Abstract
A specific bacterial gut microbiota profile with increased extraction of calories has recently been associated with obesity, which has been shown to be a transmissible phenotype by microbiota transplantation. At the same time, there is now increasing evidence that gut microbiota plays a role in the development of hepatic steatosis and its progression to non-alcoholic steatohepatitis, as well.
This review summarizes well-known as well as unexpected interacting factors leading to obesity and its related hepatic diseases, including intestinal mucosal permeability and its regulation, gut microbiota and translocation of its biological products, and gut associated lymphoid tissue. These intestinal factors dictate also the balance between tolerance and immune response which are critical for most of the complications in near and far organs or systems.
We will review novel mechanisms involving the development of gut permeability and adipose tissue plasticity, e.g. the crosstalk between the gut microbiota, lipopolysaccharide, high fat diet and the endocannabinoid system tone, which have hitherto not yet fully explored. Interactions between gut microbiota and other factors (e.g. inflammasome deficiency) will also be reviewed as emerging but far from being completely elucidated mechanisms influencing the onset of obesity and non-alcoholic fatty liver disease.
Keywords: Gut dysbiosis, gut-liver axis, inflammasome, non-alcoholic fatty liver disease, intestinal permeability, zonulin
INTRODUCTION
The term gut-liver axis in its present lexicon has been introduced for the first time in 1978 by Volta et al (1) in relation to the production of IgA antibodies directed against intestinal microorganisms and food antigens in liver cirrhosis. Since then the scientific literature has produced an increasing body of widely cited articles functionally linking the two organs (2) in health and disease. This interplay has involved from time to time an extraordinarily growing number of anticipated as well as unexpected interacting factors, including gut microbiota, intestinal barrier function, mucosal innate immune response, antigen trafficking, liver insult and, ultimately, metabolic disorders.
Since obesity and obesity-related liver disease [nonalcoholic fatty liver disease (NAFLD) and its more severe form nonalcoholic steatohepatitis (NASH)] are an established common and serious worldwide growing problem at all ages including pediatric age, our review is aimed at discussing recent advances on the role of the above mentioned protagonists in the pathogenesis of NAFLD/NASH and as possible tools for the development of innovative therapeutic approaches, particularly relevant in pediatrics. Although the majority of these findings derive from animal models, preliminary data from human studies seem to support their clinical relevance.
INTESTINAL MUCOSA
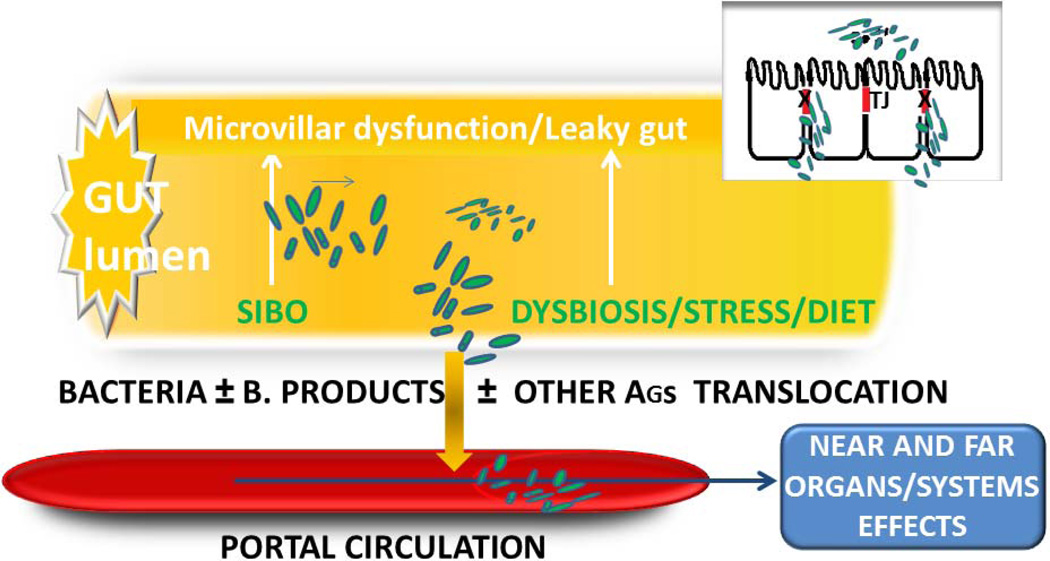
The gut epithelium represents a natural barrier which permits a selective entry of nutrients, ions, water and other useful substances present in the lumen, while keeping at bay potential harmful elements, including bacteria and their bio-products. This exquisitely regulated and discriminated trafficking is at least in part under the control of specialized intercellular structures called tight junctions (tj) (Figure 1).
Figure 1.
Events leading to bacterial and bacterial-products translocation from gut lumen to portal circulation in presence of microvillar dysfunction/leaky gut, small intestinal bacterial overgrowth (SIBO) or gut dysbiosis, stress, fat diet. Note malfunction of Tight Junctions (TJ) as one of the critical events.
