Abstract
In the developing embryo, hematopoiesis begins with the formation of primitive erythroid cells (EryP), a distinct and transient red blood cell lineage. EryP play a vital role in oxygen delivery and in generating shear forces necessary for normal vascular development. Progenitors for EryP arise as a cohort within the blood islands of the mammalian yolk sac at the end of gastrulation. As a strong heartbeat is established, nucleated erythroblasts begin to circulate and to mature in a stepwise, nearly synchronous manner. Until relatively recently, these cells were thought to be “primitive” in that they seemed to more closely resemble the nucleated erythroid cells of lower vertebrates than the enucleated erythrocytes of mammals. It is now known that mammalian EryP do enucleate, but not until several days after entering the bloodstream. I will summarize the common and distinguishing characteristics of primitive versus definitive (adult type) erythroid cells, review the development of EryP from the emergence of their progenitors through maturation and enucleation, and discuss pluripotent stem cells as models for erythropoiesis. Erythroid differentiation of both mouse and human pluripotent stem cells in vitro has thus far reproduced early but not late red blood cell ontogeny. Therefore, a deeper understanding of cellular and molecular mechanisms underlying the differences and similarities between the embryonic and adult erythroid lineages will be critical to improving methods for production of red blood cells for use in the clinic.
Keywords: primitive erythropoiesis, transgenic mice, mammalian embryo, erythroid progenitors, hemangioblast, erythroid differentiation, enucleation
Introduction
For more than a century, hematopoietic development in vertebrates has been thought to comprise “primitive” and “definitive” phases. The emergence of “primitive” erythroid cells (EryP) in the yolk sac (YS) marks the beginning of hematopoiesis in the mammalian embryo. EryP were believed to be more “primitive” than adult red blood cells because, like the macrocytic red cells of non-mammalian vertebrate embryos, they formed in the YS, circulated as nucleated erythroblasts, and were present only transiently, during embryonic stages of development [1]. With the demonstration that these large cells do eventually eliminate their nuclei [2–4] and then continue to circulate as enucleated cells until and perhaps beyond the time of birth [4], it became evident that they are not so primitive after all [1, 5]. “Primitive” is a misnomer not only because embryonic, fetal and adult mammalian erythroid cells all enucleate but also because the lineages share a number of other features, including differentiation from a unipotential progenitor, production of hemoglobin, progressive decrease in cell size and nuclear condensation during their terminal maturation, and regulation by erythroid transcription factors such as Gata1, Gata2, Eklf/Klf1, Scl/Tal, and Lmo2 [1, 6]. EryP play critical roles not only in facilitating the transport of oxygen and carbon dioxide throughout the embryo but also in vascular remodeling [7]. The importance of this lineage for normal development is suggested by its conservation among vertebrates and the observation that failure in primitive erythropoiesis invariably results in embryonic lethality [8, 9].
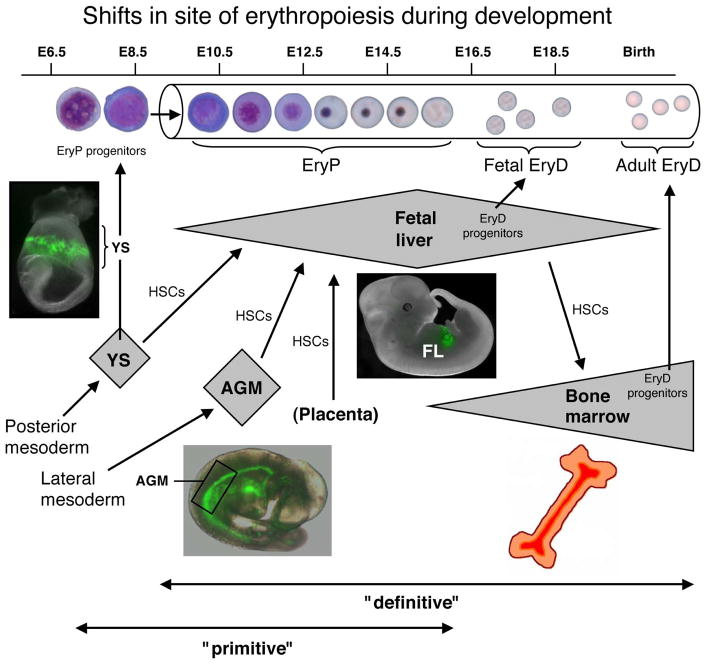
Shifts in sites of hematopoiesis during development
Mammalian hematopoiesis occurs in several waves and arises from multiple anatomic sites (Fig. 1). The first wave of hematopoietic cells appears in the YS and is largely erythropoietic, but also includes megakaryocytes and macrophages [10–14]. EryP progenitors are blast-like cells that are identified functionally on the basis of colony-forming assays in methylcellulose. They can be detected from around embryonic day (E) 7.25–8.75 in the mouse [10, 15] and within weeks 3–4 of human gestation [16] and may arise from bipotential megakaryocyte/erythroid progenitors (MEPs) [14]. Around the time primitive erythroblasts enter the circulation, progenitor activity is lost and the cells begin to differentiate. Until around midgestation, the macrocytic maturing EryP retain their nuclei [2, 3, 17], in contrast with the much smaller definitive EryD that terminally differentiate and enucleate extravascularly [18, 19], prior to entering the bloodstream. EryP are specified directly from mesodermal progenitors with restricted hematopoietic potential [20–22].
Figure 1. Shifts in the site of hematopoiesis during ontogeny.
The cartoon summarizes hematopoietic development in the mouse embryo but shifts in sites (YS, AGM region, placenta, FL, bone marrow) are closely analogous for human hematopoiesis. Primitive hematopoiesis initiates with the formation of EryP progenitors in the YS. Definitive hematopoiesis arises from HSCs that emerge in the Aorta-Gonads-Mesonephros (AGM) region, the large vessels of the embryo (not shown), and the placenta. At least in the mouse, HSCs also form in the YS, but later than primitive hematopoietic progenitors. HSCs migrate to and differentiate within the FL and, around the time of birth and throughout postnatal life, in the bone marrow. The photographs show transgenic mouse embryos that express a GFP reporter in the EryP lineage (see text). The spleen (not shown) is a site of hematopoiesis late in gestation [31].). The contributions of the thymus and spleen to lymphoid maturation are not shown in this cartoon.
