Abstract
Recent data indicate that Tau immunotherapy may be relevant for interfering with neurofibrillary degeneration in Alzheimer disease and related disorders referred to as Tauopathies. The key question for immunotherapy is the choice of the epitope to target. Abnormal phosphorylation is a well-described post-translational modification of Tau proteins and may be a good target. In the present study, we investigated the effects of active immunization against the pathological epitope phospho-Ser422 in the THY-Tau22 transgenic mouse model.
Starting from 3–6 months of age, THY-Tau22 mice develop hippocampal neurofibrillary tangle-like inclusions and exhibit phosphorylation of Tau on several AD-relevant Tau epitopes. Three month-old THY-Tau22 mice were immunized with a peptide including the phosphoserine 422 residue while control mice received the adjuvant alone.
A specific antibody response against the phospho-Ser422 epitope was observed. We noticed a decrease in insoluble Tau species (AT100- and pS422 immunoreactive) by both biochemical and immunohistochemical means correlated with a significant cognitive improvement using the Y-maze. This Tau immunotherapy may facilitate Tau clearance from the brain toward the periphery since, following immunization, an increase in Tau concentrations was observed in blood.
Overall, the present work is, to our knowledge, the first one to demonstrate that active immunotherapy targeting a real pathological epitope such as phospho-Ser422 epitope is efficient. This immunotherapy allows for Tau clearance and improves cognitive deficits promoted by Tau pathology in a well-defined Tau transgenic model.
Keywords: Age Factors; Alzheimer Disease; genetics; immunology; pathology; physiopathology; therapy; Animals; Antibodies; blood; Cognition Disorders; etiology; therapy; Disease Models, Animal; Enzyme-Linked Immunosorbent Assay; Hippocampus; metabolism; pathology; Humans; Immunotherapy, Active; methods; Male; Maze Learning; physiology; Mice; Mice, Inbred C57BL; Mice, Transgenic; Mutation; genetics; Peptides; administration & dosage; immunology; Phosphorylation; immunology; Serine; genetics; metabolism; tau Proteins; genetics; metabolism
Keywords: Alzheimer, immunotherapy, neurofibrillary degeneration, transgenic mouse model, Tau proteins
Introduction
Neurodegenerative disorders characterized by the intracellular aggregation of the microtubule-associated Tau proteins are referred to as Tauopathies [1,2]. Among them, the most common one is Alzheimer Disease (AD). Tau proteins found in these aggregates are always phosphorylated and referred to as pathological Tau. However, it should be noted that aggregated Tau proteins bear both physiological and pathological phosphorylation sites [1,2].
Pathological Tau proteins are also found extracellularly [2,3]. Extracellular pathological Tau may participate to the propagation of the pathology within the brain by contaminating neighbor neurons [4,5]. Formation of Tau aggregates in a single brain cell and their subsequent spreading may therefore be at the origin of sporadic tauopathies. Consequently, the presence of Tau aggregates in the extracellular space would be an instrumental step in the events leading to disease progression [5]. Immunization strategies are prone to prevent this process.
There are two types of approach in immunotherapy: active and passive [6–8]. In active immunotherapy, immunization is achieved by challenging the immune system with a foreign antigen. Passive immunotherapy consists in the use of a specific antibody, which targets a pathological epitope. It has the advantage of providing immediate protection. Nevertheless, it is effective only for a short duration of time and thus increases the cost of the treatment [9].
Recently, active and passive immunotherapies have been shown to be successful in rodent models of Tauopathies [10–13]. The key question for Tau immunotherapy is the choice of the epitope to target. Indeed, targeting non-phosphorylated Tau has been shown to be deleterious since Tau proteins are normal neuronal components [14]. This also suggests that Tau immunotherapy does not only affect extracellular but might also reach intracellular Tau. Therefore, it is essential to target pathological Tau species. Abnormal phosphorylation is a well-described post-translational modification of Tau proteins. Numerous phosphorylation-dependent anti-Tau antibodies (Alz50, AP422, AT100, MC1, TG-3) are known to specifically recognize pathological Tau species [1,2]. Such signature may be useful to define targets for immunotherapy. Among these epitopes, most of them are conformational and are not suitable for active immunotherapy with the exception of phospho-Ser422. AP422, 988 and pS422 antibodies have been extensively studied showing that the phospho-Ser422 residue is specific to Tau pathology and encountered in numerous neurodegenerative disorders [1,2,15–17] as well as in a number of Tau transgenic mice including the THY-Tau22 model [18–20]. Starting from 3–6 months of age, THY-Tau22 mice develop hippocampal neurofibrillary tangle-like inclusions and exhibit phosphorylation of Tau on several AD-relevant Tau epitopes [20,21]. Specifically, at early stages (3 months of age), hyperphosphorylated Tau proteins are visualized (AD2, AT270) in the hippocampus while abnormal Tau species (AT100, pS422) are faintly observed. Over the time, hippocampal pathology increases with strong AT100 and pS422 immunoreactivities at 8–10 months of age, ultimately correlated with cognitive deterioration [20–22].
In the present work, peptides containing the phospho-Ser422 residue were used to immunize the THY-Tau22 transgenic mice in order to assess Tau active immunotherapy targeting a pathological epitope.