A century ago, tj were conceptualized as a secreted extracellular cement forming an absolute and unregulated barrier within the paracellular space. The contribution of the paracellular pathway of the gastrointestinal (GI) tract to the overall molecular trafficking between environment and host, therefore, was judged to be negligible. It is now apparent that tj are extremely dynamic structures involved in developmental, physiological, and pathological circumstances. Recently, attention has been placed on the role of tj dysfunction in the pathogenesis of several diseases, particularly immune-mediated diseases. While our knowledge on tj ultrastructure and intracellular signaling events has progressed significantly during the past decade, relatively little was known about their pathophysiological regulation secondary to extracellular stimuli. The discovery of zonulin, a molecule that reversibly modulates tj permeability, shed light on how the intestinal barrier function is regulated in health and disease (3). Antigen trafficking through tj is tightly controlled in order to optimize the balance between tolerance (the active suppression of inflammatory responses) and immune response to non-self antigens, including food allergens (3). Avoiding systemic infections triggered by intestinal microorganisms is another key function of the gut mucosa. Innate immune cells as dendritic cells have a key role in sensing pathogens and subsequently engaging adaptive immune response via the modulation of T cells responses (4).
Also important are the so called pattern recognition receptors (PRRs) which sense the presence of potentially harmful bacteria present in the intestinal lumen. These prompt the gut associated lymphoid tissue (GALT) (i.e. Peyer’s patches, lamina propria lymphocytes, intraepithelial lymphocytes and mesenteric lymph nodes) to build a defensive immune response.
Specifically, there is a growing body of evidences that PRRs such as Toll like receptors (TLRs) recognize bacteria, mycobacteria, yeast membrane/wall components as well as several gut-derived products such as bacterial LPS (the active component of Gram-ve bacteria endotoxin), flagellin, peptidoglycan, viral/bacterial nucleic acid (referred as pathogen associated molecular patterns (PAMPs) on a large repertoire of immune and non-immune cells, resulting in the activation of Nuclear factor Kappa 03B2 (NFK β) and the initiation of innate immune response with transcription of all genes encoding inflammatory cytokines, chemokines and antimicrobial agents (2).
The T cell homing to the liver after priming by GALT-derived dendritic cells may be the possible basis of extra-intestinal manifestations of chronic inflammatory bowel disease. Interestingly, also the opposite scenario may be operative: T cells activated by liver sinusoidal endothelial cells (LSECs) acquire the capacity to home the intestine and the GALT, so that the Gut–Liver Axis is really close to become a true “ two way crosstalk” (5).
Derangement of the homeostasis between bacteria and host-derived signals provokes intestinal barrier malfunction leading to bacterial translocation, i.e. the bacteria (or bacterial products) transport from the intestinal lumen into the lamina propria and, eventually, to extra-intestinal sites.
GUT MICROBIOTA
Gut microbiota consists of trillions of commensal microorganisms residing in the human gut, which are essential for the preservation of the integrity of the mucosal barrier function. It is essential for the maturation of GALT, the secretion of IgA, and the production of antimicrobial peptides.
It is host specific, evolves throughout an individual's lifetime, and is susceptible to both exogenous and endogenous factors. The normal intestinal microbial population consists of 500–1000 different species and varies physiologically in terms of qualitative composition and abundance from the proximal to the distal portion, from the inside to outer, and is influenced by the subject’s age, dietary habits, geographical origin, type of birth, antibiotic therapies, and exposure to a variety of environmental stimuli. Firmicutes and Bacteroidetes species represent more than 90% of the total. The human gut microbiome is dominated by anaerobic bacteria, and outnumbers greatly human cells (by one order of magnitude) and genes (100 times the human genome).
Gut microbiota plays an important role in the gut-liver axis: it is involved in a number of aspects of normal host physiology, ranging from nutritional status to behavior and stress response. Gut microbes protect against exogenous pathogen microorganisms through active and/or competition mechanisms. In normal conditions, commensal microbes and the host share several mutual advantages still safeguarding the intestinal barrier integrity.
One of the major metabolic function is the fermentation of undigested carbohydrates and proteins with production of organic acids [(e.g. butyrate, acetate, and other short chain fatty acids (SCFAs)] and molecules, which may have important intestinal trophic effects and represent an additional energy source for the host, as well.
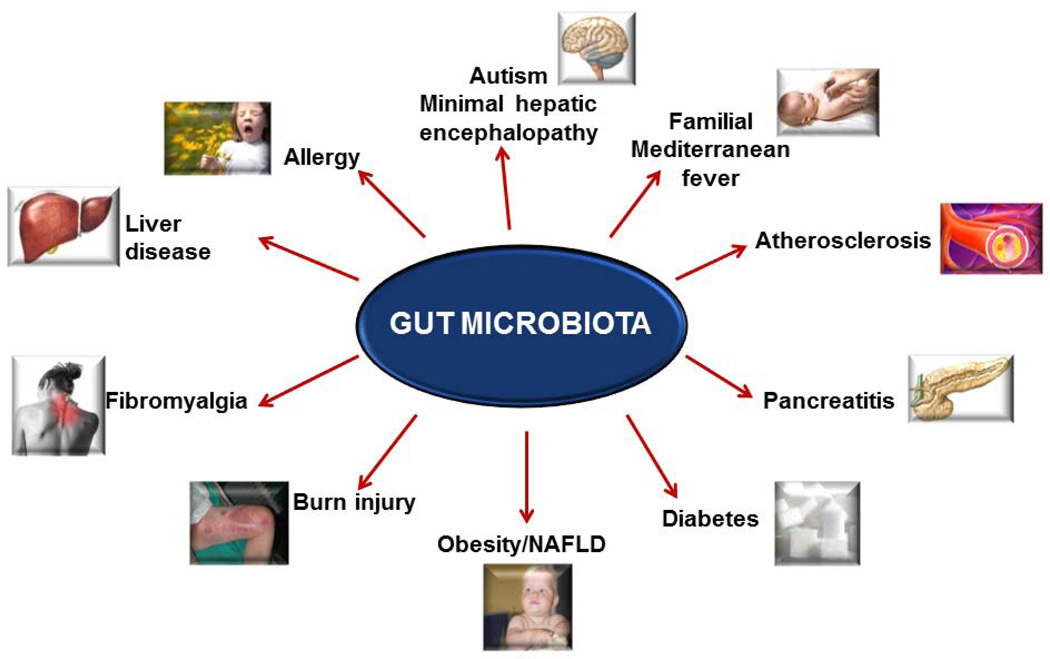
When disturbed in its composition, the gut microbiota may play a central role in contributing to many diseases, by affecting both near and far organ systems (Figure 2). Specific disease dysbiotic patterns have been recognized via improved analytical intestinal microbiology methodologies and by the focus on a most interesting condition called leaky gut (2). In this abnormal circumstance, the intestinal mucosa shows an impairment in its barrier function (increased intestinal permeability and endotoxin translocation), with subsequent increased trafficking of fat and waste materials (i.e. bacteria, fungi, parasites and their toxins, undigested proteins), from the intestinal lumen to the submucosa and from there to the bloodstream.