The second hematopoietic wave also begins in the YS (~E8.25 in mouse [10] and ~4 weeks in human[23]), is “definitive,” and comprises erythroid and myeloid lineages [10, 11, 14, 23, 24]. In the human embryo, EryD progenitors are found in the YS by ~4 weeks and in the fetal liver (FL) by 5–6 weeks [23]. Thus, in both mouse and human, there is a partial temportal overlap between primitive and definitive hematopoiesis in the YS. YS definitive hematopoietic cells are believed to arise from transient multipotent progenitors (but not hematopoietic stem cells, HSC) that seed the FL [25]. The relationship between these progenitors and the extensively self-renewing erythroid (ESRE) progenitors obtained upon culture of ~E9.5 YS or E12.5 FL cells under carefully defined conditions [26] remains to be clarified.
The third wave of hematopoiesis is seemingly more complex and arises from HSCs produced within a number of intra-embryonic sites, including the aorta-gonad-mesonephros (AGM) region (E10.5–11.5), the major blood vessels, and the placenta (reviewed in refs. [9, 27, 28]). The HSCs that form in these tissues do not differentiate there [29] but seed the developing FL, thymus, and bone marrow, producing all hematopoietic lineages, including lymphoid potentials [30, 31]. Functional HSCs have been identified in E9.0 mouse YS [32, 33]. The developmental origins of HSCs (YS, embryo or both) have been the subject of a controversy that has once again been recently reopened [34].
Definitive hematopoietic development is less well understood in humans but also arises in association with the AGM region, large vessels and placenta (reviewed in refs. [12, 28]). HSC activity has recently been found to arise in the human AGM by around 5 weeks [35]. Though present in very small numbers, these cells display extremely high self-renewal ability [35].
Primitive versus definitive erythropoiesis
Primitive erythropoiesis has been studied in a number of model vertebrate organisms. This concise review will cover the mouse and human systems. Primitive and definitive erythroid cells are distinct lineages that arise from different populations of mesoderm (posterior and lateral plate, respectively) generated during gastrulation (reviewed in ref. [36]). While the two erythroid lineages share a number of common features, as noted above, they differ in a number of important characteristics. Whereas EryP form only in the YS, EryD progenitors are found in the YS, FL and, eventually, the adult bone marrow [8–10, 25]. EryP are much larger than EryD (Fig. 2), express distinct globin genes [37], have different oxygen carrying capacities, and differ in their requirements for specific cytokines (e.g. erythropoietin [38, 39]), transcription factors, and downstream signaling pathways (for a review, see ref. [6]).
Figure 2. Primitive erythroid cells are megalocytic and are much larger than definitive erythrocytes.
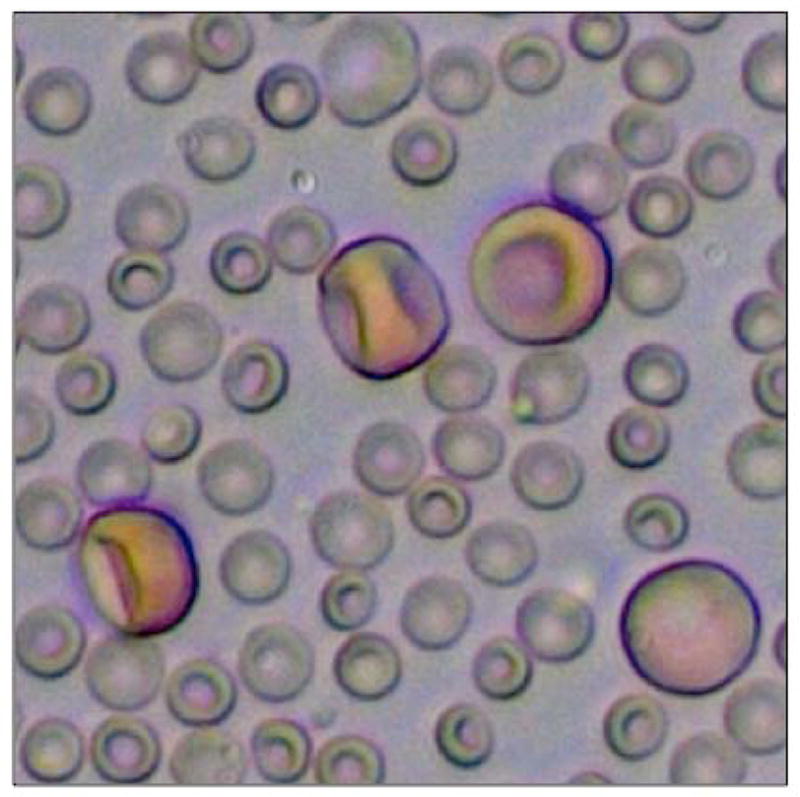
The photograph shows FACS-sorted E13.5 EryP/GFP+ cells that were mixed with maternal blood cells for the purpose of highlighting the difference in size of these cells.
The ontogeny of hematopoiesis is summarized in Fig. 2. Owing in large part to the technical challenges involved in studying the early mammalian embryo, much less is known about primitive compared with definitive erythropoiesis. In addition, after midgestation, as FL erythropoiesis becomes active, maturing EryP and adult-type erythrocytes (EryD) are present simultaneously within the circulation and EryP are rapidly outnumbered by EryD [2, 4]. At present, there are no cell surface markers that uniquely distinguish EryP from EryD. Transgenic mouse lines have been created to mark EryP, using expression of lacZ [40, 41] or green fluorescent protein (GFP) [4, 17, 41] reporters expressed under the control of human embryonic (epsilon)-globin regulatory sequences and a β-globin locus control region (ε-globin-H2B-GFP transgenic mice; photographs of such embryos are shown in Fig. 1). The fluorescent reporters have made it possible to isolate EryP progenitors [15] and maturing EryP [4, 17], track EryP enucleation [4, 17], generate a genome-wide transcriptome of this lineage at distinct stages of its development [15], and evaluate the consequences of ablating expression of a transcription factor (Eklf) specifically in the EryP lineage [42].
Hemangioblastic origins of primitive erythropoiesis
The observation that primitive erythroblasts form in close spatial and temporal relationship with vascular endothelial cells of the YS led to the proposal that they share a common progenitor termed the “hemangioblast” (reviewed in ref. [43]). It was thought that hemangioblasts gave rise to “blood islands,” clusters of EryP surrounded by endothelial cells within the mesothelial layer of the YS [13]. This view gained increased support from studies of differentiating mouse and human embryonic stem (ES) cells [44, 45] and from mouse embryos [21]. “Blast colony”-forming cells (BL-CFC) isolated from ES cell-derived embryoid bodies (EBs) displayed properties expected of the hemangioblast and were hypothesized to be its in vitro equivalent [44]. Interestingly, in the mouse embryo, most BL-CFC activity is found in the posterior primitive streak and not in the YS [21]. BL-CFC are not bipotential but, in addition to primitive and definitive hematopoietic cell types [14, 44], they can also give rise to smooth muscle [46] and non-hematopoietic mesenchymal cells [47].
