Abstract
Background
Even though more than 600 stroke treatments have been shown effective in preclinical studies, clinically proven treatment alternatives for cerebral infarction remain scarce. Amongst the reasons for the discrepancy may be methodological shortcomings, such as high mortality and outcome variability, in the preclinical studies. A common approach in animal stroke experiments is that A) focal cerebral ischemia is inflicted, B) some type of treatment is administered and C) the infarct sizes are assessed. However, within this paradigm, the researcher has to make numerous methodological decisions, including choosing rat strain and type of surgical procedure. Even though a few studies have attempted to address the questions experimentally, a lack of consensus regarding the optimal methodology remains.
Methods
We therefore meta-analyzed data from 502 control groups described in 346 articles to find out how rat strain, procedure for causing focal cerebral ischemia and the type of filament coating affected mortality and infarct size variability.
Results
The Wistar strain and intraluminal filament procedure using a silicone coated filament was found optimal in lowering infarct size variability. The direct and endothelin methods rendered lower mortality rate, whereas the embolus method increased it compared to the filament method.
Conclusions
The current article provides means for researchers to adjust their middle cerebral artery occlusion (MCAo) protocols to minimize infarct size variability and mortality.
Keywords: Brain infarction, Middle cerebral artery occlusion, Rats, Methods, Mortality, Variability
Background
Ischemic stroke is amongst the leading causes of death and disability in the world and has been the subject of massive research efforts during recent years. Even though these efforts have resulted in more than 600 treatments reported effective in preclinical studies [1], clinically proven treatment options are still few. There are reasons to believe that this apparent translational roadblock may inter-alia be due to methodological confounding factors, including high mortality and large outcome variability, in the preclinical studies [2-4].
The usual approach in experimental stroke studies, used in hundreds of publications each year, is that A) focal cerebral ischemia is inflicted in rodents [5,6], B) some type of treatment is administered and C) the infarct sizes are assessed. Even though this setup may seem straight forward, there are infinite numbers of methodological variants, and there is a profound lack of consensus regarding the ideal methodology to be used in experiments of this kind. A small number of studies aiming to optimize the infarct induction regarding the important aspects of mortality and variability, for example by testing various rodent strains and sizes [7-9], surgical procedures [10-13] or occluding intraluminal filaments [14], have been published. However, these studies have rendered conflicting results, and are too few and too small to provide comprehensive understanding of how the different methodological parameters interact.
Hence, it was thought of interest to investigate the influence of different methodological factors on infarct variability and mortality in rat stroke models using a hypothesis-driven meta-analytical approach where their interactions and complexity could be embraced rather than disregarded. The meta-analytical approach seemed attractive since such a vast number of studies with the abovementioned experimental setup are published, and since control/vehicle/placebo groups (hereby referred to as “control groups”), suitable for inter-study comparisons, are almost invariably included. A study of this kind has, to the best of our knowledge, not been published previously. Even though other animals, not least mice, are also well-used in experimental stroke research, rats were due to space restrictions chosen to be the sole focus of the current article.
The aim of the current meta-analysis was to investigate chosen methodological variables’ effects on infarct size variability and mortality. An a priori hypothesis of six main factor-outcome relations (1A-3B) was established:
1. Rat strain affects (A) infarct size variability and (B) mortality.
2. Type of focal ischemia procedure affects (A) infarct size variability and (B) mortality.
3. In studies using the intraluminal filament method, the type of filament affects (A) infarct size variability and (B) mortality.
Methods
Article inclusion
To identify articles to be included in the meta-analysis, Medline was searched with the line (mcao or “middle cerebral artery occlusion” or “MCA occlusion” or “stroke” or “cerebral ischemia” or “brain ischemia”) and (rat or rats), resulting in more than 19,000 hits. Starting with the latest article the 10th of June 2011 [15], the articles were consecutively, in order of PubMed identifier, assessed for inclusion in the study.
The inclusion criteria were:
A. Article written in English
B. Original research article
C. Experiments performed in living adolescent, adult or elderly rats
D. Animals inflicted one single focal cerebral ischemic lesion
E. Infarct size assessed and results presented
F. Inclusion of a control group, untreated except for vehicle treatments
G. Sufficient description of fundamental aspects of the experiment (after e-mail correspondence)
Data extraction
Data about the control groups were extracted from all included articles. If an article included more than one control group, differing in for example euthanasia time-point, all control groups were separately included and assessed independently of each other. When extracting the method data, we adhered strictly to the principle “If it is not described, it was not performed”. Registered factors and outcome measures are listed in Table 1.
Table 1.
Extracted factors and outcome measures
| Factor/Outcome measure | Data type | Final categories* or unit | Reference category for regression analyses |
|---|---|---|---|
|
Rat property factors | |||
|
Strain |
Category |
I. Sprague Dawley |
Sprague Dawley |
| II. Wistar | |||
| III. SHR | |||
| IV. WKY | |||
| V. Other strains | |||
|
Sex |
Category |
I. Male |
Male |
| II. Female | |||
| III. Ovx female | |||
| IV. Mix or not specified | |||
|
Elderly rats ** |
Category, Binomial |
[No] |
[No] |
| [Yes] | |||
|
Weight |
Continuous |
Grams |
NA |
|
Animal exclusion rate |
Continuous |
% |
NA |
|
Anesthesia factors | |||
|
Type of anesthetic |
Category |
I. Inhalation anesthetic |
Inhalation anesthetic |
| II. Chloral hydrate | |||
| III. Ketamine | |||
| IV. Barbiturates and benzodiazepines | |||
| V. Other anesthetics | |||
|
Intubation |
Category, Binomial |
[No] |
[No] |
| [Yes] | |||
|
Awakening of rats during occlusion |
Category, Binomial |
[No] |
[No] |
| [Yes] | |||
|
Temperature feedback system |
Category, Binomial |
[No] |
[No] |
| [Yes] | |||
|
Electroencephalographic surveillance |
Category, Binomial |
[No] |
[No] |
| [Yes] | |||
|
Blood pressure monitored |
Category, Binomial |
[No] |
[No] |
| [Yes] | |||
|
Heart rate monitored |
Category, Binomial |
[No] |
[No] |
| [Yes] | |||
|
Blood gases/O2saturation analyzed |
Category, Binomial |
[No] |
[No] |
| [Yes] | |||
|
Blood hemoglobin concentration analyzed |
Category, Binomial |
[No] |
[No] |
| [Yes] | |||
|
Blood glucose concentration analyzed |
Category, Binomial |
[No] |
[No] |
| [Yes] | |||
|
Postoperative antibiotics |
Category, Binomial |
[No] |
[No] |
| [Yes] | |||
|
Focal ischemia procedure factors | |||
|
Type of middle cerebral artery occlusion procedure |
Category |
I. Intraluminal filament |
Intraluminal filament |
| II. Direct, mechanical*** | |||
| III. Embolic | |||
| IV. Photothrombotic | |||
| V. Endothelin injection | |||
|
Occlusion duration**** |
Category |
I. Short transient (up to 60 minutes) |
Short transient (up to 60 minutes) |
| II. Long transient (>60 min) | |||
| III. Permanent | |||
|
Occluding filament type (Only studies using the intraluminal filament method) |
Category |
I. Uncoated |
Uncoated |
| II. Silicon coated | |||
| III. Poly-L-Lysine coated | |||
| IV. Other coatings | |||
|
Analysis procedure factors | |||
|
Time after focal ischemia for evaluation of damage |
Continuous |
Hours |
NA |
|
Type of infarct size evaluation |
Category |
I. TTC |
TTC |
| II. Radiology | |||
| III. Acidic/Basic stain or silver stain histology | |||
| IV. Immunohistology | |||
|
Edema correction used |
Category, Binomial |
[No] |
[No] |
| [Yes] | |||
|
Blinding of infarct size determination procedure |
Category, Binomial |
[No] |
[No] |
| [Yes] | |||
|
Criteria for excluding rats |
Category |
I. None |
None |
| II. Observed absence of cerebral blood flow reduction | |||
| III. Lack of functional deficit | |||
| IV. Too small infarct | |||
| V. Other pathology in animal | |||
|
Outcome measures | |||
|
Infarct size coefficient of variation |
Continuous |
% |
NA |
| Mortality rate | Continuous | % | NA |
* Only categories represented by at least 5 control groups were included in the analysis to avoid statistically inadequate attribution of explanatory value to too small categories. Categories represented by less than 5 control groups were in the analysis included in an Others category. Further, some other reductions in number of categories were performed, as presented below.
** Elderly rats were defined as being >12 months of age at time of ischemic insult.
*** Direct, mechanical refers to all MCAo procedures where the MCA is mechanically occluded from the outside, for example by clips, cauterization or ligation.
**** Only methods including actions taken to ensure reperfusion (such as removing the occluding intraluminal filament or arterial clip) were considered transient.
Because many of the included articles/control groups lacked information about for example mortality rate, the researchers of those articles were contacted via e-mail with a gentle request to provide this information. In total, the authors of 310 articles were e-mailed, of which 183 (59%) complied.
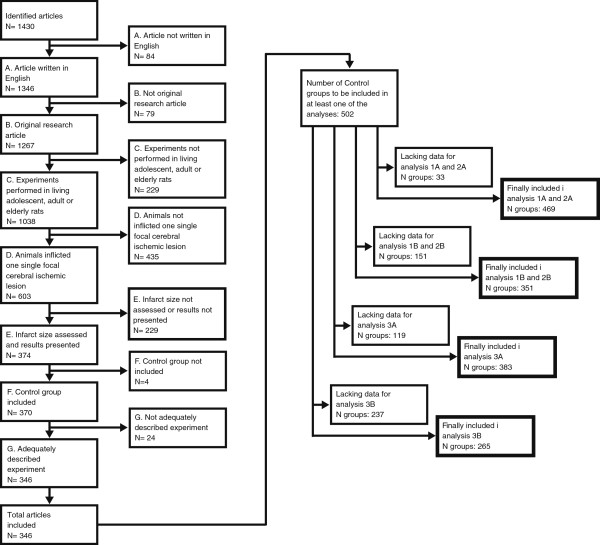
Since power calculations for large multiple regression analyses are extremely complex to perform a priori, a saturation principle was adopted to determine a sufficient number of control groups to be included. After information from 300 control groups had been extracted, an interim analysis was performed, and then re-performed every 40–50 new control groups included. When the results had stabilized (no changes in overall trends, and only minor changes in p-values), no more articles were included. 502 control groups from 346 articles were finally included in the study [11,15-359] while 1084 articles were excluded (Figure 1).
Figure 1.
When analyses were saturated, 1430 articles had been evaluated for inclusion in the meta-analysis. After exclusion due to criteria A-G, 346 studies, describing 502 control groups, remained. Due to lack of certain pieces of information in some of the articles, not all 502 control groups could be included in all four multiple regression analyses, as shown in the thick-boarded boxes to the right in the figure.
Processing of data
Category refinement
After extraction of data, categories represented by less than 5 control groups (corresponding to less than 1% of the material) were included in the Others-categories. This approach was motivated by the fact that these small categories otherwise would run the risk of being attributed high explanatory values that were not statistically substantiated, thus obscuring the influence of other categories.
In the Other strain category, the following variants were included: Long Evans rats, T-cell deficient nude rats, SHRSP, Fischer rats, Zucker rats, Hooded Wistar rats, Lewis rats, Holtzmann rats and Swiss albino rats.
The variable Sex finally contained four categories, since males formed the first category, females that were not explicitly ovariectomized were separated from ovariectomized females in a second and third category, and control groups using mixed or unspecified animals were grouped in a fourth category.
Fifteen various anesthesia regimens were reduced to four main categories and one Others category (in which for example methohexithal sodium, medetomedin and unspecified regimens were included). All inhalation anesthetics (isoflurane, halothane, sevoflurane, fluothane and enflurane) were included in the first category, while chloral hydrate was used frequently enough (and was not appreciably similar to any other category) to deserve a category of its own. The third category, Ketamine, included all ketamine containing regimens, such as ketamine/xylazine, ketamine/rumpun or ketamine only. Finally, all anesthetic regimens pertaining to the barbiturate or bensodiazepine groups, such as pentobarbital and diazepam, formed a fourth category.
The variable Blood gases/O2saturation analyzed was initially registered as the separate variables Blood pH analyzed, Blood oxygen saturation analyzed and Blood carbon dioxide analyzed, however these three were so highly correlated that they were thought better to be represented by only one variable.
Regarding the techniques for causing focal ischemic lesions, all intraluminal filament procedures were reduced to one single category. All direct occlusion techniques, based on craniectomy followed by physical occlusion of the MCA by means of a clip, suture, hook or cauterizer, formed the Direct category, while emboli techniques were clumped up in a third category. Photothrombotic procedures and methods of endothelin injection defined the fourth and fifth categories, respectively. It should however be noted that the occlusion time was accounted for in another variable and that the choice of different filaments were analyzed separately.
The filament categories, used for the analyses addressing hypotheses 3A and 3B, were also reduced. The uncoated filaments, a seemingly homogenous group, formed the first category, while silicone and resin coating were put in the Silicone category. Poly-L-Lysine formed a category of its own, while other rare coating techniques (including for example heparin coating, “glue coating” and paraffin coating) were put in a separate category together with unspecified coating techniques.
The procedures used for infarct evaluation were reduced to four categories. The most frequently used technique, 2,3,5-triphenyltetrazolium chloride staining, defined the first category, while radiologic methods (in the majority of cases magnetic resonance imaging, but in a few cases computed tomography) were put in a Radiology category. Various acidic/basic staining techniques (such as hematoxylin/eosin, cresyl violet and thionine) was, together with silver staining (used in only one of the included studies), included in category number three, while immunohistological methods were put in a fourth category.
Edema correction can be performed in different ways [360,361]. It was initially the intention to register not only if, but also which type of, edema correction was used. However, it soon turned out that this was not specified in a sufficient number of articles to perform a meaningful analysis. It was therefore only registered if edema correction had been used or not.
Concerning the exclusion procedures, the first category included all control groups in which no exclusion criteria were explicitly adopted. In the second category, control groups in which surveillance of blood flow reduction (for example using laser-doppler), with the plausible aim to exclude the absence of such, were put. The third, fourth and fifth categories contained control groups from articles in which lack of functional deficit, too small infarct size or other pathology (including intracerebral hemorrhage), respectively, were stated to be exclusion criteria. It should be noted that control groups from articles accounting for multiple exclusion criteria were registered in more than one of the exclusion categories.
Definition of continuous variables
Animal exclusion rate was defined as the percentage of rats excluded due to other reasons than mortality from induction of focal cerebral ischemia until the final infarct size assessment. Time after focal ischemia for evaluation of damage was defined as the time from cerebral blood flow obstruction until sacrifice. The outcome Infarct size coefficient of variation was defined as the standard deviation of the infarct volume divided by the average infarct volume. Irrespective of how the infarct size is presented; as percentage of the whole brain, as percentage of the hemisphere or in cubic millimeters, this calculation provides a strictly defined, and inter-comparable, measure of the infarct size variability. The other outcome, Mortality, was defined as the unintended mortality in the control group, from induction of focal cerebral ischemia until infarct size assessment, as a percentage of the whole group.
Statistical analyses
A priori, six main hypotheses (1A-3B) were put (as aforementioned):
1. Rat strain affects (A) infarct size variability and (B) mortality
2. Type of focal ischemia procedure affects (A) infarct size variability and (B) mortality.
3. In studies using the intraluminal filament method, the type of filament affects (A) infarct size variability and (B) mortality.
Obviously, several additional hypotheses could be tested in the information compiled from the studies, but the higher number of hypotheses, the higher the risk of finding falsely significant results due to multiple comparisons (type I errors). However, due to the risk of type II-errors, corrections for multiple comparisons were not performed, calling for separate assessment of the six hypotheses.
All category variables were dummy-converted before analysis (Table 1). For categorical variables with more than two categories, the most common category was chosen to be the reference category. For binary variables, it does not matter which one is made the reference, why [No] (in other words, the lack of a specific methodological ingredient) was consistently chosen. The data were subsequently analyzed using multiple regression analyses with backward variable exclusion. This step identified which factors significantly affected the outcomes Infarct size coefficient of variation and Mortality, respectively. Next, an enter model, in which the variables from the backward procedure were complemented by lacking dummy variables, was performed (the enter models with the variables found significant in the backward analyses are presented in Tables 2, 3, 4, 5). The analyses were weighted according to the number of animals used in each group; hence, a study including 5 animals in the control group was given less impact than a study including 20 animals. In total, four large multiple regression models (one for hypotheses 1A and 2A, one for hypotheses 1B and 2B, one for hypothesis 3A and one for hypothesis 3B) were set up, testing the combined effects of all available factors on the respective outcome measure (Infarct size coefficient of variation or Mortality rate). Hence, all models controlled for the factors listed in Table 1 when testing the stated hypotheses. All statistical calculations were performed in SPSS (Version 20, IBM Corporation, Armonk, NY, USA). P-values <0.05 were considered statistically significant. Data were presented as mean ± standard deviation or, when presenting results from the meta-analysis, with 0.95 confidence interval shown within brackets. It should be noted that the percent changes (regression coefficients) in Infarct size coefficient of variation and Mortality rate are presented in absolute, not relative, terms. In other words, if a certain variable decreases Mortality rate with 10%, it means that the mortality would decrease from for example 40% to 30%, and not merely from 40% to 36%.
Table 2.
Regression formula for hypotheses 1A and 2A
|
Regression formula for the effect of Strain and Type of middle cerebral artery occlusion procedure on Infarct size coefficient of variation (hypotheses 1A and 2A) | |||||
|---|---|---|---|---|---|
| Variable (reference category) | Variable categories | Regression coefficient | 0.95 confidence interval for regression coefficient | p-value | |
| Constant |
NA |
23.1 |
8.3 |
38.0 |
0.002 |
|
Strain (Sprague Dawley) |
Wistar |
−6.2 |
−11.5 |
−0.9 |
0.023 |
|
SHR |
−1.7 |
−11.0 |
7.5 |
0.710 |
|
|
WKY |
19.0 |
−0.8 |
38.8 |
0.059 |
|
|
Other Strains |
20.7 |
10.7 |
30.8 |
0.000 |
|
|
Type of middle cerebral artery occlusion procedure (Intraluminal filament) |
Direct, mechanical |
4.2 |
−3.4 |
11.8 |
0.274 |
|
Embolic |
14.7 |
3.4 |
26.0 |
0.011 |
|
|
Photothrombotic |
10.1 |
−5.2 |
25.5 |
0.196 |
|
|
Endothelin injection |
23.1 |
9.3 |
36.9 |
0.001 |
|
|
Sex (Male) |
Female |
−0.6 |
−19.7 |
18.5 |
0.951 |
|
Ovx female |
2.3 |
−16.7 |
21.4 |
0.810 |
|
|
Mix or not specified |
−11.5 |
−21.5 |
−1.5 |
0.024 |
|
|
Elderly rats |
[Yes] |
−23.6 |
−39.6 |
−7.7 |
0.004 |
|
Weight |
[Continuous; Grams] |
0.1 |
0.0 |
0.1 |
0.001 |
|
Type of anesthetic (Inhalation anesthetic) |
Chloral Hydrate |
−11.5 |
−16.9 |
−6.1 |
0.000 |
|
Ketamine |
2.9 |
−4.4 |
10.2 |
0.430 |
|
|
Barbiturates and bensodiazepines |
−8.4 |
−17.6 |
0.9 |
0.076 |
|
|
Other anesthetic |
−13.6 |
−22.6 |
−4.6 |
0.003 |
|
|
Awakening of rats during occlusion (No) |
[Yes] |
8.8 |
0.9 |
16.7 |
0.028 |
|
Temperature feedback system (No) |
[Yes] |
−6.9 |
−12.4 |
−1.5 |
0.013 |
|
Blood hemoglobin concentration analyzed (No) |
[Yes] |
10.9 |
3.0 |
18.8 |
0.007 |
|
Occlusion duration (Short transient) |
Long transient |
1.9 |
−3.9 |
7.8 |
0.519 |
|
Permanent |
−16.6 |
−24.1 |
−9.1 |
0.000 |
|
|
Time after focal ischemia for evaluation of damage |
[Continuous; Hours] |
0.0051 |
0.0005 |
0.0096 |
0.030 |
| Criteria for excluding rats (None) |
Observed absence of cerebral blood flow reduction |
−7.7 |
−12.4 |
−2.9 |
0.002 |
|
Lack of functional deficit |
−2.9 |
−8.6 |
2.7 |
0.310 |
|
|
Too small infarct |
0.1 |
−9.6 |
9.9 |
0.981 |
|
| Other pathology in animal | 18.6 | 12.7 | 24.6 | 0.000 | |
Variables excluded by statistical software due to too low explanatory value: Intubation, Postoperative antibiotics, Blood pressure monitoring, Heart rate monitoring, Blood gases/O2 saturation analyzed, Blood glucose concentration analyzed, Type of infarct size evaluation, Edema correction used, Blinding of infarct size determination procedure.
Table 3.
