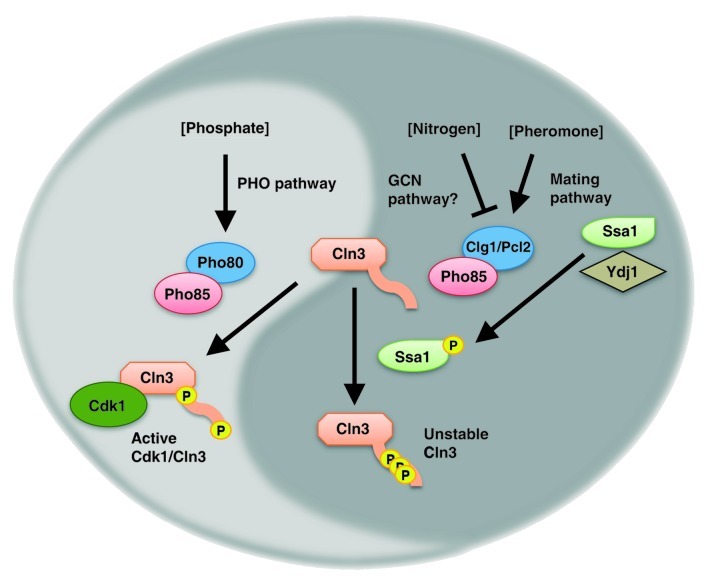
The timing and order of cell cycle progression is regulated by cyclin-dependent kinases (CDKs) to ensure accurate duplication of the genome and other cell components. In budding yeast, accumulation of the cyclin Cln3 (homolog of metazoan Cyclin D) during G1 activates the major CDK Cdk1 (homolog of CDK4, CDK2 and CDC2). The active Cdk1-Cln3 complex induces expression of late G1 cyclins (homologs of Cyclins E) that further activate Cdk1 to drive cells to pass START and initiate DNA replication in S phase.1 Since Cln3 levels fail to increase upon starvation, both the expression and stability of Cln3 have long been implicated as targets of nutrient signaling. Nonetheless, specific mechanisms by which nutrient signals impinge on the cell cycle machinery have yet to be defined. Recent studies by our groups2,3 revealed that nitrogen and phosphate availability regulate distinct phosphorylations that determine Cln3 stability. Both signals are mediated by activation of a second CDK, Pho85,4 though via different cyclins that have distinct effects on the cell cycle. Thus, the two studies converge to suggest a remarkably complex regulation of the CDK-cyclin system in response to nutrient levels.
Pho85 associated with its cyclin Pho80 is a key effector of the so-called PHO pathway,5 by which media phosphate availability is sensed. The Clotet group discovered that Cln3 becomes stabilized in the presence of adequate phosphate levels, via phosphorylation by Pho85-Pho80, on a pair of consensus CDK sites that frame the Cln3 PEST region, a CDK phosphorylation-dependent degron.6 Correspondingly, cells carrying a Cln3-aspartic mutant that mimics constitutive phosphorylation maintain high Cln3 levels irrespective of Pho85-Pho80 activity. In low phosphate, the cells fail to arrest in G1 and instead die via deregulated proliferation. The Pho80-dependent activity of Pho85 represents the yin, blocking Cln3 degradation to promote cell proliferation under favorable conditions.
In their complementary story, Kron and colleagues found that upon nitrogen starvation, Pho85 is activated by cyclins Clg1 and Pcl2 to phosphorylate the Hsp70-family chaperone Ssa1 at a conserved consensus CDK phosphorylation site. The resulting dissociation of the Hsp40-family co-chaperone Ydj1 allows Ssa1 to bind Cln3 in its place and induce Cln3 degradation. Similarly, Pho85- Clg1 and -Pcl2 phosphorylates Ssa1 in yeast cells stimulated by mating pheromone, destabilizing Cln3 to maintain G1 arrest during conjugation. Further, this mechanism appears conserved to human Hsc70, which is phosphorylated at the analogous site to destabilize Cyclin D. Whereas Ssa1 phosphorylation and binding to Cln3 may be necessary, phosphorylation of Cln3 within its PEST degron appears to be required as well. Though as yet untested, the PEST region appears likely to be a second target of Pho85-Clg1 and -Pcl2. Thus, in its complementary yang form, Pho85 promotes Cln3 destruction to block cell cycle progression when conditions favor cell cycle arrest.
Interestingly, such conflicting roles for Pho85 have previously been described in the regulation of autophagy, where its distinct activities are similarly influenced by the specific Pcls.7 However, it is still remarkable that by switching between yin and yang form, the Pho85 cyclin-dependent kinase can regulate cell cycle progression negatively or positively in response to nutrient signals (Fig. 1). Cln3 accumulation is the critical determinant of whether a cell will pass START, the “point of no return” in late G1, and it seems natural that Cln3 stability would be a common target for multiple nutrient signaling pathways.
