Abstract
The molecular mechanisms that regulate functional activation of IL-1α remain elusive. In this issue of Immunity, Zheng et al. describe a molecular system implicating interleukin-1 receptor-2 (IL-1R2) as a principal cytosolic factor that controls functional IL-1α activation during necrosis.
Inflammation is a coordinated physiological host response to cell damage, stress, or infection. In the context of “sterile inflammation”- a response to cell damage or stress in the absence of pathogens, inflammation is triggered by host-derived factors, called danger-associated molecular patterns or DAMPs, that are released from damaged cells and signal disturbances of cellular or tissue homeostasis. The principal goal of the inflammatory response to DAMPs is restoration of physiological homeostasis through tissue repair. When cell damage or stress associates with the presence of or are directly induced by pathogens, the inflammatory response can be triggered by pathogen-associated molecular patterns or PAMPs. The goals of the host response to PAMPs are pathogen elimination and establishing immunity. The interleukin-1 (IL-1) family cytokines IL-1α and IL-1β are amongst the most potent host proteins that activate inflammatory responses to both DAMPs and PAMPs (Dinarello, 2009). Upon engagement of the IL-1 receptor type 1 (IL-1R1), IL-1α and IL-1β initiate identical biological responses through a signaling cascade that conveys the message that the cellular homeostasis has been disturbed and that the recruitment of specialized cells to initiate tissue repair and/or pathogen elimination is warranted.
Apart from being synthesized as precursor pro-proteins and engaging the same receptor at the plasma membrane, the similarity between IL-1α and IL-1β is limited. Extensive efforts in trying to understand molecular processes that lead to functional activation of IL-1β has resulted in a cohesive model of initiation of inflammation via caspase-1-dependent proteolytic processing of pro-IL-1β and secretion of its mature form from the cell, where the mature p17 IL-1β form is the only biologically active form of this cytokine (Dinarello, 2009). The caspase-1-dependent processing of pro-IL-1β is tightly controlled by the inflammasome – a supramolecular complex of cytosolic proteins that sense the presence of noxious toxins or pathogen components, and, when prompted, multimerize and activate caspase-1 (Schroder and Tschopp, 2010). Caspase-1, however, appears to play no role in proteolytic processing of pro-IL-1α, but rather controls its secretion from the cell via a still poorly defined mechanism (Gross et al., 2012; Kuida et al., 1995). Importantly, and in contrast to IL-1β, both the pro-IL-1α p33 form and its mature p17 IL-1α forms are the functional biologically active ligands for IL-1RI (Mosley et al., 1987), implying that inflammation can be initiated by IL-1α in various pathological contexts independently of its proteolytic processing. Although secretion of IL-1β is thought to activate systemic inflammatory responses, IL-1α can function both as a secreted and as a membrane-bound cytokine and, thus, trigger and sustain inflammation locally. To this end, IL-1α has been shown to be the principal inducer of inflammation in response to dying and necrotic cells, where the loss of plasma membrane integrity leads to a passive release of IL-1α-containing cytosolic content to the surrounding milieu and initiation of pro-inflammatory signaling downstream of IL-1R1 (Chen et al., 2007).
Because IL-1α is expressed ubiquitously (Dinarello, 2009), these observations imply that the necrotic death of any cell type in the body should necessarily induce potent local inflammatory responses irrespective of the cell and tissue type. This, however, may not be beneficial to the host under all physiological or pathophysiological circumstances. Exacerbated cell necrosis-driven inflammation in tissues with limited regenerative capacity, that are present for instance in kidneys or heart, can lead to organ dysfunction or even death. But what can prevent pro-IL-1α from initiating inflammation in response to cellular necrosis, which is, in many cases, an uncontrolled process of catastrophic cell demise?
In this issue of Immunity, Zeng et al. have revealed an elaborate and elegant molecular system that controls pro-IL-1α biological activity post necrosis (REF). They implicate a decoy IL-1R type II (IL-1R2) as a key protein that binds pro-IL-1α in the cytosol and prevents its interaction with IL-1R1 upon release from necrotic cells. Initial observations that Zeng et al. made have shown that IL-1R1 signaling in response to necrotic cells is highly cell type-dependent. They found that although primary human monocytes-derived macrophages, aortic vascular smooth muscle cells (VSMC), or immortalized Jurkat and HeLa cells expressed similar amounts of pro-IL-1α, upon triggering necrosis, only cytosolic content from VSMC and not other cell types was able to initiate IL-1R1-dependent responses. Furthermore, the authors found that in necrotic VSMC, pro-IL-1α p33 form was readily proteolytically processed to its mature p17 IL-1α form by calpains (Kobayashi et al., 1990), and that this processing did not occur upon induction of necrosis in other cell types that they analyzed. The authors posited that a cytosolic factor may exist that associates with pre-IL-1α in “non-inflammatory” necrotic cell types that blocks both the pro-IL-1α maturation by calpains and its ability to interact with IL-1R1.