Regression formula for hypotheses 1B and 2B
|
Regression formula for the effect of Strain and Type of middle cerebral artery occlusion procedure on Mortality rate (hypotheses 1B and 2B) | |||||
|---|---|---|---|---|---|
| Variable (reference category) | Variable categories | Regression coefficient | 0.95 confidence interval for regression coefficient | p-value | |
| Constant |
NA |
17.1 |
13.9 |
20.3 |
0.000 |
|
Strain (Sprague Dawley) |
Wistar |
1.0 |
−2.4 |
4.4 |
0.551 |
|
SHR |
−6.9 |
−12.8 |
-.87 |
0.025 |
|
|
WKY |
−8.3 |
−19.3 |
2.8 |
0.141 |
|
|
Other Strains |
4.4 |
−1.1 |
9.9 |
0.113 |
|
|
Type of middle cerebral artery occlusion procedure (Intraluminal filament) |
Direct, mechanical |
−10.7 |
−15.1 |
−6.2 |
0.000 |
|
Embolic |
12.1 |
6.9 |
17.3 |
0.000 |
|
|
Photothrombotic |
−5.6 |
−15.3 |
4.2 |
0.262 |
|
|
Endothelin injection |
−9.7 |
−16.8 |
−2.6 |
0.007 |
|
|
Type of anesthetic (Inhalation anesthetic) |
Chloral Hydrate |
4.1 |
0.17 |
8.1 |
0.041 |
|
Ketamine |
3.2 |
−1.1 |
7.4 |
0.144 |
|
|
Barbiturates and bensodiazepines |
1.1 |
−4.6 |
6.8 |
0.710 |
|
|
Other anesthetic |
3.0 |
−1.9 |
8.0 |
0.230 |
|
|
Awakening of rats during occlusion (No) |
[Yes] |
9.1 |
3.9 |
14.2 |
0.001 |
|
Heart rate monitored (No) |
[Yes] |
−3.3 |
−7.5 |
0.94 |
0.127 |
|
Blood glucose concentration analyzed (No) |
[Yes] |
−2.8 |
−6.1 |
0.53 |
0.099 |
|
Type of infarct size evaluation (TTC) |
Radiology |
5.4 |
−0.11 |
10.9 |
0.055 |
|
Acidic/Basic stain or silver stain histology |
−1.2 |
−4.7 |
2.4 |
0.526 |
|
|
Immunohistology |
10.4 |
3.2 |
17.6 |
0.005 |
|
| Criteria for excluding rats (None) |
Observed absence of cerebral blood flow reduction |
−4.0 |
−6.9 |
−1.1 |
0.008 |
|
Lack of functional deficit |
0.15 |
−3.4 |
3.7 |
0.933 |
|
|
Too small infarct |
0.22 |
−5.1 |
5.6 |
0.935 |
|
| Other pathology in animal | −3.4 | −7.1 | 0.40 | 0.079 | |
Variables excluded by statistical software due to too low explanatory value: Sex, Elderly rats, Weight, Intubation, Temperature feedback system, Blood pressure monitored, Blood gases/O2 saturation analyzed, Blood hemoglobin concentration analyzed, Postoperative antibiotics, Occlusion duration, Time after focal ischemia for evaluation of damage, Edema correction used, Blinding of infarct size determination procedure.
Table 4.
Regression formula for hypothesis 3A
|
Regression formula for the effect of Occluding filament type on Infarct size coefficient of variation (hypothesis 3A) | |||||
|---|---|---|---|---|---|
| Variable (reference category) | Variable categories | Regression coefficient | 0.95 confidence interval for regression coefficient | p-value | |
| Constant |
NA |
49.1 |
38.7 |
59.4 |
0.000 |
|
Occluding filament type (Uncoated) |
Silicon coated |
−12.7 |
−18.3 |
−7.0 |
0.000 |
|
Poly-L-Lysine coated |
3.1 |
−4.8 |
11.0 |
0.444 |
|
|
Other coatings |
3.1 |
−5.0 |
11.3 |
0.451 |
|
|
Strain (Sprague Dawley) |
Wistar |
−2.1 |
−8.3 |
4.1 |
0.507 |
|
SHR |
−3.0 |
−17.2 |
11.2 |
0.679 |
|
|
WKY |
16.1 |
−5.6 |
37.8 |
0.146 |
|
|
Other Strains |
25.9 |
11.0 |
40.7 |
0.001 |
|
|
Sex (Male) |
Female |
3.3 |
−17.0 |
23.7 |
0.747 |
|
Ovx female |
12.1 |
−8.5 |
32.6 |
0.248 |
|
|
Mix or not specified |
−5.0 |
−15.1 |
5.2 |
0.335 |
|
|
Elderly rats |
[Yes] |
−17.8 |
−38.4 |
2.9 |
0.091 |
|
Type of anesthetic (inhalation anesthetic) |
Chloral Hydrate |
−12.0 |
−18.7 |
−5.4 |
0.000 |
|
Ketamine |
4.5 |
−4.3 |
13.2 |
0.315 |
|
|
Barbiturates and bensodiazepines |
−12.4 |
−24.8 |
0.00 |
0.050 |
|
|
Other anesthetic |
−14.2 |
−24.1 |
−4.4 |
0.005 |
|
|
Awakening of rats during occlusion (No) |
[Yes] |
10.4 |
2.1 |
18.6 |
0.014 |
|
Temperature feedback system (No) |
[Yes] |
−7.6 |
−14.1 |
−1.2 |
0.021 |
|
Blood pressure monitored (No) |
[Yes] |
−12.0 |
−21.4 |
−2.6 |
0.013 |
|
Heart rate monitored (No) |
[Yes] |
14.9 |
5.4 |
24.4 |
0.002 |
|
Blood gases/O2saturation analyzed (No) |
[Yes] |
11.5 |
1.8 |
21.1 |
0.021 |
|
Blood hemoglobin concentration analyzed (No) |
[Yes] |
10.7 |
1.5 |
19.9 |
0.023 |
|
Occlusion duration (Short transient) |
Long transient |
−3.3 |
−9.3 |
2.8 |
0.288 |
|
Permanent |
−21.4 |
−30.1 |
−12.8 |
0.000 |
|
|
Time after focal ischemia for evaluation of damage |
[Continuous; Hours] |
0.000036 |
−0.000013 |
0.000087 |
0.157 |
|
Blinding of infarct size determination procedure (No) |
[Yes] |
−3.8 |
−9.9 |
2.2 |
0.211 |
| Criteria for excluding rats (None) |
Observed absence of cerebral blood flow reduction |
−7.4 |
−13.1 |
−1.7 |
0.011 |
|
Lack of functional deficit |
−5.6 |
−12.0 |
0.7 |
0.083 |
|
|
Too small infarct |
7.6 |
−4.5 |
19.6 |
0.219 |
|
| Other pathology in animal | 16.2 | 8.7 | 23.6 | 0.000 | |
Variables excluded by statistical software due to too low explanatory value: Weight, Intubation, Postoperative antibiotics, Blood glucose concentration analyzed, Type of infarct size evaluation, Edema correction used.
Table 5.
Regression formula for hypotheses 3B
|
Regression formula for the effect of Occluding filament type on Mortality rate (hypothesis 3B) | |||||
|---|---|---|---|---|---|
| Variable (reference category) | Variable categories | Regression coefficient | 0.95 confidence interval for regression coefficient | p-value | |
| Constant |
NA |
16.8 |
13.2 |
20.4 |
0.000 |
|
Occluding filament type (Uncoated) |
Silicon coated |
−1.2 |
−4.7 |
2.4 |
0.516 |
|
Poly-L-Lysine coated |
3.0 |
−1.3 |
7.4 |
0.171 |
|
|
Other coatings |
−3.6 |
−9.0 |
1.8 |
0.188 |
|
|
Type of anesthetic (Inhalation anesthetic) |
Chloral Hydrate |
5.0 |
0.57 |
9.4 |
0.027 |
|
Ketamine |
3.1 |
−1.6 |
7.8 |
0.198 |
|
|
Barbiturates and bensodiazepines |
6.6 |
−0.91 |
14.1 |
0.085 |
|
|
Other anesthetic |
5.6 |
−0.04 |
11.2 |
0.052 |
|
|
Awakening of rats during occlusion (No) |
[Yes] |
9.7 |
4.5 |
14.8 |
0.000 |
|
Heart rate monitoring (No) |
[Yes] |
−6.5 |
−10.8 |
−2.2 |
0.003 |
|
Type of infarct size evaluation (TTC) |
Radiology |
13.3 |
6.5 |
20.1 |
0.000 |
|
Acidic/Basic stain or silver stain histology |
−1.2 |
−5.2 |
2.9 |
0.576 |
|
|
Immunohistology |
10.8 |
1.6 |
20.0 |
0.022 |
|
| Criteria for excluding rats (None) |
Observed absence of cerebral blood flow reduction |
−5.5 |
−8.6 |
−2.4 |
0.001 |
|
Lack of functional deficit |
.39 |
−3.5 |
4.2 |
0.843 |
|
|
Too small infarct |
−7.4 |
−14.2 |
-.56 |
0.034 |
|
| Other pathology in animal | −1.2 | −5.3 | 2.9 | 0.577 | |
Variables excluded by statistical software due to too low explanatory value: Strain, Sex, Elderly rats, Weight, Intubation, Temperature feedback system, Blood pressure monitoring, Blood gases/O2 saturation analyzed, Blood hemoglobin concentration analyzed, Blood glucose concentration analyzed, Postoperative antibiotics, Occlusion duration, Time after focal ischemia for evaluation of damage, Edema correction used, Blinding of infarct size determination procedure.
Protocol violations
It was originally planned to include the variable Exclusion rate to control for this confounder; however, too few articles presented the needed information. Even after all persistent e-mail correspondence, such a high number of studies lacked this variable that including it would have seriously hampered the analyses’ power. The variable was therefore omitted.
Electroencephalographic surveillance was only utilized in one of the included studies, and this variable could thus not be analyzed. It was therefore omitted.
Results
Impact of rat strain on infarct size coefficient of variation and mortality: Hypotheses 1A-B
Strain significantly affected both Infarct size coefficient of variation and Mortality rate. Wistar had the strongest negative regression coefficient, and rendered significantly lower variability (−6.2%, 0.95 CI: -11.5 to −0.9%, p = 0.023) than the well-used Sprague Dawley, while the category Other strains had significantly higher variability (+20.7%, 0.95 CI: +10.7 to +30.8%; p = 0.000; Figure 2; Table 2).
Figure 2.
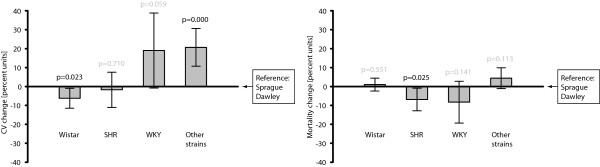
The choice of strain significantly affected the Infarct size coefficient of variation, so that the Wistar rendered lower variability than Sprague Dawley, which was chosen as the reference category. The Other strains category increased variability in comparison to Sprague Dawley. Regarding mortality rate, the effects of animal strain was limited to a slight decrease from using SHR. N = 469 and 351, respectively, in the two analyses/graphs. The bars represent 0.95 confidence intervals.
The only effect of Strain on Mortality rate was that SHR seemed to render lower percentages (−6.9%, 0.95 CI: -12.8 to −0.87%; p = 0.025; Figure 2; Table 3).
The multiple regression analysis addressing hypotheses 1A and 2A included 469 control groups, while the analysis for hypotheses 1B and 2B included 351 control groups (Figure 1). These regression formulae had r2 of 0.34 and 0.31, respectively, meaning that they explained 34% and 31% of the variation in the outcomes Infarct size coefficient of variation and Mortality rate.
Impact of focal ischemia procedure on infarct size coefficient of variation and mortality: Hypotheses 2A-B
Regarding Infarct size coefficient of variation, all analyzed surgical procedures had positive regression coefficients, indicating higher variability than in the intraluminal filament method, here chosen to be the reference. This trend was significant for the Emboli (+14.7%, 0.95 CI: +3.4 to +26.0%; p = 0.011) and Endothelin (+23.1%, 0.95 CI: +9.3 to +36.9%; p = 0.001) categories (Figure 3, Table 2).
Figure 3.
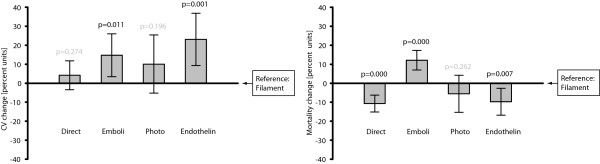
Concerning Infarct size coefficient of variation, the general trend was that the intraluminal filament procedure, here chosen to be the reference category, resulted in lower percentages than did the other methods. The emboli and endothelin injection methods rendered significantly higher variability. Mortality rate was clearly influenced by choice of induction procedure, with higher percentages in the emboli studies, while the direct and endothelin procedures had decreased numbers of deaths in comparison to the intraluminal filament method. N = 469 and 351, respectively, in the two analyses/graphs. The bars represent 0.95 confidence intervals.
The emboli (+12.1%, 0.95 CI: +6.9 to +17.3%; p = 0.000) method rendered higher mortality than the intraluminal filament method, while the direct (−10.7%, 0.95 CI: -15.1 to −6.2%; p = 0.000) and endothelin (−9.7%, 0.95 CI: -16.8 to −2.6%; p = 0.000) methods resulted in lower mortality (Figure 3, Table 3).
Impact of type of filament on infarct size coefficient of variation and mortality: Hypotheses 3A-B
In studies in which the intraluminal filament method had been used, silicone coating of the occluding filament substantially and significantly lowered Infarct size coefficient of variation compared to uncoated filaments (−12.7%, 0.95 CI: -18.3 to −7.0%; 0.000). It should also be noted that Poly-L-Lysine had a positive regression coefficient, indicating a slight trend of increased rather than decreased variability in comparison with the reference category (Figure 4, Table 4).
Figure 4.
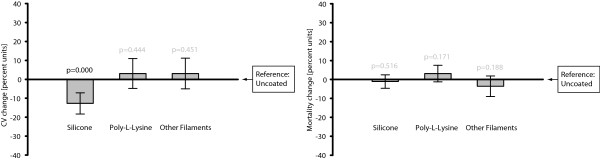
Silicone coated filaments rendered lower infarct size coefficient of variation than the uncoated filaments. Poly-L-Lysine coated and other filaments resulted in infarct size coefficients of variation comparable to the uncoated counterparts. No effect on Mortality rate from the choice of intraluminal filament type was seen. N = 383 and 265, respectively, in the two analyses/graphs. The bars represent 0.95 confidence intervals.
The choice of filament coating had no significant effects on mortality, and regression coefficients were generally small (Figure 4, Table 5).
The multiple regression analyses addressing hypotheses 3A and 3B included 383 and 265 control groups, respectively, all using the intraluminal filament technique (Figure 1). These regression formulae had r2 of 0.40 and 0.27.
Background data
In the 502 control groups finally included, the Infarct size coefficient of variation were on average 28.9 ± 21.3%, with a range from 1.7 to 148%. Mortality rate, the other outcome variable, averaged 15.1 ± 13.5%, ranging from 0 to 60.4%.
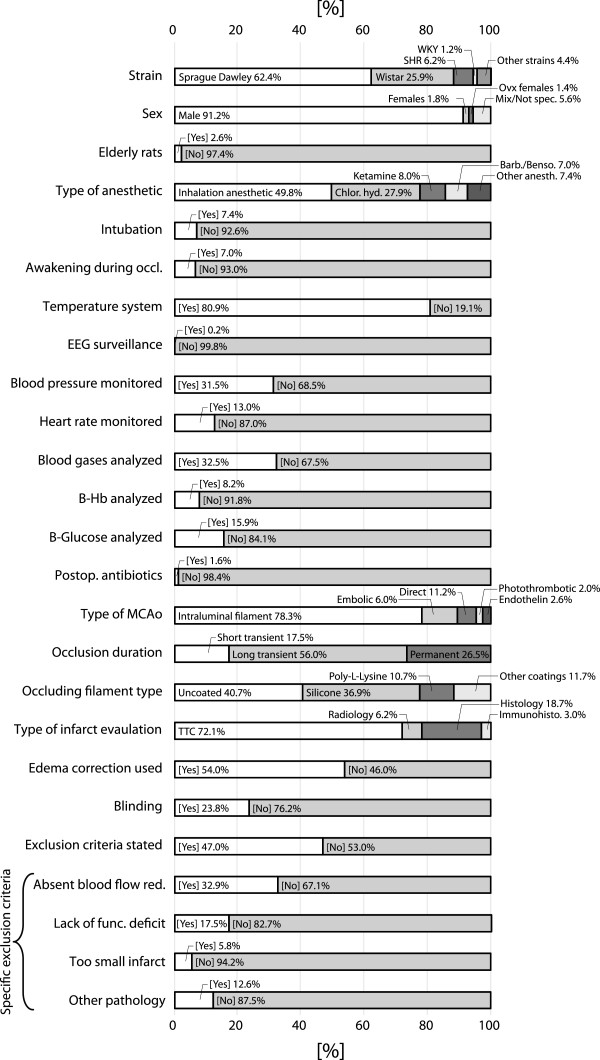
The average number of animals in the 502 control groups, which was used for weighing the studies’ impact in the analyses, was 9.0 ± 7.7, with a range from 3 to 145. Mean rat body weight in the included studies was 294.9 ± 61.0 g, ranging from group means of 190 to 779 g. Cerebral damage was evaluated on average 165.5 ± 506.3 hours after ischemic insult, but this data was heavily skewed, with a median of 24 hours. The exclusion rate (due to other reasons than mortality) averaged 8.9 ± 9.8% in the few studies in which this information was available. Frequencies of the different classifications in the registered categorical variables are presented in Figure 5.
Figure 5.
Frequencies of registered categories in the 502 included control groups. The specific exclusion criteria are presented separately in the last 4 bars. Many of the variable names are abbreviated in the figure; for extended description, see Table 1. “Histology” in the bar “Type of infarct evaluation” refers to acidic/basic stain or silver stain histology. EEG = Electroencephalography, B = Blood, Hb = Hemoglobin.
Discussion
The most important findings in the current hypothesis-driven meta-analysis was that the Wistar strain and intraluminal filament procedure using a silicone coated filament resulted in smallest infarct size variability. The direct and endothelin methods rendered the lowest mortality rates, while the emboli method increased mortality when compared to the intraluminal filament method. A number of interesting observations regarding the control variables were also made, such as the significant impact of the exclusion criterion Observed absence of cerebral blood flow reduction on variability (Table 2) and the effect of awakening the rats during occlusion on mortality (Table 3). However, since these accidental findings were not part of the original hypothesis, we refrain from drawing any conclusions about them, and refer to Tables 2, 3, 4, 5 for the interested reader.
The high infarct size variability in rodent focal ischemic models is a problem that burdens the entire experimental stroke field, and has been commented in several reviews [2,362]. The problem with high outcome variability is that a higher number of animals is needed to get an adequate statistical power, which is problematic from both an ethical and economical point of view. The pressure from ethical boards on the researcher to minimize the number of animals used may be the main reason that the power of stroke experiments is often low. In the current meta-analysis, the average infarct size coefficient of variation was 28.9%, while the average number of animals included in the control groups were 9.0. If we assume that the animals in the included studies often are equally distributed between the treatment groups and control groups, these numbers can provide an estimate of the average statistical power in the studies. Given the abovementioned numbers and an alpha of 0.05, the chance of detecting a 20% difference between the groups would be merely 54.6% (if non-parametric tests are used instead or if more than two groups are included in the comparison, the power would be even lower). Under these circumstances, a negative result is marginally more interesting that tossing a coin. The use of low power designs risks serious publication bias which also makes meta-analyses of experimental stroke studies difficult to interpret properly, since there is probably an unknown number of unpublished studies that cannot be weighed in. In addition to using means to decrease variability, it is of fundamental importance to use a sufficient number of animals/replicates to render an acceptably high power.
Mortality can be another confounding factor in experimental stroke research, at least if it is not reported in the article. With parametric statistical methods, incorporating mortality in for example the infarct size calculations is not uncomplicated from a statistical point of view, which is probably why the mortality is often simply not mentioned. Non-parametric models may offer an alternative approach [363], but irrespective of how the main outcome is statistically assessed, the importance of reporting mortality and other exclusion criteria cannot be over-emphasized. For example, if mortality rate is omitted, a substance that kills all rats with large infarcts may seem to decrease infarct sizes, since only the animals with small infarcts will survive in the treatment group. Unfortunately, mortality rate is usually not reported in experimental stroke studies. In fact, for only 35.3% of the included control groups an account of unintended deaths was provided; and this was the most frequently requested item in our e-mail correspondence.
A few previous studies have assessed the influence of the rat strain on experimental stroke outcomes, however with conflicting results. Spontaneously hypertensive rats (SHR) have, probably because of the implications of hypertension in stroke pathophysiology, attracted some attention. Since this strain often sustains larger infarcts [364], the infarct size coefficient of variation has in a few studies been shown to be relatively low [8,365,366]. Others have argued that the use of another inbred strain – Fischer-344 rats – give the most consistent results [7,367,368], however this type may because of its variable vascular anatomy be unsuitable for the well-used intraluminal filament model [369]. Long Evans rats have also been proponed as a good model animal, because of the relatively consistent decrease in cerebral blood flow after intraluminal filament MCAo [370]. Many other studies have investigated differences between rat strains, however not focusing on variability, but rather on infarct sizes per se [371-373]. Except for the study emphasizing the unsuitability of Fischer-344 for intraluminal filament MCAo [369], we are only aware of one study aiming to compare mortality between strains. In this study, an intraluminal filament model rendered higher mortality in Fischer-344 rats than in Wistar and Sprague–Dawley rats [7]. It is not easy to summarize the conclusions in the existing literature, since the mentioned experiments have been performed under such different circumstances, but it seems that SHR might be attractive because of their low variability. In the current meta-analysis, there was a slight trend towards lower variability in the SHR strain compared to the reference category Sprague–Dawley, which however was far from reaching statistical significance. Regarding Long Evans and Fischer-344, these strains were used too rarely to be analyzed separately. As abovementioned, the strain that we found to render the lowest variability was the relatively well-used Wistar strain.