Materials & methods
Animals
In the present study, we have used heterozygous male THY-Tau22 and littermate wild type mice as controls [20–22]. All animals were kept in standard animal cages under conventional laboratory conditions (12h/12h light-dark cycle, 22°C), with ad libitum access to food and water. All experiments on animals were performed in compliance with, and following the approval of the local Animal Resources Committee, standards for the care and use of laboratory animals and with French and European Community rules (Approval n° AF 06/2010, March 31, 2010).
Antigens
The two synthesized peptides were from NeoMPS (France) with a minimum of 80% purity. Both phospho-peptides contain the Tau sequence (in bold) and three amino-acids Tyr-Gly-Gly allowing for spacing and coupling: Y14T [Tyr-Gly-Gly-Ile-Asp-Met-Val-Asp-Ser(PO3H2)-Pro-Gln-Leu-Ala-Thr] and Y10A [Tyr-Gly-Gly-Val-Asp-Ser(PO3H2)-Pro-Gln-Leu-Ala]. They were conjugated to KLH through the Tyr residue.
Vaccine preparation and administration
For the first injection, antigen was mixed 1:1 with Freund’s complete adjuvant (F5881; Sigma), and 100 μg was injected intraperitoneally. For the following injections, antigen was mixed 1:1 with Freund’s incomplete adjuvant (F5506; Sigma). The first two injections were administered every two weeks, the following injections monthly. Control mice received adjuvant alone.
A first protocol was used to evaluate immune response in 3.5 month-old THY-Tau22 mice either injected by the Y10A (n=5) or the Y14T peptide (n=5) for 14 weeks. Then, Tau immunotherapy protocols using the Y10A peptide were started at the age of 15 weeks for 18 weeks (Vaccinated THY-Tau22, n=13; Control THY-Tau22, n=12; Littermate controls, n=7).
Antibody response
Mice were bled before the beginning of the study (S0) and one week following each injection (Sn). The antibody response was determined by serial dilution of sera using ELISA as follows: 96-well microtiter plates (Maxisorp F8; Nunc, Inc.) were coated overnight at 4°C with 100ng/well of S422-Tau peptide, pS422-Tau peptide and an irrelevant peptide (ATP synthase alpha chain; NeoMPS, France) in 50 mM NaHCO3, pH 9.6. After 3 washes with PBS containing 0.05% Tween (PBS-T), plates were blocked and several dilutions of sera tested by use of goat anti-mouse IgG (γ specific) horseradish peroxidase-conjugated antibody (A3673; Sigma) at 1:4000 dilution. Tetramethyl benzidine (T3405, Sigma) was the substrate. The reaction was stopped by addition of sulfuric acid, changing the color from blue to yellow. Plates were measured with a spectrophotometer (Multiskan Ascent, Thermo Labsystem) at 450 nm.
Tau assays in blood samples from Y10A vaccinated mice
Tau concentrations (pg/ml) were determined by serial dilutions of sera (S0-S4) using the INNOTEST® hTau Ag (Innogenetics, Belgium) that is a sandwich ELISA microplate assay for the quantitative determination of human Tau antigen in fluids. Capture antibody is the AT120 antibody and biotinylated antibodies HT7 and BT2 are detecting antibodies [2].
Behavioral test: Y-Maze
Mice were tested for short-term hippocampus-dependent spatial memory using a two-trial Y-maze task. The arms of the maze were 22cm long, 6.4cm wide and 15cm deep. The floor of the maze was covered with sawdust that was mixed after each trial in order to eliminate olfactory cues. Various extra-maze cues were placed on the surrounding walls. Experiments were conducted with an ambient light level of 6 lux. During the exposure phase, mice were assigned to two arms (the “start arm” and the “other arm”), which can be freely explored during 5 min, without access to the third arm of the maze (the “novel arm”), blocked by an opaque door. The assignment of arms was counterbalanced within each experimental group. Mice were then removed from the maze and returned to their home cage for 2 min. During the test phase, mice were placed at the end of the same “start arm” and allowed to freely explore all three arms during 1 min. The amount of time spent in each of the arms was recorded using EthovisionXT (Noldus, Netherlands).
Immunohistochemistry
After the completion of behavioral experiments, animals were sacrificed and brains removed. Some of the brains (Non-vaccinated, n=6; vaccinated, n=7) were post-fixed for 7 days in 4% paraformaldehyde, then incubated in 20% sucrose for 24 hours and finally kept frozen at −80°C until use. Serial free-floating coronal sections (40μm) were obtained using a cryostat (Leica Microsystems GmbH, Germany). Sections of interest were used for free floating immunohistochemistry using the following antibodies AT8 (Pierce MN-120, against pS202/T205, 1/200), AT100 (Pierce MN-1060, against pT212/S214, 1/400), and anti-Tau pS422 (Biosource 44-764G 1/500) as described [21], and finally mounted on gelatine slides. Staining was semi-quantified as previously described [23]. Photomicrographs were taken using a Leica digital camera, imported in ImageJ software (Scion) and converted to black and white images. Threshold intensity was set and kept constant and the number of pixel, expressing staining density, was determined for both THY-Tau22 and vaccinated THY-Tau22- mice. Quantifications were performed blindly by at least two observers and averaged from six to seven animals per group.