Figure 2.
Some conditions where gut microbiota has been implicated outside gastrointestinal tract (7).
Several conditions with rather specific microbiome patterns and/or leaky gut include not only the intestine (targeted in conditions like celiac disease (6), irritable bowel syndrome, and inflammatory bowel diseases (IBD)) (Table 1) but likely also a growing number of extra-intestinal organs or systems with subsequent onset of several pathologies (7), including hepatopathies, allergic diseases, diabetes type 1, familial Mediterranean fever, autism, and cardiovascular disease (Figure 2). Furthermore it has been shown that gut microbiota in germ-free mice also affect bone-mass density, intestinal angiogenesis, and immune response. Here we will focus especially on obesity and its hepatic complications, i.e. NAFLD & NASH.
Table 1.
Established gut microbiota patterns in pathologies of near and far organs/systems of pediatric interest.
Modified from (10).
| OBESITY | CELIAC DISEASE |
INFLAMMATORY BOWEL DISEASES |
IRRITABLE BOWEL SYNDROME |
ALLERGIC DISEASES |
TYPE 2 DIABETES |
|
|---|---|---|---|---|---|---|
| ENHANCED BACTERIAL GROWTH | Firmicutes | Gram negative Bacteroides-Provetella Escherichia coli | Enterobacteriacecae Bacteroidetes Enterococci C. difficile Escherichia coli Shigella flexneri Listeria spp | Vellonella Enterobacteriacecae | Staph. aureus E. coli Bifidobacterium adolescentis Lactobacilli B. Fragilis | B-Proteobacteria Bacteroides Parabacteroides |
| REDUCED BACTERIAL GROWTH | Bacteroides Bifidobacteria Staph aureus | Gram positive Bifidobacteria Clostridium histolyticum Clostridium liteseburense Faecalibacterium prausnitzii | Firmicutes Eubacterium rectale Bacteroides fragilis B. vulgatus Ruminococcus albus R. callidus R. bromii F. Prausnitzii | Bifidobacteria Collinsella aerofaciens Coprococcus eutactus Clostridium cocleatum | Bifidobacteria | Firmicutes Clostridia Bifidobacteria Bacteroides vulgatus |
MICROBIOTA, GUT-LIVER AXIS, AND OBESITY INTERACTION
Obesity develops from a prolonged imbalance of energy intake and energy expenditure, It is now clear that adipose tissue is a key endocrine organ releasing a number of adipokines with pro- or anti-inflammatory properties which contribute to the pathogenesis of obesity complications. A large body of emerging literature seems to suggest that intestinal microbiota is also involved in the development of these complications, including obesity related liver disease (i.e. NAFLD), and of obesity itself even.
Indeed, enteric bacteria contribute with the elaboration of enzymes not produced by humans to the catabolism of dietary fibers. It is in fact becoming increasingly evident from animal studies that in obesity a specific gut microbiota (e.g. Bacteroidetes thetaiotaomicron) may be responsible for a more efficient intestinal absorption of calories and increased lipid deposition by digesting and absorbing commonly poorly digestible plant polysaccharides (via glycoside hydrolyses, beta-fructosidase for fructose containing carbohydrates) (8).
A role for increased microbial extraction of calories in obese humans is hitherto still scarcely proven. However studies in children have shown that the response to slimming strategic programs were dependent on the type of initial host microbiota, and that childhood microbiota is strongly influenced by the type of delivery (mothers birth canals versus cesarean section), type of feeding (formula versus breast feeding) and exposition to antibiotics (decreased number of anti obesogenic bifidobacteriae and bacteroides) (9).
Gordon’s group pioneered the field of specific microbiome associated to obesity with the demonstration that altered gut microbiota composition (approximately 50% reduction in Bacteroidetes and consensual increase in Firmicutes) is causal and not a simple bystander phenomenon, through studies in which normal or genetically obese mice microbiota was transplanted to lean germ-free mice (10).