Very few studies have compared different methods of inducing focal cerebral ischemia. This is perhaps not surprising given that the effort of introducing an entirely new MCAo method in a laboratory is large enough to make many researchers reluctant to switch once a technique has been mastered. This lack of relevant studies underscores the importance of a meta-analysis as the current one. However, in a study by Gerriets et al. [10], an embolization technique was compared to an intraluminal filament procedure. The take-home message from that article was that even though infarct variability tended to be higher from the emboli method, as corroborated by the current study, it did not affect body temperature to the same extent as the filament method did. In another article, the use of microsurgical direct occlusion was advocated over the intraluminal filament method because the latter was thought to not only compromise blood flow to the MCA territory, but rather a larger part of the ICA territory [374].
Different types of filaments for the intraluminal method are much easier to compare, and have been assessed in several studies. Most of these studies have argued that silicone coated rather than uncoated or poly-L-lysine coated filaments should be used, because of a more consistent blood flow reduction [375], lower incidence of sub-arachnoid hemorrhage [14,376], higher success rate [376-378], lower mortality [377] and lower variability [368,376], even if arguments based on low variability also have been used to encourage the use of poly-L-lysine coating [379]. In the current analysis, it was found that silicone coated filaments are superior in terms of reducing infarct size variability, while no effects on mortality were found. Another study compared different brands of blunted nylon filaments, and found Ethilon to be superior to Nitcho [380].
Strengths and weaknesses
The main strength of the current study is that it, based on hundreds of published studies, provides a composite understanding of how different methodological factors interact to affect outcome variability and mortality. However, since this method-investigating meta-analytical approach is relatively novel, we consider it important to highlight and discuss some aspects of the design:
• A multiple regression analysis assumes that the variables are linearly related, which evidently is not always true. For example, the effect of average body weight on variability could theoretically be U-shaped, with higher variability in young, not fully developed, rats and very old animals, than in adult animals. This is an inherent drawback, but multiple regression analysis still seemed the most attractive statistical method for the current purpose.
• There is a problem in investigating coefficients of variation in published studies, and weighing the impact of the included control groups by number of animals, since important sources of bias come into play. Researchers that know that their model render large variability will compensate by including more animals, thus giving more weight in the meta-analysis to studies with larger variability. We however believe that weighing the analysis by number of animals is the fairest alternative. Another problem is publication bias, since the studies rendering the largest coefficients of variation probably to large part remain unpublished, and cannot be assessed.
• Even if this meta-analysis controls for many confounders by its broad approach, there is complexity and heterogeneity in the underlying experiments that is far beyond our reach. For example, the impact of different rat vendors [381], the skill of the surgeon and the suitability of using specific rat strains for certain surgical procedures are not accounted for. For mathematical reasons, categories have also, as stated in the Materials and Methods section, been reduced to larger categories, meaning that differences within categories may be lost. There are for example numerous variations within the different MCAo techniques, and it is likely that the best embolization procedure renders a lower variability than the worst intraluminal filament paradigm, even if the last-mentioned method proved superior on a general level.
• The meta-analysis includes 502 control groups from only 346 published reports, meaning that several studies described more than one control group. We have in the statistical analysis regarded control groups from the same study as independent, which is not statistically stringent. However, if categories had been created for all separate studies, the entire analysis would have been impossible to perform, and thus this imperfection is an inevitable problem with the chosen approach.
Conclusions
The choice of methodological parameters, such as rat strain and infarct surgical procedures, is of utmost importance for consistent and reliable results. As found in the meta-analysis, the effect sizes were large, with many parameters by themselves increasing or decreasing variability and mortality with more than 10% (in absolute terms).
Finally, it deserves to be emphasized that this analysis does not encompass all perspectives on the suitability of focal stroke models. Even if infarct size coefficient of variation and mortality are important components, other aspects, not least similarity of the model to the clinical situation, emphasizing the importance of the embolic model [382], must be taken into consideration when planning experiments.
Abbreviations
MCAo: Middle cerebral artery occlusion; MCA: Middle cerebral artery; SHR: Spontaneously hypertensive rat; WKY: Wistar Kyoto rats; Ovx: Ovariectomized; CV: Coefficient of variation, calculated as [standard deviation/average]; CI: Confidence interval; EEG: Electroencephalography; Hb: Hemoglobin.
Competing interests
The authors declared that they have no competing interests.
Authors’ contributions
JOS contributed to designing the study, extracted data, performed the outcome analyses and drafted the manuscript. EI contributed to designing the study, extracted data and revised the manuscript. AT and ET contributed to designing the study and revised the manuscript. All authors read and approved the final manuscript version before submission.
Contributor Information
Jakob O Ström, Email: jakob.strom@liu.se.
Edvin Ingberg, Email: edvin.ingberg@liu.se.
Annette Theodorsson, Email: annette.theodorsson@liu.se.
Elvar Theodorsson, Email: elvar.theodorsson@liu.se.
Acknowledgements
First and foremost, we would like to express our sincere gratitude towards our fellow researchers who embodied scientific good-will by generously sharing unpublished details concerning their experiments. We also gratefully acknowledge the expert advice of statistician Karl Wahlin PhD. This study was supported by the County Council of Östergötland, Sweden.
References
- O'Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW. 1,026 experimental treatments in acute stroke. Ann Neurol. 2006;59(3):467–477. doi: 10.1002/ana.20741. [DOI] [PubMed] [Google Scholar]
- Macleod MR, Fisher M, O'Collins V, Sena ES, Dirnagl U, Bath PM, Buchan A, van der Worp HB, Traystman RJ, Minematsu K. Reprint: Good laboratory practice: preventing introduction of bias at the bench. J Cereb Blood Flow Metab. 2009;29(2):221–223. doi: 10.1161/STROKEAHA.108.525386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Worp HB, de Haan P, Morrema E, Kalkman CJ. Methodological quality of animal studies on neuroprotection in focal cerebral ischaemia. J Neurol. 2005;252(9):1108–1114. doi: 10.1007/s00415-005-0802-3. [DOI] [PubMed] [Google Scholar]
- Philip M, Benatar M, Fisher M, Savitz SI. Methodological quality of animal studies of neuroprotective agents currently in phase II/III acute ischemic stroke trials. Stroke. 2009;40(2):577–581. doi: 10.1161/STROKEAHA.108.524330. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27(9):1616–1622. doi: 10.1161/01.str.27.9.1616. discussion 1623. [DOI] [PubMed] [Google Scholar]
- Aspey BS, Taylor FL, Terruli M, Harrison MJ. Temporary middle cerebral artery occlusion in the rat: consistent protocol for a model of stroke and reperfusion. Neuropathol Appl Neurobiol. 2000;26(3):232–242. doi: 10.1046/j.1365-2990.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- Barone FC, Price WJ, White RF, Willette RN, Feuerstein GZ. Genetic hypertension and increased susceptibility to cerebral ischemia. Neurosci Biobehav Rev. 1992;16(2):219–233. doi: 10.1016/s0149-7634(05)80182-4. [DOI] [PubMed] [Google Scholar]
- Menzies SA, Hoff JT, Betz AL. Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery. 1992;31(1):100–106. doi: 10.1227/00006123-199207000-00014. discussion 106–107. [DOI] [PubMed] [Google Scholar]
- Gerriets T, Li F, Silva MD, Meng X, Brevard M, Sotak CH, Fisher M. The macrosphere model: evaluation of a new stroke model for permanent middle cerebral artery occlusion in rats. J Neurosci Meth. 2003;122(2):201–211. doi: 10.1016/s0165-0270(02)00322-9. [DOI] [PubMed] [Google Scholar]
- Boyko M, Zlotnik A, Gruenbaum BF, Gruenbaum SE, Ohayon S, Goldsmith T, Kotz R, Leibowitz A, Sheiner E, Shapira Y. An experimental model of focal ischemia using an internal carotid artery approach. J Neurosci Meth. 2010;193(2):246–253. doi: 10.1016/j.jneumeth.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Chen ST, Hsu CY, Hogan EL, Maricq H, Balentine JD. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke. 1986;17(4):738–743. doi: 10.1161/01.str.17.4.738. [DOI] [PubMed] [Google Scholar]
- Dittmar M, Spruss T, Schuierer G, Horn M. External carotid artery territory ischemia impairs outcome in the endovascular filament model of middle cerebral artery occlusion in rats. Stroke. 2003;34(9):2252–2257. doi: 10.1161/01.STR.0000083625.54851.9A. [DOI] [PubMed] [Google Scholar]
- Schmid-Elsaesser R, Zausinger S, Hungerhuber E, Baethmann A, Reulen HJ. A critical reevaluation of the intraluminal thread model of focal cerebral ischemia: evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by laser-Doppler flowmetry. Stroke. 1998;29(10):2162–2170. doi: 10.1161/01.str.29.10.2162. [DOI] [PubMed] [Google Scholar]
- Sheng H, Spasojevic I, Tse HM, Jung JY, Hong J, Zhang Z, Piganelli JD, Batinic-Haberle I, Warner DS. Neuroprotective efficacy from a lipophilic redox-modulating Mn(III) N-Hexylpyridylporphyrin, MnTnHex-2-PyP: rodent models of ischemic stroke and subarachnoid hemorrhage. J Pharmacol Exp Ther. 2011;338(3):906–916. doi: 10.1124/jpet.110.176701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Kong X, Yang Q, Zhang Y, Han J, Li P, Xiong L, Zhao G. Ginsenoside rd in experimental stroke: superior neuroprotective efficacy with a wide therapeutic window. Neurotherapeutics. 2011;8(3):515–525. doi: 10.1007/s13311-011-0051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Li Y, Fang W, Mao L. Therapeutic time window for treatment of focal cerebral ischemia reperfusion injury with XQ-1 h in rats. Eur J Pharmacol. 2011;666(1–3):105–110. doi: 10.1016/j.ejphar.2011.05.048. [DOI] [PubMed] [Google Scholar]
- Rau TF, Kothiwal A, Zhang L, Ulatowski S, Jacobson S, Brooks DM, Cardozo-Pelaez F, Chopp M, Poulsen DJ. Low dose methamphetamine mediates neuroprotection through a PI3K-AKT pathway. Neuropharmacology. 2011;61(4):677–686. doi: 10.1016/j.neuropharm.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Maheshwari A, Badgujar L, Phukan B, Bodhankar SL, Thakurdesai P. Protective effect of Etoricoxib against middle cerebral artery occlusion induced transient focal cerebral ischemia in rats. Eur J Pharmacol. 2011;667(1–3):230–237. doi: 10.1016/j.ejphar.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Ceulemans AG, Zgavc T, Kooijman R, Hachimi-Idrissi S, Sarre S, Michotte Y. Mild hypothermia causes differential, time-dependent changes in cytokine expression and gliosis following endothelin-1-induced transient focal cerebral ischemia. J Neuroinflammation. 2011;8:60. doi: 10.1186/1742-2094-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Chen L, Wei X, Xiang Y, Liu X, Zhang X. The Akt/GSK-3beta pathway mediates flurbiprofen-induced neuroprotection against focal cerebral ischemia/reperfusion injury in rats. Biochem Biophys Res Commun. 2011;409(4):808–813. doi: 10.1016/j.bbrc.2011.05.095. [DOI] [PubMed] [Google Scholar]
- Suda S, Shimazaki K, Ueda M, Inaba T, Kamiya N, Katsura K, Katayama Y. Combination therapy with bone marrow stromal cells and FK506 enhanced amelioration of ischemic brain damage in rats. Life Sci. 2011;89(1–2):50–56. doi: 10.1016/j.lfs.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Yang J, Song TB, Zhao ZH, Qiu SD, Hu XD, Chang L. Vasoactive intestinal peptide protects against ischemic brain damage induced by focal cerebral ischemia in rats. Brain Res. 2011;1398:94–101. doi: 10.1016/j.brainres.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, McMillan EL. Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain. Brain. 2011;134(Pt 6):1777–1789. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Yin H, Chen L, Liu H, Zhao M, Zhang X. Neuroprotective effect of ginkgolide K against acute ischemic stroke on middle cerebral ischemia occlusion in rats. J Nat Med. 2012;66(1):25–31. doi: 10.1007/s11418-011-0545-7. [DOI] [PubMed] [Google Scholar]
- Lammer AB, Beck A, Grummich B, Forschler A, Krugel T, Kahn T, Schneider D, Illes P, Franke H, Krugel U. The P2 receptor antagonist PPADS supports recovery from experimental stroke in vivo. PLoS One. 2011;6(5):e19983. doi: 10.1371/journal.pone.0019983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J, Tuor UI, Zhao Z, Barber PA. Quantitative MRI reveals the elderly ischemic brain is susceptible to increased early blood–brain barrier permeability following tissue plasminogen activator related to claudin 5 and occludin disassembly. J Cereb Blood Flow Metab. 2011;31(9):1874–1885. doi: 10.1038/jcbfm.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DD, Parsons MW, Levi CR, Beautement S, Buxton D, Roworth B, Spratt NJ. Establishing a rodent stroke perfusion computed tomography model. Int J Stroke. 2011;6(4):284–289. doi: 10.1111/j.1747-4949.2010.00564.x. [DOI] [PubMed] [Google Scholar]
- Chang HC, Yang YR, Wang PS, Kuo CH, Wang RY. Insulin-like growth factor I signaling for brain recovery and exercise ability in brain ischemic rats. Med Sci Sports Exerc. 2011;43(12):2274–2280. doi: 10.1249/MSS.0b013e318223b5d9. [DOI] [PubMed] [Google Scholar]
- Nair SM, Rahman RM, Clarkson AN, Sutherland BA, Taurin S, Sammut IA, Appleton I. Melatonin treatment following stroke induction modulates L-arginine metabolism. J Pineal Res. 2011;51(3):313–323. doi: 10.1111/j.1600-079X.2011.00891.x. [DOI] [PubMed] [Google Scholar]
- Koh PO. Nicotinamide attenuates the ischemic brain injury-induced decrease of Akt activation and Bad phosphorylation. Neurosci Lett. 2011;498(2):105–109. doi: 10.1016/j.neulet.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Boyko M, Ohayon S, Goldsmith T, Douvdevani A, Gruenbaum BF, Melamed I, Knyazer B, Shapira Y, Teichberg VI, Elir A. Cell-free DNA–a marker to predict ischemic brain damage in a rat stroke experimental model. J Neurosurg Anesthesiol. 2011;23(3):222–228. doi: 10.1097/ANA.0b013e31821b536a. [DOI] [PubMed] [Google Scholar]
- Wu WN, Wu PF, Chen XL, Zhang Z, Gu J, Yang YJ, Xiong QJ, Ni L, Wang F, Chen JG. Sinomenine protects against ischaemic brain injury: involvement of co-inhibition of acid-sensing ion channel 1a and L-type calcium channels. Br J Pharmacol. 2011;164(5):1445–1459. doi: 10.1111/j.1476-5381.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Kim YJ, Kim YH, Roh J, Kim SU, Yoon BW. Effects of duplicate administration of human neural stem cell after focal cerebral ischemia in the rat. Int J Neurosci. 2011;121(8):457–461. doi: 10.3109/00207454.2011.576792. [DOI] [PubMed] [Google Scholar]
- Lin YC, Ko TL, Shih YH, Lin MY, Fu TW, Hsiao HS, Hsu JY, Fu YS. Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke. 2011;42(7):2045–2053. doi: 10.1161/STROKEAHA.110.603621. [DOI] [PubMed] [Google Scholar]
- Yamato M, Shiba T, Ide T, Honda Y, Yamada K, Tsutsui H. Nifedipine treatment reduces brain damage after transient focal ischemia, possibly through its antioxidative effects. Hypertens Res. 2011;34(7):840–845. doi: 10.1038/hr.2011.51. [DOI] [PubMed] [Google Scholar]
- Robertson CA, McCabe C, Gallagher L, Lopez-Gonzalez Mdel R, Holmes WM, Condon B, Muir KW, Santosh C, Macrae IM. Stroke penumbra defined by an MRI-based oxygen challenge technique: 1. Validation using [14C]2-deoxyglucose autoradiography. J Cereb Blood Flow Metab. 2011;31(8):1778–1787. doi: 10.1038/jcbfm.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsanen A, Hiltunen M, Jolkkonen J. Chronic ibuprofen treatment does not affect the secondary pathology in the thalamus or improve behavioral outcome in middle cerebral artery occlusion rats. Pharmacol Biochem Behav. 2011;99(3):468–474. doi: 10.1016/j.pbb.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Weng H, Shen C, Hirokawa G, Ji X, Takahashi R, Shimada K, Kishimoto C, Iwai N. Plasma miR-124 as a biomarker for cerebral infarction. Biomed Res. 2011;32(2):135–141. doi: 10.2220/biomedres.32.135. [DOI] [PubMed] [Google Scholar]
- Pires PW, Rogers CT, McClain JL, Garver HS, Fink GD, Dorrance AM. Doxycycline, a matrix metalloprotease inhibitor, reduces vascular remodeling and damage after cerebral ischemia in stroke-prone spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;301(1):H87–H97. doi: 10.1152/ajpheart.01206.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Cheng Y, Chen J. Transfection of Noggin in bone marrow stromal cells (BMSCs) enhances BMSC-induced functional outcome after stroke in rats. J Neurosci Res. 2011;89(8):1194–1202. doi: 10.1002/jnr.22662. [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhang H, Acharya AB, Cruz-Flores S, Panneton WM. The effect of heliox treatment in a rat model of focal transient cerebral ischemia. Neurosci Lett. 2011;497(2):144–147. doi: 10.1016/j.neulet.2011.04.048. [DOI] [PubMed] [Google Scholar]
- Zhang X, Deguchi S, Deguchi K, Ohta Y, Yamashita T, Shang J, Tian F, Liu N, Liu W, Ikeda Y. Amlodipine and atorvastatin exert protective and additive effects via antiapoptotic and antiautophagic mechanisms after transient middle cerebral artery occlusion in Zucker metabolic syndrome rats. J Neurosci Res. 2011;89(8):1228–1234. doi: 10.1002/jnr.22633. [DOI] [PubMed] [Google Scholar]
- Shimada K, Kitazato KT, Kinouchi T, Yagi K, Tada Y, Satomi J, Kageji T, Nagahiro S. Activation of estrogen receptor-alpha and of angiotensin-converting enzyme 2 suppresses ischemic brain damage in oophorectomized rats. Hypertension. 2011;57(6):1161–1166. doi: 10.1161/HYPERTENSIONAHA.110.167650. [DOI] [PubMed] [Google Scholar]
- Wang RY, Chang HC, Chen CH, Tsai YW, Yang YR. Effects of hyperbaric oxygenation on oxidative stress in acute transient focal cerebral ischemic rats. Eur J Appl Physiol. 2012;112(1):215–221. doi: 10.1007/s00421-011-1976-2. [DOI] [PubMed] [Google Scholar]
- Li M, Zhang X, Cui L, Yang R, Wang L, Liu L, Du W. The neuroprotection of oxymatrine in cerebral ischemia/reperfusion is related to nuclear factor erythroid 2-related factor 2 (nrf2)-mediated antioxidant response: role of nrf2 and hemeoxygenase-1 expression. Biol Pharm Bull. 2011;34(5):595–601. doi: 10.1248/bpb.34.595. [DOI] [PubMed] [Google Scholar]