The existence of the hemangioblast – or at least its unique function as a clonal source of hematopoietic and endothelial cells in the YS -- has been called into question by a number of other studies. Lineage-tracing experiments suggested that EryP and angioblasts (endothelial progenitors) in the YS do not share a common progenitor [20]. In other work involving mouse chimeric embryos expressing four different fluorescent proteins, the identification of polyclonal (multi-color) blood islands argued against a bipotential progenitor [22]. It is worth noting, however, that while cells with BL-CFC activity can give rise to either hematopoietic or endothelial cells in vitro [21, 44], it has not been claimed that the emergence of these lineages is clonal. In the embryo, hemangioblasts in the posterior streak may produce more restricted progenitors (e.g. EryP progenitors, angioblasts, megakaryocyte-erythroid progenitors) that colonize and differentiate within the YS. Yet another twist in the story came from an immunohistochemical analysis of the YS at ~E7.75 that revealed a “blood band” of CD41+ (hematopoietic progenitor) cells and occasional endothelial cells rather than discrete blood islands [13]. The authors proposed that this blood band is later subdivided by endothelial cells [13], perhaps by a second wave of angioblast development that is independent of hematopoiesis [48]. In contrast with evidence for a hemogenic endothelial origin for hematopoietic stem cell (HSC)-derived blood lineages [43, 49–51], the early wave of blood development in the YS does not progress through an endothelial intermediate [13, 52].
Properties of primitive erythroid progenitors
In the mouse, EryP progenitors can be detected in the YS (but not the embryo proper) between ~E7.25–E8.75 [10, 15]. EryP progenitors have been prospectively isolated from E7.5–E8.5 ε-globin-H2B-GFP transgenic mouse embryos using flow cytometry [15]. EryP progenitor activity was found exclusively in the FACS-sorted GFP+ population and expanded rapidly between E7.5 and E8.5 [15]. A fraction of the GFP+ cells from E7.5 embryos expressed Flk1, c-kit, VE-cadherin, and Tie-2 -- proteins normally associated with endothelial cells [15]. All progenitor activity was found in the GFP+; c-kit+ population and was enriched by 4-fold in the GFP+; Tie2+ fraction [15]. By ~E9.0, after EryP have begun to enter the bloodstream, progenitor activity is lost [discussed below and ref. 53]. However, circulating EryP continue to divide until ~E12.5–13.5 (our unpublished data and reviewed in ref. [1]).
EryP progenitors express a number of cell adhesion proteins on their surface (Pecam1, CD44 and the integrins α4, α5, β1 and β3) [15] (Fig. 3). Genes encoding other adhesion molecules as well as collagens are also expressed in these cells [15], suggesting that, within the YS, EryP form tight associations with one another and/or with surrounding endothelial cells, as suggested from electron microscopic analysis [54]. Loss of EryP surface adhesion proteins, in combination with expression of metalloproteases in the yolk sac, might help to facilitate the entry of these cells into the bloodstream [15].
Figure 3. Transient expression of α4, α5 and β1 integrins on the surface of progenitor stage EryP.
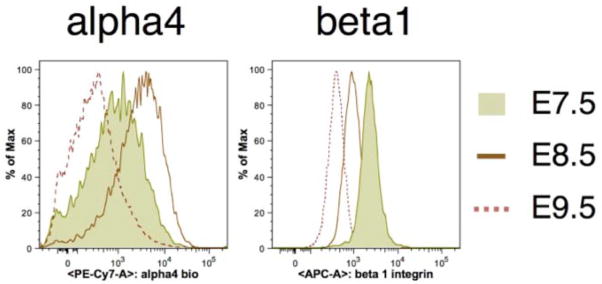
FACS histograms demonstrating that α4, α5 and β1 integrins are present on progenitor stage EryP (E7.5–8.5). By the time the cells have entered the circulation (around E9.0), little if any integrin expression is detected [15]. These integrins appear once again on the surface of EryP, later in their maturation [4, 17].
Primitive erythroid cells mature within the circulation
Circulating EryP continue to mature within the bloodstream, progressing in a stepwise, developmentally synchronized fashion from proerythroblast to orthochromatic erythroblast to reticulocyte [4]. This cellular maturation is accompanied by the loss of nucleoli (E9.5–10.5), decreased cell diameter and cross-sectional area (E10.5–11.5), condensation of nuclei to ~20% of their original size (E10.5 onwards), expression of cell adhesion proteins (E12.5–14.5 [4, 17]), and enucleation to primitive reticulocytes (E12.5 onwards [2–4]). Maturing EryP also display a progressive loss of CD71 and upregulation of Ter-119 expression [4] analogous to that observed for FL erythroblasts [55]. Selective reorganization of surface proteins such as Ter-119, CD71 and integrins occurs during enucleation of EryP [4, 17, 42]. A similar process has been reported for the partitioning of β1 integrin and phosphatidylserine onto the membrane surrounding the expelled nuclei and of Ter-119 onto the reticulocyte membrane of definitive erythroid cells [56–58].
In a study that overturned the long-held belief that EryP retain their nuclei throughout their lifetime, an antibody against a mouse embryonic β-like hemoglobin chain was used to show that EryP begin to undergo nuclear extrusion by E12.5 [2]. These findings were confirmed and extended in ε-globin-GFP transgenic mouse embryos, using a FACS analysis in which nucleated and enucleated EryP were distinguished and quantified in peripheral blood based on binding of the cell-permeable DNA-binding fluorescent dye DRAQ5 and EryP-specific expression of GFP [4]. Enucleation was essentially complete by E15.5 [4] but the total number of GFP+ EryP remained about the same from E12.5 to the end of gestation (our unpublished data and ref. [4]). Therefore, EryP remain a stable population through the rest of gestation [4].
The signals that initiate differentiation of EryP progenitors, just as the cells begin to enter the bloodstream [10, 53], are largely unknown. An important breakthrough in our understanding of this process has recently come from a screen for microRNAs (miRs) in sorted populations of differentiating ES cells that might function in early hematopoiesis [47]. miR-126 was identified as a non cell-autonomous regulator of primitive erythropoiesis that represses Vascular Cell Adhesion Molecule-1 (Vcam-1) in the YS mesenchyme [47]. Upon downregulation of miR-126, expression of Vcam-1 is induced on the surface of mesenchymal cells. Engagement of Vcam-1 with its ligands on EryP, for instance α4β1 integrin (VLA-4), which is expressed on EryP progenitors [15] (Fig. 3), initiates a maturation signal in EryP that operates via a Src Family Kinase (SFK) member that remains to be identified [47]. The Vcam-1+ mesenchymal cells have not yet been well characterized, but they appear to arise from a Flk1+ hemangioblastic progenitor [47].