Western blot analysis
In remaining brains (Non-vaccinated, n=6; vaccinated, n=6), neocortex and hippocampi were dissected out using a coronal acrylic slicer (Delta Microscopies, France) at 4°C and stored at − 80°C until use. Tissue was homogenized in 200 μl Tris buffer pH 7.4 containing 10% sucrose and protease inhibitors (Complete, Roche, France). Hundred μl of lysates were collected and centrifuged at 12000g for 5 minutes at 4°C. The resulting supernatant was collected; the volume adjusted at 100 μl with sucrose buffer and 100 μl of sodium dodecyl sulfate (SDS) buffer (10-mM Tris-HCl pH 7.4, 1M NaCl, Triton 4%, SDS 0.2% and protease inhibitors) was added. The SDS homogenate was sonicated and spun at 100,000×g during 1h at 4°C. The remaining pellet containing the SDS-insoluble Tau protein was resuspended in 50 μL of LDS 2X, sonicated and boiled 10 min at 100°C.
For Western Blot analysis, samples were diluted in NuPage sample buffer (Invitrogen) and denaturated at 100°C for 5 minutes. Then, proteins were loaded on 4–12% NuPAGE Novex gels (Invitrogen), and transferred to nitrocellulose membranes and incubated with appropriate antibodies. Signals were visualized by chemiluminescence (ECL, Amersham Biosciences). Antibodies used for western blot were AT8, AT100, pS422 and Tau5.
Statistics
Data are presented as mean ± standard error of the mean (SEM). Differences between mean values were determined using the Student’s t-test or ANOVA followed by a post-hoc Fisher’s LSD test. Differences of p<0.05 were considered significant. Data were analyzed using Statistica software (Statsoft) and graphs were plotted by Prism Graphpad (San Diego, CA, USA).
Results
THY-Tau22 mice progressively develop hippocampal Tau pathology
THY-Tau22 transgenic mice express human 4-repeats Tau mutated at sites G272V and P301S under a neuronal Thy 1.2 promoter (Fig. 1A). In contrast to many other Tau transgenic models, THY-Tau22 mice do not show any signs of motor deficits or changes in motor activity at any age investigated, therefore allowing behavioral testing without interference due to motor abnormality. THY-Tau22 mice displayed abnormal Tau phosphorylation (AT100, pS422) in the CA1 region of hippocampus, starting from 3 months of age and progressively develop over time, as observed by immunohistochemistry and western blotting (Fig. 1B–C). This age-dependent topological development of Tau pathology within the hippocampus occurred in parallel to learning and memory impairments, in absence of neuronal death and synaptic loss [20,22].
Fig. 1. Age-dependent progression of Tau pathology in the hippocampus of THY-Tau22 mice.
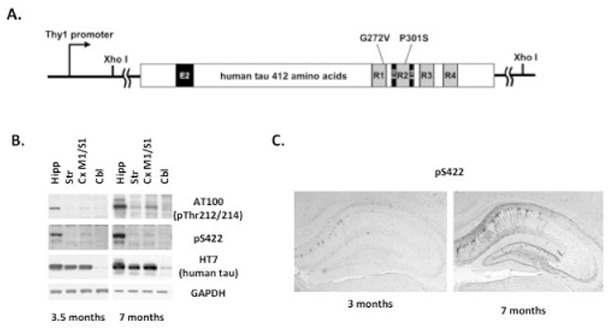
A. THY-Tau22 is a Tau transgenic mouse model, which over-expresses the human Tau isoform with one amino-terminal insert (E2) and 4 microtubule-binding domains (E10) under a neuron-specific Thy1.2-promoter. Tau is mutated at sites G272V and P301S. B. Progressive development of hippocampal Tau pathology as seen following western blot analysis from hippocampal homogenates at 3.5 and 7 month-old WT and THY-Tau22 mice, using AT100 and pS422 antibodies. Both AT100 and pS422 stainings are never observed in WT animals and total human Tau protein remains unchanged over time, as visualized using the HT7 antibody. GAPDH is used as an internal loading control. C. Immunohistochemical labelling of the pathological pS422 Tau epitope in hippocampal sagittal sections from 3 and 7 month-old THY-Tau22 mice. Tau pathology extends with time throughout the CA1 region of the hippocampus.
Specific immunological response toward phospho-Ser422 residue
Two peptides surrounding the Ser422 residue were designed in order to specifically target the phosphorylation site. The Y14T peptide corresponds to 11 amino-acids of the Tau sequence whereas the Y10A contains 7 amino-acids. Mice immunized with both immunogens in Freund’s adjuvant developed good IgG antibody response against the immunogen (pS422-Tau) compared to an irrelevant peptide (Fig. 2A–B). In the case of the Y14T peptide, these antibodies also recognized the non-phosphorylated peptide as well (Fig. 2A). Conversely, mice immunized with the shorter peptide Y10A exhibited a weaker IgG antibody response in sera than the Y14T ones. However, antibodies found in Y10A sera were more specific, towards the phosphoepitope, than the Y14T ones, making the Y10A phospho-peptide more interesting for a specific active immunotherapy. In addition, sera from both immunized mice were used for brain immunohistochemistry in THY-Tau22 mice (Fig. 2C) and AD patients (data not shown). Both recognized neurofibrillary tangles similar to those identified by immunohistochemistry with AT100- and pS422-antibodies (Fig. 2C).