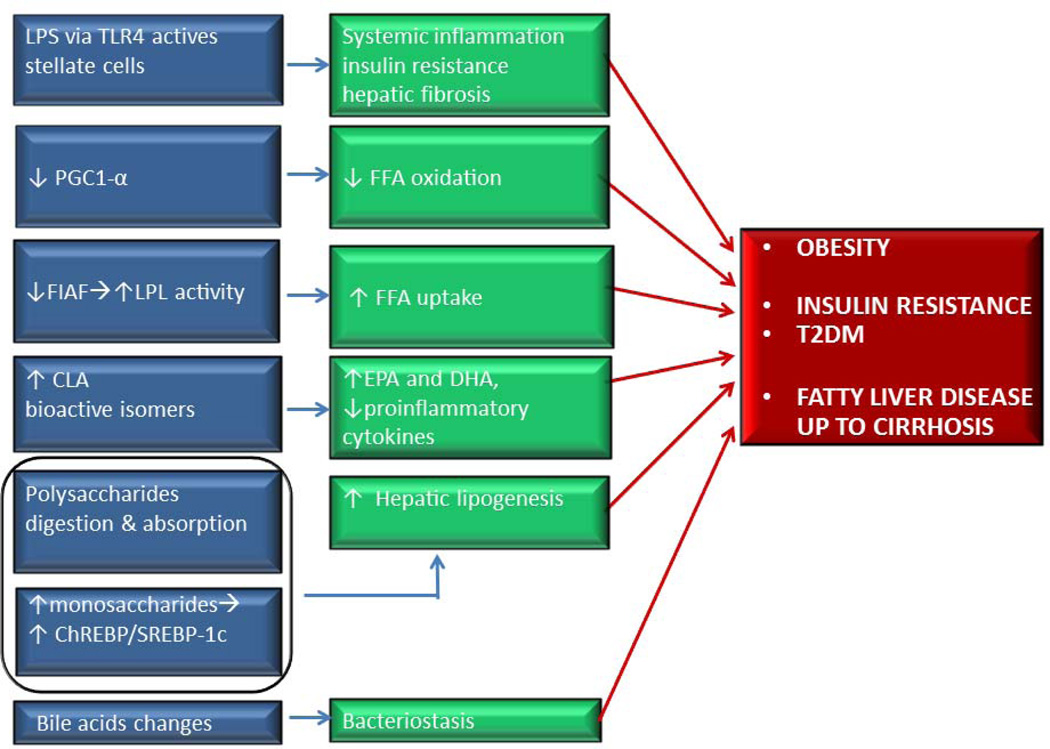
Since then, several subsequent metagenomic and metabolomic studies have confirmed that the obese gut microbioma has an increased capacity to harvest energy from the diet influencing body weight, and to interfere with metabolic changes such as increased levels of the orexigenic adipokine leptin, blood glucose and insulin levels, intestinal expression of the lipoprotein lipase (LPL) related fasting induced adipose factor (FIAF, which favors adipose tissue expansion, and interplays with fatty acid oxidation processes mediated by peroxisomal proliferator-activated receptor γ (PPAR γ)) (Figure 3). Most recently the transfer of intestinal microbiota from human lean donors has been shown to increase insulin sensitivity also in men with metabolic syndrome (11).
Figure 3.
Several mechanisms by which intestinal bacteria may interact on near and far organs or systems. Gut microbiota may be implicated in the pathogenesis of obesity, diabetes and nonalcoholic fatty liver disease. Gut dysbiosis is associated with an induction of TLR-4 in NAFLD patients that causes hepatic fibrogenesis and systemic inflammation. Gut microbiota interferes with FFA oxidation and uptake via PGC1-α and FIAF changes. Gut bacteria produce isomers of CLA which reduce the expression of proinflammatory cytokines and increase intestinal content of EPA and DHA . Microbiota also converts nondigestible carbohydrates into monosaccharides determining the activation of ChREBP and SREPB-1c transcription that promote hepatic lipogenesis. Properties of bile acids which have a bacteriostatic activity are modified, as well. Modified from (19).
Abbreviations: EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; ChREBP/SREBP, carbohydrate- responsive element -binding protein; SREPB-1c Sterolresponsive element-binding protein 1c; CLA, conjugated linoleic acid ; FFA, free fatty acids; FIAF, fasting-induced adipose factor; LPL, lipoprotein lipase; LPS, lipopolysaccharide; PGC1-α, peroxisomal proliferator-activated receptor coactivator 1α; T2DM, Type 2 Diabetes Mellitus; TLR-4, toll like receptor 4.
In obese adult patients circulating zonulin, a novel marker of intestinal permeability (see above), is increased in association with obesity-associated insulin resistance probably mediated through the obesity-related circulating IL-6, a cytokine which has been suggested to act as promotor of the zonulin gene (12).
A specific microbiota signature highly influenced by western diet and characterized by decreased Bacteroidetes (e.g. Bacteroides thetaiotaomicron)/Firmicutes (e.g. Lactobacillus, Streptococcus, Mycoplasma and Clostridium) ratio, or increase of Lactobacillus, Staphilococcus aureus, Escherichia Coli, Faecalibacterium prausnitzii has been described (Table 1).
Specifically, Faecalibacterium prausntizii may modulate systemic inflammation. Interestingly, the changes in the gut microbes can be rapidly reversed by dieting and its related weight loss. Also metabolic surgery (i.e. Roux-en-Y gastric bypass) in a non- obese rat model has shown to exert a profound influence on gutmicrobial- host metabolic cross-talk by shifting the main gut phyla towards higher concentrations of Proteobacteria with a decrease in Firmicutes (13).