Extravascular niches for primitive erythroid maturation and enucleation
The first enucleated EryP are not detected in the blood until E12.5, five days after they first appear in the YS and at least three days after they have begun to circulate [2, 4]. An explanation for this lag may be that EryP appear to collect and complete their maturation within the FL [3, 17], an organ that does not form until midgestation.
Integrins (α4-, α5-, and β1) are expressed transiently on EryP progenitors and are downregulated by the time the cells have entered the circulation [15] (Fig. 3). These and other adhesion proteins are re-expressed beginning around E12.5 [4], suggesting the possibility that EryP home to a fetal tissue such as the liver, where they continue to mature. Support for this hypothesis came from experiments using ε-globin-GFP transgenic mouse embryos [17]. Green fluorescence was detected in the FLs of transgenic embryos from E10.5 through E14.5 [17]. The FL is the site of development of definitive erythroid cells, which mature within “erythroblastic islands” (EBIs) [18]. EBIs contain a central macrophage surrounded by erythroid cells at various stages of maturation [18]. The macrophages are thought to function as nurse cells and engulf expelled erythroid nuclei [18]. EryP/GFGP+ cells were found in EBIs of the FL, along with developing definitive erythroblasts and in close association with macrophages [17]. Reconstitution experiments demonstrated that EryP can bind to FL macrophages in a developmentally regulated manner [17]. The EryP/GFP+ cells found in the FL upregulate α4, α5 and β1 integrins and CD44 and bind more readily to macrophages in reconstituted EBIs than do circulating EryP [17]. The “rosettes” formed by these interactions can be disrupted by a blocking antibody against Vcam-1, a macrophage receptor for α4β1 integrin [17], and by peptide blocking of α4 and α5 integrin binding to fibronectin (Isern and Baron, unpublished data). Confocal microscopy and FACS analyses of FLs from ε-globin-H2B-GFP (nuclear GFP) transgenic mice indicated that nuclei extruded by EryP, like those of adult red blood cells [58], undergo phagocytosis by macrophages [17]. In both adult and embryonic lineages, nuclear extrusion occurs by budding off of the highly condensed nucleus surrounded by a rim of plasma membrane [3].
EryP enucleation could be monitored and expelled nuclei isolated using flow cytometry [17]. This analysis confirmed that the extruded nuclei were highly enriched for integrins, in contrast with enucleated primitive reticulocytes, which showed little or no expression of these adhesion proteins [17]. Therefore, adhesion molecules are specifically redistributed onto the membrane surrounding the expelled nucleus, perhaps causing it to become more attractive for engulfment by FL macrophages [17]. Others have used antibodies against mouse embryonic β-hemoglobins to identify EryP and to demonstrate that these cells are present in FL and can interact with macrophages that can engulf expelled nuclei in vitro [3]. Direct evidence for maturation and enucleation of EryP within the FL will require time-lapse imaging. Although a role for macrophages in definitive erythropoiesis is well accepted [18], it is not clear that they are essential for enucleation of either erythroid lineage (reviewed in ref. [6]).
Enucleation of human primitive erythroblasts has been reported to occur within the placental villi rather than the FL [16]. The expelled nuclei are likely engulfed by macrophages, as suggested from fluorescence microscopy [16]. It is not yet known whether enucleation of EryP also occurs in the placenta of the mouse.
Transcriptional regulators of primitive erythroid development
Transcription during primitive erythropoiesis is characterized by two discrete waves that correlate with the transition from YS to circulation and with late maturation events such as nuclear condensation and enucleation, respectively [15]. The zinc finger proteins GATA1 and EKLF are central players in both primitive and definitive erythropoiesis. Deletion of both Gata1 and Gata2 genes essentially eliminates primitive erythropoiesis [59]. While EKLF (KLF1) was initially thought to function only in definitive erythropoiesis [60, 61], it later became evident that this gene functions in primitive erythropoiesis as well [42, 62, 63]. Haploinsufficiency of Eklf in the embryo results in striking abnormalities in the cell surface of EryP but not EryD, suggesting that EryP maturation may be more sensitive to gene dosage [42]. In EryD, fetal-to-adult globin gene switching is also sensitive to Eklf gene dosage [64, 65] and haploinsufficiency of EKLF in humans causes hereditary persistence of fetal hemoglobin (HPFH) [64]. Loss of Eklf in EryP causes an “identity crisis” [42] (partial change in cellular identity) within maturing EryP in the circulation, with upregulation of a subset of megakaryocyte-related surface proteins such as CD41 and Pecam-1/CD31 and transcription factors such as Fli-1 [42]. Therefore, Eklf not only regulates lineage divergence from the megakaryocyte-erythroid progenitor (MEP, reviewed in ref. [66]) but also controls various aspects of EryP maturation [15]. Cell surface abnormalities are also seen on Runx1 mutant EryP [67], suggesting that Eklf and Runx1 may regulate some of the same targets.
In the absence of both Eklf and the closely related gene Klf2, embryonic lethality occurs by ~E11.5, 2 to 4 days earlier than when either gene is deleted alone [68], suggesting that these genes function in a common pathway. Recently it has been demonstrated that c-Myc, known to be a critical regulator of erythroid progenitor survival in vivo [69], is a direct target of both Eklf/Klf1 and Klf2 [70]. These findings are particularly interesting in light of the discovery that c-Myc functions as a transcriptional amplifier of pre-existing gene expression [71, 72]
EryP and EryD differ in their requirements for a variety of other transcriptional regulators such as c-Myb, Runx1, Sox6 and ZBP-89 [73–78].
Pluripotent stem cells as models for mammalian erythropoiesis
Embryonic stem (ES) cells, isolated from the “inner cell mass” of a preimplantation embryo (the blastocyst), have gained increasing attention as an approach to exploring the induction and differentiation of each of the three germ layers [79, 80]. Under appropriate conditions, ES cells form EBs that mimic many aspects of early embryogenesis [79, 80]. The ability to move back and forth between the developing embryo and cell culture has been a particularly powerful feature of the mouse ES cell system, which is also the best studied. The temporal appearance of hematopoietic and endothelial progenitors has been well characterized in ES cell-derived EBs and mimics that observed during normal embryogenesis. For example, in differentiating ES cultures from mouse or human, EryP progenitors are found earlier than cells with definitive erythroid potential [44, 45, 81–85]. Studies using ES cells have complemented genetic analyses in the mouse and sometimes have often led to important insights such as the realization that erythroid progenitors form in the absence of Gata1 but fail to mature [86].
