Abstract
Obesity and premature adrenarche (PA) are both associated with bone age (BA) advancement of unclear etiology, which may lead to earlier puberty, suboptimal final height and obesity in adulthood. Our objective was to understand the hormonal and anthropometric characteristics of BA advancement in a spectrum of prepubertal children with and without obesity and PA.
In this cross-sectional study of 66 prepubertal children (35 PA, 31 control, 5–9 years), body mass index (BMI) z-score, hormonal values and response to an oral glucose tolerance test were the main outcome measures. Subjects were divided into tertiles by BA divided by chronological age (BA/CA), an index of BA advancement.
Subjects in the top tertile for BA/CA had the highest dehydroepiandrosterone sulfate (DHEAS), free testosterone (%), hemoglobin A1C, BMI z-score and weight (P < 0.05). BMI z-score (r = 0.47), weight (r = 0.40), free testosterone (%) (r = 0.34) and DHEAS (r = 0.30) correlated with BA/CA (P<0.02). Regression analysis showed greater BA/CA in PA compared to controls after controlling for weight (0.21 ± 0.56, p<0.004). An exploratory stepwise regression model showed that weight, estradiol and DHEAS were the strongest predictors of BA/CA accounting for 24% of its variance.
Obesity was highly associated with BA advancement in this study of prepubertal children. In addition, children with PA had greater BA/CA at any given weight when compared to controls. These findings suggest a possible hormonal factor, which potentiates the effect of obesity on BA advancement in children with obesity and/or PA.
Introduction
Precocious or premature pubarche is defined as the appearance of pubic and/or axillary hair before 8 yr in girls and 9 yr in boys unaccompanied by signs of true puberty, such as breast development in girls or testicular enlargement in boys. Premature adrenarche (PA) is a term used for individuals with precocious pubarche whose adrenal androgens are elevated for chronological age but within the range expected for the Tanner stage of pubic hair and in whom there is no evidence of enzymatic defects of steroidogenesis, precocious puberty or adrenal or gonadal malignancy (1). PA is caused by early isolated maturation of the zona reticularis of the adrenal cortex or by increased peripheral sensitivity to adrenal hormones (1). Although PA may occur in nonobese children, there is a significant association between PA and obesity (2,3).
PA was originally thought to be a benign and self-limited physiological process, but recent data suggest that children with PA are more likely to develop the metabolic syndrome during adolescence and adulthood, and affected girls are more likely to eventually develop polycystic ovary syndrome, even in the absence of obesity (2). Tall stature and advanced bone age (BA) may be present at diagnosis although adult height generally correlates with midparental height (4,5). However, a recent study showed that affected children with an advanced bone age had a predicted height below their midparental target height (3). Another recent study suggests that increased growth velocity in PA may begin as early as the first two years of life (5).
The skeletal maturation process involves transformation of the cartilaginous epiphyseal growth plate into bone, which proceeds as a child grows and matures. Once the epiphyseal plate ossifies an individual reaches final adult height. This complex cascade of events includes parathyroid hormone related peptide (PTHrP), Indian Hedgehog, matrix metalloproteinases and vascular endothelial growth factor (VEGF), which are involved in coordinating cellular growth, cellular differentiation, apoptosis, extracellular matrix remodeling and angiogenesis (6). Estrogens, androgens and the growth hormone/insulin-like growth factor-1 (GH/IGF-1) axis influence the activity of these factors and the progression of skeletal maturation (7).
BA advancement has been described both in obese children (8, 9) and in children with PA (3, 5, 10). The cause of advanced BA seen in both of these groups is still unclear; however, both obesity and PA are associated with elevations of adrenal androgens, which theoretically may be converted into estrogens either peripherally or at the growth plate.
The goals of this study were to assess the relationships of body mass index (BMI) z-score, hormonal factors and insulin resistance to BA advancement in a group of prepubertal children with and without PA who represent a wide spectrum of BMI z-scores. The data from this study will add to our understanding of the characteristics of BA advancement in obesity and PA.
Materials and Methods
Subjects
The study group comprised 66 prepubertal children (43 girls and 23 boys) ages 5 – 9 years, 35 with PA and 31 control subjects (Table 1). The PA group was 83% Hispanic, 6% white, 3% black and 8% “other” the control group was 74% Hispanic, 6.5% white, 6.5% black and 13% “other”. Hispanic subjects were primarily from the Dominican Republic. Ethnicity was determined by self report with consistent black, white or Hispanic background of both parents and all four grandparents. Subjects with ethnic backgrounds that did not fit these criteria were classified as “other”.
Table 1.