- Han Q, Li B, Feng H, Xiao Z, Chen B, Zhao Y, Huang J, Dai J. The promotion of cerebral ischemia recovery in rats by laminin-binding BDNF. Biomaterials. 2011;32(22):5077–5085. doi: 10.1016/j.biomaterials.2011.03.072. [DOI] [PubMed] [Google Scholar]
- Yang ZZ, Li J, Li SX, Feng W, Wang H. Effect of ginkgolide B on striatal extracellular amino acids in middle cerebral artery occluded rats. J Ethnopharmacol. 2011;136(1):117–122. doi: 10.1016/j.jep.2011.04.027. [DOI] [PubMed] [Google Scholar]
- Lei C, Deng J, Wang B, Cheng D, Yang Q, Dong H, Xiong L. Reactive oxygen species scavenger inhibits STAT3 activation after transient focal cerebral ischemia-reperfusion injury in rats. Anesth Analg. 2011;113(1):153–159. doi: 10.1213/ANE.0b013e31821a9fbe. [DOI] [PubMed] [Google Scholar]
- van Meer MP, van der Marel K, van der Sprenkel JW, Dijkhuizen RM. MRI of bilateral sensorimotor network activation in response to direct intracortical stimulation in rats after unilateral stroke. J Cereb Blood Flow Metab. 2011;31(7):1583–1587. doi: 10.1038/jcbfm.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Zhao L, Jacewicz M, Pulsinelli WA, Nowak TS Jr. Metabolic and perfusion responses to recurrent peri-infarct depolarization during focal ischemia in the Spontaneously Hypertensive Rat: dominant contribution of sporadic CBF decrements to infarct expansion. J Cereb Blood Flow Metab. 2011;31(9):1863–1873. doi: 10.1038/jcbfm.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejda A, Seaborn T, Bourgault S, Touzani O, Fournier A, Vaudry H, Vaudry D. PACAP and a novel stable analog protect rat brain from ischemia: Insight into the mechanisms of action. Peptides. 2011;32(6):1207–1216. doi: 10.1016/j.peptides.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Mariucci G, Taha E, Tantucci M, Spaccatini C, Tozzi A, Ambrosini MV. Intravenous administration of pravastatin immediately after middle cerebral artery occlusion reduces cerebral oedema in spontaneously hypertensive rats. Eur J Pharmacol. 2011;660(2–3):381–386. doi: 10.1016/j.ejphar.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li Y, Zhang C, Chopp M, Gosiewska A, Hong K. Delayed administration of human umbilical tissue-derived cells improved neurological functional recovery in a rodent model of focal ischemia. Stroke. 2011;42(5):1437–1444. doi: 10.1161/STROKEAHA.110.593129. [DOI] [PubMed] [Google Scholar]
- Choi Y, Kim SK, Choi IY, Ju C, Nam KW, Hwang S, Kim BW, Yoon MJ, Won MH, Park YK. Amelioration of cerebral infarction and improvement of neurological deficit by a Korean herbal medicine, modified Bo-Yang-Hwan-O-Tang. J Pharm Pharmacol. 2011;63(5):695–706. doi: 10.1111/j.2042-7158.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- Kuboyama K, Harada H, Tozaki-Saitoh H, Tsuda M, Ushijima K, Inoue K. Astrocytic P2Y(1) receptor is involved in the regulation of cytokine/chemokine transcription and cerebral damage in a rat model of cerebral ischemia. J Cereb Blood Flow Metab. 2011;31(9):1930–1941. doi: 10.1038/jcbfm.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titova E, Ostrowski RP, Adami A, Badaut J, Lalas S, Ghosh N, Vlkolinsky R, Zhang JH, Obenaus A. Brain irradiation improves focal cerebral ischemia recovery in aged rats. J Neurol Sci. 2011;306(1–2):143–153. doi: 10.1016/j.jns.2011.02.034. [DOI] [PubMed] [Google Scholar]
- Yan LG, Lu Y, Zheng SZ, Wang AY, Li MQ, Ruan JS, Zhang L. Injectable caltrop fruit saponin protects against ischemia-reperfusion injury in rat brain. Am J Chin Med. 2011;39(2):325–333. doi: 10.1142/S0192415X11008853. [DOI] [PubMed] [Google Scholar]
- Hu Q, Ma Q, Zhan Y, He Z, Tang J, Zhou C, Zhang J. Isoflurane enhanced hemorrhagic transformation by impairing antioxidant enzymes in hyperglycemic rats with middle cerebral artery occlusion. Stroke. 2011;42(6):1750–1756. doi: 10.1161/STROKEAHA.110.603142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A, Khan MM, Hoda MN, Raza SS, Khan MB, Javed H, Ishrat T, Ashafaq M, Ahmad ME, Safhi MM. Quercetin protects against oxidative stress associated damages in a rat model of transient focal cerebral ischemia and reperfusion. Neurochem Res. 2011;36(8):1360–1371. doi: 10.1007/s11064-011-0458-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhao Y, Liu C, Jiang C, Zhao C, Zhu Z. Progesterone inhibits inflammatory response pathways after permanent middle cerebral artery occlusion in rats. Mol Med Report. 2011;4(2):319–324. doi: 10.3892/mmr.2011.418. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Helps SC, Thornton E, Vink R. A substance P antagonist improves outcome when administered 4 h after onset of ischaemic stroke. Brain Res. 2011;1393:84–90. doi: 10.1016/j.brainres.2011.03.066. [DOI] [PubMed] [Google Scholar]
- Jiang M, Lv L, Ji H, Yang X, Zhu W, Cai L, Gu X, Chai C, Huang S, Sun J. Induction of pluripotent stem cells transplantation therapy for ischemic stroke. Mol Cell Biochem. 2011;354(1–2):67–75. doi: 10.1007/s11010-011-0806-5. [DOI] [PubMed] [Google Scholar]
- Ay I, Sorensen AG, Ay H. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia: an unlikely role for cerebral blood flow. Brain Res. 2011;1392:110–115. doi: 10.1016/j.brainres.2011.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata K, Kuge Y, Yokota C, Harada A, Kokame K, Inoue H, Kawashima H, Hanzawa H, Shono Y, Saji H. Gene and protein analysis of brain derived neurotrophic factor expression in relation to neurological recovery induced by an enriched environment in a rat stroke model. Neurosci Lett. 2011;495(3):210–215. doi: 10.1016/j.neulet.2011.03.068. [DOI] [PubMed] [Google Scholar]
- Allahtavakoli M, Jarrott B. Sigma-1 receptor ligand PRE-084 reduced infarct volume, neurological deficits, pro-inflammatory cytokines and enhanced anti-inflammatory cytokines after embolic stroke in rats. Brain Res Bull. 2011;85(3–4):219–224. doi: 10.1016/j.brainresbull.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Rahimian R, Daneshmand A, Mehr SE, Barzegar-Fallah A, Mohammadi-Rick S, Fakhfouri G, Shabanzadeh AP, Dehpour AR. Tropisetron ameliorates ischemic brain injury in an embolic model of stroke. Brain Res. 2011;1392:101–109. doi: 10.1016/j.brainres.2011.03.053. [DOI] [PubMed] [Google Scholar]
- Blankenship D, Niemi J, Hilow E, Karl M, Sundararajan S. Oral pioglitazone reduces infarction volume and improves neurologic function following MCAO in rats. Adv Exp Med Biol. 2011;701:157–162. doi: 10.1007/978-1-4419-7756-4_22. [DOI] [PubMed] [Google Scholar]
- Chauveau F, Cho TH, Perez M, Guichardant M, Riou A, Aguettaz P, Picq M, Lagarde M, Berthezene Y, Nighoghossian N. Brain-targeting form of docosahexaenoic acid for experimental stroke treatment: MRI evaluation and anti-oxidant impact. Curr Neurovasc Res. 2011;8(2):95–102. doi: 10.2174/156720211795495349. [DOI] [PubMed] [Google Scholar]
- Ma L, Zhu Z, Zhao Y, Hou L, Wang Q, Xiong L, Zhu X, Jia J, Chen S. Cannabinoid receptor type 2 activation yields delayed tolerance to focal cerebral ischemia. Curr Neurovasc Res. 2011;8(2):145–152. doi: 10.2174/156720211795495394. [DOI] [PubMed] [Google Scholar]
- Langdon KD, Clarke J, Corbett D. Long-term exposure to high fat diet is bad for your brain: exacerbation of focal ischemic brain injury. Neuroscience. 2011;182:82–87. doi: 10.1016/j.neuroscience.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Belayev L, Khoutorova L, Atkins KD, Eady TN, Hong S, Lu Y, Obenaus A, Bazan NG. Docosahexaenoic Acid Therapy of Experimental Ischemic Stroke. Transl Stroke Res. 2011;2(1):33–41. doi: 10.1007/s12975-010-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egi Y, Matsuura S, Maruyama T, Fujio M, Yuki S, Akira T. Neuroprotective effects of a novel water-soluble poly(ADP-ribose) polymerase-1 inhibitor, MP-124, in in vitro and in vivo models of cerebral ischemia. Brain Res. 2011;1389:169–176. doi: 10.1016/j.brainres.2011.03.031. [DOI] [PubMed] [Google Scholar]
- Svalbe B, Zvejniece L, Vavers E, Pugovics O, Muceniece R, Liepinsh E, Dambrova M. Mildronate treatment improves functional recovery following middle cerebral artery occlusion in rats. Behav Brain Res. 2011;222(1):26–32. doi: 10.1016/j.bbr.2011.03.027. [DOI] [PubMed] [Google Scholar]
- Castello-Ruiz M, Torregrosa G, Burguete MC, Salom JB, Gil JV, Miranda FJ, Jover-Mengual T, Marrachelli VG, Alborch E. Soy-derived phytoestrogens as preventive and acute neuroprotectors in experimental ischemic stroke: influence of rat strain. Phytomedicine. 2011;18(6):513–515. doi: 10.1016/j.phymed.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Boyko M, Ohayon S, Goldsmith T, Novack L, Novack V, Perry ZH, Gruenbaum BF, Gruenbaum SE, Steiner O, Shapira Y. Morphological and neuro-behavioral parallels in the rat model of stroke. Behav Brain Res. 2011;223(1):17–23. doi: 10.1016/j.bbr.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Mochizuki N, Moriyama Y, Takagi N, Takeo S, Tanonaka K. Intravenous injection of neural progenitor cells improves cerebral ischemia-induced learning dysfunction. Biol Pharm Bull. 2011;34(2):260–265. doi: 10.1248/bpb.34.260. [DOI] [PubMed] [Google Scholar]
- Yang B, Strong R, Sharma S, Brenneman M, Mallikarjunarao K, Xi X, Grotta JC, Aronowski J, Savitz SI. Therapeutic time window and dose response of autologous bone marrow mononuclear cells for ischemic stroke. J Neurosci Res. 2011;89(6):833–839. doi: 10.1002/jnr.22614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XM, Qian ZM, Zhu L, Du F, Yung WH, Gong Q, Ke Y. Neuroprotective effect of ligustilide against ischaemia-reperfusion injury via up-regulation of erythropoietin and down-regulation of RTP801. Br J Pharmacol. 2011;164(2):332–343. doi: 10.1111/j.1476-5381.2011.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Zhao Y, Gu Y, Xu C. Anti-inflammatory mechanism of taurine against ischemic stroke is related to down-regulation of PARP and NF-kappaB. Amino Acids. 2012;42(5):1735–1747. doi: 10.1007/s00726-011-0885-3. [DOI] [PubMed] [Google Scholar]
- Yao J, Xu Y, Ji F, Wang C, Zhang Y, Ni J, Wang R. Protective effects of MLIF analogs on cerebral ischemia-reperfusion injury in rats. Peptides. 2011;32(5):1047–1054. doi: 10.1016/j.peptides.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Zhao R, Shi WZ, Zhang YM, Fang SH, Wei EQ. Montelukast, a cysteinyl leukotriene receptor-1 antagonist, attenuates chronic brain injury after focal cerebral ischaemia in mice and rats. J Pharm Pharmacol. 2011;63(4):550–557. doi: 10.1111/j.2042-7158.2010.01238.x. [DOI] [PubMed] [Google Scholar]
- Mishra V, Verma R, Singh N, Raghubir R. The neuroprotective effects of NMDAR antagonist, ifenprodil and ASIC1a inhibitor, flurbiprofen on post-ischemic cerebral injury. Brain Res. 2011;1389:152–160. doi: 10.1016/j.brainres.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Yang Q, Dong H, Deng J, Wang Q, Ye R, Li X, Hu S, Xiong L. Sevoflurane preconditioning induces neuroprotection through reactive oxygen species-mediated up-regulation of antioxidant enzymes in rats. Anesth Analg. 2011;112(4):931–937. doi: 10.1213/ANE.0b013e31820bcfa4. [DOI] [PubMed] [Google Scholar]
- Liang H, Liu P, Wang Y, Song S, Ji A. Protective effects of alkaloid extract from Leonurus heterophyllus on cerebral ischemia reperfusion injury by middle cerebral ischemic injury (MCAO) in rats. Phytomedicine. 2011;18(10):811–818. doi: 10.1016/j.phymed.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Zuhayra M, Zhao Y, von Forstner C, Henze E, Gohlke P, Culman J, Lutzen U. Activation of cerebral peroxisome proliferator-activated receptors gamma (PPARgamma) reduces neuronal damage in the substantia nigra after transient focal cerebral ischaemia in the rat. Neuropathol Appl Neurobiol. 2011;37(7):738–752. doi: 10.1111/j.1365-2990.2011.01169.x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Mayhan WG, Arrick DM, Xiong W, Sun H. Dose-related influence of chronic alcohol consumption on cerebral ischemia/reperfusion injury. Alcohol Clin Exp Res. 2011;35(7):1265–1269. doi: 10.1111/j.1530-0277.2011.01461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HS, Shen H, Harvey BK, Castillo P, Lu H, Yang Y, Wang Y. Post-treatment with amphetamine enhances reinnervation of the ipsilateral side cortex in stroke rats. Neuroimage. 2011;56(1):280–289. doi: 10.1016/j.neuroimage.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Cheng S, Xu G, Ma M, Zhou Z, Liu D, Liu X. Intranasal nerve growth factor enhances striatal neurogenesis in adult rats with focal cerebral ischemia. Drug Deliv. 2011;18(5):338–343. doi: 10.3109/10717544.2011.557785. [DOI] [PubMed] [Google Scholar]
- Kam KY, Yu SJ, Jeong N, Hong JH, Jalin AM, Lee S, Choi YW, Lee CK, Kang SG. p-Hydroxybenzyl alcohol prevents brain injury and behavioral impairment by activating Nrf2, PDI, and neurotrophic factor genes in a rat model of brain ischemia. Mol Cells. 2011;31(3):209–215. doi: 10.1007/s10059-011-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H, Xia Z, Chen H, Hou C, Li W. Simple diffusion delivery via brain interstitial route for the treatment of cerebral ischemia. Sci China Life Sci. 2011;54(3):235–239. doi: 10.1007/s11427-011-4141-6. [DOI] [PubMed] [Google Scholar]
- Ryang YM, Fahlenkamp AV, Rossaint R, Wesp D, Loetscher PD, Beyer C, Coburn M. Neuroprotective effects of argon in an in vivo model of transient middle cerebral artery occlusion in rats. Crit Care Med. 2011;39(6):1448–1453. doi: 10.1097/CCM.0b013e31821209be. [DOI] [PubMed] [Google Scholar]
- Cao XL, Hu XM, Hu JQ, Zheng WX. Myocardin-related transcription factor-A promoting neuronal survival against apoptosis induced by hypoxia/ischemia. Brain Res. 2011;1385:263–274. doi: 10.1016/j.brainres.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Yang L, Zhang B, Yin L, Cai B, Shan H, Zhang L, Lu Y, Bi Z. Tanshinone IIA prevented brain iron dyshomeostasis in cerebral ischemic rats. Cell Physiol Biochem. 2011;27(1):23–30. doi: 10.1159/000325202. [DOI] [PubMed] [Google Scholar]
- Jiao H, Wang Z, Liu Y, Wang P, Xue Y. Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood–brain barrier in a focal cerebral ischemic insult. J Mol Neurosci. 2011;44(2):130–139. doi: 10.1007/s12031-011-9496-4. [DOI] [PubMed] [Google Scholar]
- Mao X, Yin W, Liu M, Ye M, Liu P, Liu J, Lian Q, Xu S, Pi R. Osthole, a natural coumarin, improves neurobehavioral functions and reduces infarct volume and matrix metalloproteinase-9 activity after transient focal cerebral ischemia in rats. Brain Res. 2011;1385:275–280. doi: 10.1016/j.brainres.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Hsu WH, Yen TL, Chang NC, Luo YJ, Hsiao G, Sheu JR. Traditional Chinese medicine, Xue-Fu-Zhu-Yu decoction, potentiates tissue plasminogen activator against thromboembolic stroke in rats. J Ethnopharmacol. 2011;134(3):824–830. doi: 10.1016/j.jep.2011.01.033. [DOI] [PubMed] [Google Scholar]
- Kanazawa M, Igarashi H, Kawamura K, Takahashi T, Kakita A, Takahashi H, Nakada T, Nishizawa M, Shimohata T. Inhibition of VEGF signaling pathway attenuates hemorrhage after tPA treatment. J Cereb Blood Flow Metab. 2011;31(6):1461–1474. doi: 10.1038/jcbfm.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi V, Pignataro G, Del Prete A, Sirabella R, Matrone C, Boscia F, Scorziello A, Sisalli MJ, Esposito E, Zambrano N. NCX1 is a novel target gene for hypoxia-inducible factor-1 in ischemic brain preconditioning. Stroke. 2011;42(3):754–763. doi: 10.1161/STROKEAHA.110.597583. [DOI] [PubMed] [Google Scholar]
- Chen TY, Tai SH, Lee EJ, Huang CC, Lee AC, Huang SY, Wu TS. Cinnamophilin offers prolonged neuroprotection against gray and white matter damage and improves functional and electrophysiological outcomes after transient focal cerebral ischemia. Crit Care Med. 2011;39(5):1130–1137. doi: 10.1097/CCM.0b013e31820a9442. [DOI] [PubMed] [Google Scholar]
- Chen J, Chen X, Qin J. Effects of polysaccharides of the Euphoria Longan (Lour.) Steud on focal cerebral ischemia/reperfusion injury and its underlying mechanism. Brain Inj. 2011;25(3):292–299. doi: 10.3109/02699052.2010.546824. [DOI] [PubMed] [Google Scholar]
- Wei Y, Yemisci M, Kim HH, Yung LM, Shin HK, Hwang SK, Guo S, Qin T, Alsharif N, Brinkmann V. Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann Neurol. 2011;69(1):119–129. doi: 10.1002/ana.22186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Park J, Yoon OJ, Kim HW, Lee do Y, Kim do H, Lee WB, Lee NE, Bonventre JV, Kim SS. Amine-modified single-walled carbon nanotubes protect neurons from injury in a rat stroke model. Nat Nanotechnol. 2011;6(2):121–125. doi: 10.1038/nnano.2010.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscher K, Shamloo M, Rickhag M, Ladunga I, Soriano L, Gisselsson L, Toresson H, Ruslim-Litrus L, Oksenberg D, Urfer R. The sigma-1 receptor enhances brain plasticity and functional recovery after experimental stroke. Brain. 2011;134(Pt 3):732–746. doi: 10.1093/brain/awq367. [DOI] [PubMed] [Google Scholar]
- Dang J, Mitkari B, Kipp M, Beyer C. Gonadal steroids prevent cell damage and stimulate behavioral recovery after transient middle cerebral artery occlusion in male and female rats. Brain Behav Immun. 2011;25(4):715–726. doi: 10.1016/j.bbi.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Park SI, Jang DK, Han YM, Sunwoo YY, Park MS, Chung YA, Maeng LS, Im R, Kim MW, Jeun SS. Effect of combination therapy with sodium ozagrel and panax ginseng on transient cerebral ischemia model in rats. J Biomed Biotechnol. 2010;2010:893401. doi: 10.1155/2010/893401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marushima A, Suzuki K, Nagasaki Y, Yoshitomi T, Toh K, Tsurushima H, Hirayama A, Matsumura A. Newly synthesized radical-containing nanoparticles enhance neuroprotection after cerebral ischemia-reperfusion injury. Neurosurgery. 2011;68(5):1418–1425. doi: 10.1227/NEU.0b013e31820c02d9. discussion 1425–1416. [DOI] [PubMed] [Google Scholar]
- Murotomi K, Takagi N, Takeo S, Tanonaka K. NADPH oxidase-mediated oxidative damage to proteins in the postsynaptic density after transient cerebral ischemia and reperfusion. Mol Cell Neurosci. 2011;46(3):681–688. doi: 10.1016/j.mcn.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Sheng R, Zhang LS, Han R, Gao B, Liu XQ, Qin ZH. Combined prostaglandin E1 and lithium exert potent neuroprotection in a rat model of cerebral ischemia. Acta Pharmacol Sin. 2011;32(3):303–310. doi: 10.1038/aps.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encarnacion A, Horie N, Keren-Gill H, Bliss TM, Steinberg GK, Shamloo M. Long-term behavioral assessment of function in an experimental model for ischemic stroke. J Neurosci Meth. 2011;196(2):247–257. doi: 10.1016/j.jneumeth.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan LB, Dong HL, Zhang HP, Zhao RN, Gong G, Chen XM, Zhang LN, Xiong L. Neuroprotective effect of orexin-A is mediated by an increase of hypoxia-inducible factor-1 activity in rat. Anesthesiology. 2011;114(2):340–354. doi: 10.1097/ALN.0b013e318206ff6f. [DOI] [PubMed] [Google Scholar]