Obese patients display increased concentrations of fecal SCFAs (e.g. butyrate) and/or blood/breath ethanol, produced by a more pronounced bacterial fermentation of indigestible dietary residues (e.g. large polysaccharides, unabsorbed sugars and alcohols) in the colon. Gut microbiota composition and amount of consumed carbohydrate determine types and quantity of SCFAs. These microbial and metabolic modifications have been object of several studies that link the microbial metabolic activity with a series of conditions such as the regulation of host lipid and carbohydrate metabolism, thermogenesis, satiety and chronic systemic inflammation. Gut microbiota, via the production of SCFAs which interplay with specific receptors, also modulate secretion of gut-derived peptides that can be responsible for reduced gut motility and transit time, i.e. two relevant risk factors for bacterial overgrowth development and increased nutrients adsorption.
Finally, data generated in rodents show that gut microbiota also modulates secretion of enteroendocrine cells-derived peptides involved in gut barrier integrity and function (14). Figure 3 summarizes a number of important interactions of intestinal bacteria on near and far organs and systems.
FRUCTOSE OBESOGENICITY AND BACTERIAL LPS TRANSLOCATION
The recent extraordinarily large increase in consumption of added refined carbohydrates (including dietary fructose and/or sucrose in soft drinks) is capable of conditioning the microbiota, with an obesogenic westernized microbiome, and an increased intestinal translocation of bacterial endotoxins. A recent pediatric pilot study showed that a moderate reduction of fructose and sugar intake may improve BMI in overweight and/or obese children (15). These results were further confirmed in a subsequent large and randomized trial on controlled sugar-sweetened beverages consumption in overweight and obese adolescents which showed a smaller BMI increase in the study group vs. controls. (16).
MICROBIOTA, GUT LIVER AXIS AND NAFLD
NAFLD is the hepatic manifestation of metabolic syndrome and the leading cause of chronic liver disease in pediatric and adult individuals living in industrialized countries.
It encompasses a disease spectrum ranging from simple steatosis to non-alcoholic steatohepatitis (NASH), which is histologically characterized by hepatocyte injury, inflammation, and variable degrees of fibrosis, eventually progressing to cirrhosis in a portion of patients.
The possible role played by gut microbiota in obesity and NAFLD has therefore triggered a series of studies aiming to better define NAFLD pathogenesis and envisaging novel treatments.
Obese/overweight pre-school children show a prevalence of Gram –ve Entero-bacteriaceae, and an amount of Bifidobacterium which is inversely correlated to alanine aminotransferase serum levels, a surrogate marker of NAFLD in obese individuals (17).
Changes prompted by specific intestinal microbiota are characterized not only by a general obesogenic and dysmetabolic framework, but also by a specific de novo hepatic lipogenesis (de novo fatty acid synthesis mediated by increased activity of acetyl Coenzyma A carboxylase and fatty acid synthase, with triglyceride accumulation in adipocytes and in the liver, as well) (18), which is typical of the first stage of NAFLD. SCFAs moreover serve as cholesterol and fatty acid precursors and further neoglucogenesis substrates in the liver.
Small intestinal bacterial overgrowth (SIBO) is defined as an increase in the number and/or alteration in the type of bacteria in the upper gastrointestinal tract, due to the loss of one or more of the several endogenous defense mechanisms. It has been described in both obese animal models of NAFLD (e.g. genetically obese ob/ob mouse) (19) and in humans with NASH (20), variably associated with increased TNF-α serum levels and/or intestinal permeability and endotoxin levels abnormalities (21, 22).
SIBO has been explained on the basis of impaired small bowel motility and prolonged intestinal transit time, which have been signaled in obese and/or cirrhotics, and in NASH, as well. Specially, the latter group of patients’ reduced intestinal motility and SIBO development might be correlated to low levels of ghrelin, a food intake and prokinetic regulator gastric hormone.
An increased intestinal permeability might be at the basis of LPS translocation and transport [bound to a specific binding protein (LBP)], to target organs (21). This process (overgrowth of LPS-containing bacteria and translocation itself) is facilitated by chylomicrons synthesized by enterocytes in response to a fat meal (so called metabolic endotoxaemia).
The increased intestinal permeability may be evaluated by differential absorption of lactulose/mannitol sugars and more precisely by means of trans-epithelial electrical resistance, and study of the pattern of tj proteins expression (23). Serum levels of zonulin have recently been reported also in obesity and obesity related liver disease (11). A busy cross-talk between the liver and gut microbiota has been invoked to explain progression of NAFLD into NASH: liver cells’ TLRs 2,4 and 5 recognize LPS and trigger the inflammatory cascade, (particularly IFN-β chemokine) and provoke injury to hepatic tissue (24).
In mice, a high fat diet reduced Bifidobacteria and increased plasma levels of LPS and liver fat, along with the expression of a number of pro-inflammatory chemokines. High fat meal related hypertriglyceridemia and concurrent acute increase in plasma endotoxins levels seem due to an increase of intestinal permeability secondary to reduced expression of two epithelial tight junctions proteins, occludin and zonula occludens-1 (ZO-1) (14).