Composition of the study population
| Girls | Boys | Total | |
|---|---|---|---|
| PA | 27 (40.9%) | 8 (12.1%) | 35 (53.0%) |
| Control | 16 (24.2%) | 15 (22.7%) | 31(47.0%) |
| Total | 43 (65.2%) | 23 (34.8%) | 66 (100%) |
Children with PA were recruited from the pediatric endocrinology ambulatory service and control subjects were recruited from general pediatrics ambulatory service and posted flyers. Both ambulatory services are located at Morgan Stanley Children’s Hospital of New York at New York Presbyterian Medical Center, Columbia University. Informed consent was obtained from a parent or guardian and assent was obtained from each subject above the age of seven. The protocol was approved by the Institutional Review Boards of Columbia University Medical Center and of St. Luke’s-Roosevelt Hospital Center.
The diagnosis of precocious pubarche was made on a clinical basis. The criteria for entry into the study for PA subjects were: 1) onset of pubic hair and/or axillary hair before age 8 yr in girls and 9 yr in boys; 2) no clitoromegaly in girls; 3) Tanner stage 1 breasts on physical exam in girls and ≤ 3 cc testes on physical exam in boys; 4) no evidence of 21-hydroxylase deficiency or other adrenal or gonadal disorder by hormonal analysis as documented by basal 17-hydroxyprogesterone levels (11). The criteria for entry into the study for control subjects were: 1) Tanner stage 1 pubic hair; 2) Tanner stage 1 breasts on physical exam in girls and ≤ 3 cc testes on physical exam in boys. Exclusion criteria included subjects who were a product of a pregnancy complicated by gestational diabetes, twin or other multiple, preterm delivery or small for gestational age, chronic medical condition and chronic use of glucocorticoids or other hormonal therapies.
Pubertal and clinical assessment
Pubertal and clinical assessment of subjects was performed on the day of testing at the Pediatric Clinical Research Center at the Irving Institute for Clinical and Translational Research, Columbia University Medical Center. Breasts and pubic hair were assessed by visual inspection and palpation using the rating scales of Tanner and Marshall (12). The volume of each testis was estimated by comparative palpation with the orchidometer of Prader (13). Height, weight, heart rate and blood pressure were measured; BMI and BMI z-score were calculated and were based on 1 SD reference data from the National Health and Nutrition Examination Survey (14). Normal weight was defined as BMI < 85th percentile for age and sex, overweight/obese was defined as BMI ≥ 85th percentile for age and sex.
Bone Age Assessment
An x-ray of the hand and wrist was taken and BA was determined by the standards of Greulich and Pyle (15). All BAs were read by three of the coinvestigators who were blinded to the identity and diagnosis of each subject. Final determination of BA was made by consensus among the three readers. BA divided by chronological age (BA/CA) was used as an index for BA advancement. Subjects were divided into tertiles based on BA/CA (Table 2).
Table 2.
Categorization of the entire study population by tertiles for BA/CA (n = 66)
| Tertile | n | Minimum BA/CA |
Mean BA/CA | Maximum BA/CA |
|---|---|---|---|---|
| 1st | 21 | 0.816 | 0.954 | 1.019 |
| 2nd | 23 | 1.020 | 1.088 | 1.152 |
| 3rd | 22 | 1.170 | 1.303 | 1.600 |
Procedures
Blood samples were drawn between 0800 h and 0900 h after an overnight fast. Basal levels of glucose (G0), insulin (I0), IGF-1, IGF binding protein 3 (IGFBP-3), DHEAS, androstenedione, testosterone, free testosterone, free testosterone (%), Sex hormone binding globulin (SHBG), estradiol, estrone, luteinizing hormone (LH), follicle stimulating hormone (FSH), hemoglobin A1C, lipids, thyroid function tests, and leptin were drawn. Leptin was measured on a subset of subjects (n = 50). Glucose and insulin levels were measured at 30 minutes, 60 minutes, 90 minutes and 120 minutes after a 1.75g/kg (maximum 75 g) oral glucose tolerance test using Glucola®.
Measures of insulin sensitivity and insulin secretion
Fasting glucose to insulin ratio (FGIR), homeostasis model assessment (HOMA), insulin sensitivity measure (SiM), early insulin response (EIR), glucose area under the curve (GAUC) and insulin area under the curve (IAUC) were calculated. FGIR, HOMA and SiM were calculated as measures of insulin sensitivity and EIR was calculated as a measure of insulin secretion. FGIR was calculated by dividing G0 by I0. This value has been validated in prepubertal girls with PA using intravenous and oral glucose tolerance tests (16). HOMA was calculated as: I0/(22.5(−ln(G0)), SiM was calculated as: ((0.137 × Sib) + Si2h)/2, (Sib = 108/I0 × G0 × 150 × wt; Si2h = 108/I120 × G120 × 150cc × wt) and EIR was calculated as: I30 − I0/G30 − G0.