- Yifeng M, Bin W, Weiqiao Z, Yongming Q, Bing L, Xiaojie L. Neuroprotective effect of sophocarpine against transient focal cerebral ischemia via down-regulation of the acid-sensing ion channel 1 in rats. Brain Res. 2011;1382:245–251. doi: 10.1016/j.brainres.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Sugiyama M, Iohara K, Wakita H, Hattori H, Ueda M, Matsushita K, Nakashima M. Dental pulp-derived CD31(−)/CD146(−) side population stem/progenitor cells enhance recovery of focal cerebral ischemia in rats. Tissue Eng Part A. 2011;17(9–10):1303–1311. doi: 10.1089/ten.TEA.2010.0306. [DOI] [PubMed] [Google Scholar]
- Ye R, Zhang X, Kong X, Han J, Yang Q, Zhang Y, Chen Y, Li P, Liu J, Shi M. Ginsenoside Rd attenuates mitochondrial dysfunction and sequential apoptosis after transient focal ischemia. Neuroscience. 2011;178:169–180. doi: 10.1016/j.neuroscience.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Jin D, Yang Z, Shen B, Liu M. Effects of 17beta-estradiol pre-treated adult neural stem cells on neuronal differentiation and neurological recovery in rats with cerebral ischemia. Brain Inj. 2011;25(2):227–236. doi: 10.3109/02699052.2010.542430. [DOI] [PubMed] [Google Scholar]
- Lee JS, Choi HS, Kang SW, Chung JH, Park HK, Ban JY, Kwon OY, Hong HP, Ko YG. Therapeutic effect of Korean red ginseng on inflammatory cytokines in rats with focal cerebral ischemia/reperfusion injury. Am J Chin Med. 2011;39(1):83–94. doi: 10.1142/S0192415X1100866X. [DOI] [PubMed] [Google Scholar]
- Jung HW, Mahesh R, Bae HS, Kim YH, Kang JS, Park YK. The antioxidant effects of Joongpoongtang 05 on brain injury after transient focal cerebral ischemia in rats. J Nat Med. 2011;65(2):322–329. doi: 10.1007/s11418-010-0497-3. [DOI] [PubMed] [Google Scholar]
- Tai SH, Hung YC, Lee EJ, Lee AC, Chen TY, Shen CC, Chen HY, Lee MY, Huang SY, Wu TS. Melatonin protects against transient focal cerebral ischemia in both reproductively active and estrogen-deficient female rats: the impact of circulating estrogen on its hormetic dose–response. J Pineal Res. 2011;50(3):292–303. doi: 10.1111/j.1600-079X.2010.00839.x. [DOI] [PubMed] [Google Scholar]
- Yang YC, Liu BS, Shen CC, Lin CH, Chiao MT, Cheng HC. Transplantation of adipose tissue-derived stem cells for treatment of focal cerebral ischemia. Curr Neurovasc Res. 2011;8(1):1–13. doi: 10.2174/156720211794520215. [DOI] [PubMed] [Google Scholar]
- Danielisova V, Burda J, Nemethova M, Gottlieb M. Aminoguanidine administration ameliorates hippocampal damage after middle cerebral artery occlusion in rat. Neurochem Res. 2011;36(3):476–486. doi: 10.1007/s11064-010-0366-1. [DOI] [PubMed] [Google Scholar]
- Wattanathorn J, Jittiwat J, Tongun T, Muchimapura S, Ingkaninan K. Zingiber officinale Mitigates Brain Damage and Improves Memory Impairment in Focal Cerebral Ischemic Rat. Evid Based Complement Alternat Med. 2011;2011:429505. doi: 10.1155/2011/429505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lu S, Yu Q, Liang W, Gao H, Li P, Gan Y, Chen J, Gao Y. Sevoflurane preconditioning confers neuroprotection via anti-inflammatory effects. Front Biosci (Elite Ed) 2011;3:604–615. doi: 10.2741/e273. [DOI] [PubMed] [Google Scholar]
- Yuan HJ, Zhu XH, Luo Q, Wu YN, Kang Y, Jiao JJ, Gao WZ, Liu YX, Lou JS. Noninvasive delayed limb ischemic preconditioning in rats increases antioxidant activities in cerebral tissue during severe ischemia-reperfusion injury. J Surg Res. 2012;174(1):176–183. doi: 10.1016/j.jss.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Zhao G, Zang SY, Jiang ZH, Chen YY, Ji XH, Lu BF, Wu JH, Qin GW, Guo LH. Postischemic administration of liposome-encapsulated luteolin prevents against ischemia-reperfusion injury in a rat middle cerebral artery occlusion model. J Nutr Biochem. 2011;22(10):929–936. doi: 10.1016/j.jnutbio.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Zhang HL, Xu M, Wei C, Qin AP, Liu CF, Hong LZ, Zhao XY, Liu J, Qin ZH. Neuroprotective effects of pioglitazone in a rat model of permanent focal cerebral ischemia are associated with peroxisome proliferator-activated receptor gamma-mediated suppression of nuclear factor-kappaB signaling pathway. Neuroscience. 2011;176:381–395. doi: 10.1016/j.neuroscience.2010.12.029. [DOI] [PubMed] [Google Scholar]
- Ye R, Yang Q, Kong X, Han J, Zhang X, Zhang Y, Li P, Liu J, Shi M, Xiong L. Ginsenoside Rd attenuates early oxidative damage and sequential inflammatory response after transient focal ischemia in rats. Neurochem Int. 2011;58(3):391–398. doi: 10.1016/j.neuint.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Richard MJ, Connell BJ, Khan BV, Saleh TM. Cellular mechanisms by which lipoic acid confers protection during the early stages of cerebral ischemia: a possible role for calcium. Neurosci Res. 2011;69(4):299–307. doi: 10.1016/j.neures.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Shono Y, Yokota C, Kuge Y, Kido S, Harada A, Kokame K, Inoue H, Hotta M, Hirata K, Saji H. Gene expression associated with an enriched environment after transient focal ischemia. Brain Res. 2011;1376:60–65. doi: 10.1016/j.brainres.2010.12.058. [DOI] [PubMed] [Google Scholar]
- Shi GD, OuYang YP, Shi JG, Liu Y, Yuan W, Jia LS. PTEN deletion prevents ischemic brain injury by activating the mTOR signaling pathway. Biochem Biophys Res Commun. 2011;404(4):941–945. doi: 10.1016/j.bbrc.2010.12.085. [DOI] [PubMed] [Google Scholar]
- Wang Q, Li X, Chen Y, Wang F, Yang Q, Chen S, Min Y, Xiong L. Activation of epsilon protein kinase C-mediated anti-apoptosis is involved in rapid tolerance induced by electroacupuncture pretreatment through cannabinoid receptor type 1. Stroke. 2011;42(2):389–396. doi: 10.1161/STROKEAHA.110.597336. [DOI] [PubMed] [Google Scholar]
- Mohagheghi F, Bigdeli MR, Rasoulian B, Hashemi P, Pour MR. The neuroprotective effect of olive leaf extract is related to improved blood–brain barrier permeability and brain edema in rat with experimental focal cerebral ischemia. Phytomedicine. 2011;18(2–3):170–175. doi: 10.1016/j.phymed.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Noh SJ, Lee SH, Shin KY, Lee CK, Cho IH, Kim HS, Suh YH. SP-8203 reduces oxidative stress via SOD activity and behavioral deficit in cerebral ischemia. Pharmacol Biochem Behav. 2011;98(1):150–154. doi: 10.1016/j.pbb.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Connell BJ, Saleh M, Khan BV, Saleh TM. Lipoic acid protects against reperfusion injury in the early stages of cerebral ischemia. Brain Res. 2011;1375:128–136. doi: 10.1016/j.brainres.2010.12.045. [DOI] [PubMed] [Google Scholar]
- Ramos-Cabrer P, Agulla J, Argibay B, Perez-Mato M, Castillo J. Serial MRI study of the enhanced therapeutic effects of liposome-encapsulated citicoline in cerebral ischemia. Int J Pharm. 2011;405(1–2):228–233. doi: 10.1016/j.ijpharm.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Mohanan A, Deshpande S, Jamadarkhana PG, Kumar P, Gupta RC, Chauthaiwale V, Dutt C. Delayed intervention in experimental stroke with TRC051384–a small molecule HSP70 inducer. Neuropharmacology. 2011;60(6):991–999. doi: 10.1016/j.neuropharm.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Moyanova SG, Mastroiacovo F, Kortenska LV, Mitreva RG, Fardone E, Santolini I, Sobrado M, Battaglia G, Bruno V, Nicoletti F. Protective role for type 4 metabotropic glutamate receptors against ischemic brain damage. J Cereb Blood Flow Metab. 2011;31(4):1107–1118. doi: 10.1038/jcbfm.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Li Z, Xu XY, Guo YL, Du F. Neuroprotective Properties of Picroside II in a Rat Model of Focal Cerebral Ischemia. Int J Mol Sci. 2010;11(11):4580–4590. doi: 10.3390/ijms11114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyagi T, Nishikawa T, Tobe Y. Neuroprotective effects and suppression of ischemia-induced glutamate elevation by beta1-adrenoreceptor antagonists administered before transient focal ischemia in rats. J Neurosurg Anesthesiol. 2011;23(2):131–137. doi: 10.1097/ANA.0b013e31820369c1. [DOI] [PubMed] [Google Scholar]
- Garcia-Bonilla L, Sosti V, Campos M, Penalba A, Boada C, Sumalla M, Hernandez-Guillamon M, Rosell A, Montaner J. Effects of acute post-treatment with dipyridamole in a rat model of focal cerebral ischemia. Brain Res. 2011;1373:211–220. doi: 10.1016/j.brainres.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Fernandez M, Rodriguez-Frutos B, Alvarez-Grech J, Vallejo-Cremades MT, Exposito-Alcaide M, Merino J, Roda JM, Diez-Tejedor E. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience. 2011;175:394–405. doi: 10.1016/j.neuroscience.2010.11.054. [DOI] [PubMed] [Google Scholar]
- Qi J, Hong ZY, Xin H, Zhu YZ. Neuroprotective effects of leonurine on ischemia/reperfusion-induced mitochondrial dysfunctions in rat cerebral cortex. Biol Pharm Bull. 2010;33(12):1958–1964. doi: 10.1248/bpb.33.1958. [DOI] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Paterniti I, Esposito E, Bramanti P, Cuzzocrea S. Modulation of NADPH oxidase activation in cerebral ischemia/reperfusion injury in rats. Brain Res. 2011;1372:92–102. doi: 10.1016/j.brainres.2010.11.088. [DOI] [PubMed] [Google Scholar]
- Adachi N, Liu K, Ninomiya K, Matsuoka E, Motoki A, Irisawa Y, Nishibori M. Reduction of the infarct size by simultaneous administration of L-histidine and diphenhydramine in ischaemic rat brains. Resuscitation. 2011;82(2):219–221. doi: 10.1016/j.resuscitation.2010.10.024. [DOI] [PubMed] [Google Scholar]
- Wang SH, Li Q, Deng ZH, Ji X, Jiang X, Ge X, Bo QQ, Cui JY, Zhang LZ, Liu JK. Neanthes japonica (Iznka) fibrinolytic enzyme reduced cerebral infarction, cerebral edema and increased antioxidation in rat models of focal cerebral ischemia. Neurosci Lett. 2011;489(1):16–19. doi: 10.1016/j.neulet.2010.11.057. [DOI] [PubMed] [Google Scholar]
- Greco R, Mangione AS, Amantea D, Bagetta G, Nappi G, Tassorelli C. IkappaB-alpha expression following transient focal cerebral ischemia is modulated by nitric oxide. Brain Res. 2011;1372:145–151. doi: 10.1016/j.brainres.2010.11.071. [DOI] [PubMed] [Google Scholar]
- Yu SS, Zhao J, Lei SP, Lin XM, Wang LL, Zhao Y. 4-hydroxybenzyl alcohol ameliorates cerebral injury in rats by antioxidant action. Neurochem Res. 2011;36(2):339–346. doi: 10.1007/s11064-010-0335-8. [DOI] [PubMed] [Google Scholar]
- Xue X, Qu XJ, Yang Y, Sheng XH, Cheng F, Jiang EN, Wang JH, Bu W, Liu ZP. Baicalin attenuates focal cerebral ischemic reperfusion injury through inhibition of nuclear factor kappaB p65 activation. Biochem Biophys Res Commun. 2010;403(3–4):398–404. doi: 10.1016/j.bbrc.2010.11.042. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Yasuhara T, Kameda M, Baba T, Kuramoto S, Kondo A, Takahashi K, Tajiri N, Wang F, Meng J. Striatal stimulation nurtures endogenous neurogenesis and angiogenesis in chronic-phase ischemic stroke rats. Cell Transplant. 2011;20(7):1049–1064. doi: 10.3727/096368910X544915. [DOI] [PubMed] [Google Scholar]
- Riegelsberger UM, Deten A, Posel C, Zille M, Kranz A, Boltze J, Wagner DC. Intravenous human umbilical cord blood transplantation for stroke: impact on infarct volume and caspase-3-dependent cell death in spontaneously hypertensive rats. Exp Neurol. 2011;227(1):218–223. doi: 10.1016/j.expneurol.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Hayward NM, Yanev P, Haapasalo A, Miettinen R, Hiltunen M, Grohn O, Jolkkonen J. Chronic hyperperfusion and angiogenesis follow subacute hypoperfusion in the thalamus of rats with focal cerebral ischemia. J Cereb Blood Flow Metab. 2011;31(4):1119–1132. doi: 10.1038/jcbfm.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CJ, Chen JT, Yen TL, Jayakumar T, Chou DS, Hsiao G, Sheu JR. Neuroprotection by the Traditional Chinese Medicine, Tao-Hong-Si-Wu-Tang, against Middle Cerebral Artery Occlusion-Induced Cerebral Ischemia in Rats. Evid Based Complement Alternat Med. 2011;2011:803015. doi: 10.1155/2011/803015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama J, Miyake S, Sasayama T, Chiba Y, Kondoh T, Kohmura E. The novel VEGF receptor antagonist, VGA1155, reduces edema, decreases infarct and improves neurological function after stroke in rats. Kobe J Med Sci. 2010;56(1):E1–E11. [PubMed] [Google Scholar]
- Qu WS, Wang YH, Ma JF, Tian DS, Zhang Q, Pan DJ, Yu ZY, Xie MJ, Wang JP, Wang W. Galectin-1 attenuates astrogliosis-associated injuries and improves recovery of rats following focal cerebral ischemia. J Neurochem. 2011;116(2):217–226. doi: 10.1111/j.1471-4159.2010.07095.x. [DOI] [PubMed] [Google Scholar]
- Chen J, Huang RB. Protective effect of Yulangsan polysaccharide on focal cerebral ischemia/reperfusion injury in rats and its underlying mechanism. Neurosciences (Riyadh) 2009;14(4):343–348. [PubMed] [Google Scholar]
- Koya-Miyata S, Ohta H, Akita K, Arai S, Ohta T, Kawata T, Fukuda S. Cyanine dyes attenuate cerebral ischemia and reperfusion injury in rats. Biol Pharm Bull. 2010;33(11):1872–1877. doi: 10.1248/bpb.33.1872. [DOI] [PubMed] [Google Scholar]
- Jin YC, Kim SW, Cheng F, Shin JH, Park JK, Lee S, Lee JE, Han PL, Lee M, Kim KK. The effect of biodegradable gelatin microspheres on the neuroprotective effects of high mobility group box 1 A box in the postischemic brain. Biomaterials. 2011;32(3):899–908. doi: 10.1016/j.biomaterials.2010.09.054. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Wei N, Lu T, Zhu J, Xu G, Liu X. Intranasal brain-derived neurotrophic factor protects brain from ischemic insult via modulating local inflammation in rats. Neuroscience. 2011;172:398–405. doi: 10.1016/j.neuroscience.2010.10.054. [DOI] [PubMed] [Google Scholar]
- Kim JY, Jeong HY, Lee HK, Yoo JK, Bae K, Seong YH. Protective effect of Ilex latifolia, a major component of "kudingcha", against transient focal ischemia-induced neuronal damage in rats. J Ethnopharmacol. 2011;133(2):558–564. doi: 10.1016/j.jep.2010.10.037. [DOI] [PubMed] [Google Scholar]
- Yeh SH, Ou LC, Gean PW, Hung JJ, Chang WC. Selective inhibition of early–but not late–expressed HIF-1alpha is neuroprotective in rats after focal ischemic brain damage. Brain Pathol. 2011;21(3):249–262. doi: 10.1111/j.1750-3639.2010.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Collins VE, Macleod MR, Cox SF, Van Raay L, Aleksoska E, Donnan GA, Howells DW. Preclinical drug evaluation for combination therapy in acute stroke using systematic review, meta-analysis, and subsequent experimental testing. J Cereb Blood Flow Metab. 2011;31(3):962–975. doi: 10.1038/jcbfm.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X, Wei J, Feng M, Lu S, Li G, Dou W, Ma W, Ma S, An Y, Qin C. Transplantation of human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and endogenous neurogenesis after cerebral ischemia in rats. Brain Res. 2011;1367:103–113. doi: 10.1016/j.brainres.2010.10.063. [DOI] [PubMed] [Google Scholar]
- Gan X, Luo Y, Ling F, Ji X, Chen J, Ding Y. Outcome in acute stroke with different intra-arterial infusion rate of urokinase on thrombolysis. Interv Neuroradiol. 2010;16(3):290–296. doi: 10.1177/159101991001600311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L, Theodorsson E, Hokfelt T, Theodorsson A. Effects of intracerebroventricular galanin or a galanin receptor 2/3 agonist on the lesion induced by transient occlusion of the middle cerebral artery in female rats. Neuropeptides. 2011;45(1):17–23. doi: 10.1016/j.npep.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhou W, Heiland S, Marti HH, Veltkamp R. A translationally relevant thromboembolic stroke model for the study of secondary hemorrhage after thrombolysis in rats. Brain Res. 2011;1368:346–354. doi: 10.1016/j.brainres.2010.10.067. [DOI] [PubMed] [Google Scholar]
- Kawai H, Deguchi S, Deguchi K, Yamashita T, Ohta Y, Shang J, Tian F, Zhang X, Liu N, Liu W. Synergistic benefit of combined amlodipine plus atorvastatin on neuronal damage after stroke in Zucker metabolic rat. Brain Res. 2011;1368:317–323. doi: 10.1016/j.brainres.2010.10.046. [DOI] [PubMed] [Google Scholar]
- Nagel S, Heinemann PV, Heiland S, Koziol J, Gardner H, Wagner S. Selective MMP-inhibition with Ro 28–2653 in acute experimental stroke–a magnetic resonance imaging efficacy study. Brain Res. 2011;1368:264–270. doi: 10.1016/j.brainres.2010.10.057. [DOI] [PubMed] [Google Scholar]
- Bora KS, Shri R, Monga J. Cerebroprotective effect of Ocimum gratissimum against focal ischemia and reperfusion-induced cerebral injury. Pharm Biol. 2011;49(2):175–181. doi: 10.3109/13880209.2010.506489. [DOI] [PubMed] [Google Scholar]
- Shang J, Deguchi K, Ohta Y, Liu N, Zhang X, Tian F, Yamashita T, Ikeda Y, Matsuura T, Funakoshi H. Strong neurogenesis, angiogenesis, synaptogenesis, and antifibrosis of hepatocyte growth factor in rats brain after transient middle cerebral artery occlusion. J Neurosci Res. 2011;89(1):86–95. doi: 10.1002/jnr.22524. [DOI] [PubMed] [Google Scholar]
- Hu Q, Chen C, Khatibi NH, Li L, Yang L, Wang K, Han J, Duan W, Zhang JH, Zhou C. Lentivirus-mediated transfer of MMP-9 shRNA provides neuroprotection following focal ischemic brain injury in rats. Brain Res. 2011;1367:347–359. doi: 10.1016/j.brainres.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Loh KP, Qi J, Tan BK, Liu XH, Wei BG, Zhu YZ. Leonurine protects middle cerebral artery occluded rats through antioxidant effect and regulation of mitochondrial function. Stroke. 2010;41(11):2661–2668. doi: 10.1161/STROKEAHA.110.589895. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Guo Q, Ye Z, Pingping X, Wang N, Song Z. Ischemic postconditioning protects brain from ischemia/reperfusion injury by attenuating endoplasmic reticulum stress-induced apoptosis through PI3K-Akt pathway. Brain Res. 2011;1367:85–93. doi: 10.1016/j.brainres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Tanaka S, Hirooka K, Toyoshima T, Kawai N, Tamiya T, Shiraga F, Tokuda M, Keep RF, Itano T. Anti-oxidative effects of d-allose, a rare sugar, on ischemia-reperfusion damage following focal cerebral ischemia in rat. Neurosci Lett. 2011;487(1):103–106. doi: 10.1016/j.neulet.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Cespedes-Rubio A, Jurado FW, Cardona-Gomez GP. p120 catenin/alphaN-catenin are molecular targets in the neuroprotection and neuronal plasticity mediated by atorvastatin after focal cerebral ischemia. J Neurosci Res. 2010;88(16):3621–3634. doi: 10.1002/jnr.22511. [DOI] [PubMed] [Google Scholar]
- Britton GL, Kim H, Kee PH, Aronowski J, Holland CK, McPherson DD, Huang SL. In vivo therapeutic gas delivery for neuroprotection with echogenic liposomes. Circulation. 2010;122(16):1578–1587. doi: 10.1161/CIRCULATIONAHA.109.879338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Kim BK, Ko YJ, Bang MS, Kim MH, Han TR. Functional and histologic changes after repeated transcranial direct current stimulation in rat stroke model. J Korean Med Sci. 2010;25(10):1499–1505. doi: 10.3346/jkms.2010.25.10.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y, Yu S, Kaneko Y, Tajiri N, Bae EC, Chheda SH, Stahl CE, Yang T, Fang L, Hu K. Intravenous infusion of GDNF gene-modified human umbilical cord blood CD34+ cells protects against cerebral ischemic injury in spontaneously hypertensive rats. Brain Res. 2010;1366:217–225. doi: 10.1016/j.brainres.2010.09.098. [DOI] [PubMed] [Google Scholar]