More in details, intestinal dysbiosis through bacterial products (LPS endotoxins, ethanol) interacting with TLRs favors NF-kB synthesis by stimulating the production of interleukin 1β, that is primarily responsible for the pro-inflammatory response in obesity (mild chronic inflammatory systemic status). This pathway of activation, especially as for as TNF-α is regarded, has been shown to also lead to target tissue dysfunction and the development of insulin resistance, which has a key role in NAFLD pathogenesis, and to progression of its fibrogenetic component through TLRs (in particular TLR4) expressed on Kupffer cells (proinflammatory and profibrogenetic mediators) and hepatic stellate cells (extra cellular matrix fibrogenesis). Moreover, cell wall components of gram –ve bacteria modulate the effects also of transforming growth factor beta on hepatic stellate cells (24). Steps of the inflammatory pathway induced by LPS as suggested by studies in genetically modified animal models have been quite well summarized by the Abu-Shanab group (20).
Due to fructose-obesity-microbiota interactions, one can expect that fructose and/or sugar sweetened foods or beverages may be involved in NAFLD development and progression as well. In this regard, studies from the Bergheim group in Germany have shown that hepatic fat accumulation in fructose-fed mice were associated with induction of TLR 1–4 and 6–8. Fructose effects could be halted/prevented by antibiotic or probiotics (25). Metformin therapy also resulted effective probably through positively altering intestinal permeability and subsequent endotoxin-dependent activation of hepatic Kupffer cells (26). A pilot study in humans, has shown that a dietary intervention focusing on fructose reduced Insulin resistance and helped to decrease intrahepatic fat content in NAFLD adult patients. This was paralleled by improvement of intestinal permeability, blood ethanol level, endotoxin and PAI-1 plasma concentration. However, because this happened without affecting the examined SIBO parameters, further studies are necessary to better understand the exact underlying pathomechanism (27).
Inflammasome-dysbiosis relationship
Most recently inflammasome, a molecular macrocomplex which includes the enzyme caspase-1, whose activation causes the release of bioactive IL-1β and/or IL-18, has been involved not only in the mild chronic inflammatory status of obesity, but also in NAFLD/NASH progression, as well as multiple aspects of metabolic syndrome via modulation of the gut microbiota (28). In particular, NOD-like receptor protein 3 (NLRP3) inflammasome is involved in the caspase-1 activation and caspase-1-mediated IL-1β and IL-18 release. The consequent activation of IL-1 β promotes insulin resistance but also β cell death and atherosclerotic plaque process (28).
It has recently been shown that the combination of genetic inflammasome deficiency-associated dysbiosis determines high concentration of bacterial products, such as PAMPs, in portal blood which may further damage the liver made steatotic by an high fat diet (28).
Bacterial translocation participates also in the up regulation of nitric oxide synthesis (NOS), a molecule involved in inflammation. NOS may also influence adipogenicity through the lipoprotein lipase (LPL) activity responsible for the increase in circulating free fatty acids uptake by adipocytes.
Microbiota driven Choline deficiency status
Gut microbiota produces enzymes able to catalyze the conversion of dietary choline to dymethylamine and trimethylamine (TMA) which are then cleared by the liver. Dumas et al. have recently shown in a NAFLD susceptible insulin-resistant mouse model that gut microbiota may mimic choline-deficient diets. The indirect mechanism of hepatotoxicity could involve in first instance microbial conversion of choline into TMA, thus reducing bioavailability of choline, followed by influx of fatty acids in the liver, and generation of radical oxidative species via reprocessing of fatty acids and oxidative stress (29). Similarly, elevated levels of trimethylamine-N-oxide are also correlated with cardiovascular disease.
Farnesoid X receptor (FXR): control of bile acid dependent intestinal bacteriostasis
Bile acids have a bacteriostatic activity. The nuclear bile acid receptor FXR is strongly expressed at sites of bile acid excretion (i.e. liver) as well as absorption (i.e. intestine). Its activation, in addition to reduce the amount of circulating bile acids in a feedback mechanism, plays an important role in the critical control of the bacterial flora, and also in the gut-liver axis feedback regulation of lipids and glucose homeostasis. All of these are factors involved in the pathogenesis of obesity and obesity-related conditions, including hepatic fat content, hepatic inflammation and fibrogenesis (30).
Gut-brain-liver axis: the lipopolysaccharide-endocannabinoid (LPS-eCB) system regulatory loop
Although energy and glucose homeostasis are regulated by food intake and liver glucose production, in rats it has been shown that lipids in the upper intestine may activate an intestine-brain-liver neural axis to inhibit glucose production (31). The endocannabinoid (eCB) system, consisting of the cannabinoid receptors, endogenous cannabinoid ligands and their biosynthetic and degradative enzymes, seems to play critical roles in the control of energy balance, the control of intestinal permeability and immunity. Through direct and indirect actions throughout the body, the endocannabinoid system controls the development of obesity and its inflammatory complications. Data in the murine model indicate that gut microbiota modulates the intestinal eCB system tone, which in turn regulates gut permeability and plasma lipopolysaccharide levels. Gut microbiota may therefore determine adipose tissue physiology through LPS-eCB system regulatory loops and may have critical functions in adipose tissue plasticity during obesity (32).
Alcoholic liver disease and Gut liver axis
Altered intestinal bacterial flora and gut-associated endotoxinemia are increasingly recognized as critical components also of alcoholic liver disease (ALD). Experimental studies in animals and in humans showed that consumption of alcohol alters, in the same fashion observed in NAFLD, the competency of intestinal tight junctions (increased gut permeability) allowing an increased influx of LPS and bacterial endotoxins into the portal and peripheral blood, which may be reverted by probiotics and antibiotics (33).
