Abstract
The human UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyl-transferase 2 (GalNAc-T2) is one of the key enzymes that initiate synthesis of hinge-region O-linked glycans of human immunoglobulin A1 (IgA1). We designed secreted soluble form of human GalNAc-T2 as a fusion protein containing mouse immunoglobulin light chain kappa secretory signal and expressed it using baculovirus and mammalian expression vectors. The recombinant protein was secreted by insect cells Sf9 and human HEK 293T cells in the culture medium. The protein was purified from the media using affinity Ni-NTA chromatography followed by stabilization of purified protein in 50 mM Tris-HCl buffer at pH 7.4. Although the purity of recombinant GalNAc-T2 was comparable in both expression systems, the yield was higher in Sf9 insect expression system (2.5 mg of GalNAc-T2 protein per 1 L culture medium). The purified soluble recombinant GalNAc-T2 had an estimated molecular mass of 65.8 kDa and its amino-acid sequence was confirmed by mass-spectrometric analysis. The enzymatic activity of Sf9-produced recombinant GalNAc-T2 was determined by the quantification of enzyme-mediated attachment of GalNAc to synthetic IgA1 hinge-region peptide as the acceptor and UDP-GalNAc as the donor. In conclusion, murine immunoglobulin kappa secretory signal was used for production of secreted enzymatically active GalNAc-T2 in insect baculovirus expression system.
Keywords: UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyl-transferase 2GalNAc-T2, immunoglobulin A1 (IgA1), baculovirus expression system, immunoglobulin light chain κ secretory signal
Introduction
Alterations in glycan moieties of cell-surface or secreted glycoproteins are now recognized as factors that can cause, or contribute to, defects in organs or cell development and function, autoimmune diseases as well as some types of cancer [1–5]. Human UDP-GalNAc: polypeptide N-acetylgalactosaminyltransferase 2 (GalNAc-T2) is member of the large GalNAc-transferases family of at least 20 different enzymes that initiate O-glycosylation. Each member differs in the tissue expression, transcriptional regulation, and substrate specificity, especially amino-acid motifs surrounding the Ser/Thr, protein folding, and the sensitivity to the already glycosylated neighboring sites. Human GalNAc-T2 was initially purified from human placenta [6]. The full-length GalNAc-T2 cDNA encodes a 571 amino-acid long protein with estimated molecular mass of 64.7 kDa. GalNAc-T2 is a type II transmembrane protein with a hydrophobic, transmembrane domain (amino acids 7–24) [6]. GalNAc-T2 initiates the first step of mucin-type O-glycan biosynthesis by attaching the N-acetylgalactosamine (GalNAc) to the hydroxyl groups of target Ser/Thr residues in glycoproteins such as mucins and immunoglobulin A1 (IgA1) [7].
Here, we report purification and analysis of recombinant GalNAc-T2 fused with mouse immunoglobulin kappa secretory signal that mediates efficient secretion of enzymatically active soluble human GalNAc-T2 by the insect Sf9 cells.
Materials and Methods
All chemicals, unless otherwise specified, were from Sigma (St. Louis, MO, USA); tissue culture media and reagents were from Gibco (Invitrogen, Carlsbad, CA, USA).
Sf9 cells
The Sf9 insect cell line is a clonal isolate derived from the parental Spodoptera frugiperda cell line. Sf9 cells were grown as adherent cells or in suspension in serum-free Sf-900 II SFM at 28°C ± 0.5°C in non-humidified, ambient-air incubator.
Preparation of recombinant baculovirus Igκ-T2
Recombinant bacmid coding for secreted form of human GalNAc-T2 was cloned in two steps. First, the synthetic oligonucleotide coding for murine Ig kappa secretory signal (derived from first 21 amino acids METDTLLLWVLLLWVPGSTGDAA) was cloned into pFastBacHT A vector (Invitrogen, Carlsbad, CA) in front of His tag-encoding sequence using RsrII restriction site. Next, human GalNAc-T2 cDNA without the transmembrane domain-encoding sequence (corresponding to amino acids 52–571, NCBI Acc. No. NP_004472) was isolated from human monocyte cell line U937 by PCR (downstream primer 5’-AAAAAGAAAGACCTTCATCAC-3’ and upstream primer 5’-CTACTGCTGCAGGTTGAGC-3’) a further PCR modified by adding EcoRI and XhoI sites on 5’ and 3’ ends, respectively. Furthermore, the modified GalNAc-T2 cDNA was cloned in frame behind the His tag and Tobacco Etch Virus protease (TEV) recognition sites of pFastBacHT A using EcoRI and XhoI restriction enzymes and the recombinant vector was designed pIgκ-T2-FastBac (Figure 1). Then, the construct was transformed into DH10Bac E. coli (Invitrogen) where the Igκ-T2 insert was spontaneously transposed into bacmid. The resultant recombinant bacmid designated BacIgκ-T2 was purified by Large-Construct Kit (Qiagen, Hilden, Germany) and transfected into Sf9 cells using Cellfectin reagents (Invitrogen). Infectious recombinant baculoviruses designated BaculoIgκ-T2 driving expression of secreted soluble GalNAc-T2 designated κGalNAc-T2 were amplified to reach at least 1 × 108 plaque-forming units (PFU)/ml of the viral stock and subsequently used for production and isolation of κGalNAc-T2 using Sf9 cells.