- Hyun H, Lee J, Hwang do W, Kim S, Hyun DK, Choi JS, Lee JK, Lee M. Combinational therapy of ischemic brain stroke by delivery of heme oxygenase-1 gene and dexamethasone. Biomaterials. 2011;32(1):306–315. doi: 10.1016/j.biomaterials.2010.08.116. [DOI] [PubMed] [Google Scholar]
- Jin Z, Wu J, Oh SY, Kim KW, Shin BS. The effect of stress on stroke recovery in a photothrombotic stroke animal model. Brain Res. 2010;1363:191–197. doi: 10.1016/j.brainres.2010.09.081. [DOI] [PubMed] [Google Scholar]
- Chan SJ, Wong WS, Wong PT, Bian JS. Neuroprotective effects of andrographolide in a rat model of permanent cerebral ischaemia. Br J Pharmacol. 2010;161(3):668–679. doi: 10.1111/j.1476-5381.2010.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calloni RL, Winkler BC, Ricci G, Poletto MG, Homero WM, Serafini EP, Corleta OC. Transient middle cerebral artery occlusion in rats as an experimental model of brain ischemia. Acta Cir Bras. 2010;25(5):428–433. doi: 10.1590/s0102-86502010000500008. [DOI] [PubMed] [Google Scholar]
- Spratt NJ, Donnan GA, McLeod DD, Howells DW. 'Salvaged' stroke ischaemic penumbra shows significant injury: studies with the hypoxia tracer FMISO. J Cereb Blood Flow Metab. 2011;31(3):934–943. doi: 10.1038/jcbfm.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Li J, Rosenbaum DM, Barone FC. Thrombopoietin protects the brain and improves sensorimotor functions: reduction of stroke-induced MMP-9 upregulation and blood–brain barrier injury. J Cereb Blood Flow Metab. 2011;31(3):924–933. doi: 10.1038/jcbfm.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao X, Zhou J, Chen T, Liu W, Dong W, Qu Y, Jiang X, Ji X, Zhen H, Fei Z. Neuroprotective effect of osthole against acute ischemic stroke on middle cerebral ischemia occlusion in rats. Brain Res. 2010;1363:206–211. doi: 10.1016/j.brainres.2010.09.052. [DOI] [PubMed] [Google Scholar]
- Li H, Yan Z, Zhu J, Yang J, He J. Neuroprotective effects of resveratrol on ischemic injury mediated by improving brain energy metabolism and alleviating oxidative stress in rats. Neuropharmacology. 2011;60(2–3):252–258. doi: 10.1016/j.neuropharm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Sun M, Gu Y, Zhao Y, Xu C. Protective functions of taurine against experimental stroke through depressing mitochondria-mediated cell death in rats. Amino Acids. 2011;40(5):1419–1429. doi: 10.1007/s00726-010-0751-8. [DOI] [PubMed] [Google Scholar]
- Tu XK, Yang WZ, Shi SS, Chen Y, Wang CH, Chen CM, Chen Z. Baicalin inhibits TLR2/4 signaling pathway in rat brain following permanent cerebral ischemia. Inflammation. 2011;34(5):463–470. doi: 10.1007/s10753-010-9254-8. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Zhu P, Cao F, Kikuchi-Taura A, Kasahara Y, Stern DM, Soma T, Matsuyama T, Hata R. Reduced ischemic brain injury by partial rejuvenation of bone marrow cells in aged rats. J Cereb Blood Flow Metab. 2011;31(3):855–867. doi: 10.1038/jcbfm.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Chopp M, Cui Y, Wang L, Zhang R, Zhang L, Lu M, Szalad A, Doppler E, Hitzl M. Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. J Neurosci Res. 2010;88(15):3275–3281. doi: 10.1002/jnr.22495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard K, Rasmussen RS, Johansen FF. The site of embolization related to infarct size, oedema and clinical outcome in a rat stroke model - further translational stroke research. Exp Transl Stroke Med. 2010;2(1):17. doi: 10.1186/2040-7378-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YP, Liu SY, Sun H, Wu XM, Li JJ, Zhu L. Neuroprotective effect of astaxanthin on H(2)O(2)-induced neurotoxicity in vitro and on focal cerebral ischemia in vivo. Brain Res. 2010;1360:40–48. doi: 10.1016/j.brainres.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Han Z, Zhang HX, Tian JS, Zheng RY, Hou ST. 2-(−2-benzofuranyl)-2-imidazoline induces Bcl-2 expression and provides neuroprotection against transient cerebral ischemia in rats. Brain Res. 2010;1361:86–92. doi: 10.1016/j.brainres.2010.09.029. [DOI] [PubMed] [Google Scholar]
- Cao W, Glushakov A, Shah HP, Mecca AP, Sumners C, Shi P, Seubert CN, Martynyuk AE. Halogenated aromatic amino acid 3,5-dibromo-D: -tyrosine produces beneficial effects in experimental stroke and seizures. Amino Acids. 2011;40(4):1151–1158. doi: 10.1007/s00726-010-0739-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Zhang S, Fu F, Zhu H, Hou J. Inhibition of nuclear factor-kappaB by 6-O-acetyl shanzhiside methyl ester protects brain against injury in a rat model of ischemia and reperfusion. J Neuroinflammation. 2010;7:55. doi: 10.1186/1742-2094-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou YC, Liou KT, Chern CM, Wang YH, Liao JF, Chang S, Chou YH, Shen YC. Preventive effect of silymarin in cerebral ischemia-reperfusion-induced brain injury in rats possibly through impairing NF-kappaB and STAT-1 activation. Phytomedicine. 2010;17(12):963–973. doi: 10.1016/j.phymed.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Fu A, Hui EK, Lu JZ, Boado RJ, Pardridge WM. Neuroprotection in experimental stroke in the rat with an IgG-erythropoietin fusion protein. Brain Res. 2010;1360:193–197. doi: 10.1016/j.brainres.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Hyun H, Won YW, Kim KM, Lee J, Lee M, Kim YH. Therapeutic effects of a reducible poly (oligo-D-arginine) carrier with the heme oxygenase-1 gene in the treatment of hypoxic-ischemic brain injury. Biomaterials. 2010;31(34):9128–9134. doi: 10.1016/j.biomaterials.2010.08.038. [DOI] [PubMed] [Google Scholar]
- Wang T, Wang J, Yin C, Liu R, Zhang JH, Qin X. Down-regulation of Nogo receptor promotes functional recovery by enhancing axonal connectivity after experimental stroke in rats. Brain Res. 2010;1360:147–158. doi: 10.1016/j.brainres.2010.08.101. [DOI] [PubMed] [Google Scholar]
- Karki K, Knight RA, Shen LH, Kapke A, Lu M, Li Y, Chopp M. Chronic brain tissue remodeling after stroke in rat: a 1-year multiparametric magnetic resonance imaging study. Brain Res. 2010;1360:168–176. doi: 10.1016/j.brainres.2010.08.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone and allopregnanolone attenuate blood–brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp Neurol. 2010;226(1):183–190. doi: 10.1016/j.expneurol.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Chio CC, Chang CH, Kuo JR, Chang CP. Beneficial effect of agmatine on brain apoptosis, astrogliosis, and edema after rat transient cerebral ischemia. BMC Pharmacol. 2010;10:11. doi: 10.1186/1471-2210-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra V, Verma R, Raghubir R. Neuroprotective effect of flurbiprofen in focal cerebral ischemia: the possible role of ASIC1a. Neuropharmacology. 2010;59(7–8):582–588. doi: 10.1016/j.neuropharm.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Du W, Huang J, Yao H, Zhou K, Duan B, Wang Y. Inhibition of TRPC6 degradation suppresses ischemic brain damage in rats. J Clin Invest. 2010;120(10):3480–3492. doi: 10.1172/JCI43165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SY, Shin JH, Hwang JS, Kim SY, Shin JA, Oh ES, Oh S, Kim JB, Lee JK, Han IO. Glucosamine exerts a neuroprotective effect via suppression of inflammation in rat brain ischemia/reperfusion injury. Glia. 2010;58(15):1881–1892. doi: 10.1002/glia.21058. [DOI] [PubMed] [Google Scholar]
- Jiang WL, Zhang SP, Zhu HB, Hou J. Effect of 8-O-acetyl shanzhiside methylester increases angiogenesis and improves functional recovery after stroke. Basic Clin Pharmacol Toxicol. 2011;108(1):21–27. doi: 10.1111/j.1742-7843.2010.00620.x. [DOI] [PubMed] [Google Scholar]
- Matsuda F, Sakakima H, Yoshida Y. The effects of early exercise on brain damage and recovery after focal cerebral infarction in rats. Acta Physiol (Oxf) 2011;201(2):275–287. doi: 10.1111/j.1748-1716.2010.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckl J, Baker W, Sun ZH, Durduran T, Yodh AG, Greenberg JH. The biological effect of contralateral forepaw stimulation in rat focal cerebral ischemia: a multispectral optical imaging study. Front Neuroenergetics. 2010;2:pii: 19. doi: 10.3389/fnene.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kelly-Cobbs AI, Mezzetti EM, Fagan SC, Ergul A. Endothelin-1-mediated cerebrovascular remodeling is not associated with increased ischemic brain injury in diabetes. Can J Physiol Pharmacol. 2010;88(8):788–795. doi: 10.1139/Y10-040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G, Jiang Q, Li L, Zhang L, Wang Y, Zhang ZG, Lu M, Panda S, Li Q, Ewing JR. Cerebral tissue repair and atrophy after embolic stroke in rat: a magnetic resonance imaging study of erythropoietin therapy. J Neurosci Res. 2010;88(14):3206–3214. doi: 10.1002/jnr.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheyuo C, Wu R, Zhou M, Jacob A, Coppa G, Wang P. Ghrelin suppresses inflammation and neuronal nitric oxide synthase in focal cerebral ischemia via the vagus nerve. Shock. 2011;35(3):258–265. doi: 10.1097/SHK.0b013e3181f48a37. [DOI] [PubMed] [Google Scholar]
- Zhang F, Jia J, Wu Y, Hu Y, Wang Y. The effect of treadmill training pre-exercise on glutamate receptor expression in rats after cerebral ischemia. Int J Mol Sci. 2010;11(7):2658–2669. doi: 10.3390/ijms11072658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Shang YC, Hou J, Maiese K. Wnt1 neuroprotection translates into improved neurological function during oxidant stress and cerebral ischemia through AKT1 and mitochondrial apoptotic pathways. Oxid Med Cell Longev. 2010;3(2):153–165. doi: 10.4161/oxim.3.2.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wu Y, Jia J, Hu YS. Pre-ischemic treadmill training induces tolerance to brain ischemia: involvement of glutamate and ERK1/2. Molecules. 2010;15(8):5246–5257. doi: 10.3390/molecules15085246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B, Bach S, Blevins TL, Campbell M, Meijer L, Timsit S. Delayed treatment with systemic (S)-roscovitine provides neuroprotection and inhibits in vivo CDK5 activity increase in animal stroke models. PLoS One. 2010;5(8):e12117. doi: 10.1371/journal.pone.0012117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JK, Yu LN, Zhang FJ, Yang MJ, Yu J, Yan M, Chen G. Postconditioning with sevoflurane protects against focal cerebral ischemia and reperfusion injury via PI3K/Akt pathway. Brain Res. 2010;1357:142–151. doi: 10.1016/j.brainres.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Yao H, Nabika T. Characterizing photothrombotic distal middle cerebral artery occlusion and YAG laser-induced reperfusion model in the Izumo strain of spontaneously hypertensive rats. Cell Mol Neurobiol. 2011;31(1):57–63. doi: 10.1007/s10571-010-9553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savos AV, Gee JM, Zierath D, Becker KJ. alpha-MSH: a potential neuroprotective and immunomodulatory agent for the treatment of stroke. J Cereb Blood Flow Metab. 2011;31(2):606–613. doi: 10.1038/jcbfm.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL, Grider M. The effect of mild post-stroke exercise on reactive neurogenesis and recovery of somatosensation in aged rats. Exp Neurol. 2010;226(1):58–67. doi: 10.1016/j.expneurol.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Gamboa J, Blankenship DA, Niemi JP, Landreth GE, Karl M, Hilow E, Sundararajan S. Extension of the neuroprotective time window for thiazolidinediones in ischemic stroke is dependent on time of reperfusion. Neuroscience. 2010;170(3):846–857. doi: 10.1016/j.neuroscience.2010.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airavaara M, Chiocco MJ, Howard DB, Zuchowski KL, Peranen J, Liu C, Fang S, Hoffer BJ, Wang Y, Harvey BK. Widespread cortical expression of MANF by AAV serotype 7: localization and protection against ischemic brain injury. Exp Neurol. 2010;225(1):104–113. doi: 10.1016/j.expneurol.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong ZT, Gong XJ, Sun HB, Li YM, Ji H. Protective effects of oleanolic acid on cerebral ischemic damage in vivo and H(2)O(2)-induced injury in vitro. Pharm Biol. 2011;49(1):78–85. doi: 10.3109/13880209.2010.499130. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xing S, Zhang J, Li J, Li C, Pei Z, Zeng J. Reduction of beta-amyloid deposits by gamma-secretase inhibitor is associated with the attenuation of secondary damage in the ipsilateral thalamus and sensory functional improvement after focal cortical infarction in hypertensive rats. J Cereb Blood Flow Metab. 2011;31(2):572–579. doi: 10.1038/jcbfm.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guluma KZ, Lapchak PA. Comparison of the post-embolization effects of tissue-plasminogen activator and simvastatin on neurological outcome in a clinically relevant rat model of acute ischemic stroke. Brain Res. 2010;1354:206–216. doi: 10.1016/j.brainres.2010.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Chopp M, Zhang L, Lu M, Zhang Z. Erythropoietin in combination of tissue plasminogen activator exacerbates brain hemorrhage when treatment is initiated 6 hours after stroke. Stroke. 2010;41(9):2071–2076. doi: 10.1161/STROKEAHA.110.586198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr TD, Seehafer JU, Nelles M, Hoehn M. Challenges towards MR imaging of the peripheral inflammatory response in the subacute and chronic stages of transient focal ischemia. NMR Biomed. 2011;24(1):35–45. doi: 10.1002/nbm.1553. [DOI] [PubMed] [Google Scholar]
- Narantuya D, Nagai A, Sheikh AM, Masuda J, Kobayashi S, Yamaguchi S, Kim SU. Human microglia transplanted in rat focal ischemia brain induce neuroprotection and behavioral improvement. PLoS One. 2010;5(7):e11746. doi: 10.1371/journal.pone.0011746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Wang Q, Chen Y, Du J, Zhu X, Lu Y, Xiong L, Chen S. Neuroprotective effect of WIN 55,212-2 pretreatment against focal cerebral ischemia through activation of extracellular signal-regulated kinases in rats. Eur J Pharmacol. 2010;645(1–3):102–107. doi: 10.1016/j.ejphar.2010.07.024. [DOI] [PubMed] [Google Scholar]
- Zhao H, Mayhan WG, Arrick DM, Xiong W, Sun H. Alcohol-induced exacerbation of ischemic brain injury: role of NAD(P)H oxidase. Alcohol Clin Exp Res. 2010;34(11):1948–1955. doi: 10.1111/j.1530-0277.2010.01284.x. [DOI] [PubMed] [Google Scholar]
- Du J, Wang Q, Hu B, Peng Z, Zhao Y, Ma L, Xiong L, Lu Y, Zhu X, Chen S. Involvement of ERK 1/2 activation in electroacupuncture pretreatment via cannabinoid CB1 receptor in rats. Brain Res. 2010;1360:1–7. doi: 10.1016/j.brainres.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Pei L, Zhang Y, Chu X, Zhang J, Wang R, Liu M, Zhu X, Yu W. Peroxisome proliferator-activated receptor gamma promotes neuroprotection by modulating cyclin D1 expression after focal cerebral ischemia. Can J Physiol Pharmacol. 2010;88(7):716–723. doi: 10.1139/y10-058. [DOI] [PubMed] [Google Scholar]
- Letourneur A, Roussel S, Toutain J, Bernaudin M, Touzani O. Impact of genetic and renovascular chronic arterial hypertension on the acute spatiotemporal evolution of the ischemic penumbra: a sequential study with MRI in the rat. J Cereb Blood Flow Metab. 2011;31(2):504–513. doi: 10.1038/jcbfm.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Kim MY, Leem KH, Moon S, Jamakattel-Pandit N, Choi H, Kim H, Bu Y. Key compound groups for the neuroprotective effect of roots of Polygonum cuspidatum on transient middle cerebral artery occlusion in Sprague–Dawley rats. Nat Prod Res. 2010;24(13):1214–1226. doi: 10.1080/14786410902992157. [DOI] [PubMed] [Google Scholar]
- Briyal S, Gulati A. Endothelin-A receptor antagonist BQ123 potentiates acetaminophen induced hypothermia and reduces infarction following focal cerebral ischemia in rats. Eur J Pharmacol. 2010;644(1–3):73–79. doi: 10.1016/j.ejphar.2010.06.071. [DOI] [PubMed] [Google Scholar]
- Wang W, Xu J, Li L, Wang P, Ji X, Ai H, Zhang L. Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain Res Bull. 2010;83(5):196–201. doi: 10.1016/j.brainresbull.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Cui L, Zhang X, Yang R, Liu L, Wang L, Li M, Du W. Baicalein is neuroprotective in rat MCAO model: role of 12/15-lipoxygenase, mitogen-activated protein kinase and cytosolic phospholipase A2. Pharmacol Biochem Behav. 2010;96(4):469–475. doi: 10.1016/j.pbb.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Liu W, Liu B, Schnell A, Liu KJ. Normobaric hyperoxia delays and attenuates early nitric oxide production in focal cerebral ischemic rats. Brain Res. 2010;1352:248–254. doi: 10.1016/j.brainres.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalal FY, Bohlke M, Maher TJ. Acetyl-L-carnitine reduces the infarct size and striatal glutamate outflow following focal cerebral ischemia in rats. Ann N Y Acad Sci. 2010;1199:95–104. doi: 10.1111/j.1749-6632.2009.05351.x. [DOI] [PubMed] [Google Scholar]
- Pino-Figueroa A, Nguyen D, Maher TJ. Neuroprotective effects of Lepidium meyenii (Maca) Ann N Y Acad Sci. 2010;1199:77–85. doi: 10.1111/j.1749-6632.2009.05174.x. [DOI] [PubMed] [Google Scholar]
- Ma Y, Sullivan JC, Schreihofer DA. Dietary genistein and equol (4', 7 isoflavandiol) reduce oxidative stress and protect rats against focal cerebral ischemia. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R871–R877. doi: 10.1152/ajpregu.00031.2010. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Esposito E, Cuomo O, Sirabella R, Boscia F, Guida N, Di Renzo G, Annunziato L. The NCX3 isoform of the Na+/Ca2+ exchanger contributes to neuroprotection elicited by ischemic postconditioning. J Cereb Blood Flow Metab. 2011;31(1):362–370. doi: 10.1038/jcbfm.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepramaniam S, Armugam A, Lim KY, Karolina DS, Swaminathan P, Tan JR, Jeyaseelan K. MicroRNA 320a functions as a novel endogenous modulator of aquaporins 1 and 4 as well as a potential therapeutic target in cerebral ischemia. J Biol Chem. 2010;285(38):29223–29230. doi: 10.1074/jbc.M110.144576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DC, Chopp M, Zhang L, Lu M, Zhang ZG. Thymosin beta4 improves functional neurological outcome in a rat model of embolic stroke. Neuroscience. 2010;169(2):674–682. doi: 10.1016/j.neuroscience.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Zhang X, Yang R, Wang L, Liu L, Li M, Du W. Neuroprotection and underlying mechanisms of oxymatrine in cerebral ischemia of rats. Neurol Res. 2011;33(3):319–324. doi: 10.1179/016164110X12759951866876. [DOI] [PubMed] [Google Scholar]
- Gao B, Cam E, Jaeger H, Zunzunegui C, Sarnthein J, Bassetti CL. Sleep disruption aggravates focal cerebral ischemia in the rat. Sleep. 2010;33(7):879–887. doi: 10.1093/sleep/33.7.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Lu TS, Yuan HY. Neuroprotective effects of ultra-low-molecular-weight heparin in vitro and vivo models of ischemic injury. Fundam Clin Pharmacol. 2011;25(3):300–303. doi: 10.1111/j.1472-8206.2010.00845.x. [DOI] [PubMed] [Google Scholar]
