Abstract
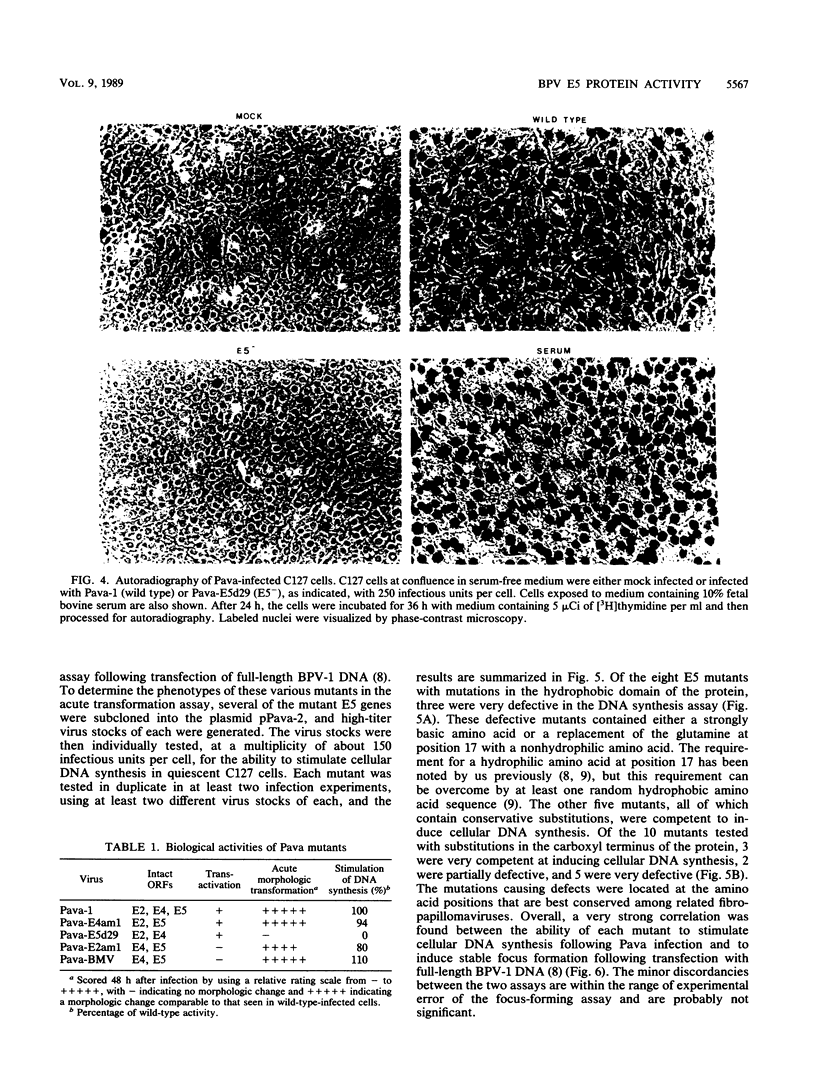
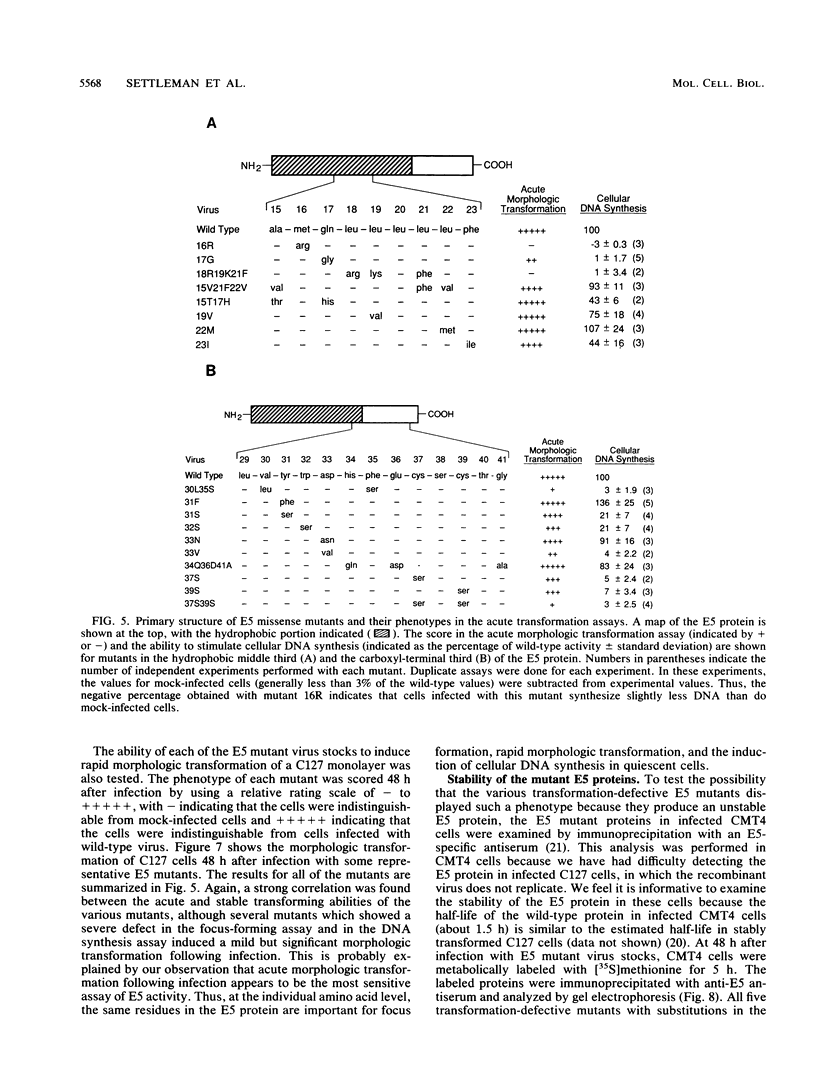
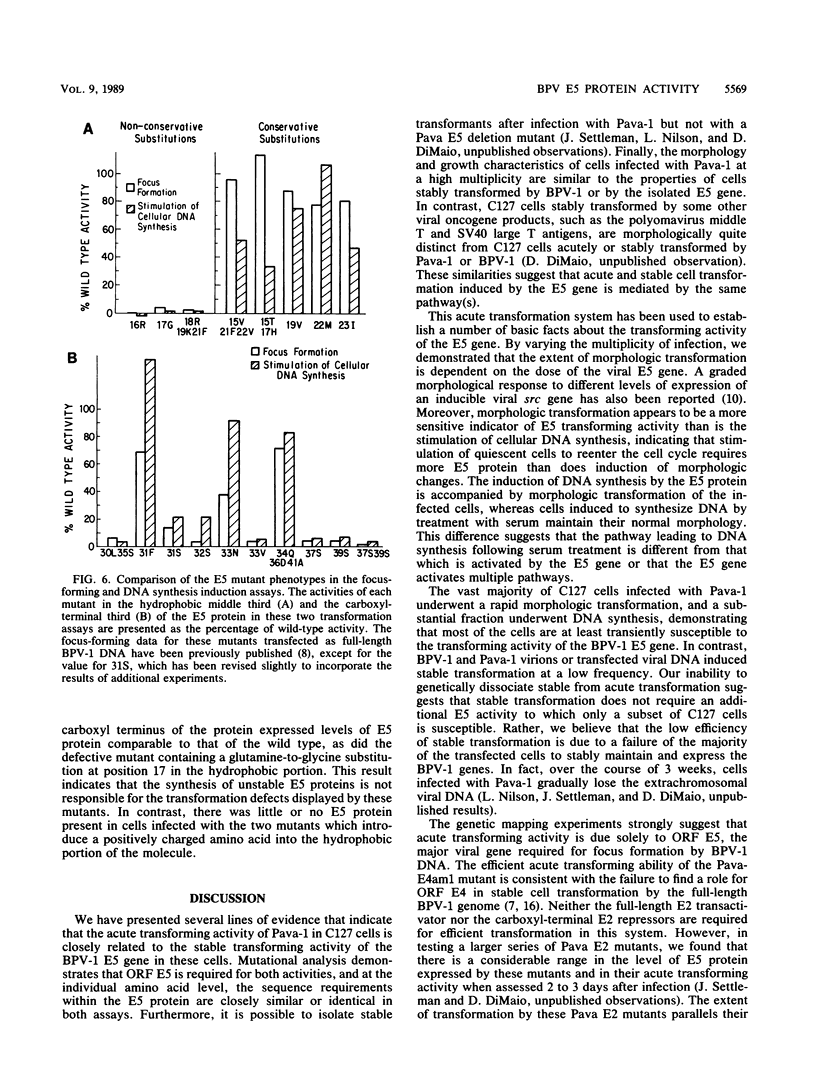
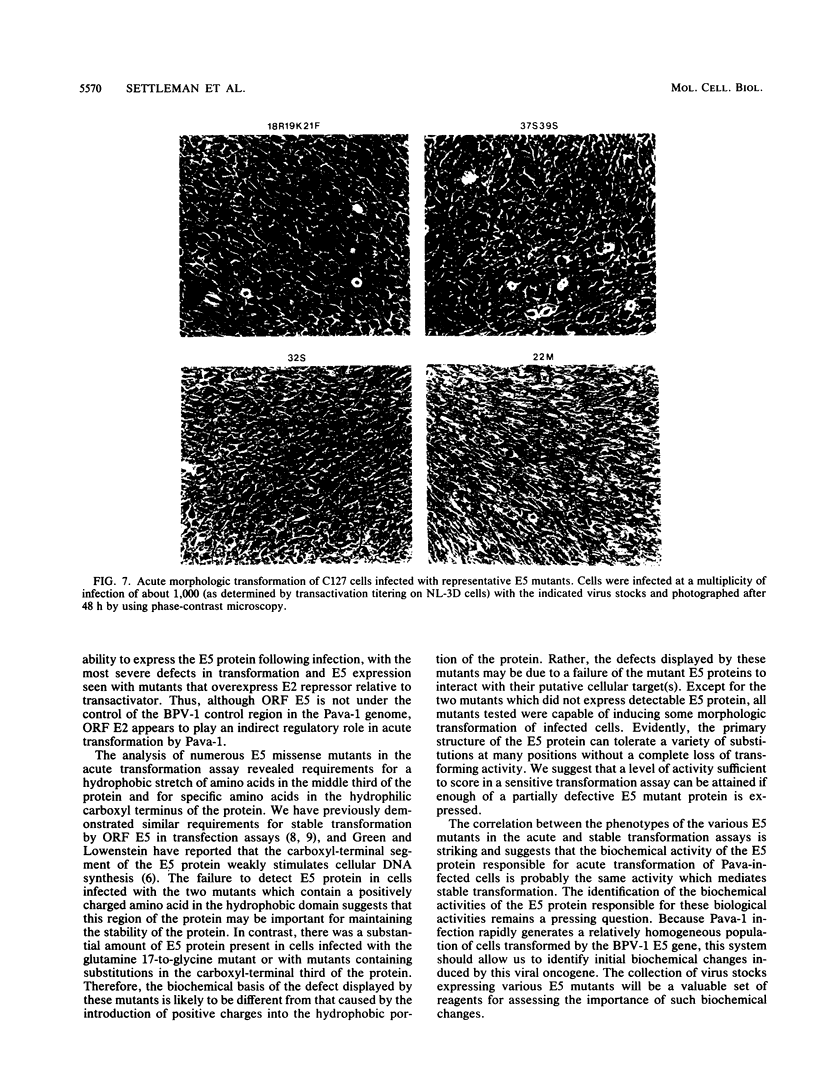
The bovine papillomavirus (BPV) type 1 E5 gene encodes a 44-amino-acid protein that can stably transform cultured rodent cells when expressed in the absence of all other viral genes. We have previously constructed a BPV-simian virus 40 recombinant virus (Pava-1) which efficiently expresses the BPV type 1 E5 gene in infected cells (J. Settleman and D. DiMaio, Proc. Natl. Acad. Sci. USA 85:9007-9011, 1988). Within 48 h of Pava-1 infection, the vast majority of mouse C127 cells underwent a dramatic morphologic transformation which was accompanied by cell proliferation. Infection of C127 cells made quiescent by contact inhibition and serum starvation caused a great induction of cellular DNA synthesis. These morphologic and mitogenic responses were proportional to the virus multiplicity of infection. Mutational analysis indicated that the E5 gene is both necessary and sufficient for these activities. Analysis of a variety of E5 missense mutants revealed a strong correlation between their phenotypes in the acute transformation assays following infection and in the stable focus-forming assay following transfection. Most of the defective mutants expressed normal levels of E5 protein following infection, indicating that their defective phenotypes are not due to the synthesis of an unstable protein. The failure to genetically resolve these E5 activities suggests that the ability of the E5 protein to cause acute morphologic transformation and reentry into the cell cycle may be intimately related to its ability to cause stable cell transformation and that these functions are probably mediated by a single biochemical activity of the E5 protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergman P., Ustav M., Sedman J., Moreno-Lopéz J., Vennström B., Pettersson U. The E5 gene of bovine papillomavirus type 1 is sufficient for complete oncogenic transformation of mouse fibroblasts. Oncogene. 1988 May;2(5):453–459. [PubMed] [Google Scholar]