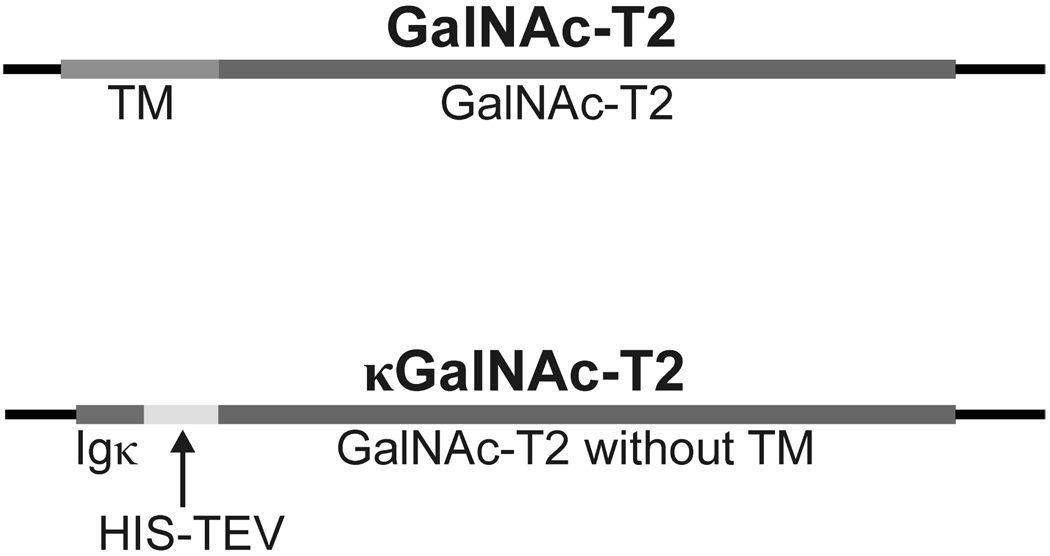
Figure 1. Structure of inserts in vectors encoding native and secreted GalNAc-T2 proteins.
Human GalNAc-T2 cDNA (NCBI Acc. No. NP_004472) isolated by RT-PCR from monocyte cell line U937 was cloned into pCRII-Blunt TOPO cloning vector and subcloned into expression vectors together with Igκ secretory signal. The comparison of full length GalNAc-T2 and recombinant secreted form of κGalNAc-T2 is shown. Igκ secretory signal, GalNAc-T2 transmembrane domain, GalNAc-T2 in red, His tag and Tobacco Etch Virus protease (HIC-TEV) recognition site are marked.
Production of recombinant κGalNAc-T2 in Sf9 cells
After optimizing the growth conditions, recombinant κGalNAc-T2 was produced in 2-L culture with 2 × 106 Sf9 cell/ml infected with recombinant BaculoIgκ-T2 at the multiplicity of infection (MOI) 2–5 PFU per one Sf9 cell using SF-900 serum-free culture medium (Invitrogen). The cells were grown at 27°C on the orbital shaker (130 RPM) for 72 h.
Production of recombinant κGalNAc-T2 in HEK 293T cells
The cDNA coding for κGalNAc-T2 was PCR cloned from pIgκ-T2-FastBac into mammalian expression plasmid pcDNA3.1D/V5-His-TOPO (Invitrogen) and designated pcDNAIgκ-T2. The recombinant κGalNAc-T2 protein was produced in 293T cells transfected with pcDNAIgκ-T2 plasmid using Superfect transfection reagent (Qiagen). The cells were grown in RPMI 1640 with L-glutamine, 10% fetal bovine serum, penicillin, streptomycin [8].
Purification of recombinant κGalNAc-T2 on Ni-NTA agarose column
The recombinant κGalNAc-T2 was purified by NiNTA affinity chromatography under native conditions. All purification steps were performed on ice or at 4°C. The Sf9 culture-medium (SF-900 SFM) supernatant was depleted of cells and debris by centrifugation at 5,000 rpm for 10 min. The binding buffer was mixed with supernatant (1:9 v:v; 50 mM NaH2PO4 pH 8, 300 mM NaCl, 10 mM imidazole, and 0.05% Tween 20) and the pH was adjusted to 6.8 using 500 mM NaH2PO4 pH 8.0. Next, 1 ml of 50% Ni-NTA agarose (Qiagen) was added per 250 ml of culture-medium supernatant and gently mixed on a roller mixer overnight at 4°C. The Ni-NTA agarose was transferred to a glass chromatographic column and washed with 10 volumes of washing buffer (50 mM NaH2PO4 pH 6.8, 300 mM NaCl, 2 mM imidazole, and 0.05% Tween 20). The recombinant κGalNAc-T2 was eluted with the 6 column volumes of elution buffer (50 mM NaH2PO4 pH 7.4, 300 mM NaCl, 200 mM imidazole and 0.05% Tween 20). Elution fraction was transferred to 50 mM Tris-HCl pH 7.4 and concentrated using Amicon Ultracell 10K (Millipore, Billerica, MA) to reach the κGalNAc-T2 protein concentration >1 mg/ml, as determined by BCA assay (Pierce, Rockford, IL).
The concentration of the protein was determined by BCA method and by densitometry of bands after SDS-PAGE separation of various loads of the κGalNAc-T2 and BSA (BSA served as the standard) followed by staining with Coomassie Blue R-250. Densitometric analysis was performed using the ImageJ 1.41a software and BSA standard curve was used to calculate κGalNAc-T2 protein concentrations.
To assess the purity of the κGalNAc-T2, the protein preparation was separated by 10% SDS-PAGE and stained with Silver Stain Kit (Pierce), or blotted on PVDF membrane (BioRad, Hercules, CA), developed with anti-His tag HRP-conjugated antibody (Qiagen), and detected with SuperSignal West Pico reagents (Pierce) followed by visualization using a cooled CCD camera (Roche, Indianapolis, IN).
Identification of isolated κGalNAc-T2 preparation by high-resolution tandem mass spectrometry (MS)
The identity of purified protein was confirmed by use of LC coupled to a high-resolution linear quadrupole ion trap Fourier transform ion cyclotron resonance mass spectrometer (LTQ FT, Thermo Fisher Scientific, San Jose, CA) using BioWorks 3.2 software (Thermo Fisher Scientific) with the NCBI database (Acc. No. NP_004472). Protein bands from Coomassie-stained SDS-PAGE gels were excised, cut into small pieces and in-gel digested with trypsin at 37°C for 12 h [9]. On-line LC was performed by use of an Eksigent MicroAS autosampler and 2D LC nanopump (Eksigent, Dublin, CA). In-gel digested sample was loaded onto a 100-µm-diameter, 11-cm-long column pulled tip packed with Jupiter 5-µm C18 reversed phase beads (Phenomenex, Torrance, CA). The digests were then eluted with an acetonitrile gradient from 5 to 30% in 0.1% formic acid over 50 min at 650 nl min−1. LTQ FT parameters were set as described previously [10]. The mass spectrometer alternated between a full FT MS scan (m/z 400–2,000) and four subsequent tandem MS scans of the four most abundant precursor ions.