- Choi HS, Ko YG, Lee JS, Kwon OY, Kim SK, Cheong C, Jang KH, Kang SA. Neuroprotective effects of consuming bovine colostrum after focal brain ischemia/reperfusion injury in rat model. Nutr Res Pract. 2010;4(3):196–202. doi: 10.4162/nrp.2010.4.3.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohagheghi F, Bigdeli MR, Rasoulian B, Zeinanloo AA, Khoshbaten A. Dietary virgin olive oil reduces blood brain barrier permeability, brain edema, and brain injury in rats subjected to ischemia-reperfusion. Sci World J. 2010;10:1180–1191. doi: 10.1100/tsw.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen CM, Leu S, Lee FY, Yen CH, Lin YC, Chua S, Chung SY, Chai HT, Sheu JJ, Ko SF. Erythropoietin markedly attenuates brain infarct size and improves neurological function in the rat. J Investig Med. 2010;58(7):893–904. doi: 10.231/JIM.0b013e3181e80c40. [DOI] [PubMed] [Google Scholar]
- Xiang J, Tang YP, Wu P, Gao JP, Cai DF. Chinese medicine Nao-Shuan-Tong attenuates cerebral ischemic injury by inhibiting apoptosis in a rat model of stroke. J Ethnopharmacol. 2010;131(1):174–181. doi: 10.1016/j.jep.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Zhou ZY, Tang YP, Xiang J, Wua P, Jin HM, Wang Z, Mori M, Cai DF. Neuroprotective effects of water-soluble Ganoderma lucidum polysaccharides on cerebral ischemic injury in rats. J Ethnopharmacol. 2010;131(1):154–164. doi: 10.1016/j.jep.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Kitamura E, Hamada J, Kanazawa N, Yonekura J, Masuda R, Sakai F, Mochizuki H. The effect of orexin-A on the pathological mechanism in the rat focal cerebral ischemia. Neurosci Res. 2010;68(2):154–157. doi: 10.1016/j.neures.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Senda DM, Franzin S, Mori MA, de Oliveira RM, Milani H. Acute, post-ischemic sensorimotor deficits correlate positively with infarct size but fail to predict its occurrence and magnitude after middle cerebral artery occlusion in rats. Behav Brain Res. 2011;216(1):29–35. doi: 10.1016/j.bbr.2010.06.026. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bi F, Xiao C, Liu J, Wang Z, Liu JN, Zhang J. The Kringle-2 domain of tissue plasminogen activator significantly reduces mortality and brain infarction in middle cerebral artery occlusion rats. Int J Mol Med. 2010;26(2):225–230. doi: 10.3892/ijmm_00000456. [DOI] [PubMed] [Google Scholar]
- Kondoh T, Kameishi M, Mallick HN, Ono T, Torii K. Lysine and arginine reduce the effects of cerebral ischemic insults and inhibit glutamate-induced neuronal activity in rats. Front Integr Neurosci. 2010;4:18. doi: 10.3389/fnint.2010.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Uchida M, Zhang W, Grafe MR, Herson PS, Hurn PD. Role of salt-induced kinase 1 in androgen neuroprotection against cerebral ischemia. J Cereb Blood Flow Metab. 2011;31(1):339–350. doi: 10.1038/jcbfm.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lay CC, Davis MF, Chen-Bee CH, Frostig RD. Mild sensory stimulation completely protects the adult rodent cortex from ischemic stroke. PLoS One. 2010;5(6):e11270. doi: 10.1371/journal.pone.0011270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Xue H, Bai C, Fu R, Wu A. The effects of Tanshinone IIA on blood–brain barrier and brain edema after transient middle cerebral artery occlusion in rats. Phytomedicine. 2010;17(14):1145–1149. doi: 10.1016/j.phymed.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Shen CC, Lin CH, Yang YC, Chiao MT, Cheng WY, Ko JL. Intravenous implanted neural stem cells migrate to injury site, reduce infarct volume, and improve behavior after cerebral ischemia. Curr Neurovasc Res. 2010;7(3):167–179. doi: 10.2174/156720210792231822. [DOI] [PubMed] [Google Scholar]
- Shehadah A, Chen J, Zacharek A, Cui Y, Ion M, Roberts C, Kapke A, Chopp M. Niaspan treatment induces neuroprotection after stroke. Neurobiol Dis. 2010;40(1):277–283. doi: 10.1016/j.nbd.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho GW, Koh SH, Kim MH, Yoo AR, Noh MY, Oh S, Kim SH. The neuroprotective effect of erythropoietin-transduced human mesenchymal stromal cells in an animal model of ischemic stroke. Brain Res. 2010;1353:1–13. doi: 10.1016/j.brainres.2010.06.013. [DOI] [PubMed] [Google Scholar]
- Tang X, Liu KJ, Ramu J, Chen Q, Li T, Liu W. Inhibition of gp91(phox) contributes towards normobaric hyperoxia afforded neuroprotection in focal cerebral ischemia. Brain Res. 2010;1348:174–180. doi: 10.1016/j.brainres.2010.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama N, Yagita Y, Sasaki T, Omura-Matsuoka E, Terasaki Y, Sugiyama Y, Sakoda S, Kitagawa K. An angiotensin II type 1 receptor blocker can preserve endothelial function and attenuate brain ischemic damage in spontaneously hypertensive rats. J Neurosci Res. 2010;88(13):2889–2898. doi: 10.1002/jnr.22441. [DOI] [PubMed] [Google Scholar]
- Jia J, Hu YS, Wu Y, Yu HX, Liu G, Zhu DN, Xia CM, Cao ZJ, Zhang X, Guo QC. Treadmill pre-training suppresses the release of glutamate resulting from cerebral ischemia in rats. Exp Brain Res. 2010;204(2):173–179. doi: 10.1007/s00221-010-2320-5. [DOI] [PubMed] [Google Scholar]
- Moubarik C, Guillet B, Youssef B, Codaccioni JL, Piercecchi MD, Sabatier F, Lionel P, Dou L, Foucault-Bertaud A, Velly L. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev. 2011;7(1):208–220. doi: 10.1007/s12015-010-9157-y. [DOI] [PubMed] [Google Scholar]
- Lapergue B, Moreno JA, Dang BQ, Coutard M, Delbosc S, Raphaeli G, Auge N, Klein I, Mazighi M, Michel JB. Protective effect of high-density lipoprotein-based therapy in a model of embolic stroke. Stroke. 2010;41(7):1536–1542. doi: 10.1161/STROKEAHA.110.581512. [DOI] [PubMed] [Google Scholar]
- Homi HM, Sheng H, Arepally GM, Mackensen GB, Grocott HP. Aprotinin improves functional outcome but not cerebral infarct size in an experimental model of stroke during cardiopulmonary bypass. Anesth Analg. 2010;111(1):38–45. doi: 10.1213/ANE.0b013e3181e0549f. [DOI] [PubMed] [Google Scholar]
- Bar-Shir A, Shemesh N, Nossin-Manor R, Cohen Y. Late stimulation of the sphenopalatine-ganglion in ischemic rats: improvement in N-acetyl-aspartate levels and diffusion weighted imaging characteristics as seen by MR. J Magn Reson Imaging. 2010;31(6):1355–1363. doi: 10.1002/jmri.22110. [DOI] [PubMed] [Google Scholar]
- Saad MA, Abbas AM, Boshra V, Elkhateeb M, El Aal IA. Effect of angiotensin II type 1 receptor blocker, candesartan, and beta 1 adrenoceptor blocker, atenolol, on brain damage in ischemic stroke. Acta Physiol Hung. 2010;97(2):159–171. doi: 10.1556/APhysiol.97.2010.2.2. [DOI] [PubMed] [Google Scholar]
- Beltran EJ, Papadopoulos CM, Tsai SY, Kartje GL, Wolf WA. Long-term motor improvement after stroke is enhanced by short-term treatment with the alpha-2 antagonist, atipamezole. Brain Res. 2010;1346:174–182. doi: 10.1016/j.brainres.2010.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin YY, Li WP, Gong HL, Zhu FF, Li WZ, Wu GC. Protective effect of astragaloside on focal cerebral ischemia/reperfusion injury in rats. Am J Chin Med. 2010;38(3):517–527. doi: 10.1142/S0192415X10008020. [DOI] [PubMed] [Google Scholar]
- Kao TK, Ou YC, Raung SL, Chen WY, Yen YJ, Lai CY, Chou ST, Chen CJ. Graptopetalum paraguayense E. Walther leaf extracts protect against brain injury in ischemic rats. Am J Chin Med. 2010;38(3):495–516. doi: 10.1142/S0192415X10008019. [DOI] [PubMed] [Google Scholar]
- Xiao X, Liu Y, Qi C, Qiu F, Chen X, Zhang J, Yang P. Neuroprotection and enhanced neurogenesis by tetramethylpyrazine in adult rat brain after focal ischemia. Neurol Res. 2010;32(5):547–555. doi: 10.1179/174313209X414533. [DOI] [PubMed] [Google Scholar]
- Kim YK, Leem JG, Sim JY, Jeong SM, Joung KW. The effects of gabapentin pretreatment on brain injury induced by focal cerebral ischemia/reperfusion in the rat. Korean J Anesthesiol. 2010;58(2):184–190. doi: 10.4097/kjae.2010.58.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Leem JG, Shin JW, Joung KW. Ischemic postconditioning may not influence early brain injury induced by focal cerebral ischemia/reperfusion in rats. Korean J Anesthesiol. 2010;58(2):176–183. doi: 10.4097/kjae.2010.58.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratap R, Pillai KK, Khanam R, Islam F, Ahmad SJ, Akhtar M. Protective effect of irbesartan, an angiotensin II receptor antagonist, alone and in combination with aspirin on middle cerebral artery occlusion model of focal cerebral ischemia in rats. Hum Exp Toxicol. 2011;30(5):354–362. doi: 10.1177/0960327110371257. [DOI] [PubMed] [Google Scholar]
- Tang LL, Zheng JS. Effects of tetrahydrobiopterin on cerebral infarction after transient focal ischemia in rats. Neurol Res. 2011;33(10):1064–1067. doi: 10.1179/016164110X12700393823651. [DOI] [PubMed] [Google Scholar]
- Klaus JA, Kibler KK, Abuchowski A, Koehler RC. Early treatment of transient focal cerebral ischemia with bovine PEGylated carboxy hemoglobin transfusion. Artif Cells Blood Substit Immobil Biotechnol. 2010;38(5):223–229. doi: 10.3109/10731199.2010.488635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L, Wang YK, Fang YN, Zhang AW, Li XL. Effect of electroacupuncture therapy on the expression of Na(v)1.1 and Na(v)1.6 in rat after acute cerebral ischemia. Neurol Res. 2010;32(10):1110–1116. doi: 10.1179/016164110X12700393823453. [DOI] [PubMed] [Google Scholar]
- Wang F, Luo Y, Ling F, Wu H, Chen J, Yan F, He Z, Goel G, Ji X, Ding Y. Comparison of neuroprotective effects in ischemic rats with different hypothermia procedures. Neurol Res. 2010;32(4):378–383. doi: 10.1179/016164110X12670144526183. [DOI] [PubMed] [Google Scholar]
- Peng S, Kuang Z, Zhang Y, Xu H, Cheng Q. The protective effects and potential mechanism of Calpain inhibitor Calpeptin against focal cerebral ischemia-reperfusion injury in rats. Mol Biol Rep. 2011;38(2):905–912. doi: 10.1007/s11033-010-0183-2. [DOI] [PubMed] [Google Scholar]
- Kawanishi K, Koshino H, Toyoshita Y, Tanaka M, Hirai T. Effect of mastication on functional recoveries after permanent middle cerebral artery occlusion in rats. J Stroke Cerebrovasc Dis. 2010;19(5):398–403. doi: 10.1016/j.jstrokecerebrovasdis.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Jiang WL, Tian JW, Fu FH, Zhu HB, Hou J. Neuroprotective efficacy and therapeutic window of Forsythoside B: in a rat model of cerebral ischemia and reperfusion injury. Eur J Pharmacol. 2010;640(1–3):75–81. doi: 10.1016/j.ejphar.2010.04.055. [DOI] [PubMed] [Google Scholar]
- Chang CZ, Kwan AL, Howng SL. 6-Mercaptopurine exerts an immunomodulatory and neuroprotective effect on permanent focal cerebral occlusion in rats. Acta Neurochir (Wien) 2010;152(8):1383–1390. doi: 10.1007/s00701-010-0608-7. discussion 1390. [DOI] [PubMed] [Google Scholar]
- Goyagi T, Horiguchi T, Nishikawa T, Tobe Y. Post-treatment with selective beta1 adrenoceptor antagonists provides neuroprotection against transient focal ischemia in rats. Brain Res. 2010;1343:213–217. doi: 10.1016/j.brainres.2010.04.079. [DOI] [PubMed] [Google Scholar]
- Shehadah A, Chen J, Cui X, Roberts C, Lu M, Chopp M. Combination treatment of experimental stroke with Niaspan and Simvastatin, reduces axonal damage and improves functional outcome. J Neurol Sci. 2010;294(1–2):107–111. doi: 10.1016/j.jns.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Liu Y, Zhao Z, Xue Y. Effects of green tea polyphenols on caveolin-1 of microvessel fragments in rats with cerebral ischemia. Neurol Res. 2010;32(9):963–970. doi: 10.1179/016164110X12700393823570. [DOI] [PubMed] [Google Scholar]
- Bora KS, Sharma A. Neuroprotective effect of Artemisia absinthium L. on focal ischemia and reperfusion-induced cerebral injury. J Ethnopharmacol. 2010;129(3):403–409. doi: 10.1016/j.jep.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Connell BJ, Saleh TM. A novel rodent model of reperfusion injury following occlusion of the middle cerebral artery. J Neurosci Meth. 2010;190(1):28–33. doi: 10.1016/j.jneumeth.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Cai H, Xu X, Liu Z, Wang Q, Feng G, Li Y, Xu C, Liu G, Li Z. The effects of calcitonin gene-related peptide on bFGF and AQP4 expression after focal cerebral ischemia reperfusion in rats. Pharmazie. 2010;65(4):274–278. [PubMed] [Google Scholar]
- Hung YC, Chou YS, Chang CH, Lin HW, Chen HY, Chen TY, Tai SH, Lee EJ. Early reperfusion improves the recovery of contralateral electrophysiological diaschisis following focal cerebral ischemia in rats. Neurol Res. 2010;32(8):828–834. doi: 10.1179/016164109X12581096870032. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhou W, Mueller C, Sommer C, Heiland S, Bauer AT, Marti HH, Veltkamp R. Oxygen therapy reduces secondary hemorrhage after thrombolysis in thromboembolic cerebral ischemia. J Cereb Blood Flow Metab. 2010;30(9):1651–1660. doi: 10.1038/jcbfm.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Manaenko A, Zhan Y, Liu WW, Ostrowki RP, Tang J, Zhang JH. Hydrogen gas reduced acute hyperglycemia-enhanced hemorrhagic transformation in a focal ischemia rat model. Neuroscience. 2010;169(1):402–414. doi: 10.1016/j.neuroscience.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma R, Mishra V, Sasmal D, Raghubir R. Pharmacological evaluation of glutamate transporter 1 (GLT-1) mediated neuroprotection following cerebral ischemia/reperfusion injury. Eur J Pharmacol. 2010;638(1–3):65–71. doi: 10.1016/j.ejphar.2010.04.021. [DOI] [PubMed] [Google Scholar]
- Osmond JM, Mintz JD, Stepp DW. Preventing increased blood pressure in the obese Zucker rat improves severity of stroke. Am J Physiol Heart Circ Physiol. 2010;299(1):H55–H61. doi: 10.1152/ajpheart.01111.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang X, Wang L, Yang R, Cui L, Li M, Du W, Wang S. The neuroprotective effects of Tanshinone IIA are associated with induced nuclear translocation of TORC1 and upregulated expression of TORC1, pCREB and BDNF in the acute stage of ischemic stroke. Brain Res Bull. 2010;82(3–4):228–233. doi: 10.1016/j.brainresbull.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Zhang X, Deguchi K, Yamashita T, Ohta Y, Shang J, Tian F, Liu N, Panin VL, Ikeda Y, Matsuura T. Temporal and spatial differences of multiple protein expression in the ischemic penumbra after transient MCAO in rats. Brain Res. 2010;1343:143–152. doi: 10.1016/j.brainres.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Silva E, Ali O, Mooney D, Bell W, Yu SJ, Kaneko Y, Borlongan C. Injectable VEGF hydrogels produce near complete neurological and anatomical protection following cerebral ischemia in rats. Cell Transplant. 2010;19(9):1063–1071. doi: 10.3727/096368910X498278. [DOI] [PubMed] [Google Scholar]
- Greenhalgh AD, Galea J, Denes A, Tyrrell PJ, Rothwell NJ. Rapid brain penetration of interleukin-1 receptor antagonist in rat cerebral ischaemia: pharmacokinetics, distribution, protection. Br J Pharmacol. 2010;160(1):153–159. doi: 10.1111/j.1476-5381.2010.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin GH, Lin L, Liang HW, Ma X, Wang JY, Wu LP, Jiang HD, Bruce IC, Xia Q. Antioxidant action of a Chrysanthemum morifolium extract protects rat brain against ischemia and reperfusion injury. J Med Food. 2010;13(2):306–311. doi: 10.1089/jmf.2009.1184. [DOI] [PubMed] [Google Scholar]
- Zhuang P, Ji H, Zhang YH, Min ZL, Ni QG, You R. ZJM-289, a novel nitric oxide donor, alleviates the cerebral ischaemic-reperfusion injury in rats. Clin Exp Pharmacol Physiol. 2010;37(3):e121–e127. doi: 10.1111/j.1440-1681.2010.05353.x. [DOI] [PubMed] [Google Scholar]
- Lim SH, Kim HS, Kim YK, Kim TM, Im S, Chung ME, Hong BY, Ko YJ, Kim HW, Lee JI. The functional effect of epigallocatechin gallate on ischemic stroke in rats. Acta Neurobiol Exp (Wars) 2010;70(1):40–46. doi: 10.55782/ane-2010-1772. [DOI] [PubMed] [Google Scholar]
- Porritt MJ, Chen M, Rewell SS, Dean RG, Burrell LM, Howells DW. ACE inhibition reduces infarction in normotensive but not hypertensive rats: correlation with cortical ACE activity. J Cereb Blood Flow Metab. 2010;30(8):1520–1526. doi: 10.1038/jcbfm.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel S, Papadakis M, Chen R, Hoyte LC, Brooks KJ, Gallichan D, Sibson NR, Pugh C, Buchan AM. Neuroprotection by dimethyloxalylglycine following permanent and transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2011;31(1):132–143. doi: 10.1038/jcbfm.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nategh M, Shaveisi K, Shabanzadeh AP, Sadr S, Parviz M, Ghabaei M. Systemic hyperthermia masks the neuroprotective effects of MK-801, but not rosiglitazone in brain ischaemia. Basic Clin Pharmacol Toxicol. 2010;107(3):724–729. doi: 10.1111/j.1742-7843.2010.00570.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Ye XH, Guo PP, Xu SP, Wang J, Yuan SY, Yao SL, Shang Y. Neuroprotective effect of lipoxin A4 methyl ester in a rat model of permanent focal cerebral ischemia. J Mol Neurosci. 2010;42(2):226–234. doi: 10.1007/s12031-010-9355-8. [DOI] [PubMed] [Google Scholar]
- Zhang T, Pan BS, Sun GC, Sun X, Sun FY. Diabetes synergistically exacerbates poststroke dementia and tau abnormality in brain. Neurochem Int. 2010;56(8):955–961. doi: 10.1016/j.neuint.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Henninger N, Urbanek C, Schwab S. G-CSF, rt-PA and combination therapy after experimental thromboembolic stroke. Exp Transl Stroke Med. 2010;2:9. doi: 10.1186/2040-7378-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu XK, Yang WZ, Shi SS, Wang CH, Zhang GL, Ni TR, Chen CM, Wang R, Jia JW, Song QM. Spatio-temporal distribution of inflammatory reaction and expression of TLR2/4 signaling pathway in rat brain following permanent focal cerebral ischemia. Neurochem Res. 2010;35(8):1147–1155. doi: 10.1007/s11064-010-0167-6. [DOI] [PubMed] [Google Scholar]
- Martin A, Boisgard R, Kassiou M, Dolle F, Tavitian B. Reduced PBR/TSPO expression after minocycline treatment in a rat model of focal cerebral ischemia: a PET study using [(18)F]DPA-714. Mol Imaging Biol. 2011;13(1):10–15. doi: 10.1007/s11307-010-0324-y. [DOI] [PubMed] [Google Scholar]
- Komatsu K, Honmou O, Suzuki J, Houkin K, Hamada H, Kocsis JD. Therapeutic time window of mesenchymal stem cells derived from bone marrow after cerebral ischemia. Brain Res. 2010;1334:84–92. doi: 10.1016/j.brainres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Kelsen J, Larsen MH, Sorensen JC, Moller A, Frokiaer J, Nielsen S, Nyengaard JR, Mikkelsen JD, Ronn LC. Neuronal precursor cell proliferation in the hippocampus after transient cerebral ischemia: a comparative study of two rat strains using stereological tools. Exp Transl Stroke Med. 2010;2:8. doi: 10.1186/2040-7378-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov EY, Silachev DN, Chupyrkina AA, Danshina MI, Jankauskas SS, Morosanova MA, Stelmashook EV, Vasileva AK, Goryacheva ES, Pirogov YA. New-generation Skulachev ions exhibiting nephroprotective and neuroprotective properties. Biochemistry (Mosc) 2010;75(2):145–150. doi: 10.1134/s0006297910020045. [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Stocker K, Balseanu AT, Rogalewski A, Diederich K, Minnerup J, Margaritescu C, Schabitz WR. Effects of granulocyte-colony stimulating factor after stroke in aged rats. Stroke. 2010;41(5):1027–1031. doi: 10.1161/STROKEAHA.109.575621. [DOI] [PubMed] [Google Scholar]