- Burkhardt A., DiMaio D., Schlegel R. Genetic and biochemical definition of the bovine papillomavirus E5 transforming protein. EMBO J. 1987 Aug;6(8):2381–2385. doi: 10.1002/j.1460-2075.1987.tb02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D., Guralski D., Schiller J. T. Translation of open reading frame E5 of bovine papillomavirus is required for its transforming activity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1797–1801. doi: 10.1073/pnas.83.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMaio D. Nonsense mutation in open reading frame E2 of bovine papillomavirus DNA. J Virol. 1986 Feb;57(2):475–480. doi: 10.1128/jvi.57.2.475-480.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard R. D., Gluzman Y. New host cell system for regulated simian virus 40 DNA replication. Mol Cell Biol. 1985 Nov;5(11):3231–3240. doi: 10.1128/mcb.5.11.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Loewenstein P. M. Demonstration that a chemically synthesized BPV1 oncoprotein and its C-terminal domain function to induce cellular DNA synthesis. Cell. 1987 Dec 4;51(5):795–802. doi: 10.1016/0092-8674(87)90102-4. [DOI] [PubMed] [Google Scholar]
- Hermonat P. L., Howley P. M. Mutational analysis of the 3' open reading frames and the splice junction at nucleotide 3225 of bovine papillomavirus type 1. J Virol. 1987 Dec;61(12):3889–3895. doi: 10.1128/jvi.61.12.3889-3895.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. H., Burkhardt A. L., Schlegel R., DiMaio D. 44-amino-acid E5 transforming protein of bovine papillomavirus requires a hydrophobic core and specific carboxyl-terminal amino acids. Mol Cell Biol. 1988 Oct;8(10):4071–4078. doi: 10.1128/mcb.8.10.