Assays
Insulin (by immunochemiluminometric assay), IGF-1 and IGFBP-3 (by competitive binding radioimmuno assay (RIA)), DHEAS (by RIA after enzymolysis), androstenedione (by high performance liquid chromatography (HPLC), tandem mass spectrometry), testosterone (by HPLC, tandem mass spectrometry), free testosterone (by equilibrium dialysis), estradiol (by HPLC, tandem mass spectrometry), SHBG, follicle stimulating hormone, lutenizing hormone, and hemoglobin A1C were measured by Esoterix Inc. Laboratory Services, (Calabasas Hills, California). Plasma glucose levels were assayed by the glucose hexokinase method. Plasma total cholesterol, high density lipoprotein (HDL) cholesterol and triglycerides were measured using the analyzer (Hitachi, Japan) in the Core Laboratory of the general CRC at New York Presbyterian Medical Center and low density lipoprotein (LDL) was calculated. Leptin was measured with a human radioimmunosorbant assay (RIA) kit (LINCO Research Inc, St. Charles, MO) using a 125I human leptin tracer.
Statistical Analysis
Student’s t-test was used to compare PA and control subjects for the following continuous measures: age, height, weight, BMI z-score, BA, BA/CA, testosterone, free testosterone, free testosterone (%), DHEAS, androstenedione, estradiol, estrone, SHBG, IGF-1, IGFBP-3, leptin, hemoglobin A1C, glucose (0 min), glucose (AUC), insulin (0 min), insulin (AUC), FGIR, HOMA, SiM and EIR. ANOVA was used to assess the tertile analysis of BA advancement for all of the mentioned continuous variables. In addition, distribution of overweight/obese subjects and PA subjects by BA/CA tertile were compared.
BMI z-score and age adjusted Pearson correlations were performed on the entire group. Correlations assessed the strength of association between BA/CA and estradiol, age, weight, testosterone, free testosterone, free testosterone (%), androstenedione, DHEAS, SHBG, IGF-1, HOMA and FGIR. A multivariate exploratory stepwise regression model was then used to predict the factors most likely to advance BA. Multiple regression analysis was used to assess differences between the PA and control groups in the association of BA/CA to weight.
P values < 0.05 were considered to represent statistical significance for all statistical analyses.
Results
Clinical Characteristics
Age, height, weight, BMI z-score and BA/CA were similar in the PA and control groups (Table 3).
Table 3.
| a. Clinical characteristics of the study population | |||
|---|---|---|---|
|
| |||
| PA (n=35) | Control (n=31) | P value | |
| Age (years) | 7.5 ± 1.2 | 7.1 ± 1.5 | NS |
| Height (cm) | 128.6 ± 8.8 | 125.1 ± 8.8 | NS |
| Weight (kg) | 33.0 ± 9.0 | 33.6 ± 12.6 | NS |
| BMI z-score | 1.29 ± 0.97 | 1.48 ± 1.19 | NS |
| BA (years) | 8.3 ± 1.8 | 7.7± 1.8 | NS |
| BA/CA | 1.13 ± 0.15 | 1.10 ± 0.17 | NS |
|
| |||
| b. Clinical characteristics of the study population divided by obesity status | |||
|
| |||
| Obese (n=43) | Nonobese (n=23) | P value | |
|
| |||
| Age (years) | 7.4±1.4 | 7.2±1.3 | NS |
| Height (cm) | 128.4±8.3 | 124.3±9.6 | 0.076 |
| Weight (kg) | 38.0±10.3 | 24.6±4.4 | <0.0001 |
| BMI z-score | 2.06±0.56 | 0.10±0.48 | <0.0001 |
| BA (years) | 8.4±1.9 | 7.4±1.4 | 0.037 |
| BA/CA | 1.16±0.17 | 1.04±0.12 | 0.005 |
The results are expressed as mean ± SD.
NS; not significant
Hormonal and Metabolic Characteristics
Testosterone, free testosterone, androstenedione, DHEAS, IGF-1, I0, and IAUC were all higher and SHBG and SiM were lower in the PA group (P<0.04 for all) (Table 4). These differences persisted with adjustment for BMI z-score. In a subgroup of patients (n=50), leptin was higher in the control group (21.7 ± 20.3 vs. 12.0 ± 8.4; P=0.04); however, there was no significant difference in leptin between the two groups when adjusted for BMI z-score.
Table 4.