Assessing the enzymatic activity of κGalNAc-T2
The activity of purified κGalNAc-T2 was determined based on the addition of GalNAc to the acceptor synthetic peptide Val-Pro-Ser-Thr-Pro-Pro-Thr-Pro-Ser-Pro-Ser-Thr-Pro-Pro-Thr-Pro-Ser-Pro-Ser-Cys-NH2 (1931.934 Da), corresponding to the IgA1 hinge-region amino acid sequence, designated HR. The peptide was synthesized by Bachem and covalently linked to BSA, to facilitate subsequent analyses [11]. About 20 mols of HR peptide was linked to 1 mol of BSA. UDP-GalNAc served as a GalNAc donor. The attachment of GalNAc was determined by the reactivity with biotin-labeled lectin from Helix aspersa (HAA, specific for GalNAc) [12–13] in a dot-blot assay and in parallel by on-line LC FT-ICR MS analysis. The reaction was performed for 24 h at 37°C with 1 or 4 µg of recombinant κGalNAc-T2 and serial dilution of the acceptor HR peptide (0.5, 0.25, 0.1, and 0.05 µg per reaction) in the reaction buffer (250 µM uridine 5′-diphospho-N-acetylgalactosamine, 25 mM Tris-HCl pH 7.4, 5 mM MnCl2) in total volume of 25 µl. As a positive control for HAA dot-blot assay, a glycosylated HR synthesized by Bachem with three attached GalNAc residues (asterisks mark the sites with GalNAc: Val-Pro-Ser-Thr-Pro-Pro-*Thr-Pro-*Ser-Pro-*Ser-Thr-Pro-Pro-Thr-Pro-Ser-Pro-Ser-Cys-NH2) and cross-linked to BSA [11]. About 20 mols of HR glycopeptide was linked to 1 mol of BSA. The HR incubated with recombinant κGalNAc-T2 without addition of UDP-GalNAc served as a negative control. Mass spectra were analyzed by use of the Xcalibur Qual Browser 2.0 software (Thermo Fisher Scientific). The number of GalNAc residues was assigned manually based on the masses of the HR (1931.934 Da) and GalNAc (203.079 Da), as described previously for IgA1 [10, 14–15]. The minimal threshold for HR glycopeptide identification was a signal to noise ratio of at least 5:1 with >3 isotopic peaks. The mass tolerance of peak assignment is within 10 ppm.
One Unit of the enzyme was defined as the amount of κGalNAc-T2 protein that catalyzed transfer of 1 nmol of GalNAc to HR peptide in 15 min in the standard reaction using 250 uM UDP-GalNAc as donor and 15 uM HR peptide as acceptor. The reactions were performed with serial dilutions of enzyme and the amounts of glycopeptides were determined by high-resolution mass spectrometry as described above.
Results
Construction of vectors for production of recombinant GalNAc-T2
Recombinant baculovirus and mammalian expression plasmids coding for secreted soluble form of GalNAc-T2 protein (devoid of transmembrane domain, amino acids 1–51) N-terminally fused with immunoglobulin kappa chain secretory signal (amino acids 1–21) were constructed by cloning GalNAc-T2 cDNA without the transmembrane domain-coding sequence behind the synthetic mouse immunoglobulin κ secretory signal cDNA and His tag-coding sequence and designated κGalNAc-T2. The identity of cDNA constructs was confirmed by sequencing (the GalNAc-T2 moiety is identical to NCBI Acc. No. NM_004481). Expected molecular mass of the fusion recombinant protein κGalNAc-T2 was calculated by ProtParam tool to be 65.8 kDa, whereas the native GalNAc-T2 has 64.7 kDa (http://www.expasy.ch/tools/protparam.html).