Erythroid differentiation of mouse and human ES cell cultures has resulted in the formation of megalocytic nucleated erythroblasts that resemble yolk sac-derived cells but do not enucleate [87, 88]. In one report, terminal differentiation of mouse ES-derived erythroid cells was observed after a multistep, prolonged culture period, but other hematopoietic cell types also formed and the “progenitor” population used was not well characterized [89]. Extended coculture of hESCs with immortalized fetal hepatocytes led to the differentiation of more mature fetal-like erythroblasts that expressed mostly fetal but not adult hemoglobin; some of these cells did enucleate [90].
Over the past few years, a wide variety of mouse and human embryonic, fetal and adult somatic cell types have been reprogrammed in vitro to “induced Pluripotent Stem (iPS)” cells that are very similar, and sometimes essentially identical, to ES cells [91–95]. Like ES cells, iPS cells can be induced to differentiate to a variety of lineages, including hematopoietic cells. However, in contrast with mouse ES cells, human ES [87, 88, 90, 96] and iPS [95, 97, 98] cell-derived red blood cells express little or no adult β-globin and enucleation is generally incomplete. Thus, differentiation of pluripotential cells in vitro produces erythroid cells that are phenotypically most similar to embryonic/fetal stages of ontogeny. Comparisons between the mouse and human systems will be important for understanding molecular mechanisms underlying the differentiation of the erythroid lineages and for the development of efficient methods for the production of adult type red blood cells for clinical use.
Perspectives
Our understanding of embryonic erythropoiesis has progressed over the past decade, but important questions remain unresolved. Why have vertebrates evolved to produce two erythroid lineages? Studies in the mouse embryo hint that the extraembryonic (YS) production of EryP may allow the embryo proper to conserve resources for the formation of other tissues. EryP progenitors constitute a transient amplifying pool from which large numbers of primitive erythroblasts are generated [15]. These cells enter the bloodstream and continue to divide for several days as they circulate and carry oxygen throughout the embryo. EryP are essential to support the transition from embryo to fetus, as indicated by the lethal phenotype of Gata1 mutants [99]. A second transient wave of erythroid cells (ESREs [26]) may serve to support the fetus until HSC-derived erythropoiesis is underway.
Cell size is a striking distinguishing feature of EryP and EryD (Fig. 2). FL EryD are intermediate in size between EryP and postnatal EryD [6]. What controls erythroid cell size? Cyclin D3 [100] and its targets Cdk4 and 6 [101] may be the key to this question, but more work will be required to determine whether there are different requirements for these cell cycle regulators during erythroid ontogeny.
What signals trigger the differentiation of EryP progenitors at the onset of active circulation? The implication of miR-126 in regulating the transition between EryP progenitor and differentiating erythroblast through Vcam-1 [47] provides an important clue but raises a new question. Vcam-1(+) mesenchymal cells were proposed to provide a signal to EryP progenitors, but how? The erythroid progenitors are in contact with one another and with vascular endothelium, but there is no evidence for a direct interaction with YS mesenchyme. It seems unlikely that interruptions in vascular integrity during remodeling would provide sufficient opportunity for EryP-mesenchyme interactions. Conceivably the Vcam-1 signal is soluble and can cross the endothelial barrier. Alternatively, another cell type may be the direct source of the signal in vivo. Experiments using purified EryP progenitors and other YS cell types may provide additional insights.
What is the significance of the relatively late (~E12.5–15.5) enucleation of EryP? Is their enucleation essential for normal development? It is possible that loss of the nuclei from these very large cells provides the necessary cellular flexibility for navigation through the narrowing capillary beds of later stage embryos.
What is the function of macrophages in erythropoiesis? Several lines of evidence argue against a requirement for macrophages in erythropoiesis [6]. However, these cells may stimulate proliferation of progenitors [102] and/or play other supportive roles [18].
Progenitors for primitive erythropoiesis are the first hematopoietic cells of the embryo and are found in the YS only briefly; terminally differentiated primitive erythrocytes are present in the circulation through the end of gestation and probably for a short time after birth [4]. The ontogeny of erythropoiesis is much more complex than previously appreciated. For example, at least in the mouse, three distinct erythroid lineages can be identified (EryP, YS-derived EryD, and HSC-derived EryD), each with a unique pattern of β-globin gene expression [25]. Interpretation of observations made not only in vivo but also in cell culture systems such as ES and iPS cells must take this complexity into consideration. Our understanding of red blood cell biology will continue to be enriched by comparative studies of erythropoiesis at different stages of development, in different tissues within the same stage, and across species.
Acknowledgments
I apologize to those whose work could not be cited due to space limitations. Work in my lab has been supported by grants from the National Institutes of Health (RO1 HL62248, DK52191, and EB02209), the Roche Foundation for Anemia Research (grant 9699367999, cycle X) and the New York State Department of Health (NYSTEM grant N08G-024).
Footnotes
Authorship:
Contribution: M.H.B. wrote the paper.
Disclosure of potential Conflicts of Interest:
The author declares no conflict of interest.