Hormonal and metabolic characteristics of the study populationa
| PA (n=35) | Control (n=31) | P value | |
|---|---|---|---|
| Testosterone (ng/dL) | 5.8 ± 2.3 | 3.5 ± 0.7 | <0.0001 |
| Free Testosterone (pg/mL) | 0.5 ± 0.3 | 0.3 ± 0.3 | 0.03 |
| Free Testosterone % | 0.9 ± 0.3 | 0.8 ± 0.5 | NS |
| DHEAS (ug/dL) | 50.4 ± 42.8 | 20.3 ± 17.4 | 0.0003 |
| Androstenedione (ng/dL) | 42.3 ± 15.9 | 27.3 ± 8.2 | <0.0001 |
| Estradiol (ng/dL) | 0.23 ± 0.19 | 0.35 ± 0.90 | NS |
| Estrone (ng/dL) | 0.89 ± 3.0 | 1.2 ± 4.9 | NS |
| SHBG (nmol/L) | 79.0 ± 35.4 | 107.8 ± 59.1 | 0.009 |
| IGF-1 (ng/mL) | 193.4 ± 10.6 | 139.2 ± 56.4 | 0.0005 |
| IGFBP-3 (mg/L) | 3.1 ± 0.6 | 3.5 ± 4.2 | NS |
| Hemoglobin A1C (%Hb) | 5.1 ± 0.3 | 5.1 ± 0.4 | NS |
| Glucose (mg/dL, 0 min) | 89.4 ± 6.5 | 89.4 ± 7.5 | NS |
| Glucose (mg/dL, AUC) | 233.3 ± 38.9 | 227.6 ± 49.2 | NS |
| Insulin (mg/dL, 0 min) | 6.9 ± 5.0 | 6.7 ± 5.8 | NS |
| Insulin (mg/dL, AUC) | 82.4 ± 63.7 | 54.6 ± 36.1 | 0.03 |
| FGIR | 20.6 ± 20.2 | 24.4 ± 20.6 | NS |
| HOMA | 1.6 ± 1.2 | 1.5 ± 1.3 | NS |
| SiM | 8.2 ± 7.0 | 16.0 ± 17.3 | 0.008 |
| EIR | 1.1 ± 1.0 | 0.8 ± 0.7 | NS |
Results are expressed as mean ± SD.
BA/CA Tertile Analysis
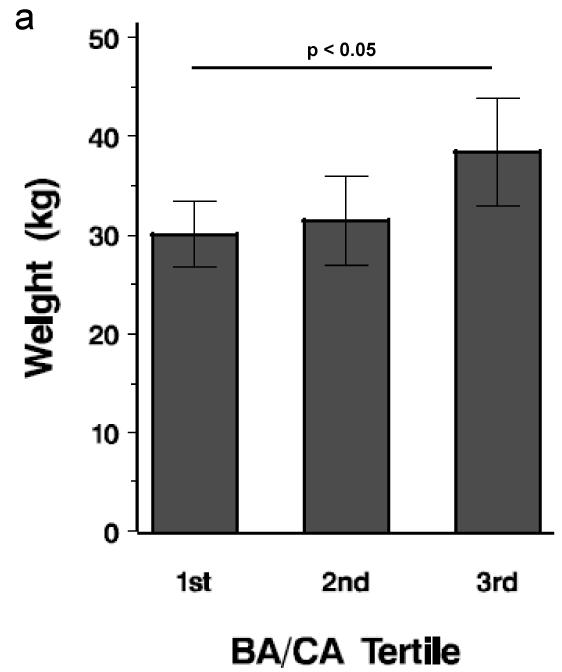
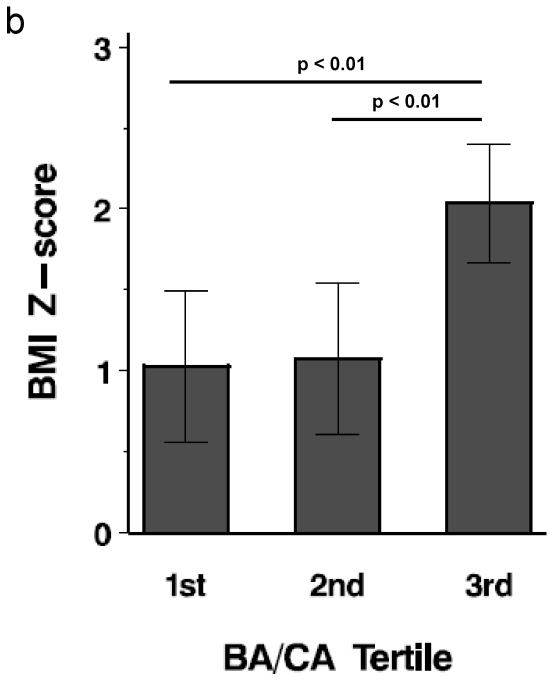
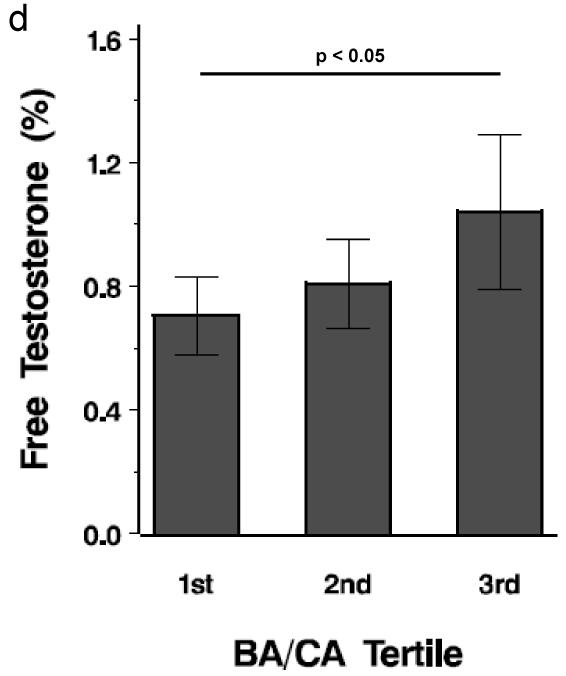
PA and control subjects in the top tertile for BA/CA had the highest DHEAS, weight, BMI z-score, free testosterone (%) and hemoglobin A1C (P < 0.05) (Figure 1). When all subjects were divided by obesity status, overweight/obese subjects (20/43, 46.5%) were more likely to be in the highest BA/CA tertile than nonobese subjects (2/23, 8.7%) (P < 0.004); however, when all subjects were divided by PA status, PA subjects were not more likely to be in the highest BA/CA tertile than non-PA subjects. PA subjects in the top tertile demonstrated a trend for higher DHEAS than control subjects in the top tertile (P = 0.082).
