Abstract
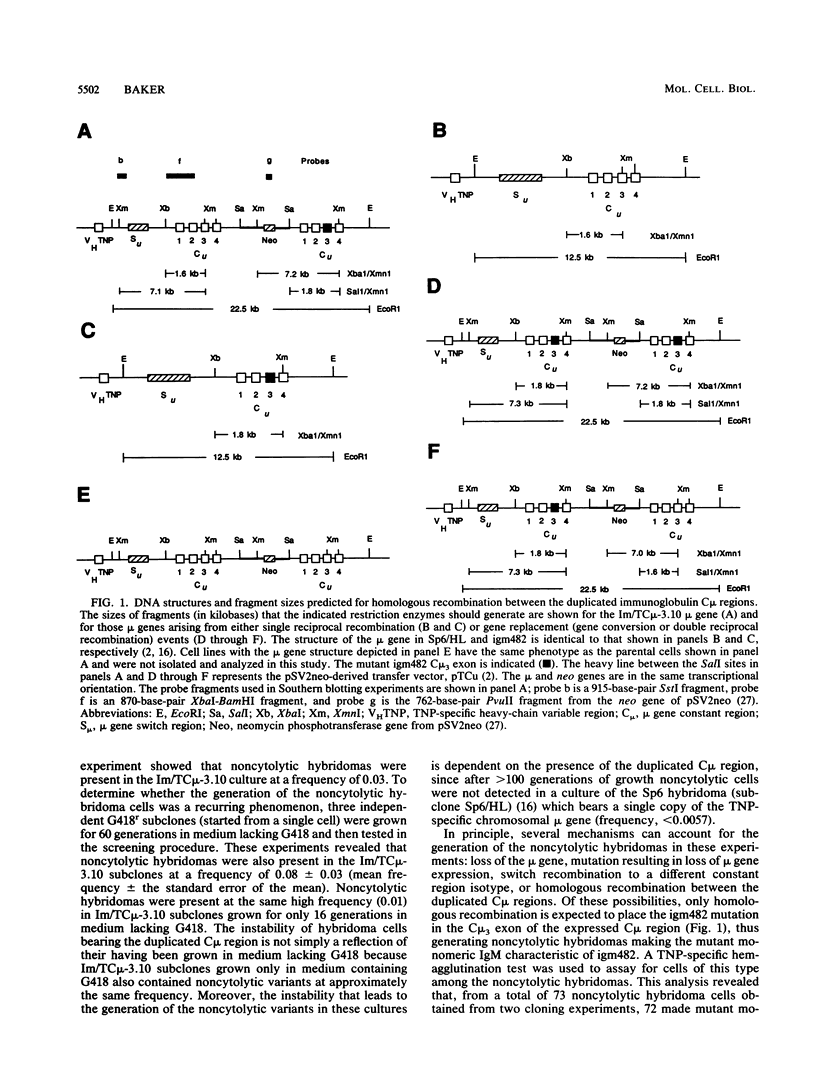
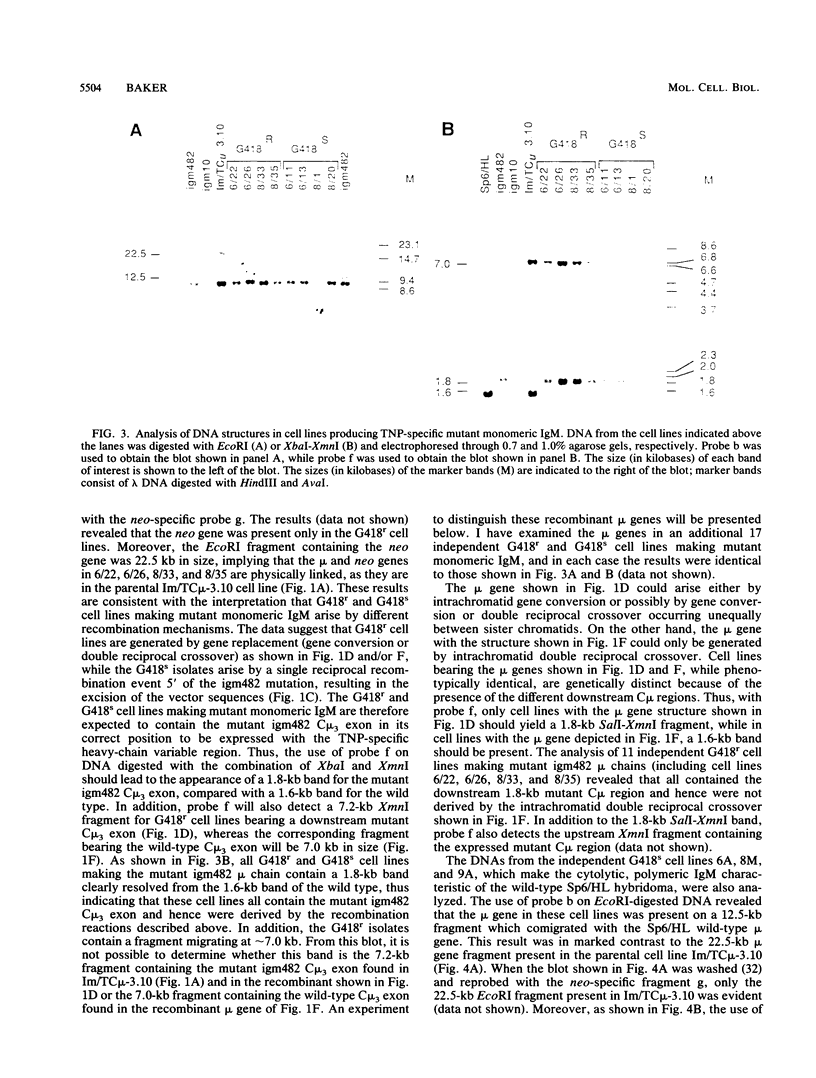
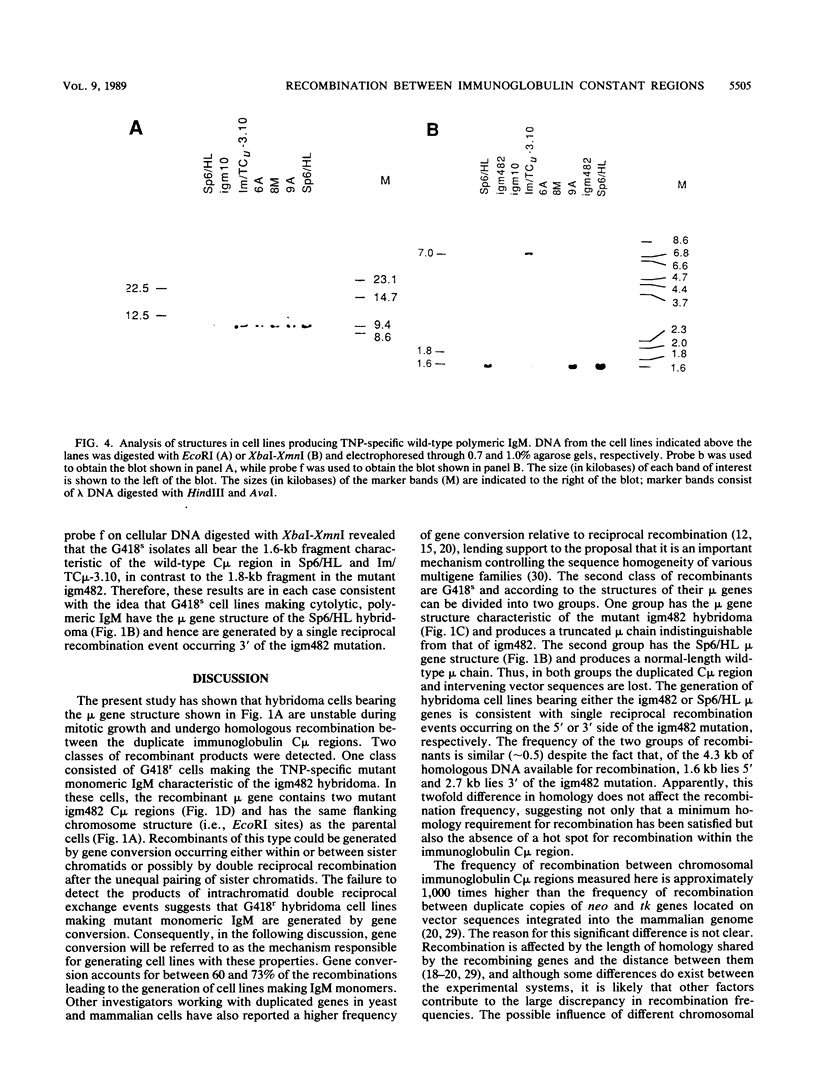
Homologous recombination was used in a previous study to correct a 2-base-pair deletion in the third constant domain (Cmu3) of the haploid chromosomal mu gene in a mutant hybridoma cell line by transfer of a pSV2neo vector bearing a subfragment of the normal Cmu region (M.D. Baker, N. Pennell, L. Bosnoyan, and M.J. Shulman, Proc. Natl. Acad. Sci. USA 85:6432-6436, 1988). In these experiments, both gene replacement and single reciprocal crossover events were found to restore normal, cytolytic 2,4,6-trinitrophenyl-specific immunoglobulin M production to the mutant cells. In the cases of single reciprocal recombination, the structure of the recombinant mu gene is such that the normal Cmu region, in its correct position 3' of the expressed 2,4,6-trinitrophenyl-specific heavy-chain variable region, is separated from the mutant Cmu region by the integrated vector sequences. I report here that homologous recombination occurs with high frequency between the duplicate Cmu regions in mitotically growing hybridoma cells. The homologous recombination events were easily detected since they generated hybridomas that were phenotypically different from the parental cells. Analysis of the recombinant cells suggests that gene conversion is the most frequent event, occurring between 60 and 73% of the time. The remaining events consisted of single reciprocal crossovers. Intrachromatid double reciprocal recombination was not detected. The high frequency of recombination, the ability to isolate and analyze the participants in the recombination reactions, and the capacity to generate specific modifications in the immunoglobulin Cmu regions by gene targeting suggest that this system will be useful for studying mammalian chromosomal homologous recombination. Moreover, the ability to specifically modify the chromosomal immunoglobulin genes by homologous recombination should facilitate studies of immunoglobulin gene regulation and expression and provide a more convenient of engineering specifically modified antibody.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Kemp D. J., Bernard O., Gough N., Webb E., Tyler B., Gerondakis S., Cory S. Organization and expression of murine immunoglobulin genes. Immunol Rev. 1981;59:5–32. doi: 10.1111/j.1600-065x.1981.tb00454.x. [DOI] [PubMed] [Google Scholar]