References
- 1.Palis J, Malik J, McGrath KE, et al. Primitive erythropoiesis in the mammalian embryo. Int J Dev Biol. 2010;54:1011–1018. doi: 10.1387/ijdb.093056jp. [DOI] [PubMed] [Google Scholar]
- 2.Kingsley PD, Malik J, Fantauzzo KA, et al. Yolk Sac-Derived Primitive Erythroblasts Enucleate During Mammalian Embryogenesis. Blood. 2004;104:19–25. doi: 10.1182/blood-2003-12-4162. [DOI] [PubMed] [Google Scholar]
- 3.McGrath KE, Kingsley PD, Koniski AD, et al. Enucleation of primitive erythroid cells generates a transient population of “pyrenocytes” in the mammalian fetus. Blood. 2008;111:2409–2417. doi: 10.1182/blood-2007-08-107581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser ST, Isern J, Baron MH. Maturation and enucleation of primitive erythroblasts is accompanied by changes in cell surface antigen expression patterns during mouse embryogenesis. Blood. 2007;109:343–352. doi: 10.1182/blood-2006-03-006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mullner E. Erythropoiesis: early, not primitive. Blood. 2011;117:4685–4686. doi: 10.1182/blood-2011-02-334573. [DOI] [PubMed] [Google Scholar]
- 6.Baron MH, Isern J, Fraser ST. The embryonic origins of erythropoiesis in mammals. Blood. 2012;119:4828–4837. doi: 10.1182/blood-2012-01-153486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucitti JL, Jones EA, Huang C, et al. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lensch MW, Daley GQ. Origins of mammalian hematopoiesis: in vivo paradigms and in vitro models. Curr Top Dev Biol. 2004;60:127–196. doi: 10.1016/S0070-2153(04)60005-6. [DOI] [PubMed] [Google Scholar]
- 9.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palis J, Robertson S, Kennedy M, et al. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 11.Bertrand JY, Jalil A, Klaine M, et al. Three pathways to mature macrophages in the early mouse yolk sac. Blood. 2005;106:3004–3011. doi: 10.1182/blood-2005-02-0461. [DOI] [PubMed] [Google Scholar]
- 12.Tavian M, Peault B. Embryonic development of the human hematopoietic system. Int J Dev Biol. 2005;49:243–250. doi: 10.1387/ijdb.041957mt. [DOI] [PubMed] [Google Scholar]
- 13.Ferkowicz MJ, Yoder MC. Blood island formation: longstanding observations and modern interpretations. Exp Hematol. 2005;33:1041–1047. doi: 10.1016/j.exphem.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Tober J, Koniski A, McGrath KE, et al. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 2007;109:1433–1441. doi: 10.1182/blood-2006-06-031898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isern J, He Z, Fraser ST, et al. Single lineage transcriptome analysis reveals key regulatory pathways in primitive erythroid progenitors in the mouse embryo. Blood. 2011;117:4924–4934. doi: 10.1182/blood-2010-10-313676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Handel B, Prashad SL, Hassanzadeh-Kiabi N, et al. The first trimester human placenta is a site for terminal maturation of primitive erythroid cells. Blood. 2010;116:3321–3330. doi: 10.1182/blood-2010-04-279489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isern J, Fraser ST, He Z, et al. The fetal liver is a niche for maturation of primitive erythroid cells. Proc Natl Acad Sci US A. 2008;105:6662–6667. doi: 10.1073/pnas.0802032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chasis JA, Mohandas N. Erythroblastic islands: niches for erythropoiesis. Blood. 2008;112:470–478. doi: 10.1182/blood-2008-03-077883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji P, Murata-Hori M, Lodish HF. Formation of mammalian erythrocytes: chromatin condensation and enucleation. Trends Cell Biol. 2011;21:409–415. doi: 10.1016/j.tcb.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kinder SJ, Tsang TE, Quinlan GA, et al. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Develop. 1999;126:4691–4701. doi: 10.1242/dev.126.21.4691. [DOI] [PubMed] [Google Scholar]
- 21.Huber TL, Kouskoff V, Fehling HJ, et al. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–630. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 22.Ueno H, Weissman IL. Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell. 2006;11:519–533. doi: 10.1016/j.devcel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Migliaccio G, Migliaccio AR, Petti S, et al. Human embryonic hemopoiesis. Kinetics of progenitors and precursors underlying the yolk sac----liver transition. J Clin Invest. 1986;78:51–60. doi: 10.1172/JCI112572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palis J, Chan RJ, Koniski A, et al. Spatial and temporal emergence of high proliferative potential hematopoietic precursors during murine embryogenesis. Proc Natl Acad Sci US A. 2001;98:4528–4533. doi: 10.1073/pnas.071002398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGrath KE, Frame JM, Fromm GJ, et al. A transient definitive erythroid lineage with unique regulation of the beta-globin locus in the mammalian embryo. Blood. 2011;117:4600–4608. doi: 10.1182/blood-2010-12-325357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.England SJ, McGrath KE, Frame JM, et al. Immature erythroblasts with extensive ex vivo self-renewal capacity emerge from the early mammalian fetus. Blood. 2011;117:2708–2717. doi: 10.1182/blood-2010-07-299743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee LK, Ueno M, Van Handel B, et al. Placenta as a newly identified source of hematopoietic stem cells. Curr Opin Hematol. 2010;17:313–318. doi: 10.1097/MOH.0b013e328339f295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Godin I, Garcia-Porrero JA, Dieterlen-Lievre F, et al. Stem cell emergence and hemopoietic activity are incompatible in mouse intraembryonic sites. J Exp Med. 1999;190:43–52. doi: 10.1084/jem.190.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumaravelu P, Hook L, Morrison AM, et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- 31.Christensen JL, Wright DE, Wagers AJ, et al. Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biol. 2004;2:E75. doi: 10.1371/journal.pbio.0020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoder MC, Hiatt K. Engraftment of embryonic hemopoietic cells in conditioned newborn recepients. Blood. 1997;89:2176–2183. [PubMed] [Google Scholar]
- 33.Yoder MC, Hiatt K, Mukherjee P. In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proc Natl Acad Sci US A. 1997;94:6776–6780. doi: 10.1073/pnas.94.13.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka Y, Hayashi M, Kubota Y, et al. Early ontogenic origin of the hematopoietic stem cell lineage. Proc Natl Acad Sci U S A. 2012;109:4515–4520. doi: 10.1073/pnas.1115828109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ivanovs A, Rybtsov S, Welch L, et al. Highly potent human hematopoietic stem cells first emerge in the intraembryonic aorta-gonad-mesonephros region. J Exp Med. 2011;208:2417–2427. doi: 10.1084/jem.20111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baron MH. Early Patterning of the Mouse Embryo: Implications for Hematopoietic Commitment and Differentiation. Exper Hematol. 2005;33:1015–1020. doi: 10.1016/j.exphem.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Sankaran VG, Xu J, Orkin SH. Advances in the understanding of haemoglobin switching. Br J Haematol. 2010;149:181–194. doi: 10.1111/j.1365-2141.2010.08105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu H, Liu X, Jaenisch R, et al. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 39.Lin C-S, Lim S-K, D’Agati V, et al. Differential effects of an erythropoietin receptor gene disruption on primitive and definitive erythropoiesis. Genes & Develop. 1996;10:154–164. doi: 10.1101/gad.10.2.154. [DOI] [PubMed] [Google Scholar]