Production of recombinant κGalNAc-T2 by Sf9 and 293 cells
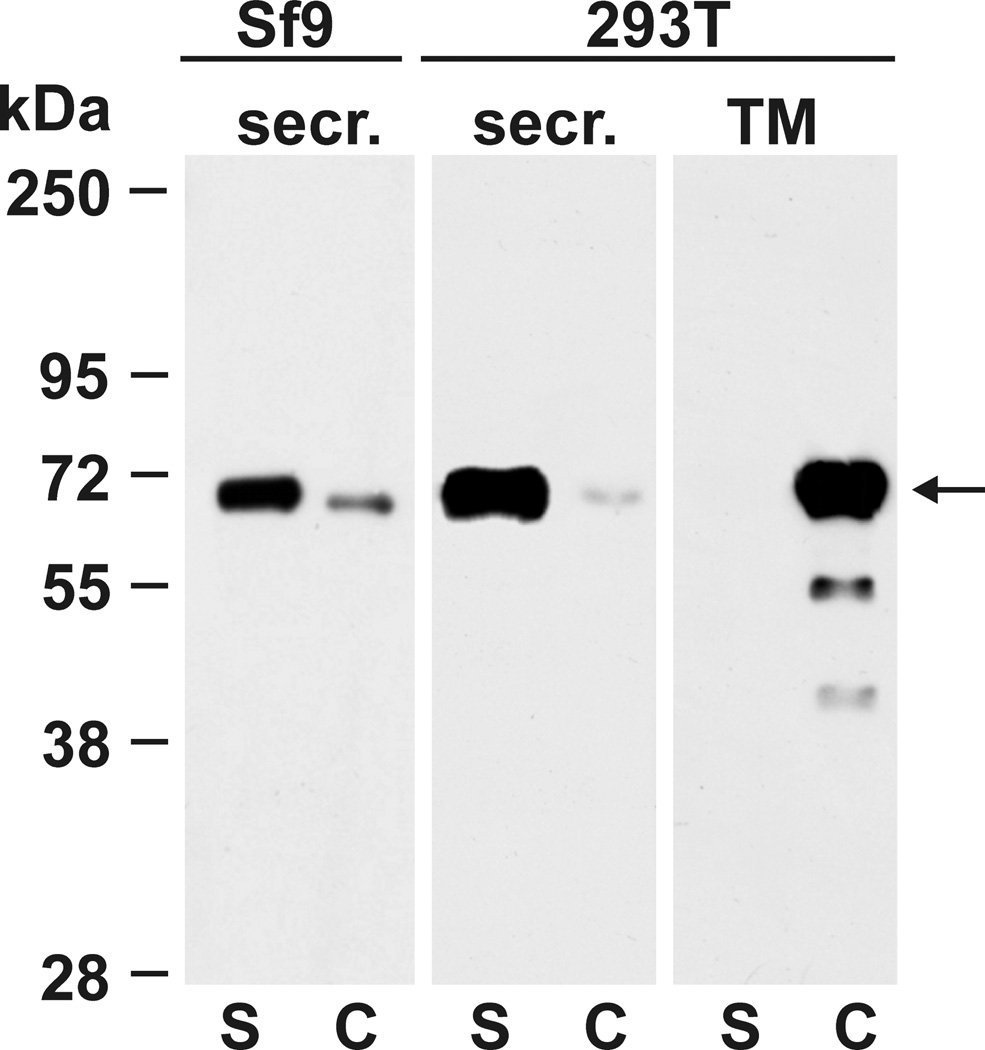
Transfection of both the mammalian cell line HEK 293T with pcDNAIgκ-T2 and insect cells Sf9 with BaculoIgκ-T2 lead to efficient secretion of recombinant κGalNAc-T2 into culture medium, with only weak accumulation of the recombinant protein in cells. This was evident from comparison of protein bands densities on western blot of supernatant and cellular pellet from centrifuged aliquots of the suspension of Sf9 or trypsinized 293T cultures. Recombinant κGalNAc-T2 was detected using anti-His tag HRP-conjugated antibody (Figure 2). The full length GalNAc-T2 cDNA was cloned in parallel into pcDNA3.1D/V5-His-TOPO plasmid and the protein GalNAc-T2 was expressed in 293T cells for comparison. In contrast to the secreted κGalNAc-T2 form, the native GalNAc-T2 protein remains exclusively within the 293T cells, as expected (Figure 2).
Figure 2. Recombinant κGalNAc-T2 protein is secreted by Sf9 cells and 293T cells.
Recombinant protein κGalNAc-T2 was expressed in human HEK 293T cells and in insect Sf9 cells. Cell-culture medium supernatants (S) and cell lysates (C) were analyzed by SDS-PAGE followed by western blotting with anti-His tag HRP-conjugated antibody. For comparison, native GalNAc-T2 (TM) containing transmembrane domain was expressed in 293T cells and analyzed in parallel. κGalNAc-T2 was secreted by both cell types, whereas the full length, transmembrane domain-containing form remained in 293T cells as expected. Predicted molecular mass of κGalNAc-T2 is 65.8 kDa and that of GalNAc-T2 is 64.7 kDa. For analyses of GalNAc-T2 from insect cells (Sf9), 50-µl aliquots of cell suspension were centrifuged and the supernatant and cell pellet were separated and mixed with SDS-PAGE sample buffer. For analysis of GalNAc-T2 from adherent cells, cell-culture supernatant was removed, adherent cells were trypsinized and then resuspended in the original volume of fresh culture medium. Equal aliquots of supernatant and trypsinized cells were mixed with SDS-PAGE sample buffer and equal volumes were used for western blot analysis.
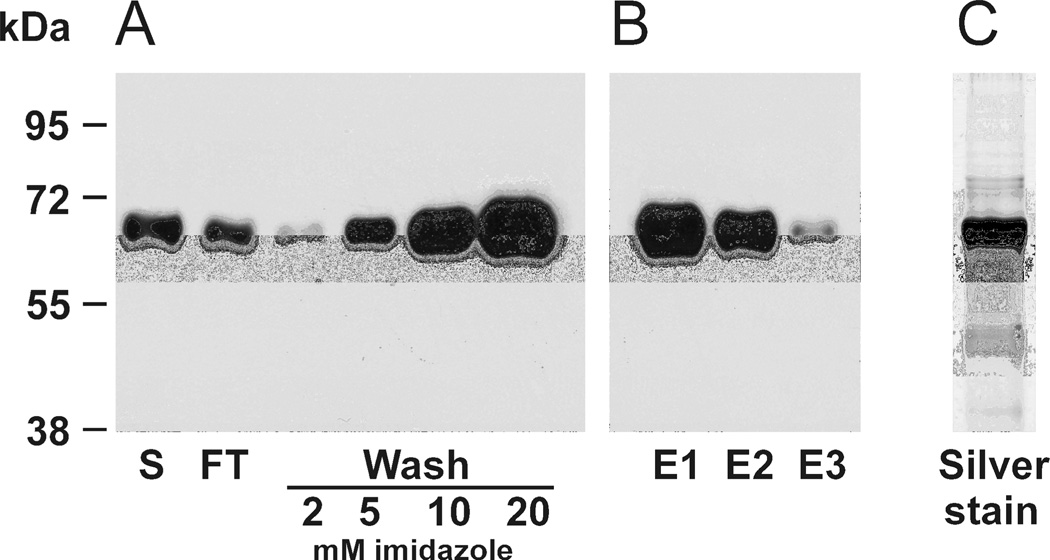
After baculovirus-driven expression, the recombinant protein was purified from Sf9 supernatant using affinity Ni-NTA agarose column under various conditions. The recombinant κGalNAc-T2 binds the Ni-NTA column with relatively poor affinity, as was demonstrated by the release of the κGalNAc-T2 when the imidazole concentration in washing buffer exceeded 2 mM (Figure 3). Similarly, we tested effect of pH (pH 6.0 to 8.0) on the binding to Ni-NTA agarose. We found that optimal pH value (6.8) was lower than expected and that pH values > 6.8 resulted in less binding, based on western blotting with anti-His tag antibody (data not shown). Therefore, for large-scale purification of κGalNAc-T2 the washing buffer was adjusted to 2 mM imidazole, pH 6.8, which ensured maximum yield and purity of the protein preparation. The elution buffer was adjusted to 200 mM imidazole, which resulted in quantitative protein elution with minimum 4 column volumes (Figure 3). Purity of the resultant preparation was assessed by SDS-PAGE with silver staining (Figure 3).
