Abstract
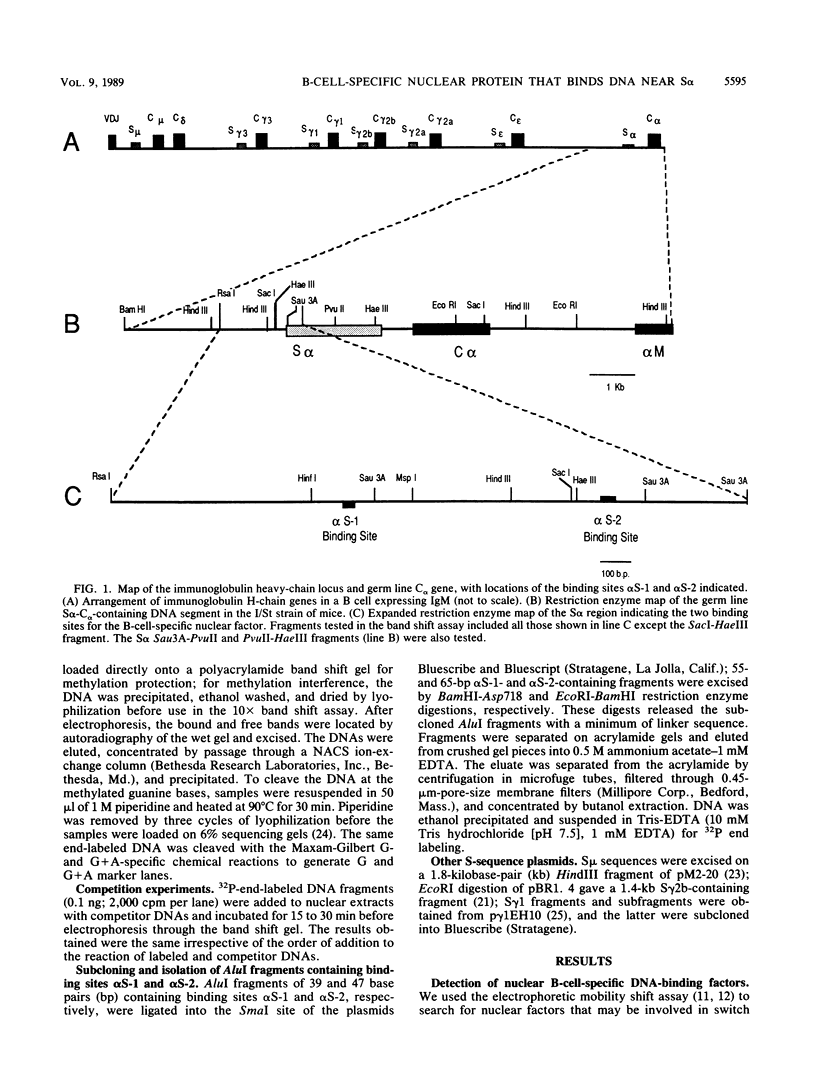
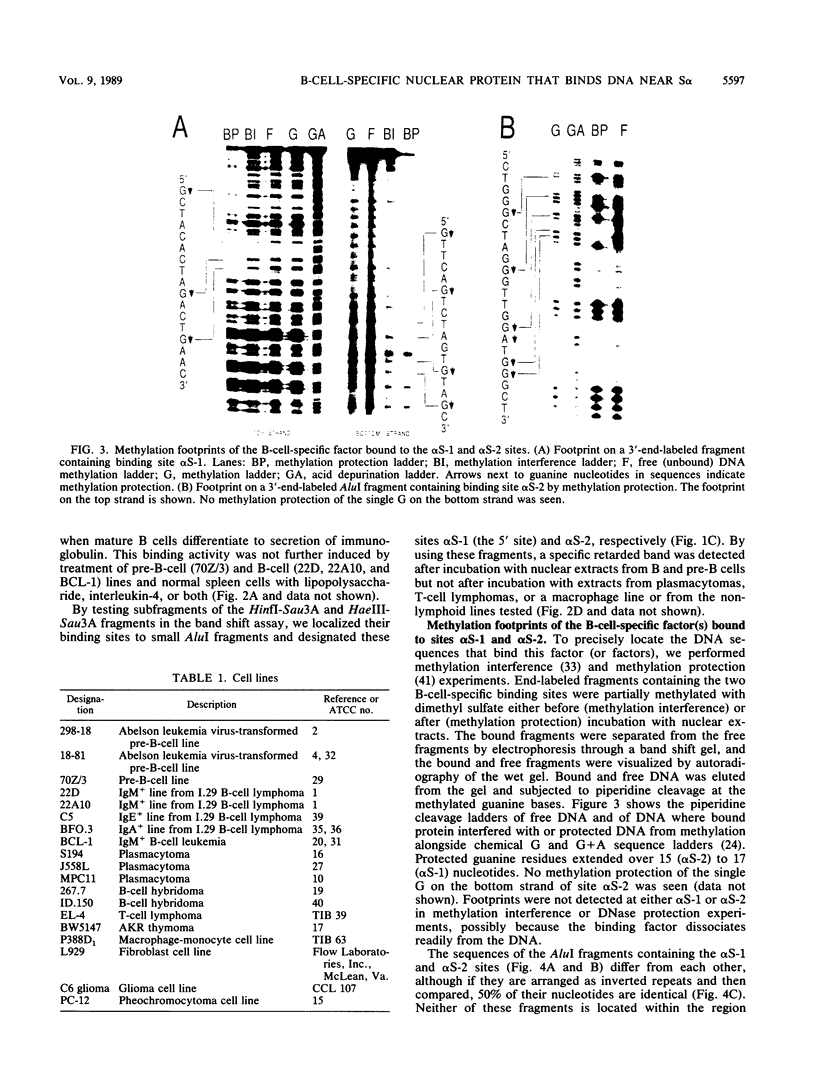
Immunoglobulin heavy-chain switching is effected by recombination events between sites associated with tandemly repeated switch sequences located 5' to immunoglobulin heavy-chain genes. Using the band mobility shift assay, we have identified two distinct sites 5' to the alpha heavy-chain switch sequence with affinity for a single B-cell-specific DNA-binding protein, S alpha-BP. S alpha-BP was present in nuclear extracts from pre-B and B cells but was not detected in extracts from plasmacytomas, B-cell hybridomas, T-cell lymphomas, or a macrophage cell line. It was also not detectable in other nonlymphoid cells tested. Evidence suggests there are S alpha-BP-binding sites near other immunoglobulin switch sequences. As with the S alpha sites, these sites appear to be distinct from the consensus tandem repeats characteristic of immunoglobulin switch sequences. The possible functions of S alpha-BP on contacting its binding sites are discussed in the context of immunoglobulin heavy-chain switch recombination.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberini C., Biassoni R., DeAmbrosis S., Vismara D., Sitia R. Differentiation in the murine B cell lymphoma I.29: individual mu + clones may be induced by lipopolysaccharide to both IgM secretion and isotype switching. Eur J Immunol. 1987 Apr;17(4):555–562. doi: 10.1002/eji.1830170419. [DOI] [PubMed] [Google Scholar]
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Boothby M., Liou H. C., Glimcher L. H. Differences in DNA sequence specificity among MHC class II X box binding proteins. J Immunol. 1989 Feb 1;142(3):1005–1014. [PubMed] [Google Scholar]
- Burrows P. D., Beck-Engeser G. B., Wabl M. R. Immunoglobulin heavy-chain class switching in a pre-B cell line is accompanied by DNA rearrangement. Nature. 1983 Nov 17;306(5940):243–246. doi: 10.1038/306243a0. [DOI] [PubMed] [Google Scholar]
- Davidson I., Xiao J. H., Rosales R., Staub A., Chambon P. The HeLa cell protein TEF-1 binds specifically and cooperatively to two SV40 enhancer motifs of unrelated sequence. Cell. 1988 Sep 23;54(7):931–942. doi: 10.1016/0092-8674(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Calame K., Early P. W., Livant D. L., Joho R., Weissman I. L., Hood L. An immunoglobulin heavy-chain gene is formed by at least two recombinational events. Nature. 1980 Feb 21;283(5749):733–739. doi: 10.1038/283733a0. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Kim S. K., Hood L. E. DNA sequences mediating class switching in alpha-immunoglobulins. Science. 1980 Sep 19;209(4463):1360–1365. doi: 10.1126/science.6774415. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick W., Wilson M., Stavnezer J. Mutations, duplication, and deletion of recombined switch regions suggest a role for DNA replication in the immunoglobulin heavy-chain switch. Mol Cell Biol. 1989 May;9(5):1850–1856. doi: 10.1128/mcb.9.5.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt L. A., Birshtein B. K. Independent immunoglobulin class-switch events occurring in a single myeloma cell line. Mol Cell Biol. 1985 Apr;5(4):856–868. doi: 10.1128/mcb.5.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M. G., Crothers D. M. Equilibrium studies of the cyclic AMP receptor protein-DNA interaction. J Mol Biol. 1984 Jan 25;172(3):241–262. doi: 10.1016/s0022-2836(84)80025-x. [DOI] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart P. J., Sigal N. H., Klinman N. R. Production of antibodies of identical idiotype but diverse immunoglobulin classes by cells derived from a single stimulated B cell. Proc Natl Acad Sci U S A. 1975 May;72(5):1707–1711. doi: 10.1073/pnas.72.5.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E. S., Doss C. A., Howard F. D., Morrison D. C., Strober S. An in vitro line of the B cell tumor BCL1 can be activated by LPS to secrete IgM1. J Immunol. 1980 Sep;125(3):976–980. [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T., Miyata T., Honjo T. Repetitive sequences in class-switch recombination regions of immunoglobulin heavy chain genes. Cell. 1981 Feb;23(2):357–368. doi: 10.1016/0092-8674(81)90131-8. [DOI] [PubMed] [Google Scholar]
- Klein S., Sablitzky F., Radbruch A. Deletion of the IgH enhancer does not reduce immunoglobulin heavy chain production of a hybridoma IgD class switch variant. EMBO J. 1984 Nov;3(11):2473–2476. doi: 10.1002/j.1460-2075.1984.tb02158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp M. R., Severinson-Gronowicz E., Schröder J., Strober S. Characterization of a spontaneous murine B cell leukemia (BCL1). II. Tumor cell proliferation and IgM secretion after stimulation by LPS. J Immunol. 1979 Sep;123(3):1000–1006. [PubMed] [Google Scholar]