- 40.Belaoussoff M, Farrington SM, Baron MH. Hematopoietic Induction and Respecification of AP Identity by Visceral Endoderm Signaling in the Mouse Embryo. Development. 1998;125:5009–5018. doi: 10.1242/dev.125.24.5009. [DOI] [PubMed] [Google Scholar]
- 41.Dyer MA, Farrington SM, Mohn D, et al. Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neurectodermal cell fate in the mouse embryo. Development. 2001;128:1717–1730. doi: 10.1242/dev.128.10.1717. [DOI] [PubMed] [Google Scholar]
- 42.Isern J, Fraser ST, He Z, et al. Dose-dependent regulation of primitive erythroid maturation and identity by the transcription factor Eklf. Blood. 2010;116:3972–3980. doi: 10.1182/blood-2010-04-281196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirschi KK. Hemogenic endothelium during development and beyond. Blood. 2012;119:4823–4827. doi: 10.1182/blood-2011-12-353466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi K, Kennedy M, Kazarov A, et al. A common precursor for hematopoietic and endothelial cells. Develop. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 45.Zambidis ET, Peault B, Park TS, et al. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2005;106:860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ema M, Faloon P, Zhang WJ, et al. Combinatorial effects of Flk1 and Tal1 on vascular and hematopoietic development in the mouse. Genes & Develop. 2003;17:380–393. doi: 10.1101/gad.1049803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sturgeon CM, Chicha L, Ditadi A, et al. Primitive Erythropoiesis Is Regulated by miR-126 via Nonhematopoietic Vcam-1(+) Cells. Dev Cell. 2012 doi: 10.1016/j.devcel.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 48.Furuta C, Ema H, Takayanagi S, et al. Discordant developmental waves of angioblasts and hemangioblasts in the early gastrulating mouse embryo. Development. 2006;133:2771–2779. doi: 10.1242/dev.02440. [DOI] [PubMed] [Google Scholar]
- 49.Lancrin C, Sroczynska P, Stephenson C, et al. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eilken HM, Nishikawa S, Schroeder T. Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature. 2009;457:896–900. doi: 10.1038/nature07760. [DOI] [PubMed] [Google Scholar]
- 51.Chen MJ, Li Y, De Obaldia ME, et al. Erythroid/Myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell. 2011;9:541–552. doi: 10.1016/j.stem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iacovino M, Chong D, Szatmari I, et al. HoxA3 is an apical regulator of haemogenic endothelium. Nat Cell Biol. 2011;13:72–78. doi: 10.1038/ncb2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGrath KE, Koniski AD, Malik J, et al. Circulation is established in a stepwise pattern in the mammalian embryo. Blood. 2003;101:1669–1676. doi: 10.1182/blood-2002-08-2531. [DOI] [PubMed] [Google Scholar]
- 54.Haar J, Ackerman GA. A phase and electron microscopic study of vasculogenesis and erythropoiesis in the yolk sac of the mouse. Anat Rec. 1971;170:199–224. doi: 10.1002/ar.1091700206. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J, Socolovsky M, Gross AW, et al. Role of Ras signaling in erythroid differentiation of mouse fetal liver cells: functional analysis by a flow cytometry-based novel culture system. Blood. 2003;102:3938–3946. doi: 10.1182/blood-2003-05-1479. [DOI] [PubMed] [Google Scholar]
- 56.Geiduschek JB, Singer SJ. Molecular changes in the membranes of mouse erythroid cells accompanying differentiation. Cell. 1979;16:149–163. doi: 10.1016/0092-8674(79)90196-x. [DOI] [PubMed] [Google Scholar]
- 57.Lee JC, Gimm JA, Lo AJ, et al. Mechanism of protein sorting during erythroblast enucleation: role of cytoskeletal connectivity. Blood. 2004;103:1912–1919. doi: 10.1182/blood-2003-03-0928. [DOI] [PubMed] [Google Scholar]
- 58.Yoshida H, Kawane K, Koike M, et al. Phosphatidylserine-dependent engulfment by macrophages of nuclei from erythroid precursor cells. Nature. 2005;437:754–758. doi: 10.1038/nature03964. [DOI] [PubMed] [Google Scholar]
- 59.Fujiwara Y, Chang AN, Williams AM, et al. Functional overlap of GATA-1 and GATA-2 in primitive hematopoietic development. Blood. 2004;103:583–585. doi: 10.1182/blood-2003-08-2870. [DOI] [PubMed] [Google Scholar]
- 60.Nuez B, Michalovich D, Bygrave A, et al. Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature. 1995;375:316–318. doi: 10.1038/375316a0. [DOI] [PubMed] [Google Scholar]
- 61.Perkins AC, Sharpe AH, Orkin SH. Lethal β-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature. 1995;375:318–322. doi: 10.1038/375318a0. [DOI] [PubMed] [Google Scholar]
- 62.Drissen R, von Lindern M, Kolbus A, et al. The erythroid phenotype of EKLF-null mice: defects in hemoglobin metabolism and membrane stability. Mol Cell Biol. 2005;25:5205–5214. doi: 10.1128/MCB.25.12.5205-5214.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hodge D, Coghill E, Keys J, et al. A global role for EKLF in definitive and primitive erythropoiesis. Blood. 2006;107:3359–3370. doi: 10.1182/blood-2005-07-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borg J, Papadopoulos P, Georgitsi M, et al. Haploinsufficiency for the erythroid transcription factor KLF1 causes hereditary persistence of fetal hemoglobin. Nat Genet. 2010;42:801–805. doi: 10.1038/ng.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou D, Liu K, Sun CW, et al. KLF1 regulates BCL11A expression and gamma- to beta-globin gene switching. Nat Genet. 2010;42:742–744. doi: 10.1038/ng.637. [DOI] [PubMed] [Google Scholar]
- 66.Siatecka M, Bieker JJ. The multifunctional role of EKLF/KLF1 during erythropoiesis. Blood. 2011;118:2044–2054. doi: 10.1182/blood-2011-03-331371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yokomizo T, Hasegawa K, Ishitobi H, et al. Runx1 is involved in primitive erythropoiesis in the mouse. Blood. 2008;111:4075–4080. doi: 10.1182/blood-2007-05-091637. [DOI] [PubMed] [Google Scholar]
- 68.Basu P, Lung TK, Lemsaddek W, et al. EKLF and KLF2 have compensatory roles in embryonic {beta}-globin gene expression and primitive erythropoiesis. Blood. 2007;110:3417–3425. doi: 10.1182/blood-2006-11-057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dubois NC, Adolphe C, Ehninger A, et al. Placental rescue reveals a sole requirement for c-Myc in embryonic erythroblast survival and hematopoietic stem cell function. Development. 2008;135:2455–2465. doi: 10.1242/dev.022707. [DOI] [PubMed] [Google Scholar]