Figure 3. Optimizing the conditions for κGalNAc-T2 isolation using Ni-NTA affinity chromatography.
Recombinant κGalNAc-T2 was expressed in Sf9 cells and purified by Ni-NTA affinity chromatography under native conditions. Binding buffer (1/9 v/v) was added to the cell-culture supernatant (S) and incubated with Ni-NTA agarose (1 ml of 50% slurry per 250 ml) over night at 4°C on roller. The suspension was loaded in a glass column and the flow-through (FT) was collected. Ni-NTA column was washed successively with 10-column volumes of washing buffer with increasing imidazole concentrations (2, 5, 10, and 20 mM). Aliquots corresponding to 5 µl from each fraction were analyzed by SDS-PAGE and western blotting with anti-His tag HRP-conjugated antibody. Washing with buffers containing 5, 10, and 20 mM imidazole lead to significant losses of κGalNAc-T2 protein (A). To optimize elution conditions, the purification was optimized, using 2 mM imidazole washing buffer followed by elution with six-column volumes of 200 mM imidazole elution buffer and two-column-volume samples were collected and aliquots corresponding to 5 µl of each fraction were analyzed (E1, E2, and E3); the results showed that four-column volumes (E2) of elution buffer released most κGalNAc-T2 protein (B). Elution fractions E1 – E3 were pooled and concentrated into 50 mM Tris-HCl pH 7.4 to >1 mg protein/ml. Purity of the final κGalNAc-T2 preparation under conditions specified in (B) was assessed by SDS-PAGE analysis of sample corresponding to 1 µl of final κGalNAc-T2 preparation followed by silver staining (C).
For further experiments the baculovirus Sf9 expression system was scaled up to 2 L of Sf9 culture (2 × 106 cells / ml) followed by affinity purification, as described in Material and methods. In total, 4 mg of κGalNAc-T2 were isolated from 2 L of Sf9 culture, as determined by the BCA method and confirmed by densitometry of κGalNAc-T2 and BSA bands in Coomassie Blue R-250 stained SDS-PAGE gels (data not shown). Furthermore, the protein was identified by SDS-PAGE separation followed by silver staining and by western blotting with anti-His-tag HRP-conjugated antibody (Figure 3). The identity of recombinant κGalNAc-T2 was confirmed by use of high-resolution LTQ FT tandem mass spectrometry with a tryptic digest of the excised SDS-PAGE band. Twenty-two unique peptides representing 41.9% of the amino-acid sequence of the human GalNAc-T2 (NCBI database Acc. No NP_004472) were confirmed with the mass error for all peptides ≤5 ppm.
κGalNAc-T2 was stored at −20°C in 50 mM Tris-HCl buffer (pH 7.4) with 30% glycerol. The protein was stable without any evidence of protein degradation or loss of enzyme activity. Also, no signs of protein degradation were observed after storage on ice or at +4°C for up to a month in Tris-HCl buffer (7.4).