Interestingly, it has been shown that NASH microbiome contains an increased abundance of endogenous alcohol producing bacteria leading to elevated blood ethanol levels, thus suggesting a leading role of gut microbiota in both ALD and NAFLD (34).
NOVEL TREATMENT STRATEGIES OF NAFLD
Weight loss, dietary modification, and the treatment of underlying metabolic syndrome remain the mainstays of therapy which are however burdened by very frequent failure rates due to poor compliance (35). A series of pharmacological treatments (e.g. antioxidants, insulin-sensitizers, and cytoprotective agents) have therefore been developed over recent years in the attempt of modifying one or more of the major factors involved in pathogenesis, but results are still puzzling and/or unsatisfactory both in adults and in children (36, 37).
Here we summarize some evidences available on gut microbiota manipulation by means of agents like antibiotics, probiotics, prebiotics and symbiotics. .
Antibiotics- Early studies with antibiotics treatment such as polimixine B, tetracycline and metronidazole have shown beneficial effects on SIBO related liver damage including total parenteral nutrition and intestinal by-pass related hepatic steatosis, which confirm the role of bacteria on liver damage and suggests their rational use also for future studies in human NAFLD (38).
Probiotics are live microorganisms which when consumed in adequate amounts, confer a healthy benefit to the host; prebiotics (e.g. inulin and fructo-oligosaccharides) are non-digestible food ingredients that beneficially affect the host by selectively stimulating growth and/or modifying the metabolic activity of selected intestinal bacteria; symbiotics are combinations of a probiotic with a prebiotic. Due to their influence on the gut-liver axis, including modulation of the intestinal microflora, modification of intestinal barrier function, and immunomodulation, along with anti-inflammatory and metabolic effects on near and far organs and systems, probiotics may be reasonably proposed as a potential treatment of NAFLD.
However, it is important to clearly mention that different probiotics species may produce different effects on changes of gut microbiota-dependent energy balance. For example, among Lactobacillus spp different strains are associated with different pro- or anti-obesity consequences, in animal and in human studies, as well (39). In this respect, a recent study in murine diet-induced obesity from Shanahan group (40) showed comparable microbiota changes but divergent metabolic outcomes arising from different targeted gut microbiota manipulation with vancomycin vs. probiotic Lactobacillus salivarius. In humans, a most recent study investigating the effect of the probiotic strain Lactobacillus salivarius Ls-33 on a series of biomarkers related to inflammation and the metabolic syndrome in obese adolescents could not detect any significant change (41). These results suggest that the specificity of the antimicrobial agent employed is critical and deserves future accurate studies. Taking this in mind, that no generalization can be done, here we will report a number of evidences from animal and human NASH studies.
a. Animal studies
Early studies with probiotics VSL # 3 (a multistrain mixture of Bifidobacteria, Lactobacilli, and Streptococci) in ob/ob genetically obese mice suffering from NASH showed a significant reduction in transaminase levels and in histological liver steatosis probably mediated by JNK (TNF regulated kinase promoting insulin resistance) and NF-KB correlated with TNF-α regulation and insulin resistance (19). Similar direction results were obtained in studies with the same probiotics in diet-induced obese rats, achieving in particular an improvement of insulin resistance (42) and as shown also by us (43) a reduction of oxidative and inflammatory liver abnormalities.
Lactobacillus strains also display beneficial effects on animal models of obesity related hepatic abnormalities, but have been object of fewer studies (44, 45).
b. Human studies
The Cochrane review (46) and a collaborative pediatric study of ESPGHAN (36) stated that, due to absence of RCTs, they could not support probiotics for patients with non-alcoholic fatty liver disease and nonalcoholic steatohepatitis. Refusal was not possible as well because preliminary data from two pilot nonrandomized studies (47, 48) suggested that probiotics may be well tolerated, may improve conventional liver function tests, and may decrease markers of lipid peroxidation and/or TNF-α. Since these meta-analyses were published, more recently 2 RCTs appeared in the literature. Aller et al carried out a double blind RCT to evaluate the effects of a 12 week course of treatment with 500 million of Lactobacillus bulgaricus and Streptococcus thermophiles/day in adult patients with NAFLD. Although anthropometric parameters and cardiovascular risk factors remained unchanged in both treated and control groups, treatment with probiotics resulted in a significant aminotransferases levels improvement (49).
With a double blind RCT in obese children of 10.7 ± 2.1 years with NAFLD, unable to comply with lifestyle interventions, our group showed that a short (8 weeks) course of probiotic treatment with Lactobacillus GG (12 billion CFU/day), irrespective of changes in BMI z score and visceral fat, also determined a significant decrease in alanine aminotransferase (with normalization in 80% of cases). This was associated to a significant reduction of antipeptidoglycan-polysaccharide antibodies (i.e. a SIBO marker), while TNF-α, and ultrasonographic bright liver parameters remained fairly stable (50).