- 70.Pang CJ, Lemsaddek W, Alhashem YN, et al. Kruppel-like factor 1 (KLF1), KLF2, and Myc control a regulatory network essential for embryonic erythropoiesis. Mol Cell Biol. 2012;32:2628–2644. doi: 10.1128/MCB.00104-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin CY, Loven J, Rahl PB, et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151:56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nie Z, Hu G, Wei G, et al. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell. 2012;151:68–79. doi: 10.1016/j.cell.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mucenski ML, McLain K, Kier AB, et al. A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell. 1991;65:677–689. doi: 10.1016/0092-8674(91)90099-k. [DOI] [PubMed] [Google Scholar]
- 74.Okuda T, van Deursen J, Hiebert SW, et al. AML1, the Target of Multiple Chromosomal Translocations in Human Leukemia, Is Essential for Normal Fetal Liver Hematopoiesis. Cell. 1996;84:321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 75.Wang Q, Stacy T, Binder M, et al. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci US A. 1996;93:3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dumitriu B, Bhattaram P, Dy P, et al. Sox6 is necessary for efficient erythropoiesis in adult mice under physiological and anemia-induced stress conditions. PLoS One. 2006;5:e12088. doi: 10.1371/journal.pone.0012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Woo AJ, Moran TB, Schindler YL, et al. Identification of ZBP-89 as a novel GATA-1-associated transcription factor involved in megakaryocytic and erythroid development. Mol Cell Biol. 2008;28:2675–2689. doi: 10.1128/MCB.01945-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mancini E, Sanjuan-Pla A, Luciani L, et al. FOG-1 and GATA-1 act sequentially to specify definitive megakaryocytic and erythroid progenitors. Embo J. 2011 doi: 10.1038/emboj.2011.390. advance online publication 8 November 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 80.Murry CE, Keller G. Differentiation of Embryonic Stem Cells to Clinically Relevant Populations: Lessons from Embryonic Development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 81.Kennedy M, Firpo M, Choi K, et al. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386:488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- 82.Robertson SM, Kennedy M, Shannon JM, et al. A transitional stage in the commitment of mesoderm to hematopoiesis requiring the transcription factor SCL/tal-1. Develop. 2000;127:2447–2459. doi: 10.1242/dev.127.11.2447. [DOI] [PubMed] [Google Scholar]
- 83.Keller GA, Webb S, Kennedy M. Hematopoietic development of ES cells in culture. In: Klug CA, Jordan CT, editors. Hematopoietic Stem Cell Protocols. Humana Press; 2001. pp. 209–230. [Google Scholar]
- 84.Fehling HJ, Lacaud G, Kubo A, et al. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Develop. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- 85.Kennedy M, D’Souza SL, Lynch-Kattman M, et al. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–2687. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weiss MJ, Keller G, Orkin SH. Novel insights into erythroid development revealed through in vitro differentiation of GATA-1- embryonic stem cells. Genes & Develop. 1994;8:1184–1197. doi: 10.1101/gad.8.10.1184. [DOI] [PubMed] [Google Scholar]
- 87.Chang KH, Nelson AM, Cao H, et al. Definitive-like erythroid cells derived from human embryonic stem cells co-express high levels of embryonic and fetal globins with little or no adult globin. Blood. 2006;108:1515–1523. doi: 10.1182/blood-2005-11-011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Olivier EN, Qiu C, Velho M, et al. Large-scale production of embryonic red blood cells from human embryonic stem cells. Exp Hematol. 2006;34:1635–1642. doi: 10.1016/j.exphem.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 89.Carotta S, Pilat S, Mairhofer A, et al. Directed differentiation and mass cultivation of pure erythroid progenitors from mouse embryonic stem cells. Blood. 2004;104:1873–1880. doi: 10.1182/blood-2004-02-0570. [DOI] [PubMed] [Google Scholar]
- 90.Qiu C, Olivier EN, Velho M, et al. Globin switches in yolk sac-like primitive and fetal-like definitive red blood cells produced from human embryonic stem cells. Blood. 2008;111:2400–2408. doi: 10.1182/blood-2007-07-102087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pera MF. Stem cells. A new year and a new era. Nature. 2008;451:135–136. doi: 10.1038/451135a. [DOI] [PubMed] [Google Scholar]
- 92.Maherali N, Hochedlinger K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell. 2008;3:595–605. doi: 10.1016/j.stem.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 93.Rossant J. The impact of developmental biology on pluripotent stem cell research: successes and challenges. Dev Cell. 2011;21:20–23. doi: 10.1016/j.devcel.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 94.Carey BW, Markoulaki S, Hanna JH, et al. Reprogramming factor stoichiometry influences the epigenetic state and biological properties of induced pluripotent stem cells. Cell Stem Cell. 2011;9:588–598. doi: 10.1016/j.stem.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 95.Chang CJ, Mitra K, Koya M, et al. Production of embryonic and fetal-like red blood cells from human induced pluripotent stem cells. PLoS One. 2011;6:e25761. doi: 10.1371/journal.pone.0025761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qiu C, Hanson E, Olivier E, et al. Differentiation of human embryonic stem cells into hematopoietic cells by coculture with human fetal liver cells recapitulates the globin switch that occurs early in development. Exp Hematol. 2005;33:1450–1458. doi: 10.1016/j.exphem.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 97.Chang KH, Huang A, Hirata RK, et al. Globin phenotype of erythroid cells derived from human induced pluripotent stem cells. Blood. 2010;115:2553–2554. doi: 10.1182/blood-2009-11-252650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lapillonne H, Kobari L, Mazurier C, et al. Red blood cell generation from human induced pluripotent stem cells: perspectives for transfusion medicine. Haematologica. 2011;95:1651–1659. doi: 10.3324/haematol.2010.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pevny L, Lin C-S, D’Agati V, et al. Development of hematopoietic cells lacking transcription factor GATA-1. Development. 1995;121:163–172. doi: 10.1242/dev.121.1.163. [DOI] [PubMed] [Google Scholar]
- 100.Sankaran VG, Ludwig LS, Sicinska E, et al. Cyclin D3 coordinates the cell cycle during differentiation to regulate erythrocyte size and number. Genes Dev. 2012;26:2075–2087. doi: 10.1101/gad.197020.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166. doi: 10.1038/nrc2602. [DOI] [PubMed] [Google Scholar]
- 102.Rhodes MM, Kopsombut P, Bondurant MC, et al. Adherence to macrophages in erythroblastic islands enhances erythroblast proliferation and increases erythrocyte production by a different mechanism than erythropoietin. Blood. 2008;111:1700–1708. doi: 10.1182/blood-2007-06-098178. [DOI] [PMC free article] [PubMed] [Google Scholar]