Assessment of κGalNAc-T2 enzyme activity
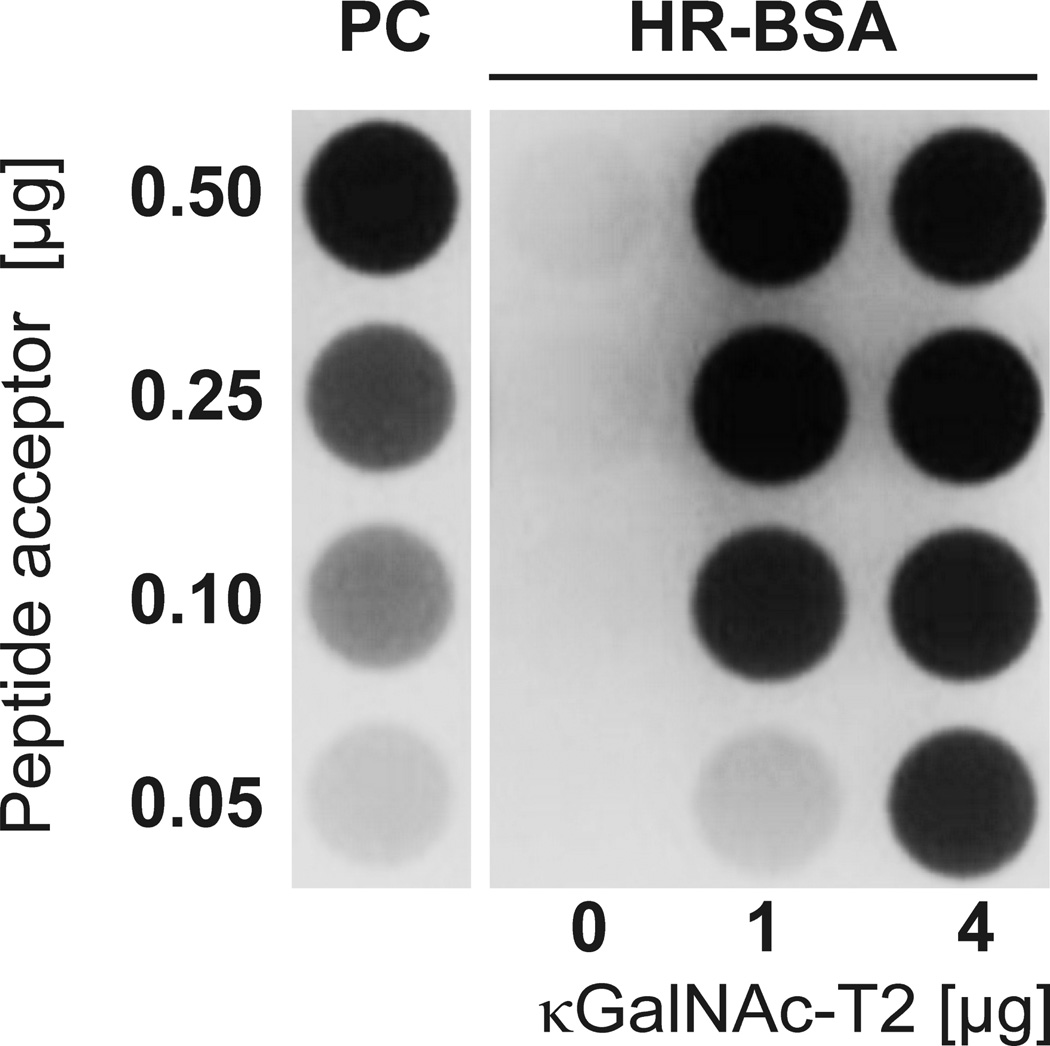
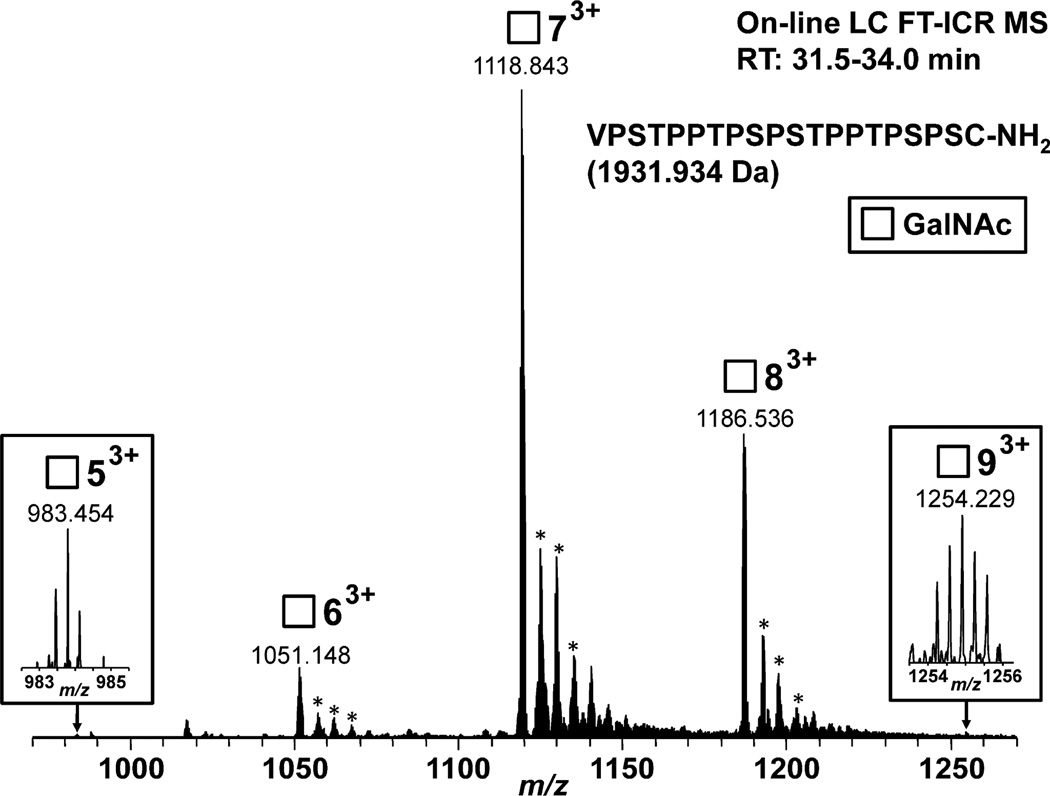
The activity of κGalNAc-T2 preparation was measured by dot-blot assay based on binding of HAA, a GalNAc-specific lectin, to HR peptide linked to BSA (HR-BSA) after the incubation with κGalNAc-T2 in the presence UDP-GalNAc. Synthetic HR glycopeptide with three GalNAc residues was linked to BSA and served as a positive control for lectin dot-blot assay (PC). Lectin dot-blot assay revealed that κGalNAc-T2 was enzymatically active (Figure 4). To confirm and extend these observations, a high-resolution mass spectrometry analysis of the HR acceptor (substrate) was performed. Five glycopeptides corresponding to HR peptide with 5 to 9 GalNAc residues were observed; the variant with seven GalNAc residues was the predominant ion species (Figure 5). The number of GalNAc residues was assigned based on the masses of the HR peptide (1931.934 Da) and GalNAc (203.079 Da) and confirmed the activity of the κGalNAc-T2.
Figure 4. Detection of κGalNAc-T2 enzyme activity.
The enzymatic activity of κGalNAc-T2 was determined using a synthetic IgA1 hinge-region peptide covalently linked to BSA as acceptor (HR-BSA, 0.05, 0.10, 0.25 and 0.50 µg per reaction) and UDP-GalNAc as the donor in the reaction performed for 24 h at 37°C. Two different amounts of κGalNAc-T2 (1 or 4 µg protein per reaction, i.e., 1.85 and 3.7 Units of enzyme) were tested. Negative control had all components except κGalNAc-T2 (0 µg). The IgA1 hinge-region glycopeptide with three attached GalNAc residues and linked to BSA served as a positive control (PC) [11]. Addition of GalNAc after the enzyme reaction was analyzed by dot blot with biotin-labeled HAA lectin followed by neutravidine-HRP and chemiluminescence detection [11].
Figure 5. FT-ICR MS spectrum of the products of GalNAc-T2 reaction after overnight incubation.
Hinge-region peptide was used as acceptor with 1 protein, corresponding to 1.85 Units of enzyme. The reaction products were purified from the mixture by LC coupled on-line with LTQ FT-ICR MS. Predominant ion species corresponded to hinge-region glycopeptides with 6, 7, and 8 GalNAc residues. Minor species with 5 and 9 residues are shown in the insets. The number next to square symbol indicates number of GalNAc residues attached. All glycopeptides were detected at LC retention time of 31.5–34.0 min. All glycopeptides were detected as 3+ charged ions. *adducts with NH3 and matched the theoretical mass values for the expected hinge-region glycopeptides within 2 ppm or less.