Figure 1.
Tertile analysis of BA/CA. Each bar depicts mean value. a. Mean weight by tertile; b. Mean BMI z-score by tertile; c. Mean DHEAS by tertile; d. Mean free testosterone (%) by tertile. Horizontal lines represent differences and significance among the tertiles.
Relationships between BA/CA and Hormones
Pearson correlation analysis was performed on the entire group of PA and control subjects to determine factors that correlated with BA/CA. BA/CA correlated with BMI z-score (r=0.47), weight (r = 0.40), free testosterone (%) (r=0.33) and DHEAS (r=0.30) (P< 0.02). When adjusted for BMI z-score, weight, free testosterone (%) and DHEAS no longer correlated with BA/CA.
A multivariate analysis was used in an exploratory stepwise regression model for predicting BA/CA. Weight was the top predictor of BA/CA, estradiol was the predictor selected second and DHEAS was selected third (P = 0.0013). Separately, weight predicted 16.7%, estradiol predicted 3.8%, and DHEAS predicted 3.5% of the variability of BA/CA. This model accounted for 24% of the variance in BA/CA in our study population.
Prediction of BA/CA for weight by PA status
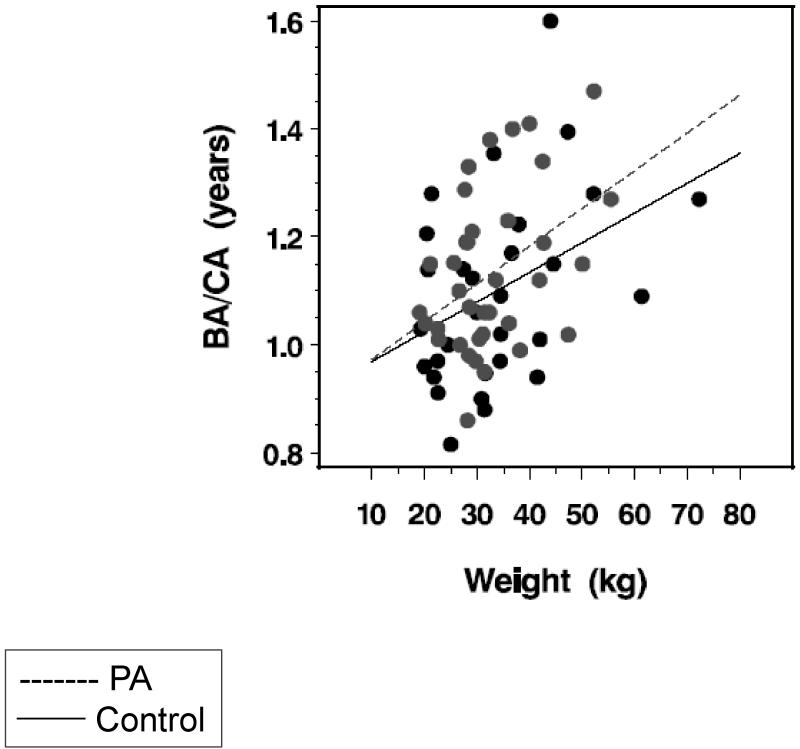
While mean BA/CA did not differ between PA and control subjects, in a multiple regression analysis, PA subjects had higher BA/CA than control subjects after controlling for group differences in weight (P<0.004) (Figure 2). The PA subjects also showed a statistically higher slope relating BA/CA to weight (0.325 ± 0.014) than control subjects (0.0294 ± 0.018), with an approximate 11% difference in the slopes of the two regression lines (P<0.01). In this linear model, children with PA who weigh 20 kg would have BA/CA advancement of 1.84%; those who weigh 30 kg would have BA advancement of 4.3% and those who weigh 60 kg would have BA advancement of 6.3%. Specifically, a child with PA who weighs 33.0 kg would have the same degree of BA advancement as an unaffected child who weighs 40 kg.