- Baker M. D., Pennell N., Bosnoyan L., Shulman M. J. Homologous recombination can restore normal immunoglobulin production in a mutant hybridoma cell line. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6432–6436. doi: 10.1073/pnas.85.17.6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. D., Shulman M. J. Homologous recombination between transferred and chromosomal immunoglobulin kappa genes. Mol Cell Biol. 1988 Oct;8(10):4041–4047. doi: 10.1128/mcb.8.10.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M. D., Wu G. E., Toone W. M., Murialdo H., Davis A. C., Shulman M. J. A region of the immunoglobulin-mu heavy chain necessary for forming pentameric IgM. J Immunol. 1986 Sep 1;137(5):1724–1728. [PubMed] [Google Scholar]
- Baumann B., Potash M. J., Köhler G. Consequences of frameshift mutations at the immunoglobulin heavy chain locus of the mouse. EMBO J. 1985 Feb;4(2):351–359. doi: 10.1002/j.1460-2075.1985.tb03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Moore M. W., Yancopoulos G. D., Suh H., Lutzker S., Selsing E., Alt F. W. Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature. 1986 Dec 11;324(6097):585–589. doi: 10.1038/324585a0. [DOI] [PubMed] [Google Scholar]
- Cunningham A. J., Szenberg A. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology. 1968 Apr;14(4):599–600. [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Hawley R. G., Shulman M. J., Murialdo H., Gibson D. M., Hozumi N. Mutant immunoglobulin genes have repetitive DNA elements inserted into their intervening sequences. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7425–7429. doi: 10.1073/pnas.79.23.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Nakai S., Nishida Y., Kataoka T., Yamawaki-Kataoka Y., Takahashi N., Obata M., Shimizu A., Yaoita Y., Nikaido T. Rearrangements of immunoglobulin genes during differentiation and evolution. Immunol Rev. 1981;59:33–67. doi: 10.1111/j.1600-065x.1981.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Jackson J. A., Fink G. R. Gene conversion between duplicated genetic elements in yeast. Nature. 1981 Jul 23;292(5821):306–311. doi: 10.1038/292306a0. [DOI] [PubMed] [Google Scholar]