Discussion
Mechanisms involved in the formation of aberrant O-glycans involve abnormal expression of glycosyltransferases, their abnormal localization, or mutation of the respective genes [16]. In IgA nephropathy, aberrant O-glycosylation in the hinge region of IgA1 is directly involved in the pathogenesis of this autoimmune disease [17–19]. This aberrant glycosylation consists of a truncation of mono- or di-sialylated Core 1 structure (GalNAc with a β1,3-linked Gal) and formation of Tn antigen (GalNAc linked to Ser or Thr) or its sialylated form (sialyl-Tn antigen) [14, 18–19]. Mechanisms and pathways involved in the synthesis of Tn antigen on IgA1 are not completely understood. Preparations of recombinant, enzymatically active glycosyltransferases likely involved in the formation of IgA1 hinge-region O-glycans could thus provide an important tool for in vitro studies of the IgA1 O-glycan formation. GalNAc-T2 has been identified among the first twelve described GalNAc-transferases (GalNAc-T1 to T12) as the enzyme responsible for the initiation of O-glycosylation of IgA1 [7]. More recently, it was shown that other GalNAc-transferases may also participate in the IgA1 hinge-region glycosylation [20].
Human GalNAc-T2 has been produced previously using Pichia expression system for structural studies [21]. This construct was based on amino-acid residues 75–571 of GalNAc-T2 fused with α-factor secretion signal sequence, His tag, and TEV protease cleavage site. Consequently, GalNAc-T2 was purified from culture medium as a fusion protein and, after TEV protease cleavage, pure GalNAc-T2 was isolated and crystallized. However, Pichia expression system requires selection of stable transfectans and, thus requires experience with this time-consuming analysis and screening of multiple clones. Here, we demonstrated that the N-terminal fusion of soluble form of GalNAc-T2 (lacking the transmembrane N-terminal domain) with secretion signal peptide from murine immunoglobulin κ light chain leads to efficient secretion of the recombinant κGalNAc-T2 in the culture medium both in insect (Sf9) and mammalian (HEK 293T) cells. Notably, using our vector in mammalian cells allowed transient transfection resulting in an ample protein production in the medium. As GalNAc-T2 is not glycosylated, all three systems, i.e., Pichia, insect cells, and mammalian cells are suitable for the production of active enzyme.
Igκ secretion signal-driven secretion of recombinant immunoglobulins was demonstrated in baculovirus expression systems more than 20 years ago. Since then, several secretory signals have been used for secretion of recombinant proteins: N-terminal sequence of the gene encoding the insulin-like peptide bombyxin [22], baculovirus GP67 signal sequence [23], mouse V47 VH signal sequence [24], and honeybee melittin (HBM) secretion signal [25]. Murine immunoglobulin κ and V47 VH signals were successfully used for secretion of immunoglobulin molecules. An advantage of this signal peptide is that this peptide is not recognized as a foreign sequence by the mammalian hosts. The construction of secreted κGalNAc-T2 protein confirmed applicability of such signals for effective secretion of nonhomologous proteins from insect cells for biotechnology application. It needs to be stressed that secretion of such fusion proteins must be experimentally confirmed for each construct because even strong insect secretory signals like HBM are not efficiently mediating production and secretion of every construct [26]. Expression of recombinant κGalNAc-T2 from insect cells Sf9 followed by purification on NiNTA column we described here provides an easy and fast approach for high-yield production of active enzyme. After establishing optimal parameters for infection, cultivation, and purification conditions, yields can reach 2.5 mg protein from 1 L of the culture medium.
> Murine Igκ secretory signal drives secretion of fusion GalNAc-T2 in insect system. > Secreted soluble human GalNAc-T2 was enzymatically active. > The yield in Sf9 cells was 2.5 mg of GalNAc-T2 protein per 1 L culture. >Recombinant GalNAc-T2 attaches GalNAc to all possible O-glycosylation sites in IgA1.
Acknowledgement
This work was supported by grant: MSM6198959223 Ministry of School, Youth, and Sport, GAP302/10/1055 Czech Science Foundation, NT11081 Grant Agency of the Ministry of the Health Czech Republic, and by grants from the National Institutes of Health DK082753, DK078244, DK083663, DK075868, DK077279, and GM098539.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 2006;7:599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chui D, Sellakumar G, Green R, Sutton-Smith M, McQuistan T, Marek K, Morris H, Dell A, Marth J. Genetic remodeling of protein glycosylation in vivo induces autoimmune disease. Proc Natl Acad Sci U S A. 2001;98:1142–1147. doi: 10.1073/pnas.98.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ju T, Cummings RD. Protein glycosylation: chaperone mutation in Tn syndrome. Nature. 2005;437:1252. doi: 10.1038/4371252a. [DOI] [PubMed] [Google Scholar]
- 4.Kobata A. A retrospective and prospective view of glycopathology. Glycoconj J. 1998;15:323–331. doi: 10.1023/a:1006961532182. [DOI] [PubMed] [Google Scholar]
- 5.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 6.White T, Bennett EP, Takio K, Sorensen T, Bonding N, Clausen H. Purification and cDNA cloning of a human UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase. J Biol Chem. 1995;270:24156–24165. doi: 10.1074/jbc.270.41.24156. [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki H, Zhang Y, Tachibana K, Gotoh M, Kikuchi N, Kwon YD, Togayachi A, Kudo T, Kubota T, Narimatsu H. Initiation of O-glycan synthesis in IgA1 hinge region is determined by a single enzyme, UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 2. J Biol Chem. 2003;278:5613–5621. doi: 10.1074/jbc.M211097200. [DOI] [PubMed] [Google Scholar]