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. H., Weinstat D. L., DiMaio D. Transforming activity of a 16-amino-acid segment of the bovine papillomavirus E5 protein linked to random sequences of hydrophobic amino acids. J Virol. 1989 Nov;63(11):4515–4519. doi: 10.1128/jvi.63.11.4515-4519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits E. B., Majors J. E., Varmus H. E. Hormonal regulation of the Rous sarcoma virus src gene via a heterologous promoter defines a threshold dose for cellular transformation. Cell. 1984 Oct;38(3):757–765. doi: 10.1016/0092-8674(84)90271-x. [DOI] [PubMed] [Google Scholar]
- Jaskulski D., Kaczmarek L., DiMaio D. Stimulation of cellular DNA synthesis by wild type and mutant bovine papillomavirus DNA. Biochem Biophys Res Commun. 1987 Oct 14;148(1):86–91. doi: 10.1016/0006-291x(87)91079-5. [DOI] [PubMed] [Google Scholar]
- Lambert P. F., Spalholz B. A., Howley P. M. A transcriptional repressor encoded by BPV-1 shares a common carboxy-terminal domain with the E2 transactivator. Cell. 1987 Jul 3;50(1):69–78. doi: 10.1016/0092-8674(87)90663-5. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Bradley M. K. The simian virus 40 large T antigen. A lot packed into a little. Mol Biol Med. 1987 Apr;4(2):63–80. [PubMed] [Google Scholar]
- Markland W., Smith A. E. Mutants of polyomavirus middle-T antigen. Biochim Biophys Acta. 1987 Nov 25;907(3):299–321. doi: 10.1016/0304-419x(87)90011-4. [DOI] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Neary K., Horwitz B. H., DiMaio D. Mutational analysis of open reading frame E4 of bovine papillomavirus type 1. J Virol. 1987 Apr;61(4):1248–1252. doi: 10.1128/jvi.61.4.1248-1252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipas J. M., Peden K. W., Nathans D. Mutational analysis of simian virus 40 T antigen: isolation and characterization of mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983 Feb;3(2):203–213. doi: 10.1128/mcb.3.