Fig. 2. Evaluation of the immune response in vaccinated THY-Tau22 mice.
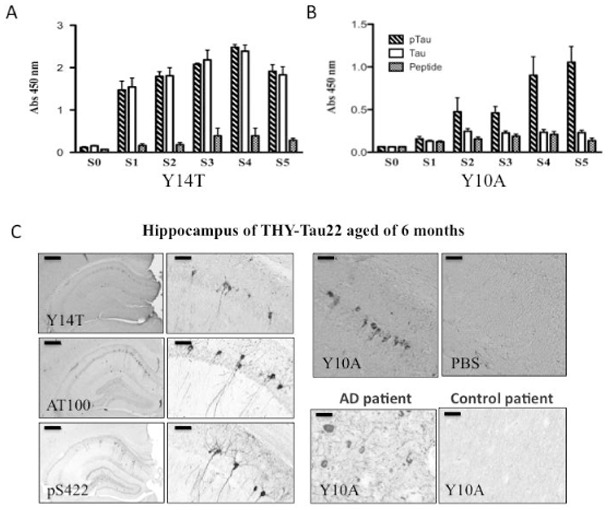
THY-Tau22 mice were immunized with either the Y14T phospho-Tau peptide (A) or the Y10A phospho-Tau peptide (B). Serum samples (Pre-immune (S0) and S1–S5) were collected. A, B, Shown is the generation of IgG antibodies (1:1500 serum dilution) against the immunogen at various time points as determined by ELISA assay with phospho-Ser422 Tau peptide (pTau), non-phosphorylated Tau peptide (Tau) and an irrelevant peptide (Peptide) [A, B, 3.5–6.5 months: S0, S1, S2, S3, S4, and S5 = 14, 15, 17, 19, 24 and 29 weeks]. Mice immunized with the Y10A phospho-Tau peptide gave the most specific immune response. Error bars indicate SEM.
C: Hippocampal brain sections of THY Tau22 aged of 6 months labeled with serum from Y14T- (top, left panel) and Y10A-immunized mouse (right panel) and with antibodies against abnormally phosphorylated Tau species (AT100, pS422; bottom, left panel). As a control, pre-immune serum from Y10A-immunized was used (S0, right panel). Representative human brain sections labeled with serum from Y10A-immunized mouse are also shown (Control and AD cases; bottom, right panel).
Left panels: Scale bar = 300μm. Medium panels: Scale bar = 50μm. Top right panels (Y10A; PBS): Scale bar = 50μm. Bottom right panels (human brain sections): Scale bar = 25 μm.
Knowing that we were able to induce a specific immune response against the pathological Tau epitope pS422 using the Y10A peptide, a Tau immunotherapy protocol was developed. THY-Tau22 mice were first injected at the age of 15 weeks for 18 weeks. Mice were sacrificed at 36 weeks of age at completion of behavioural experiments.
Active immunotherapy and cognition
THY-Tau22 mice have strong deficits in learning and memory in the Morris water maze at 12 months of age [22]. Around 7–8 months, we previously showed that a Y-maze is more appropriate to assess short-term memory in this model [21]. Thus, memory was assessed using a two-trial Y-maze task. During the exposure phase, all groups explored the maze equally, spending a similar amount of time in each available arm (Fig. 3A). No differences were found comparing distance moved (Fig. 3B). During the test phase, WT littermate controls (n=7) and vaccinated THY-Tau22 mice (n=13) spent a significantly greater proportion of time in the novel arm than expected by chance (p<0.05) whereas non-vaccinated THY-Tau22 mice (n=12) did not (Fig. 3C). In this test, the behavior of littermate controls was similar to that of vaccinated THY-Tau22 mice (Fig. 3C).
Fig. 3. Effects of vaccination with Y-10-A on spatial memory of THY-Tau22 aged of 8 months.
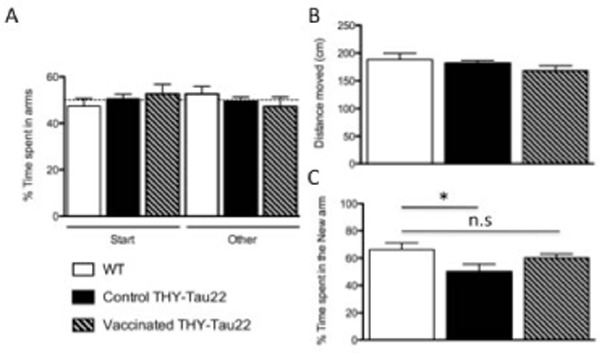
A-B: Working memory in the Y-maze. During exposure, WT and Tau transgenic mice equally explored the single open (other) arm.
C: During the test, WT mice spent significantly more time in the new arm compared to control THY-Tau22 (n=12). Such defect is prevented by vaccination as both vaccinated THY-Tau22 (n=13) and WT mice (n=7) spent a comparable time exploring the novel arm.
