Identification of novel sequences and codon usage in Strongyloides stercoralis. Mol Biochem Parasitol. 1996;79:243–248. doi: 10.1016/0166-6851(96)02659-x. [DOI] [PubMed] [Google Scholar]
- Morris JZ, Tissenbaum HA, Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- Nasrin N, Ogg S, Cahill CM, Biggs W, Nui S, Dore J, Calvo D, Shi Y, Ruvkun G, Alexander-Bridges MC. DAF-16 recruits the CREB-binding protein coactivator complex to the insulin-like growth factor binding protein 1 promoter in HepG2 cells. Proc Natl Acad Sci U S A. 2000;97:10412–10417. doi: 10.1073/pnas.190326997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble ER, Noble GA, Schad GA, MacInnes AJ. Parasitology: The Biology of Animal Parasites. Lea and Febiger; Philadelphia, PA: 1989. [Google Scholar]
- Nolan TJ, Megyeri Z, Bhopale VM, Schad G. Strongyloides stercoralis: the first rodent model for uncomplicated and hyperinfective strongyloidiasis, the mongolian gerbil (Meriones unguiculatus) J Infect Dis. 1993;168:1479–1484. doi: 10.1093/infdis/168.6.1479. [DOI] [PubMed] [Google Scholar]
- Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- Ogg S, Ruvkun G. The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway. Mol Cell. 1998;2:887–893. doi: 10.1016/s1097-2765(00)80303-2. [DOI] [PubMed] [Google Scholar]
- Okkema PG, Harrison SW, Plunger V, Aryana A, Fire A. Sequence requirements for myosin gene expression and regulation in Caenorhabditis elegans. Genetics. 1993;135:385–404. doi: 10.1093/genetics/135.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S, Ailion M, Toker A, Thomas JH, Ruvkun G. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev. 1999;13:1438–1452. doi: 10.1101/gad.13.11.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle DL, Albert PS. In: Genetic and Environmental Regulation of Dauer Larva Development, C. elegans II. Riddle DL, et al., editors. Cold Spring Harbor Press; Plainview, NY: 1997. pp. 739–768. [PubMed] [Google Scholar]
- Schad GA, Hellman ME, Muncey DW. Strongyloides stercoralis: hyperinfection in immunosuppressed dogs. Exp Parasitol. 1984;57:287–296. doi: 10.1016/0014-4894(84)90103-6. [DOI] [PubMed] [Google Scholar]
- Siddiqui AA, Stanley CS, Skelly PJ, Berk SL. A cDNA encoding a nuclear hormone receptor of the steroid/thyroid hormone-receptor superfamily from the human parasitic nematode Strongyloides stercoralis. Parasitol Res. 2000;86:24–29. doi: 10.1007/pl00008502. [DOI] [PubMed] [Google Scholar]
- Snow MI, Larsen PL. Structure and expression of daf-12: a nuclear hormone receptor with three isoforms that are involved in development and aging in Caenorhabditis elegans. Biochim Biophys Acta. 2000;1494:104–116. doi: 10.1016/s0167-4781(00)00224-4. [DOI] [PubMed] [Google Scholar]
- Thomas JH, Birnby DA, Vowels JJ. Evidence for parallel processing of sensory information controlling dauer formation in Caenorhabditis elegans. Genetics. 1993;134:1105–1117. doi: 10.1093/genetics/134.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum HA, Hawdon J, Perregaux M, Hotez P, Guarente L, Ruvkun G. A common muscarinic pathway for diapause recovery in the distantly related nematode species Caenorhabditis elegans and Ancylostoma caninum. Proc Natl Acad Sci U S A. 2000;97:460–465. doi: 10.1073/pnas.97.1.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viney ME, Matthews BE, Walliker D. On the biological and biochemical nature of cloned populations of Strongyloides ratti. J Helminthol. 1992;66:45–52. doi: 10.1017/s0022149x00012554. [DOI] [PubMed] [Google Scholar]
- Vowels JJ, Thomas JH. Genetic analysis of chemosensory control of dauer formation in Caenorhabditis elegans. Genetics. 1992;130:105–123. doi: 10.1093/genetics/130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski N, Weinstein PP. Growth and development of Brugia pahangi larvae under various in vitro conditions. J Parasitol. 1993;79:390–398. [PubMed] [Google Scholar]