- Jäck H. M., Wabl M. Immunoglobulin mRNA stability varies during B lymphocyte differentiation. EMBO J. 1988 Apr;7(4):1041–1046. doi: 10.1002/j.1460-2075.1988.tb02911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil R. L., Roeder G. S. Cis-acting, recombination-stimulating activity in a fragment of the ribosomal DNA of S. cerevisiae. Cell. 1984 Dec;39(2 Pt 1):377–386. doi: 10.1016/0092-8674(84)90016-3. [DOI] [PubMed] [Google Scholar]
- Klar A. J., Strathern J. N., Hicks J. B. A position-effect control for gene transposition: state of expression of yeast mating-type genes affects their ability to switch. Cell. 1981 Aug;25(2):517–524. doi: 10.1016/0092-8674(81)90070-2. [DOI] [PubMed] [Google Scholar]
- Klein H. L., Petes T. D. Intrachromosomal gene conversion in yeast. Nature. 1981 Jan 15;289(5794):144–148. doi: 10.1038/289144a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Potash M. J., Lehrach H., Shulman M. J. Deletions in immunoglobulin mu chains. EMBO J. 1982;1(5):555–563. doi: 10.1002/j.1460-2075.1982.tb01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liskay R. M., Letsou A., Stachelek J. L. Homology requirement for efficient gene conversion between duplicated chromosomal sequences in mammalian cells. Genetics. 1987 Jan;115(1):161–167. doi: 10.1093/genetics/115.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liskay R. M., Stachelek J. L. Information transfer between duplicated chromosomal sequences in mammalian cells involves contiguous regions of DNA. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1802–1806. doi: 10.1073/pnas.83.6.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liskay R. M., Stachelek J. L., Letsou A. Homologous recombination between repeated chromosomal sequences in mouse cells. Cold Spring Harb Symp Quant Biol. 1984;49:183–189. doi: 10.1101/sqb.1984.049.01.021. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Rohdewohld H., Weiher H., Reik W., Jaenisch R., Breindl M. Retrovirus integration and chromatin structure: Moloney murine leukemia proviral integration sites map near DNase I-hypersensitive sites. J Virol. 1987 Feb;61(2):336–343. doi: 10.1128/jvi.61.2.336-343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz M., Freisem-Rabien U., Jessberger R., Doerfler W. Transcriptional activities of mammalian genomes at sites of recombination with foreign DNA. J Virol. 