Active immunotherapy and Tau pathology
To assess the impact of Tau immunotherapy on the development of Tau pathology, we carried out an immunohistochemical analysis of phosphorylated (AT8; pSer202/205) and abnormally phosphorylated (AT100; pSer212/pThr214; pSer422) Tau species. While there was no striking difference in the level of phosphorylated Tau transgene at AT8 epitope in vaccinated THY-Tau22 mice as compared to control THY-Tau22 mice (Fig. 4A), vaccination reduced the level of pathological Tau species not only at the targeted epitope (pS422) but also at the AT100 epitope (Fig. 4A). AT100 reflects mainly aggregated Tau [19]. To further explore Tau aggregation, biochemical fractionation was performed and soluble and insoluble fractions were analyzed. If a degradation process targeting pathological Tau species is activated, it would reduce the aggregation rate and thus the amount of insoluble materials as visualized by AT100 immunoreactivity. As expected, the most striking differences were observed in the insoluble fractions with decreased AT100- and Phospho-Ser422-immunoreactive insoluble Tau materials (Fig. 4B).
Fig. 4. Effects of vaccination on Tau proteins in THY-Tau22 mice aged of 8 months.
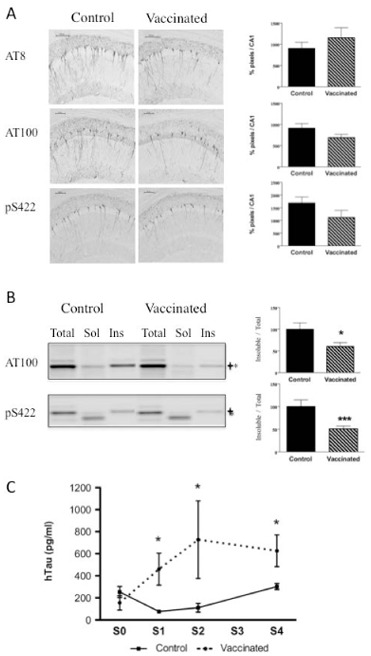
A: There was no significant difference in the levels of phosphorylated Tau species as revealed by AT8 immunoreactivity between THY-Tau22-vaccinated mice (n=7) and control THY-Tau22 mice (n=6), (p=0.6282 using Student’s t-test). Abnormally phosphorylated Tau species as revealed by AT100 and pS422 immunoreactivity are decreased in THY-Tau22-vaccinated mice as compared to THY-Tau22 controls even if it did not reach significance (p=0.0516 for AT100 and p=0.0842 for pS422 using Student’s t-test). B: Insoluble Tau species (AT100 and pS422-immunoreactive) were significantly decreased by vaccination against pS422 in THY-Tau22 (n=6 in each group).
C: Tau concentration (pg/ml) from sera of vaccinated THY-Tau22 was significantly increased by vaccination [S0, S1, S2, S3, and S4 = 15, 16, 18, 20, and 25 week-old]. The results are expressed mean ± SEM * p<0,05, *** p<0,001.
Active immunotherapy and Tau clearance
We also analyzed Tau amounts in blood during the immunization protocol. Interestingly, we observed an increase in Tau concentrations in sera following the number of immunizations (from pre-immune serum S0 to sera S1–S4) in vaccinated THY-Tau22 compared to control THY-Tau22 mice. It suggests that increasing antibodies titer may enhance Tau clearance from brain to blood (Fig. 4C).
Discussion
In the present work, we investigated the targeting of a particular Tau epitope by active immunotherapy in the THY-Tau22 mouse model. A specific immune response was obtained against the targeted epitope pS422. Interestingly, a trend to decreasing AT100- and AP422-immunoreactivities was observed on brain sections in vaccinated animals compared to control THY-Tau22 mice. These data were further supported by biochemical data showing a decrease in aggregated Tau proteins in vaccinated animals. Finally, this active immunotherapy also likely delayed cognitive deficits in the THY-Tau22 model as observed in the Y-maze test.
Ser422 is one of the 17 Ser/Thr-Pro sites found on Tau proteins. Ser422 phosphorylation may be linked to the activation of stress-activated protein kinases [24]. It is one of the rare phosphorylation sites that is not observed on Tau in physiological conditions [16,25]. Phosphorylation of Ser422 has been described in starved wild-type mice [26] but it is always found in Tauopathies and experimental models of Tau pathology [17,19,27–29]. It is also exacerbated after Aβ exposure [30–32]. In the THY-Tau22 model, as observed in other Tau models [19], this epitope is found when Tau aggregates into sarkosyl-insoluble materials [20,21].
Targeting pS422 leads to a decrease in insoluble Tau species (AT100- and pS422-immunoreactive). One can ask what are the mechanisms leading to this decrease. Active immunization leads to a specific immune response toward phospho-Ser422. It is known that a small percentage of antibodies is able to cross the blood-brain barrier [33,34]. Once in the brain, anti-Tau antibodies may easily recognize extracellular epitopes found in ghost tangles. However, they may also enter the cell through the endosome-lysosome pathway and activate mechanisms of Tau clearance [35]. Phosphorylation at Ser422 also blocks Asp421 caspase cleavage [36]. This latter may lead to formation of truncated Tau species, which act as nucleation agent [37]. In the THY- Tau models, Asp421 caspase cleavage site is only found after 12 months [38]. However, in humans, phospho-Ser422 active imunotherapy might protect Tau from this caspase cleavage and thus limit the aggregation process. Moreover, recent data suggest that Tau is also secreted [2–4]. This extracellular Tau may participate to the propagation of Tau pathology [4]. Clearance of extracellular Tau by antibodies may also reduce Tau pathology, either by direct antibody-mediated disassembly or by microglia activation [39]. Finally, as observed with some anti-Aβ antibodies, the peripheral sink hypothesis may also be raised [40]. Sera (S1-S4) were collected one week after each peptide immunization (I1-I4). Since we observed an increase in Tau concentrations in collected sera from vaccinated animals (Fig. 3C), it strongly suggests that circulating antibodies sequester Tau and favor efflux of Tau from the brain.