- Lang R. B., Stanton L. W., Marcu K. B. On immunoglobulin heavy chain gene switching: two gamma 2b genes are rearranged via switch sequences in MPC-11 cells but only one is expressed. Nucleic Acids Res. 1982 Jan 22;10(2):611–630. doi: 10.1093/nar/10.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzker S., Rothman P., Pollock R., Coffman R., Alt F. W. Mitogen- and IL-4-regulated expression of germ-line Ig gamma 2b transcripts: evidence for directed heavy chain class switching. Cell. 1988 Apr 22;53(2):177–184. doi: 10.1016/0092-8674(88)90379-0. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Banerji J., Penncavage N. A., Lang R., Arnheim N. 5' flanking region of immunoglobulin heavy chain constant region genes displays length heterogeneity in germlines of inbred mouse strains. Cell. 1980 Nov;22(1 Pt 1):187–196. doi: 10.1016/0092-8674(80)90167-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mowatt M. R., Dunnick W. A. DNA sequence of the murine gamma 1 switch segment reveals novel structural elements. J Immunol. 1986 Apr 1;136(7):2674–2683. [PubMed] [Google Scholar]
- Nikaido T., Yamawaki-Kataoka Y., Honjo T. Nucleotide sequences of switch regions of immunoglobulin C epsilon and C gamma genes and their comparison. J Biol Chem. 1982 Jul 10;257(13):7322–7329. [PubMed] [Google Scholar]
- Oi V. T., Morrison S. L., Herzenberg L. A., Berg P. Immunoglobulin gene expression in transformed lymphoid cells. Proc Natl Acad Sci U S A. 1983 Feb;80(3):825–829. doi: 10.1073/pnas.80.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott D. E., Alt F. W., Marcu K. B. Immunoglobulin heavy chain switch region recombination within a retroviral vector in murine pre-B cells. EMBO J. 1987 Mar;6(3):577–584. doi: 10.1002/j.1460-2075.1987.tb04793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C. J., Kincade P. W., Ralph P. Murine B cell leukemia line with inducible surface immunoglobulin expression. J Immunol. 1978 Aug;121(2):641–647. [PubMed] [Google Scholar]
- Pfeifer K., Kim K. S., Kogan S., Guarente L. Functional dissection and sequence of yeast HAP1 activator. Cell. 1989 Jan 27;56(2):291–301. doi: 10.1016/0092-8674(89)90903-3. [DOI] [PubMed] [Google Scholar]
- Siden E. J., Baltimore D., Clark D., Rosenberg N. E. Immunoglobulin synthesis by lymphoid cells transformed in vitro by Abelson murine leukemia virus. Cell. 1979 Feb;16(2):389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Gilbert W. Contacts between Escherichia coli RNA polymerase and an early promoter of phage T7. Proc Natl Acad Sci U S A. 1980 Jan;77(1):122–126. doi: 10.1073/pnas.77.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Sen R., Baltimore D., Sharp P. A. A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature. 1986 Jan 9;319(6049):154–158. doi: 10.1038/319154a0. [DOI] [PubMed] [Google Scholar]