1987 Feb;61(2):344–353. doi: 10.1128/jvi.61.2.344-353.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M. J., Heusser C., Filkin C., Köhler G. Mutations affecting the structure and function of immunoglobulin M. Mol Cell Biol. 1982 Sep;2(9):1033–1043. doi: 10.1128/mcb.2.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J., Berg P. Homologous recombination between defective neo genes in mouse 3T6 cells. Cold Spring Harb Symp Quant Biol. 1984;49:171–181. doi: 10.1101/sqb.1984.049.01.020. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Strathern J. N., Klar A. J., Hicks J. B., Abraham J. A., Ivy J. M., Nasmyth K. A., McGill C. Homothallic switching of yeast mating type cassettes is initiated by a double-stranded cut in the MAT locus. Cell. 1982 Nov;31(1):183–192. doi: 10.1016/0092-8674(82)90418-4. [DOI] [PubMed] [Google Scholar]
- Subramani S., Rubnitz J. Recombination events after transient infection and stable integration of DNA into mouse cells. Mol Cell Biol. 1985 Apr;5(4):659–666. doi: 10.1128/mcb.5.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B. J., Rothstein R. Elevated recombination rates in transcriptionally active DNA. Cell. 1989 Feb 24;56(4):619–630. doi: 10.1016/0092-8674(89)90584-9. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Trimble W. S., Baker M. D., Boulianne G. L., Murialdo H., Hozumi N., Shulman M. J. Analysis of hybridoma mutants defective in synthesis of immunoglobulin M. Somat Cell Mol Genet. 1986 Sep;12(5):467–477. doi: 10.1007/BF01539918. [DOI] [PubMed] [Google Scholar]
- Voelkel-Meiman K., Keil R. L., Roeder G. S. Recombination-stimulating sequences in yeast ribosomal DNA correspond to sequences regulating transcription by RNA polymerase I. Cell. 1987 Mar 27;48(6):1071–1079. doi: 10.1016/0092-8674(87)90714-8. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Regulation of the assembly and expression of variable-region genes. Annu Rev Immunol. 1986;4:339–368. doi: 10.1146/annurev.iy.04.040186.002011. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Blackwell T. K., Suh H., Hood L., Alt F. W. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986 Jan 31;44(2):251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]