Figure 2.
Linear regression of weight on BA/CA in PA (grey symbols, dotted line) and control (black symbols, solid line) subjects: PA (BA/CA = 0.90244 + (weight × 0.00700), p < 0.02); controls (BA/CA = 0.91345 + (weight × 0.00550), p < 0.03). PA subjects had higher BA/CA than control subjects after controlling for group differences in weight (p<0.004). The PA subjects also showed a statistically higher slope relating BA/CA to weight (0.325 ± 0.014) than control subjects (0.0294 ± 0.018), with an approximate 11% difference in the slopes of the two regression lines (p<0.01). Thus, for example, if a control and a PA subject both weigh 20 kg, then the predicted advancement of bone age in PA is 1.84% compared to control; in order to have the same BA/CA as a control subject, a PA subject would weigh only 17.3 kg (2.7 kg less than control). If a control and a PA subject both weigh 60 kg then the predicted advancement of bone age is 6.32%; in order to have the same BA/CA as a control subject, a PA subject would weigh only 48.8 kg (11.2 kg less than control).
Discussion
In this study of prepubertal children with and without obesity and/or PA, obesity was highly associated with BA advancement. In addition, children with PA appeared to be affected by a BA advancing factor that potentiated the effect of obesity on BA/CA. Possible potentiating factors include adrenal hormones and insulin resistance, both of which are elevated in PA, and estrogen at the growth plate. The findings of this study support prior reports that cite advanced BA to be independently associated with obesity and PA (2,3,9). This is the first study that demonstrates a synergistic effect between PA and obesity on BA advancement. Leptin and IGF-1 did not correlate with BA advancement in this study. Lack of correlation of leptin with BA/CA is consistent with other studies; however two other studies did show correlation between IGF-1and BA advancement, whereas our study showed no correlation (5,17).
DHEAS may represent a candidate hormone responsible for potentiating BA advancement. In our study, both PA and controls subjects in the top tertile had elevated DHEAS. DHEAS correlated with BA/CA. Our findings are consistent with past studies. Increased levels of adrenal androgens are associated with both PA and obesity (18). Adrenal androgens may also be higher in obese children and it is thought that obesity may influence the onset of PA in some children, although the mechanism is not clear (19,20). Weight loss in a group of obese pediatric subjects was associated with a significant decrease in subsequent BA advancement without change in predicted adult height (21). Additionally, DHEAS was shown to be associated with height SDS in girls with and without PA (5).
Although traditionally estrogen is thought to be a BA advancer, adrenal androgens may be converted into estrogens by aromatase either peripherally or at the growth plate, which explains a common mechanism in the two conditions that may advance BA (22,23). Both BA and weight have been shown to be significant covariates of DHEAS levels (9).
Whereas estradiol was selected as a predictor of BA/CA, it did not correlate with BA/CA. An explanation for the lack of correlation of estradiol with BA/CA in this study is that estradiol levels measured by most standard RIAs are not sensitive enough in children (24). The assay used for this study has a lower limit of detection for estradiol of 1 pg/mL. However, we propose that the androgens that correlate with BA advancement are converted into estradiol either peripherally or at the growth plate and may be immeasurable using standard assays. In fact, DHEAS may be a marker for estradiol production as both of these substances are end products of the sex steroid pathway (18). A recent study reported that DHEA supplementation given to older adults resulted in an increase in DHEAS and estradiol. The elevated estradiol was associated with increased bone mineral density, whereas DHEAS and testosterone were not (25).
This study has limitations including use of the Greulich and Pyle method of BA determination (26). One of the authors of this paper (JB) has observed that the BA x-rays of children with obesity and/or PA are difficult to interpret because of discrepancies in maturation among the distal, middle and proximal interphalangeal epiphyses. These discrepancies, in our experience, are generally not frequently encountered in children who do not have PA or obesity. Another limitation is that there is no measure of adiposity in this study (e.g percentage body fat, waist circumference, visceral adipose tissue measurement); therefore, our categorization of obesity is based on BMI percentile. In addition, this study does not reveal direct cause of BA advancement and only shows associations.
The findings of this study, nonetheless, have several implications. Advanced BA is associated with earlier puberty and increased adult BMI and central adiposity (27,28). As puberty is a state of relative insulin resistance, children with earlier puberty may be exposed to insulin resistance at an earlier point in life, which may increase risk of obesity and development of type 2 diabetes mellitus, polycystic ovary syndrome or the metabolic syndrome. In addition, children with advanced BA tend to reach their adult height at an earlier age, which may result in suboptimal final height attainment (3).