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S. S., Horwitz B. H., Zibello T., Settleman J., DiMaio D. Bovine papillomavirus E2 gene regulates expression of the viral E5 transforming gene. J Virol. 1988 Oct;62(10):3608–3613. doi: 10.1128/jvi.62.10.3608-3613.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J. T., Vass W. C., Vousden K. H., Lowy D. R. E5 open reading frame of bovine papillomavirus type 1 encodes a transforming gene. J Virol. 1986 Jan;57(1):1–6. doi: 10.1128/jvi.57.1.1-6.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settleman J., DiMaio D. Efficient transactivation and morphologic transformation by bovine papillomavirus genes expressed from a bovine papillomavirus/simian virus 40 recombinant virus. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9007–9011. doi: 10.1073/pnas.85.23.9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soprano K. J., Galanti N., Jonak G. J., McKercher S., Pipas J. M., Peden K. W., Baserga R. Mutational analysis of simian virus 40 T antigen: stimulation of cellular DNA synthesis and activation of rRNA genes by mutants with deletions in the T-antigen gene. Mol Cell Biol. 1983 Feb;3(2):214–219. doi: 10.1128/mcb.3.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalholz B. A., Yang Y. C., Howley P. M. Transactivation of a bovine papilloma virus transcriptional regulatory element by the E2 gene product. Cell. 1985 Aug;42(1):183–191. doi: 10.1016/s0092-8674(85)80114-8. [DOI] [PubMed] [Google Scholar]
- Sugano S., Yamaguchi N. Two classes of transformation-deficient, immortalization-positive simian virus 40 mutants constructed by making three-base insertions in the T antigen gene. J Virol. 1984 Dec;52(3):884–891. doi: 10.1128/jvi.52.3.884-891.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Okayama H., Howley P. M. Bovine papillomavirus contains multiple transforming genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1030–1034. doi: 10.1073/pnas.82.4.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]