- 8.Raska M, Takahashi K, Czernekova L, Zachova K, Hall S, Moldoveanu Z, Elliott MC, Wilson L, Brown R, Jancova D, Barnes S, Vrbkova J, Tomana M, Smith PD, Mestecky J, Renfrow MB, Novak J. Glycosylation patterns of HIV-1 gp120 depend on the type of expressing cells and affect antibody recognition. J Biol Chem. 2010;285:20860–20869. doi: 10.1074/jbc.M109.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joo HY, Jones A, Yang C, Zhai L, Smith ADt, Zhang Z, Chandrasekharan MB, Sun ZW, Renfrow MB, Wang Y, Chang C, Wang H. Regulation of histone H2A and H2B deubiquitination and Xenopus development by USP12 and USP46. J Biol Chem. 2011;286:7190–7201. doi: 10.1074/jbc.M110.158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renfrow MB, Mackay CL, Chalmers MJ, Julian BA, Mestecky J, Kilian M, Poulsen K, Emmett MR, Marshall AG, Novak J. Analysis of O-glycan heterogeneity in IgA1 myeloma proteins by Fourier transform ion cyclotron resonance mass spectrometry: implications for IgA nephropathy. Anal Bioanal Chem. 2007;389:1397–1407. doi: 10.1007/s00216-007-1500-z. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J. Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest. 2009;119:1668–1677. doi: 10.1172/JCI38468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore JS, Kulhavy R, Tomana M, Moldoveanu Z, Suzuki H, Brown R, Hall S, Kilian M, Poulsen K, Mestecky J, Julian BA, Novak J. Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Mol Immunol. 2007;44:2598–2604. doi: 10.1016/j.molimm.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes MM, Suzuki H, Brooks MT, Tomana M, Moldoveanu Z, Mestecky J, Julian BA, Novak J, Herr AB. Recognition of galactose-deficient O-glycans in the hinge region of IgA1 by N-acetylgalactosamine-specific snail lectins: a comparative binding study. Biochemistry. 2010;49:5671–5682. doi: 10.1021/bi9019498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renfrow MB, Cooper HJ, Tomana M, Kulhavy R, Hiki Y, Toma K, Emmett MR, Mestecky J, Marshall AG, Novak J. Determination of aberrant O-glycosylation in the IgA1 hinge region by electron capture dissociation fourier transform-ion cyclotron resonance mass spectrometry. J Biol Chem. 2005;280:19136–19145. doi: 10.1074/jbc.M411368200. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K, Wall SB, Suzuki H, Smith ADt, Hall S, Poulsen K, Kilian M, Mobley JA, Julian BA, Mestecky J, Novak J, Renfrow MB. Clustered O-glycans of IgA1: defining macro- and microheterogeneity by use of electron capture/transfer dissociation. Mol Cell Proteomics. 2010;9:2545–2557. doi: 10.1074/mcp.M110.001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill DJ, Clausen H, Bard F. Location, location, location: new insights into O-GalNAc protein glycosylation. Trends Cell Biol. 2011;21:149–158. doi: 10.1016/j.tcb.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Tomana M, Novak J, Julian BA, Matousovic K, Konecny K, Mestecky J. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M, Julian BA, Mestecky J, Huang WQ, Anreddy SR, Hall S, Hastings MC, Lau KK, Cook WJ, Novak J. Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int. 2007;71:1148–1154. doi: 10.1038/sj.ki.5002185. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki H, Moldoveanu Z, Hall S, Brown R, Vu HL, Novak L, Julian BA, Tomana M, Wyatt RJ, Edberg JC, Alarcon GS, Kimberly RP, Tomino Y, Mestecky J, Novak J. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest. 2008;118:629–639. doi: 10.1172/JCI33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wandall HH, Irazoqui F, Tarp MA, Bennett EP, Mandel U, Takeuchi H, Kato K, Irimura T, Suryanarayanan G, Hollingsworth MA, Clausen H. The lectin domains of polypeptide GalNAc-transferases exhibit carbohydrate-binding specificity for GalNAc: lectin binding to GalNAc-glycopeptide substrates is required for high density GalNAc-O-glycosylation. Glycobiology. 2007;17:374–387. doi: 10.1093/glycob/cwl082. [DOI] [PubMed] [Google Scholar]
- 21.Fritz TA, Raman J, Tabak LA. Dynamic association between the catalytic and lectin domains of human UDP-GalNAc:polypeptide α-N-acetylgalactosaminyltransferase-2. J Biol Chem. 2006;281:8613–8619. doi: 10.1074/jbc.M513590200. [DOI] [PubMed] [Google Scholar]
- 22.Congote LF, Li Q. Accurate processing and secretion in the baculovirus expression system of an erythroid-cell-stimulating factor consisting of a chimaera of insulin-like growth factor II and an insect insulin-like peptide. Biochem J. 1994;299(Pt 1):101–107. doi: 10.1042/bj2990101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kretzschmar T, Aoustin L, Zingel O, Marangi M, Vonach B, Towbin H, Geiser M. High-level expression in insect cells and purification of secreted monomeric single-chain Fv antibodies. J Immunol Methods. 1996;195:93–101. doi: 10.1016/0022-1759(96)00093-2. [DOI] [PubMed] [Google Scholar]
- 24.Lemeulle C, Chardes T, Montavon C, Chaabihi H, Mani JC, Pugniere M, Cerutti M, Devauchelle G, Pau B, Biard-Piechaczyk M. Anti-digoxin scFv fragments expressed in bacteria and in insect cells have different antigen binding properties. FEBS Lett. 1998;423:159–166. doi: 10.1016/s0014-5793(98)00029-5. [DOI] [PubMed] [Google Scholar]
- 25.Tessier DC, Thomas DY, Khouri HE, Laliberte F, Vernet T. Enhanced secretion from insect cells of a foreign protein fused to the honeybee melittin signal peptide. Gene. 1991;98:177–183. doi: 10.1016/0378-1119(91)90171-7. [DOI] [PubMed] [Google Scholar]
- 26.Reavy B, Ziegler A, Diplexcito J, Macintosh SM, Torrance L, Mayo M. Expression of functional recombinant antibody molecules in insect cell expression systems. Protein Expr Purif. 2000;18:221–228. doi: 10.1006/prep.1999.1191. [DOI] [PubMed] [Google Scholar]