- Li L, Ke Z, Tong KY, Ying M. Evaluation of cerebral blood flow changes in focal cerebral ischemia rats by using transcranial Doppler ultrasonography. Ultrasound Med Biol. 2010;36(4):595–603. doi: 10.1016/j.ultrasmedbio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Langdon KD, Maclellan CL, Corbett D. Prolonged, 24-h delayed peripheral inflammation increases short- and long-term functional impairment and histopathological damage after focal ischemia in the rat. J Cereb Blood Flow Metab. 2010;30(8):1450–1459. doi: 10.1038/jcbfm.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homi HM, Jones WL, de Lange F, Mackensen GB, Grocott HP. Exacerbation of systemic inflammation and increased cerebral infarct volume with cardiopulmonary bypass after focal cerebral ischemia in the rat. J Thorac Cardiovasc Surg. 2010;140(3):660–666. doi: 10.1016/j.jtcvs.2009.10.063. 666 e661. [DOI] [PubMed] [Google Scholar]
- Strom JO, Theodorsson E, Holm L, Theodorsson A. Different methods for administering 17beta-estradiol to ovariectomized rats result in opposite effects on ischemic brain damage. BMC Neurosci. 2010;11:39. doi: 10.1186/1471-2202-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggraf D, Vosko MR, Schubert M, Stassen JM, Hamann GF. Different therapy options protecting microvasculature after experimental cerebral ischaemia and reperfusion. Thromb Haemost. 2010;103(5):891–900. doi: 10.1160/TH09-07-0500. [DOI] [PubMed] [Google Scholar]
- Haelewyn B, Risso JJ, Abraini JH. Human recombinant tissue-plasminogen activator (alteplase): why not use the 'human' dose for stroke studies in rats? J Cereb Blood Flow Metab. 2010;30(5):900–903. doi: 10.1038/jcbfm.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe E, Fujiki M, Nagai Y, Shiqi K, Kubo T, Ishii K, Abe T, Kobayashi H. The phosphatidylinositol-3 kinase/Akt pathway mediates geranylgeranylacetone-induced neuroprotection against cerebral infarction in rats. Brain Res. 2010;1330:151–157. doi: 10.1016/j.brainres.2010.02.074. [DOI] [PubMed] [Google Scholar]
- Tu XK, Yang WZ, Wang CH, Shi SS, Zhang YL, Chen CM, Yang YK, Jin CD, Wen S. Zileuton reduces inflammatory reaction and brain damage following permanent cerebral ischemia in rats. Inflammation. 2010;33(5):344–352. doi: 10.1007/s10753-010-9191-6. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang ZG, Buller B, Jiang J, Jiang Y, Zhao D, Liu X, Morris D, Chopp M. Combination treatment with VELCADE and low-dose tissue plasminogen activator provides potent neuroprotection in aged rats after embolic focal ischemia. Stroke. 2010;41(5):1001–1007. doi: 10.1161/STROKEAHA.109.577288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diansan S, Shifen Z, Zhen G, Heming W, Xiangrui W. Resection of the nerves bundle from the sphenopalatine ganglia tend to increase the infarction volume following middle cerebral artery occlusion. Neurol Sci. 2010;31(4):431–435. doi: 10.1007/s10072-010-0238-0. [DOI] [PubMed] [Google Scholar]
- Batra A, Latour LL, Ruetzler CA, Hallenbeck JM, Spatz M, Warach S, Henning EC. Increased plasma and tissue MMP levels are associated with BCSFB and BBB disruption evident on post-contrast FLAIR after experimental stroke. J Cereb Blood Flow Metab. 2010;30(6):1188–1199. doi: 10.1038/jcbfm.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prajapati KD, Sharma SS, Roy N. Upregulation of albumin expression in focal ischemic rat brain. Brain Res. 2010;1327:118–124. doi: 10.1016/j.brainres.2010.02.063. [DOI] [PubMed] [Google Scholar]
- Chen SJ, Chang CM, Tsai SK, Chang YL, Chou SJ, Huang SS, Tai LK, Chen YC, Ku HH, Li HY. Functional improvement of focal cerebral ischemia injury by subdural transplantation of induced pluripotent stem cells with fibrin glue. Stem Cells Dev. 2010;19(11):1757–1767. doi: 10.1089/scd.2009.0452. [DOI] [PubMed] [Google Scholar]
- Maddahi A, Edvinsson L. Cerebral ischemia induces microvascular pro-inflammatory cytokine expression via the MEK/ERK pathway. J Neuroinflammation. 2010;7:14. doi: 10.1186/1742-2094-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GH, Jiang ZL, Chen ZQ, Li X, Peng LL. Neuroprotective effect of L-serine against temporary cerebral ischemia in rats. J Neurosci Res. 2010;88(9):2035–2045. doi: 10.1002/jnr.22365. [DOI] [PubMed] [Google Scholar]
- Shang J, Deguchi K, Yamashita T, Ohta Y, Zhang H, Morimoto N, Liu N, Zhang X, Tian F, Matsuura T. Antiapoptotic and antiautophagic effects of glial cell line-derived neurotrophic factor and hepatocyte growth factor after transient middle cerebral artery occlusion in rats. J Neurosci Res. 2010;88(10):2197–2206. doi: 10.1002/jnr.22373. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Dohare P, Ray M, Panda G. Design, synthesis and biological evaluation of new ionone derivatives as potential neuroprotective agents in cerebral ischemia. Eur J Med Chem. 2010;45(5):1964–1971. doi: 10.1016/j.ejmech.2010.01.039. [DOI] [PubMed] [Google Scholar]
- Cui L, Zhang X, Yang R, Wang L, Liu L, Li M, Du W. Neuroprotection of early and short-time applying atorvastatin in the acute phase of cerebral ischemia: down-regulated 12/15-LOX, p38MAPK and cPLA2 expression, ameliorated BBB permeability. Brain Res. 2010;1325:164–173. doi: 10.1016/j.brainres.2010.02.036. [DOI] [PubMed] [Google Scholar]
- Chen C, Ostrowski RP, Zhou C, Tang J, Zhang JH. Suppression of hypoxia-inducible factor-1alpha and its downstream genes reduces acute hyperglycemia-enhanced hemorrhagic transformation in a rat model of cerebral ischemia. J Neurosci Res. 2010;88(9):2046–2055. doi: 10.1002/jnr.22361. [DOI] [PubMed] [Google Scholar]
- Ya BL, Li CY, Zhang L, Wang W, Li L. Cornel iridoid glycoside inhibits inflammation and apoptosis in brains of rats with focal cerebral ischemia. Neurochem Res. 2010;35(5):773–781. doi: 10.1007/s11064-010-0134-2. [DOI] [PubMed] [Google Scholar]
- Bu Y, Kwon S, Kim YT, Kim MY, Choi H, Kim JG, Jamarkattel-Pandit N, Dore S, Kim SH, Kim H. Neuroprotective effect of HT008-1, a prescription of traditional Korean medicine, on transient focal cerebral ischemia model in rats. Phytother Res. 2010;24(8):1207–1212. doi: 10.1002/ptr.2908. [DOI] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF. Protection by D609 through cell-cycle regulation after stroke. Mol Neurobiol. 2010;41(2–3):206–217. doi: 10.1007/s12035-010-8100-1. [DOI] [PubMed] [Google Scholar]
- Prongay KD, Lewis AD, Hurn PD, Murphy SJ. Dietary soy may not confound acute experimental stroke infarct volume outcomes in ovariectomized female rats. Lab Anim. 2010;44(3):238–246. doi: 10.1258/la.2009.009031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink C, Roy S, Khan M, Ananth P, Kuppusamy P, Sen CK, Khanna S. Oxygen-sensitive outcomes and gene expression in acute ischemic stroke. J Cereb Blood Flow Metab. 2010;30(7):1275–1287. doi: 10.1038/jcbfm.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye XH, Wu Y, Guo PP, Wang J, Yuan SY, Shang Y, Yao SL. Lipoxin A4 analogue protects brain and reduces inflammation in a rat model of focal cerebral ischemia reperfusion. Brain Res. 2010;1323:174–183. doi: 10.1016/j.brainres.2010.01.079. [DOI] [PubMed] [Google Scholar]
- Kang KA, Shin ES, Hur J, Hasan MR, Lee H, Park HJ, Park HK, Kim YJ. Acupuncture attenuates neuronal cell death in middle cerebral artery occlusion model of focal ischemia. Neurol Res. 2010;32(Suppl 1):84–87. doi: 10.1179/016164109X12537002794246. [DOI] [PubMed] [Google Scholar]
- Tang NY, Liu CH, Hsieh CT, Hsieh CL. The anti-inflammatory effect of paeoniflorin on cerebral infarction induced by ischemia-reperfusion injury in Sprague–Dawley rats. Am J Chin Med. 2010;38(1):51–64. doi: 10.1142/S0192415X10007786. [DOI] [PubMed] [Google Scholar]
- Lu J, Cheng C, Zhao X, Liu Q, Yang P, Wang Y, Luo G. PEG-scutellarin prodrugs: synthesis, water solubility and protective effect on cerebral ischemia/reperfusion injury. Eur J Med Chem. 2010;45(5):1731–1738. doi: 10.1016/j.ejmech.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Sun M, Zhao Y, Gu Y, Xu C. Neuroprotective actions of aminoguanidine involve reduced the activation of calpain and caspase-3 in a rat model of stroke. Neurochem Int. 2010;56(4):634–641. doi: 10.1016/j.neuint.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Wang J, Jiang C, Liu C, Li X, Chen N, Hao Y. Neuroprotective effects of progesterone following stroke in aged rats. Behav Brain Res. 2010;209(1):119–122. doi: 10.1016/j.bbr.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Gao F, Wang S, Guo Y, Wang J, Lou M, Wu J, Ding M, Tian M, Zhang H. Protective effects of repetitive transcranial magnetic stimulation in a rat model of transient cerebral ischaemia: a microPET study. Eur J Nucl Med Mol Imaging. 2010;37(5):954–961. doi: 10.1007/s00259-009-1342-3. [DOI] [PubMed] [Google Scholar]
- Ishii T, Asai T, Urakami T, Oku N. Accumulation of macromolecules in brain parenchyma in acute phase of cerebral infarction/reperfusion. Brain Res. 2010;1321:164–168. doi: 10.1016/j.brainres.2010.01.039. [DOI] [PubMed] [Google Scholar]
- Zwagerman N, Sprague S, Davis MD, Daniels B, Goel G, Ding Y. Pre-ischemic exercise preserves cerebral blood flow during reperfusion in stroke. Neurol Res. 2010;32(5):523–529. doi: 10.1179/016164109X12581096796431. [DOI] [PubMed] [Google Scholar]
- Yamane K, Kitamura Y, Yanagida T, Takata K, Yanagisawa D, Taniguchi T, Taira T, Ariga H. Oxidative neurodegeneration is prevented by UCP0045037, an allosteric modulator for the reduced form of DJ-1, a wild-type of familial Parkinson's disease-linked PARK7. Int J Mol Sci. 2009;10(11):4789–4804. doi: 10.3390/ijms10114789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David HN, Haelewyn B, Risso JJ, Colloc'h N, Abraini JH. Xenon is an inhibitor of tissue-plasminogen activator: adverse and beneficial effects in a rat model of thromboembolic stroke. J Cereb Blood Flow Metab. 2010;30(4):718–728. doi: 10.1038/jcbfm.2009.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang N, Yun Y, Li H, Hou L, Han M, Li G. SO2 inhalation contributes to the development and progression of ischemic stroke in the brain. Toxicol Sci. 2010;114(2):226–236. doi: 10.1093/toxsci/kfq010. [DOI] [PubMed] [Google Scholar]
- Liebelt B, Papapetrou P, Ali A, Guo M, Ji X, Peng C, Rogers R, Curry A, Jimenez D, Ding Y. Exercise preconditioning reduces neuronal apoptosis in stroke by up-regulating heat shock protein-70 (heat shock protein-72) and extracellular-signal-regulated-kinase 1/2. Neuroscience. 2010;166(4):1091–1100. doi: 10.1016/j.neuroscience.2009.12.067. [DOI] [PubMed] [Google Scholar]
- Ruscher K, Erickson A, Kuric E, Inacio AR, Wieloch T. Effects of chronic Clozapine administration on apolipoprotein D levels and on functional recovery following experimental stroke. Brain Res. 2010;1321:152–163. doi: 10.1016/j.brainres.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Zhan X, Ander BP, Liao IH, Hansen JE, Kim C, Clements D, Weisbart RH, Nishimura RN, Sharp FR. Recombinant Fv-Hsp70 protein mediates neuroprotection after focal cerebral ischemia in rats. Stroke. 2010;41(3):538–543. doi: 10.1161/STROKEAHA.109.572537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Fan X, Yu Z, Liu J, Murata Y, Lu J, Zhao S, Hajjar KA, Lo EH, Wang X. Annexin A2 combined with low-dose tPA improves thrombolytic therapy in a rat model of focal embolic stroke. J Cereb Blood Flow Metab. 2010;30(6):1137–1146. doi: 10.1038/jcbfm.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewell SS, Fernandez JA, Cox SF, Spratt NJ, Hogan L, Aleksoska E, van Raay L, Liberatore GT, Batchelor PE, Howells DW. Inducing stroke in aged, hypertensive, diabetic rats. J Cereb Blood Flow Metab. 2010;30(4):729–733. doi: 10.1038/jcbfm.2009.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu Fanne R, Nassar T, Yarovoi S, Rayan A, Lamensdorf I, Karakoveski M, Vadim P, Jammal M, Cines DB, Higazi AA. Blood–brain barrier permeability and tPA-mediated neurotoxicity. Neuropharmacology. 2010;58(7):972–980. doi: 10.1016/j.neuropharm.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Kim YJ, Kim YH, Roh J, Kim SU, Yoon BW. Using a neodymium magnet to target delivery of ferumoxide-labeled human neural stem cells in a rat model of focal cerebral ischemia. Hum Gene Ther. 2010;21(5):603–610. doi: 10.1089/hum.2009.144. [DOI] [PubMed] [Google Scholar]
- Zacharek A, Shehadah A, Chen J, Cui X, Roberts C, Lu M, Chopp M. Comparison of bone marrow stromal cells derived from stroke and normal rats for stroke treatment. Stroke. 2010;41(3):524–530. doi: 10.1161/STROKEAHA.109.568881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Wu J, Xu K, Cai F, Gu J, Ma L, Chen J. Neuroprotection by baicalein in ischemic brain injury involves PTEN/AKT pathway. J Neurochem. 2010;112(6):1500–1512. doi: 10.1111/j.1471-4159.2009.06561.x. [DOI] [PubMed] [Google Scholar]
- Cao W, Shah HP, Glushakov AV, Mecca AP, Shi P, Sumners C, Seubert CN, Martynyuk AE. Efficacy of 3,5-dibromo-L-phenylalanine in rat models of stroke, seizures and sensorimotor gating deficit. Br J Pharmacol. 2009;158(8):2005–2013. doi: 10.1111/j.1476-5381.2009.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan M, Constantinescu AO, Balseanu A, Oprescu N, Zagrean L, Popa-Wagner A. Sleep deprivation attenuates experimental stroke severity in rats. Exp Neurol. 2010;222(1):135–143. doi: 10.1016/j.expneurol.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10(2):290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Leach MJ, Swan JH, Eisenthal D, Dopson M, Nobbs M. BW619C89, a glutamate release inhibitor, protects against focal cerebral ischemic damage. Stroke. 1993;24(7):1063–1067. doi: 10.1161/01.str.24.7.1063. [DOI] [PubMed] [Google Scholar]
- Schlattmann P, Dirnagl U. Statistics in experimental cerebrovascular research-comparison of two groups with a continuous outcome variable. J Cereb Blood Flow Metab. 2010;30(3):474–479. doi: 10.1038/jcbfm.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strom JO, Strid T, Hammarstrom S. Disruption of the alox5ap gene ameliorates focal ischemic stroke: possible consequence of impaired leukotriene biosynthesis. BMC Neurosci. 2012;13:146. doi: 10.1186/1471-2202-13-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hom S, Fleegal MA, Egleton RD, Campos CR, Hawkins BT, Davis TP. Comparative changes in the blood–brain barrier and cerebral infarction of SHR and WKY rats. Am J Physiol Regul Integr Comp Physiol. 2007;292(5):R1881–R1892. doi: 10.1152/ajpregu.00761.2005. [DOI] [PubMed] [Google Scholar]
- Dogan A, Baskaya MK, Rao VL, Rao AM, Dempsey RJ. Intraluminal suture occlusion of the middle cerebral artery in Spontaneously Hypertensive rats. Neurol Res. 1998;20(3):265–270. doi: 10.1080/01616412.1998.11740517. [DOI] [PubMed] [Google Scholar]
- Brint S, Jacewicz M, Kiessling M, Tanabe J, Pulsinelli W. Focal brain ischemia in the rat: methods for reproducible neocortical infarction using tandem occlusion of the distal middle cerebral and ipsilateral common carotid arteries. J Cereb Blood Flow Metab. 1988;8(4):474–485. doi: 10.1038/jcbfm.1988.88. [DOI] [PubMed] [Google Scholar]
- Duverger D, MacKenzie ET. The quantification of cerebral infarction following focal ischemia in the rat: influence of strain, arterial pressure, blood glucose concentration, and age. J Cereb Blood Flow Metab. 1988;8(4):449–461. doi: 10.1038/jcbfm.1988.86. [DOI] [PubMed] [Google Scholar]
- Aspey BS, Cohen S, Patel Y, Terruli M, Harrison MJ. Middle cerebral artery occlusion in the rat: consistent protocol for a model of stroke. Neuropathol Appl Neurobiol. 1998;24(6):487–497. doi: 10.1046/j.1365-2990.1998.00146.x. [DOI] [PubMed] [Google Scholar]
- Dittmar MS, Vatankhah B, Fehm NP, Schuierer G, Bogdahn U, Horn M, Schlachetzki F. Fischer-344 rats are unsuitable for the MCAO filament model due to their cerebrovascular anatomy. J Neurosci Meth. 2006;156(1–2):50–54. doi: 10.1016/j.jneumeth.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Prieto R, Carceller F, Roda JM, Avendano C. The intraluminal thread model revisited: rat strain differences in local cerebral blood flow. Neurol Res. 2005;27(1):47–52. doi: 10.1179/016164105X18214. [DOI] [PubMed] [Google Scholar]
- Walberer M, Stolz E, Muller C, Friedrich C, Rottger C, Blaes F, Kaps M, Fisher M, Bachmann G, Gerriets T. Experimental stroke: ischaemic lesion volume and oedema formation differ among rat strains (a comparison between Wistar and Sprague–Dawley rats using MRI) Lab Anim. 2006;40(1):1–8. doi: 10.1258/002367706775404426. [DOI] [PubMed] [Google Scholar]
- McCabe C, Gallagher L, Gsell W, Graham D, Dominiczak AF, Macrae IM. Differences in the evolution of the ischemic penumbra in stroke-prone spontaneously hypertensive and Wistar-Kyoto rats. Stroke. 2009;40(12):3864–3868. doi: 10.1161/STROKEAHA.109.559021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardutzky J, Shen Q, Henninger N, Bouley J, Duong TQ, Fisher M. Differences in ischemic lesion evolution in different rat strains using diffusion and perfusion imaging. Stroke. 2005;36(9):2000–2005. doi: 10.1161/01.STR.0000177486.85508.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemitsu H, Nakagomi T, Tamura A, Tsuchiya T, Kono G, Sano K. Differences in the extent of primary ischemic damage between middle cerebral artery coagulation and intraluminal occlusion models. J Cereb Blood Flow Metab. 2002;22(10):1196–1204. doi: 10.1097/01.wcb.0000037992.07114.95. [DOI] [PubMed] [Google Scholar]
- Bouley J, Fisher M, Henninger N. Comparison between coated vs. uncoated suture middle cerebral artery occlusion in the rat as assessed by perfusion/diffusion weighted imaging. Neurosci Lett. 2007;412(3):185–190. doi: 10.1016/j.neulet.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Laing RJ, Jakubowski J, Laing RW. Middle cerebral artery occlusion without craniectomy in rats. Which method works best? Stroke. 1993;24(2):294–297. doi: 10.1161/01.str.24.2.294. discussion 297–298. [DOI] [PubMed] [Google Scholar]
- Spratt NJ, Fernandez J, Chen M, Rewell S, Cox S, van Raay L, Hogan L, Howells DW. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J Neurosci Meth. 2006;155(2):285–290. doi: 10.1016/j.jneumeth.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Bavik CO, Eriksson U, Allen RA, Peterson PA. Identification and partial characterization of a retinal pigment epithelial membrane receptor for plasma retinol-binding protein. J Biol Chem. 1991;266(23):14978–14985. [PubMed] [Google Scholar]
- Lourbopoulos A, Karacostas D, Artemis N, Milonas I, Grigoriadis N. Effectiveness of a new modified intraluminal suture for temporary middle cerebral artery occlusion in rats of various weight. J Neurosci Meth. 2008;173(2):225–234. doi: 10.1016/j.jneumeth.2008.06.018. [DOI] [PubMed] [Google Scholar]
- Kuge Y, Minematsu K, Yamaguchi T, Miyake Y. Nylon monofilament for intraluminal middle cerebral artery occlusion in rats. Stroke. 1995;26(9):1655–1657. doi: 10.1161/01.str.26.9.1655. discussion 1658. [DOI] [PubMed] [Google Scholar]
- Oliff HS, Weber E, Eilon G, Marek P. The role of strain/vendor differences on the outcome of focal ischemia induced by intraluminal middle cerebral artery occlusion in the rat. Brain Res. 1995;675(1–2):20–26. doi: 10.1016/0006-8993(95)00033-m. [DOI] [PubMed] [Google Scholar]
- Sena ES, Briscoe CL, Howells DW, Donnan GA, Sandercock PA, Macleod MR. Factors affecting the apparent efficacy and safety of tissue plasminogen activator in thrombotic occlusion models of stroke: systematic review and meta-analysis. J Cereb Blood Flow Metab. 2010;30(12):1905–1913. doi: 10.1038/jcbfm.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]