Active immunization, as seen in the present study and described by other groups, is a therapeutic strategy which acts on Tau pathology and behavioural alterations [10–12]. There are some discrepancies among studies but, as learnt from the Aβ immunotherapies experience, such differences rely on both the type of vaccination, the targeted antigen and the model used [6,7,39,40]. Regarding models, THY-Tau22 mice have many advantages since they do not display motor deficits and they have late neurodegeneration. When comparing the THY-Tau22 to other tau transgenic animal models, motor deficits are due to either spinal cord pathology or loss of motor cortex input and have been described in P301L [18], P301S [19], rTg4510 [41], and THY-Tau30 (Tg30)[38]. Moreover, lack of motor deficits is also an asset to perform behavior in THY-Tau22 mice [20–22]. Finally, the Ser422 epitope has not been analyzed in other available models similar to THY-Tau22 such as the htau model [42, 43].
To be used as a therapeutic strategy, immunotherapy needs to target a specific pathological antigen [44,45]. Most of the studies on Tau immunization have chosen irrelevant epitopes, which are not only hyperphosphorylated in AD but are also found in physiological conditions [10–13,46]. In fact, synthetic phospho-peptides used as antigens in active immunization studies [10–12] included physiologically epitopes [47] and thus, it is necessary to target a pathological phosphorylation. However, most of the abnormal phospho-sites on aggregated Tau display a specific conformation, which is not found in synthetic phospho-Tau peptides with the exception of phospho-Ser422 [15,48,49]. We previously developed a number of polyclonal antibodies raised against phosphorylation-dependent epitopes including phospho-Ser422 [16]. One of the key issues of this approach is the specificity of the immune response. Indeed, using a phospho-peptide, immunization leads to a polyclonal response targeting not only the phospho-residue but also its neighbouring sites. For instance, Boutajangout et coll. showed in their protocol, that immune response is also directed against non-phosphorylated Tau [12]. With our experience, we designed two phospho-peptides for immunization: the Y14T peptide corresponds to 11 amino-acids of the Tau sequence whereas the Y10A contains 7 amino-acids, both surrounding the Ser422 residue. We showed that the small peptide allows for a specific immune response against the phosphorylated epitope and does not recognize the non-phosphorylated peptide. Such peptides may thus be useful for active immunization in humans since the immune response would only target a pathological epitope (pSer422) fully homologous to murine and human proteins. Finally, on the safety side, such short peptides are not able to induce a T-cell response and induction of pro-inflammatory cytokines.
Acknowledgments
Supported by Inserm, CNRS, DN2M, FEDER, IMPRT, University of Lille 2, Lille Regional Hospital (CHRU), the Région Nord/Pas-de-Calais, LECMA, MEDIALZ and the ANR (AMYTOXTAU and ADONTAGE grants), and the European Community (MEMOSAD; FP7 contract 200611). LT and SB were recipients from scholarships from the Région Nord/Pas-de-Calais as well as the CHRU (SB) and the University of Lille 2 (LT). FJFG is a recipient of a fellowship from the Communidad Castilla-La-Mancha, Spain.
Footnotes
The authors disclose no relevant financial relationships.