- Sitia R., Rubartelli A., Hammerling U. Expression of 2 immunoglobulin isotypes, IgM and IgA, with identical idiotype in the B cell lymphoma I.29. J Immunol. 1981 Oct;127(4):1388–1394. [PubMed] [Google Scholar]
- Stavnezer-Nordgren J., Sirlin S. Specificity of immunoglobulin heavy chain switch correlates with activity of germline heavy chain genes prior to switching. EMBO J. 1986 Jan;5(1):95–102. doi: 10.1002/j.1460-2075.1986.tb04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer J., Marcu K. B., Sirlin S., Alhadeff B., Hammerling U. Rearrangements and deletions of immunoglobulin heavy chain genes in the double-producing B cell lymphoma I.29. Mol Cell Biol. 1982 Aug;2(8):1002–1013. doi: 10.1128/mcb.2.8.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer J., Radcliffe G., Lin Y. C., Nietupski J., Berggren L., Sitia R., Severinson E. Immunoglobulin heavy-chain switching may be directed by prior induction of transcripts from constant-region genes. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7704–7708. doi: 10.1073/pnas.85.20.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer J., Sirlin S., Abbott J. Induction of immunoglobulin isotype switching in cultured I.29 B lymphoma cells. Characterization of the accompanying rearrangements of heavy chain genes. J Exp Med. 1985 Mar 1;161(3):577–601. doi: 10.1084/jem.161.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada N., Hämmerling U. Secretion of either of a pair of immunoglobulins, IgM or IgX, in somatic hybrid cells derived by fusion of a B-cell lymphoma cell line carrying both immunoglobulin isotypes. Immunogenetics. 1980 Jul;11(1):7–19. doi: 10.1007/BF01567765. [DOI] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Tucker P. W., Liu C. P., Mushinski J. F., Blattner F. R. Mouse immunoglobulin D: messenger RNA and genomic DNA sequences. Science. 1980 Sep 19;209(4463):1353–1360. doi: 10.1126/science.6968091. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., DePinho R. A., Zimmerman K. A., Lutzker S. G., Rosenberg N., Alt F. W. Secondary genomic rearrangement events in pre-B cells: VHDJH replacement by a LINE-1 sequence and directed class switching. EMBO J. 1986 Dec 1;5(12):3259–3266. doi: 10.1002/j.1460-2075.1986.tb04637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoita Y., Honjo T. Deletion of immunoglobulin heavy chain genes from expressed allelic chromosome. Nature. 1980 Aug 28;286(5776):850–853. doi: 10.1038/286850a0. [DOI] [PubMed] [Google Scholar]