The observation that PA and obesity may potentiate the effect on BA advancement when they occur together further identifies obese children with PA as particularly high risk. A recent study of ours shows that insulin secretion is greatest in children with both PA and obesity when compared to children with PA alone, obesity alone and with neither condition (29). The findings of these two studies potentially identify obese children with PA as a group with uniquely increased risk in two different metabolic realms, insulin metabolism and BA advancement.
In conclusion, this study supports obesity as an important predictor of BA advancement in prepubertal children and demonstrates a potentiating effect between PA and obesity on BA advancement. Although our data do not directly prove specific BA advancing factors, we have identified potential factors that may contribute to this effect. Indeed, DHEAS may represent a common factor, which is associated with BA advancement in these two groups. It is unclear whether DHEAS acts directly on the growth plate to advance BA, as a marker of estrogen activity, or both. Further studies are needed both at the clinical level and at the cellular level in order to better understand the factors that advance BA and their mechanisms of action in these groups.
Acknowledgments
We would like to thank Dr. Lenore Levine for her help with manuscript preparation and Dr. Judith Korner for performance of the leptin assays for this study.
This manuscript was supported in part by:
-
NIH/NIDDK T32 DK065522 (PI, SE Oberfield)
Training Grant Pediatric Endocrinology
National Institutes of Health (SEO)
General Clinical Research Center grant: RR00645
-
Irving Institute for Clinical and Translational Research
CTSA grant 1UL1RR024156 (AS)
Footnotes
This work was presented in part at ENDO 08, San Francisco, CA
There is no potential, perceived or real conflict of interest among any of the authors who have contributed to this study and manuscript. The first draft was written by the above stated authors. No honorarium or other form of payment was given to anyone to produce this manuscript.
References
- 1.Korth-Schutz A, Levine LS, New MI. Evidence for the adrenal source of androgens in precocious adenarche. Acta Endocrinol (Copenh) 1976;82:242–252. doi: 10.1530/acta.0.0820342. [DOI] [PubMed] [Google Scholar]
- 2.Ibanez L, Jimenez R, Zegher F. Early puberty-menarche after precocious pubarche: relation to prenatal growth. Pediatrics. 2006;117:117–121. doi: 10.1542/peds.2005-0664. [DOI] [PubMed] [Google Scholar]
- 3.Diaz A, Bhandari S, Sison C, Vogiatzi M. Characteristics of children with premature pubarche in the New York Metropolitan Area. Horm Res. 2008;70:150–154. doi: 10.1159/000137661. [DOI] [PubMed] [Google Scholar]
- 4.Ibanez L, Verdis R, Potau N, Zampolli M, Ghizzoni L, Albisu MA, et al. Natural history of premature pubarche: an auxological study. J Clin Endocrinol Metab. 1992;74:254–257. doi: 10.1210/jcem.74.2.1730803. [DOI] [PubMed] [Google Scholar]
- 5.Utriainen P, Voutilainen R, Jaaskelainen J. Girls with premature adrenarche have accelerated early childhood growth. J Pediatr. 2009;154:882–7. doi: 10.1016/j.jpeds.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 6.Haeusler G, Wakter I, Helmreich M, Egerbacher M. Location of matrix metalloproteinases (MMPs), their tissue inhibitors, and vascular endothelial growth factor (VEGF) in growth plates of children and adolescents indicates a role for MMPs in human postnatal growth and skeletal maturation. Cacif Tissue Int. 2005;76:326–335. doi: 10.1007/s00223-004-0161-6. [DOI] [PubMed] [Google Scholar]
- 7.Grumbach MM. Estrogen, bone, growth and sex: a sea change in conventional wisdom. J Pediatr Endocrinol Metab. 2000;13(Suppl 6):1439–1455. doi: 10.1515/jpem-2000-s619. [DOI] [PubMed] [Google Scholar]
- 8.Klein KO, Lamore KA, DeLancey E, Brown JM, Considine RV, Hassink SG. Effect of obesity on estradiol level, and its relationship to leptin, bone maturation, and bone mineral density in children. J Clin Endocrinol Metab. 1998;83:3469–3474. doi: 10.1210/jcem.83.10.5204. [DOI] [PubMed] [Google Scholar]
- 9.Russell DL, Keil MF, Bonat SH, Uwaifo GI, Nicholson JC, McDuffie JR, et al. The relation between skeletal maturation and adiposity in African American and Caucasian children. J Pediatr. 2001;139:844–848. doi: 10.1067/mpd.2001.119446. [DOI] [PubMed] [Google Scholar]