References
- 1.Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau isoforms in neurodegenerative disorders. Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 2.Schraen-Maschke S, Sergeant N, Dhaenens CM, et al. Tau as biomarker of neurodegenerative diseases. Biomarkers in Medicine. 2008;2:363–384. doi: 10.2217/17520363.2.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gómez-Ramos A, Díaz-Hernández M, Cuadros R, Hernández F, Avila J. Extracellular Tau is toxic to neuronal cells. FEBS Lett. 2006;580(20):4842–50. doi: 10.1016/j.febslet.2006.07.078. [DOI] [PubMed] [Google Scholar]
- 4.Frost B, Jacks RL, Diamond MI. Propagation of Tau misfolding from the outside to the inside of a cell. J Biol Chem. 2009;284:12845–52. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clavaguera F, Bolmont T, Crowther RA, et al. Transmission and spreading of Tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–13. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–7. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 7.Brody DL, Holtzman DM. Active and passive immunotherapy for neurodegenerative disorders. Annu Rev Neurosci. 2008;31:175–93. doi: 10.1146/annurev.neuro.31.060407.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wisniewski T, Boutajangout A. Vaccination as a therapeutic approach to Alzheimer’s disease. Mt Sinai J Med. 2010;77:17–31. doi: 10.1002/msj.20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillis D, Yetiv N, Gdalevich M, et al. Active versus passive immunization against hepatitis A in the Israel defence forces: a cost-benefit analysis. Vaccine. 2000;18:3005–10. doi: 10.1016/s0264-410x(00)00091-8. [DOI] [PubMed] [Google Scholar]
- 10.Asuni AA, Boutajangout A, Quatermain A, Sigurdsson EM. Immunotherapy targeting pathological Tau conforms in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci. 2007;27:9115–29. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boimel M, Grigoriadis N, Lourbopoulos A, Haber E, Abramsky O, Rosenmann H. Efficacy and safety of immunization with phosphorylated Tau against neurofibrillary tangles in mice. Exp Neurol. 2010;224:472–85. doi: 10.1016/j.expneurol.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological Tau prevents cognitive decline in a new tangle mouse model. J Neurosci. 2010;30:16559–66. doi: 10.1523/JNEUROSCI.4363-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-Tau protein in a mouse model reduces functional decline and clears Tau aggregates from the brain. J Neurochem. 2011;118:658–67. doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenmann H, Grigoriadis N, Karussis D, et al. Tauopathy-like abnormalities and neurologic deficits in mice immunized with neuronal Tau protein. Arch Neurol. 2006;63:1459–67. doi: 10.1001/archneur.63.10.1459. [DOI] [PubMed] [Google Scholar]
- 15.Hasegawa M, Jakes R, Crowther RA, Lee VM, Ihara Y, Goedert M. Characterization of mAb AP422, a novel phosphorylation-dependent monoclonal antibody against Tau protein. FEBS Lett. 1996;384:25–30. doi: 10.1016/0014-5793(96)00271-2. [DOI] [PubMed] [Google Scholar]
- 16.Bussière T, Hof PR, Mailliot C, et al. Phosphorylated serine422 on Tau proteins is a pathological epitope found in several diseases with neurofibrillary degeneration. Acta Neuropathol (Berl) 1999;97:221–30. doi: 10.1007/s004010050978. [DOI] [PubMed] [Google Scholar]
- 17.Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific Tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol (Berl) 2002;103:26–35. doi: 10.1007/s004010100423. [DOI] [PubMed] [Google Scholar]
- 18.Sahara N, Lewis J, DeTure M, et al. Assembly of Tau in transgenic animals expressing P301L Tau: alteration of phosphorylation and solubility. J Neurochem. 2002;83:1498–508. doi: 10.1046/j.1471-4159.2002.01241.x. [DOI] [PubMed] [Google Scholar]
- 19.Allen B, Ingram E, Takao M, et al. Abundant Tau filaments and nonapoptotic neurodegeneration in transgenic mide expressing human P301S Tau protein. J Neurosci. 2002;22:9340–51. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schindowski K, Bretteville A, Leroy K, et al. Alzheimer disease-like Tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated Tau transgenic mouse without any motor deficits. Am J Pathol. 2006;169:599–616. doi: 10.2353/ajpath.2006.060002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belarbi K, Burnouf S, Fernandez-Gomez FJ, et al. Beneficial effects of exercise in a transgenic mouse model of Alzheimer’s disease-like Tau pathology. Neurobiol Dis. 2011 doi: 10.1016/j.nbd.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 22.Van der Jeugd A, Ahmed T, Burnouf S, et al. Hippocampal Tauopathy in Tau transgenic mice coincides with impaired hippocampus-dependent learning and memory, and attenuated late-phase long-term depression of synaptic transmission. Neurobiol Learn Mem. 2011;95:296–304. doi: 10.1016/j.nlm.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and Tau pathology in a mouse model of Alzheimer’s disease. J Neurosci. 2006;26:9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buée-Scherrer V, Goedert M. Phosphorylation of microtubule-associated protein Tau by stress-activated protein kinases in intact cells. FEBS Lett. 2002;515:151–154. doi: 10.1016/s0014-5793(02)02460-2. [DOI] [PubMed] [Google Scholar]
- 25.Caillet-Boudin ML, Delacourte A. Induction of a specific Tau Alzheimer epitope in SY-5Y neuroblastoma cells. NeuroReport. 1996;8:307–10. doi: 10.1097/00001756-199612200-00061. [DOI] [PubMed] [Google Scholar]