Figure 1. Nutrient signals converge on Cln3 via divergent activities of Pho85. Phosphate availability activates the PHO pathway, wherein the cyclin Pho80 activates the CDK Pho85 to directly phosphorylate Cln3 on the borders of the PEST degron, thereby protecting Cln3 from ubiquitin-proteasomal degradation. The resulting Cln3 stabilization permits Cdk1-Cln3 activity to accumulate and promote G1/S progression. By contrast, nitrogen starvation or sensing of mating pheromone induce cyclins Clg1 or Pcl2 to activate Pho85 to phosphorylate the chaperone Ssa1. Cln3 binding and PEST phosphorylation induce Cln3 degradation. Thus, nitrogen or phosphate starvation similarly block cell cycle progression via regulation of opposing functions of Pho85.
Paradoxically, a cln3∆ strain remains viable with only modest delay in G1 progression. In this context, the regulation of Cln3 appears dispensable. However, hyperactivation of Cln3 as in mutants lacking the PEST degron has a far more deleterious effect. Though tolerated on rich media or upon starvation, hyperactivation of Cdk1-Cln3 leads to inappropriate cell division and death,8 much like the Pho85-independent Cln3 mutant under phosphate starvation. Moreover, cells that cannot correctly sense the restoration of nutrients upon re-feeding show a delay in Cln3 accumulation and lag behind in restarting cell cycle machinery (Jimenez and Clotet, unpublished results). While Cln3 regulation may appear dispensable in the lab, in the wild, yeast face a fluctuating environment. Without mechanisms to tightly match proliferation to nutrient availability, it would not be possible to compensate for daily variation, let alone survive from one season to the next.
In summary, these two studies contribute significantly to our understanding of how cell signaling can be transduced to regulation of cell cycle progression but raise new questions. Together, the papers establish a broader role for Pho85 as a signaling CDK mediating cell responses to environmental conditions and reinforce the model that regulation of Cln3 instability provides a mechanistic link between nutrient signaling and START. It will be critical to define how nutrient starvation and other stresses determine expression of specific cyclins, leading to differential activation of Pho85 to target distinct sites impinging on Cln3 stability. The two stories raise a new paradox, insofar as cells exposed to adequate phosphate, which inactivates the PEST, can respond to other signals that lead to Cln3 destruction, requiring PEST function. As such, discovering mechanisms by which conflicts between the yin and tang forms of Pho85 can be resolved offers one of many new challenges to be addressed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/24217
References
- 1.Bloom J, Cross FR. Multiple levels of cyclin specificity in cell-cycle control. Nat Rev Mol Cell Biol. 2007;8:149–60. doi: 10.1038/nrm2105. [DOI] [PubMed] [Google Scholar]
- 2.Truman AW, Kristjansdottir K, Wolfgeher D, Hasin N, Polier S, Zhang H, et al. CDK-dependent Hsp70 Phosphorylation controls G1 cyclin abundance and cell-cycle progression. Cell. 2012;151:1308–18. doi: 10.1016/j.cell.2012.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menoyo S, Ricco N, Bru S, Hernández-Ortega S, Escoté X, Aldea M, et al. Phosphate-activated CDK stabilizes G1 cyclin to trigger cell cycle entry. Mol Cell Biol. 2013 doi: 10.1128/MCB.01556-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang D, Friesen H, Andrews B. Pho85, a multifunctional cyclin-dependent protein kinase in budding yeast. Mol Microbiol. 2007;66:303–14. doi: 10.1111/j.1365-2958.2007.05914.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee YS, Huang K, Quiocho FA, O’Shea EK. Molecular basis of cyclin-CDK-CKI regulation by reversible binding of an inositol pyrophosphate. Nat Chem Biol. 2008;4:25–32. doi: 10.1038/nchembio.2007.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landry BD, Doyle JP, Toczyski DP, Benanti JA. F-box protein specificity for g1 cyclins is dictated by subcellular localization. PLoS Genet. 2012;8:e1002851. doi: 10.1371/journal.pgen.1002851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Z, Geng J, Yen WL, Wang K, Klionsky DJ. Positive or negative roles of different cyclin-dependent kinase Pho85-cyclin complexes orchestrate induction of autophagy in Saccharomyces cerevisiae. Mol Cell. 2010;38:250–64. doi: 10.1016/j.molcel.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberger M, Feng L, Paul A, Smith DL, Jr., Hontz RD, Smith JS, et al. DNA replication stress is a determinant of chronological lifespan in budding yeast. PLoS ONE. 2007;2:e748. doi: 10.1371/journal.pone.0000748. [DOI] [PMC free article] [PubMed] [Google Scholar]