Although an association of pro-IL-1α with IL-1R2 in the cytosol has been previously reported (Kawaguchi et al., 2006), the role of this interaction in regulating inflammation to necrotic cells was not revealed until now. Furthermore, it still remains controversial whether proteolytic processing of pro-IL-1α is ultimately required for its functional activation or whether both the p33 pro-IL-1α and its mature p17 forms are equally potent at inducing IL-1R1-dependent inflammatory responses. Amongst the reasons that prevented definitive resolution of this question was a poor solubility of recombinant p33 pro-IL-1α form, that complicated comparative analyses of biological activities of the two IL-1α forms. To address this question, Zheng et al expressed two variants of p33 pre-IL-1α, one as a His-tagged form and the other was conjugated with GST. Through extensive sets of analyses, the authors demonstrated that calpain-dependent processing significantly increased biological activity of IL-1α. Furthermore, using an IL-1 receptor antagonist (IL-1RA) competition approach to evaluate the relative affinity of p33 and p17 IL-1α forms to IL-1RI, the authors concluded that the mature p17 IL-1α form was more efficient at binding IL-1RI and was 46.3-fold more physiologically active than its p33 pre-IL-1α form. Although these data are in line with recent findings by Afonina et al., who demonstrated that pro-IL-1α proteolysis with Granzyme B, calpain, and elastase significantly enhances its biological activity (Afonina et al., 2011), the ultimate answer to the question on whether pro-IL-1α is a potent biologically active ligand for IL-1R1 could come from analyzing inflammatory responses to necrotic IL-1α-deficient VSMCs that have been reconstituted to express calpain-cleavable or calpain cleavage-resistant forms of pro-IL-1α.
The remarkable finding of Zheng et al. is that IL-1R2-mediated inhibition of pro-IL-1α availability to processing by calpain and other proteases, including Granzyme B, elastase, and chymase, can be relieved by caspase-1. Specifically, the authors showed that the addition of caspase-1 to IL-1R2 resulted in the proteolytic processing of the latter into two major products. Importantly, upon cleavage of IL-1R2, it dissociated from pro-IL-1α which then became available to induce IL-1RI-dependent inflammation either directly or after maturation with calpain or other cellular proteases.
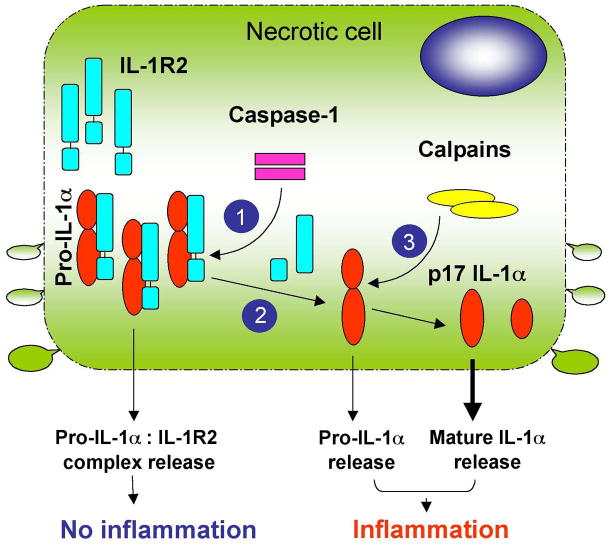
The designation of caspase-1 as a point of control for functional IL-1α activation is highly significant. Caspase-1 activation is under the tight control of the inflammasomes that sense both DAMPs and PAMPs in the cytosol (Schroder and Tschopp, 2010). Currently accepted concepts indicate that the pro-inflammatory function of inflammasomes is enabled through caspase-1-dependent proteolytic processing of pro-forms of IL-1β and IL-18, which, unlike a constitutively-expressed pro-IL-1α, are inducible cytokines. In the context of sterile inflammation, where IL-1β expression may not be induced and only the ubiquitously-expressed pro-IL-1α is present, inflammasome activation in response to noxious metabolic or damage-derived stimuli will lead to activation of caspase-1, which will target IL-1R2 for degradation and release pro-IL-1α for functional activation by a second protease-calpain, thus, triggering local inflammatory response through IL-1RI (Figure 1).