- 26.Yanagisawa M, Planel E, Ishiguro K, Fujita SC. Starvation induces Tau hyperphosphorylation in mouse brain: implication for Alzheimer’s disease. FEBS Lett. 1999;461:329–33. doi: 10.1016/s0014-5793(99)01480-5. [DOI] [PubMed] [Google Scholar]
- 27.Deters N, Ittner LM, Götz J. Divergent phosphorylation pattern of Tau in P301L Tau transgenic mice. Eur J Neurosci. 2008;28:137–47. doi: 10.1111/j.1460-9568.2008.06318.x. [DOI] [PubMed] [Google Scholar]
- 28.Ferrer I, Hernández I, Puig B, et al. Ubiquitin-negative mini-pick-like bodies in the dentate gyrus in P301L Tauopathy. J Alzheimers Dis. 2003;5:445–54. doi: 10.3233/jad-2003-5604. [DOI] [PubMed] [Google Scholar]
- 29.Ferrer I, Hernández I, Boada M, et al. Primary progressive aphasia as the initial manifestation of corticobasal degeneration and unusual Tauopathies. Acta Neuropathol. 2003;106:419–35. doi: 10.1007/s00401-003-0756-4. [DOI] [PubMed] [Google Scholar]
- 30.Götz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301L Tau transgenic mice induced by Abeta 42 fibrils. Science. 2001;293:1491–5. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 31.Ma QL, Yang F, Rosario ER, et al. Beta-amyloid oligomers induce phosphorylation of Tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci. 2009;29:9078–89. doi: 10.1523/JNEUROSCI.1071-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grueninger F, Bohrmann B, Czech C, et al. Phosphorylation of Tau at S422 is enhanced by Abeta in TauPS2APP triple transgenic mice. Neurobiol Dis. 2010;37:294–306. doi: 10.1016/j.nbd.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Sas A, Jones R, Tyor W. Intra-peritoneal injection of polyclonal anti-interferon alpha antibodies cross the blood brain barrier and neutralize interferon alpha. Neurochem Res. 2008;33:2281–7. doi: 10.1007/s11064-008-9715-8. [DOI] [PubMed] [Google Scholar]
- 34.Patel MM, Goyal BR, Bhadada SV, Bhatt JS, Amin AF. Getting into the brain: approaches to enhance brain drug delivery. CNS Drugs. 2009;23:35–58. doi: 10.2165/0023210-200923010-00003. [DOI] [PubMed] [Google Scholar]
- 35.Sigurdsson EM. Immunotherapy targeting pathological Tau protein in Alzheimer’s disease and related Tauopathies. J Alzheimers Dis. 2008;15:157–68. doi: 10.3233/jad-2008-15202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guillozet-Bongaarts AL, Cahill ME, Cryns VL, Reynolds MR, Berry RW, Binder LI. Pseudophosphorylation of Tau at serine 422 inhibits caspase cleavage: in vitro evidence and implications for tangle formation in vivo. J Neurochem. 2006;97(4):1005–14. doi: 10.1111/j.1471-4159.2006.03784.x. [DOI] [PubMed] [Google Scholar]
- 37.Yin H, Kuret J. C-terminal truncation modulates both nucleation and extension phases of Tau fibrillization. FEBS Lett. 2006;580(1):211–5. doi: 10.1016/j.febslet.2005.11.077. [DOI] [PubMed] [Google Scholar]
- 38.Leroy K, Bretteville A, Schindowski K, et al. Early axonopathy preceding neurofibrillary tangles in a mutant Tau transgenic mice. Am J Pathol. 2007;171:976–92. doi: 10.2353/ajpath.2007.070345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan D. Mechanisms of A beta plaque clearance following passive A beta immunization. Neurodegener Dis. 2005;2:261–6. doi: 10.1159/000090366. [DOI] [PubMed] [Google Scholar]
- 40.Wisniewski T, Sigurdsson EM. Murine models of Alzheimer’s disease and their use in developing immunotherapies. Biochim Biophys Acta. 2010;1802:847–59. doi: 10.1016/j.bbadis.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, Guimaraes A, Yue M, Lewis J, Carlson G, Hutton M, Ashe KH. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L) J Neurosci. 2005;25:10637–47. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci. 2005;25:5446–54. doi: 10.1523/JNEUROSCI.4637-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polydoro M, Acker CM, Duff K, Castillo PE, Davies P. Age-dependent impairment of cognitive and synaptic function in the htau mouse model of tau pathology. J Neurosci. 2009;29:10741–9. doi: 10.1523/JNEUROSCI.1065-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chai X, Wu S, Murray TK, Kinley R, Cella CV, Sims H, Buckner N, Hanmer J, Davies P, O’Neill MJ, Hutton ML, Citron M. Passive Immunization with Anti-Tau Antibodies in Two Transgenic Models: reduction of Tau pathology and delay of disease progression. J Biol Chem. 2011;286:34457–67. doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lasagna-Reeves CA, Castillo-Carranza DL, Jackson GR, Kayed R. Tau Oligomers as Potential Target for Immunotherapy for Alzheimer Disease and Tauopathies. Curr Alzheimer Res. 2011;8:659–65. doi: 10.2174/156720511796717177. [DOI] [PubMed] [Google Scholar]
- 46.Boutajangout A, Sigurdsson EM, Krishnamurthy PK. Tau as A Therapeutic Target for Alzheimer’s Disease. Curr Alzheimer Res. 2011;8:666–77. doi: 10.2174/156720511796717195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuo ES, Shin RW, Billingsley ML, et al. Biopsy-derived adult human brain Tau is phosphorylated at many of the same sites as Alzheimer’s disease paired helical filament Tau. Neuron. 1994;13:989–1002. doi: 10.1016/0896-6273(94)90264-x. [DOI] [PubMed] [Google Scholar]
- 48.Jicha GA, Lane E, Vincent I, Otvos L, Jr, Hoffmann R, Davies P. A conformation- and phosphorylation-dependent antibody recognizing the paired helical filaments of Alzheimer’s disease. J Neurochem. 1997;69:2087–95. doi: 10.1046/j.1471-4159.1997.69052087.x. [DOI] [PubMed] [Google Scholar]
- 49.Daly NL, Hoffmann R, Otvos L, Jr, Craik DJ. Role of phosphorylation in the conformation of Tau peptides implicated in Alzheimer’s disease. Biochemistry. 2000;39:9039–46. doi: 10.1021/bi0004807. [DOI] [PubMed] [Google Scholar]