- 10.Sopher AB, Thornton JC, Silfen ME, Manibo A, Oberfield SE, Wang J, et al. Prepubertal girls with premature adrenarche have greater bone mineral content and density than controls. J Clin Endocrinol Metab. 2001;86:5269–5272. doi: 10.1210/jcem.86.11.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armegaud J, Charkaluk M, Trivin C, Tardy V, Breart G, Brauner R, Chalumeau Precocious pubarche: Distinguishing late-onset congenital adrenal hyperplasia from premature adrenarche. J Clin Endocrinol Metab. 2009;94:2835–2840. doi: 10.1210/jc.2009-0314. [DOI] [PubMed] [Google Scholar]
- 12.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prader A. Testicular size: assessment and clinical importance. Triangle. 1966;7:240–243. [PubMed] [Google Scholar]
- 14.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. http://www.cdc.gov/nccdphp/dnpa/growthcharts/sas.htm. [PubMed] [Google Scholar]
- 15.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist. Stanford University Press; Stanford: 1966. [Google Scholar]
- 16.Silfen ME, Manibo AM, McMahon DJ, Levine LS, Murphy AR, Oberfield SE. Comparison of simple measures of insulin sensitivity in young girls with premature adrenarche: the fasting glucose to insulin ratio may be a simple and useful measure. J Clin Endocrinol Metab. 2001;86:2863–2868. doi: 10.1210/jcem.86.6.7537. [DOI] [PubMed] [Google Scholar]
- 17.Reinehr T, De Sousa G, Wabitsch M. Relationships of IGF-1 and androgens to skeletal maturation in obese children and adolescents. J Pediatric Endocrinol Metab. 2006;19:1133–1140. doi: 10.1515/jpem.2006.19.9.1133. [DOI] [PubMed] [Google Scholar]
- 18.Miller WL. Androgen synthesis in adrenarche. Rev Endocrinol Metab Disord. 2009;10:3–17. doi: 10.1007/s11154-008-9102-4. [DOI] [PubMed] [Google Scholar]
- 19.L’Allemand D, Schmidt S, Rousson V, Brabant D, Gasser T, Gruters A. Associations between body mass, leptin, IGF-1 and circulating adrenal androgens in children with obesity and premature adrenarche. Eur J Endocrinol. 2002;146:537–543. doi: 10.1530/eje.0.1460537. [DOI] [PubMed] [Google Scholar]
- 20.Charkaluk M, Trivin C, Brauner R. Premature pubarche as an indicator of how body weight influences the onset of adrenarche. Eur J Pediatr. 2004;163:89–93. doi: 10.1007/s00431-003-1358-9. [DOI] [PubMed] [Google Scholar]
- 21.Bermudez de la Vega JA, Vasquez MA, Bernal S, Gentil FJ, Gonzalez-Hachero J, Montoya MJ, et al. Anthropometric, bone age, and bone mineral density changes after a family-based treatment for obese children. Calcif Tissue Int. 2007;81:279–284. doi: 10.1007/s00223-007-9071-8. [DOI] [PubMed] [Google Scholar]
- 22.Chagin AS, Savendahl L. GPR30 estrogen receptor expression in the growth plate declines as puberty progresses. J Clin Endocrinol Metab. 2007;92:4873–4877. doi: 10.1210/jc.2007-0814. [DOI] [PubMed] [Google Scholar]
- 23.Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 24.Klein KO, Baron J, Colli MJ, McDonnell DP, Cutler GB., Jr Estrogen levels in childhood determined by an ultrasensitive recombinant cell bioassay. J Clin Invest. 1994;94:2475–2480. doi: 10.1172/JCI117616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jankowski CM, Gozansky WS, Kittelson JM, Van Pelt RE, Schwartz RS, Kohrt WM. Increases in bone mineral density in response to oral dehydroepiandrosterone replacement in older adults appear to be mediated by serum estrogens. J Clin Endocrinol Metab. 2008;93:4767–4773. doi: 10.1210/jc.2007-2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bull RK, Edwards PD, Kemp PM, Fry S, Hughes IA. Bone age assessment: a large scale comparison of the Greulich and Pyle, and Tanner and Whitehouse (TW2) methods. Arch Dis Child. 1999;81:172–173. doi: 10.1136/adc.81.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flor-Cisernos A, Roemmich JN, Rogol AD, Baron J. Bone age and onset of puberty in normal boys. Mol Cell Endocrinol. 2006;254-255:202–206. doi: 10.1016/j.mce.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kindblom JM, Lorentzon M, Nojavaara E, Lonn L, Brandberg J, Angelhed J, et al. Pubertal timing is an independent predictor of central adiposity in young adult males. Diabetes. 2006;55:3047–3052. doi: 10.2337/db06-0192. [DOI] [PubMed] [Google Scholar]
- 29.Jean A, Hassoun A, Hughes J, Pomeranz C, Fennoy I, McMahon DJ, et al. Utility of EIR and proinsulin to assess at risk prepubertal populations with premature adrenarche and obesity for insulin resistance. J Pediatr. 2009;155:893–899. doi: 10.1016/j.jpeds.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]