Figure 1. The model of IL-1R2-dependent control of IL-1α activation.
IL-1α is synthesized as a pro-protein p33 form that is sequestered in the cytosol by IL-1R2. While in complex with IL-1R2, the maturation of pro-IL-1α through proteolytic processing with cellular proteases, including calpain, elastase, Granzyme B, or chymase, is blocked. Upon loss of the plasma membrane integrity during necrosis, the release from cells of pro-IL-1α:IL-1R2 complexes cannot activate IL-1RI-signaling. However, following inflammasome activationcaspase-1 can cleave IL-1R2 (1) and liberate pro-IL-1α (2), which now becomes available for proteolytic processing by calpains (3), giving rise to a highly biologically active IL-1α p17 form. Necrosis induction in cells that express pro-IL-1α but lack IL-1R2 will lead to the release of both pro- and mature-IL-1α forms, which will activate inflammatory responses via IL-1RI. The mature IL-1α p17 form is a more potent ligand for IL-1RI than the p33 pre-IL-1α form, and its release triggers stronger biological responses.
This model linking IL-1R2 and caspase-1 to functional activation of IL-1α may have broad implications. This may be the principal mechanism that underlays the pathology of numerous human diseases where local and chronic IL-1R1- and/or caspase-1-dependent inflammation leads to tissue dysfunction in a sterile environment without evident transcriptional IL-1β activation. Moreover, because ligation of the Toll-like receptors leads to potent transcriptional IL-1β activation and the downstream signaling adaptors for IL-1R1 and Toll-like receptors are the same (Dinarello, 2009), inflammasome-dependent licensing of functional activation of IL-1α by “sterile” stimuli may lead to pro-IL-1β production that will be followed by its caspase-1-dependent processing and release, thus leading to exacerbation of inflammation. In this context, in response to damage or noxious stimulus assault, the initial pro-inflammatory IL-1R1 signaling can be mediated by IL-1α, while chronic inflammatory response even without any pathogen presence may then be sustained by both IL-1α and IL-1β. Collectively, this data reveals functional redundancy in mechanisms of inflammasome-dependent activation of IL-1R1-mediated pro-inflammatory responses. The unraveling of relevance of these findings to the explaining mechanistic underpinning for various pathological conditions associated with sustained inflammation in humans now appears as a manageable challenge. Thus, what can make necrosis “silent” by keeping a lid on IL-1α? Now, the answer is clear – it is IL-1R2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afonina IS, Tynan GA, Logue SE, Cullen SP, Bots M, Luthi AU, Reeves EP, McElvaney NG, Medema JP, Lavelle EC, Martin SJ. Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1alpha. Mol Cell. 2011;44:265–278. doi: 10.1016/j.molcel.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nature Medicine. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Gross O, Yazdi AS, Thomas CJ, Masin M, Heinz LX, Guarda G, Quadroni M, Drexler SK, Tschopp J. Inflammasome activators induce interleukin-1alpha secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Nishimagi E, Tochimoto A, Kawamoto M, Katsumata Y, Soejima M, Kanno T, Kamatani N, Hara M. Intracellular IL-1alpha-binding proteins contribute to biological functions of endogenous IL-1alpha in systemic sclerosis fibroblasts. Proc Natl Acad Sci U S A. 2006;103:14501–14506. doi: 10.1073/pnas.0603545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Yamamoto K, Saido T, Kawasaki H, Oppenheim JJ, Matsushima K. Identification of Calcium-Activated Neutral Protease as a Processing Enzyme of Human Interleukin-1-Alpha. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:5548–5552. doi: 10.1073/pnas.87.14.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuida K, Lippke JA, Ku G, Harding MW, Livingston DJ, Su MSS, Flavell RA. Altered Cytokine Export and Apoptosis in Mice Deficient in Interleukin-1-Beta Converting-Enzyme. Science. 1995;267:2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- Mosley B, Urdal DL, Prickett KS, Larsen A, Cosman D, Conlon PJ, Gillis S, Dower SK. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. J Biol Chem. 1987;262:2941–2944. [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The Inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]