Prebiotics treatment is another potential therapeutic strategy. In general, in addition to alter the composition and activity of gut microbiota, they may also reduce the risk of obesity by promoting satiety and weight loss, as well. In rats, prebiotic fibers along with modulation of gut microbiota by increasing Firmicutes and decreasing Bacteroidetes (leaner phenotype), yielded beneficial effects on metabolic parameters and also on hepatic cholesterol and triglyceride deposition (51). Lactulose, a prebiotic able to stimulate Lactobacilli, Bifidobacteria and Gram +ve bacteria growth, and inhibit Gram −ve bacteria, has been shown to protect against endotoxinemia. Recent data show that prebiotics promote increased selective levels of Bifidobacteria that are associated with a reduction of adiposity and of LPS in diet-induced obese mice.
CONCLUSIONS AND PERSPECTIVES
It is clear that obesity is mostly secondary to excessive calorie intake vs. limited energy expenditure, both often co-existing in unhealthy lifestyles. However, intestinal factors influencing intestinal mucosal biological and immunological functions, including modification of microbiota composition, seem to play a crucial role as well in causing obesity and associated metabolic conditions including NAFLD.
It is possible that a healthy lifestyle starting from infancy (breast feeding, appropriate use of antibiotics, moderate dietary fat and sucrose intake) will be able to ensure a friendly gut microbiota and have a positive impact on prevention and treatment of obesity, and its related metabolic disorders (14).
The possibility of manipulating gut microbiota by a number of simple means has opened novel opportunities for the treatment of obesity and NAFLD although no generalization can be made at this moment.
Novel mechanisms regulating gut permeability and adipose tissue plasticity, e.g. the crosstalk between the gut microbiota, LPS, high fat diet and the endocannabinoid system tone, have not been fully explored yet and, therefore, deserve further attention.
The synthetic FXR agonist INT-747 is still under evaluation in an ongoing trial in adults and no preliminary data are currently available (30).
Interactions between gut microbiota and other factors influencing the onset of obesity and NAFLD (e.g. inflammasome deficiency) (28) are far from being completely clear and deserve further investigation. Similarly, fructose- microbiota- obesity- NAFLD interactions warrants future insights (15, 16, 25, 27).
Acknowledgments
FUNDING: This article was partially supported by the national Institutes of health grant DK-48373 to A.F. and FARB-ex 60% 2012 grant to P.V.
Footnotes
Authors certificate that there are no conflict of interest
Authors certificate that there are no financial competing interests
References
- 1.Volta U, Bonazzi C, Bianchi FB, et al. IgA antibodies to dietary antigens in liver cirrhosis. Ric Clin Lab. 1987;17:235–242. doi: 10.1007/BF02912537. [DOI] [PubMed] [Google Scholar]
- 2.Ilan Y. Leaky gut and the liver: A role for bacterial translocation in nonalcoholic steatohepatitis. World J Gastroenterol. 2012;18:2609–2618. doi: 10.3748/wjg.v18.i21.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151–175. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 4.Szabo G, Mandrekar P, Dolganiuc A. Innate immune response and hepatic inflammation. Semin Liver Dis. 2007;27:339–350. doi: 10.1055/s-2007-991511. [DOI] [PubMed] [Google Scholar]
- 5.Mehal WZ. The gut-liver axis: a busy two-way street. Hepatology. 2012;55:1647–1649. doi: 10.1002/hep.25704. [DOI] [PubMed] [Google Scholar]
- 6.Sellitto M, Bai G, Serena G, et al. Proof of concept of microbiome-metabolome analysis and delayed gluten exposure on celiac disease autoimmunity in genetically at-risk infants. PLoS One. 2012;7:e33387. doi: 10.1371/journal.pone.0033387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 8.Bruel L, Sulzenbacher G, CerveraTison M, et al. α-Galactosidase/sucrose kinase (AgaSK), a novel bifunctional enzyme from the human microbiome coupling galactosidase and kinase activities. J Biol Chem. 2011;286:40814–40823. doi: 10.1074/jbc.M111.286039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santacruz A, Marcos A, Wärnberg J, et al. EVASYON Study Group. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity (Silver Spring) 2009;17:1906–1915. doi: 10.1038/oby.2009.112. [DOI] [PubMed] [Google Scholar]
- 10.Turnbaugh PJ, Ley RE, Mahowald MA, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 11.Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–916. doi: 10.1053/j.gastro.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Moreno-Navarrete JM, Sabater M, Ortega F, et al. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS One. 2012;7:e37160. doi: 10.1371/journal.pone.0037160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li JV, Ashrafian H, Bueter M, et al. Metabolic surgery profoundly influences gut microbialhost metabolic cross-talk. Gut. 2011;60:1214–1223. doi: 10.1136/gut.2010.234708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musso G, Gambino R, Cassader M. Gut microbiota as a regulator of energy homeostasis and ectopic fat deposition: mechanisms and implications for metabolic disorders. Curr Opin Lipidol. 2010;21:76–83. doi: 10.1097/MOL.0b013e3283347ebb. [DOI] [PubMed] [Google Scholar]
- 15.Maier IB, Stricker L, Ozel Y, Wagnerberger S, Bischoff SC, Bergheim I. A low fructose diet in the treatment of pediatric obesity: a pilot study. Pediatr Int. 2011;53:303–308. doi: 10.1111/j.1442-200X.2010.03248.x. [DOI] [PubMed] [Google Scholar]
- 16.Ebbeling CB, Feldman HA, Chomitz VR, et al. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med. 2012;367:1407–1416. doi: 10.1056/NEJMoa1203388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlsson CL, Onnerfält J, Xu J, et al. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity (Silver Spring) 2012:2257–2261. doi: 10.1038/oby.2012.110. [DOI] [PubMed] [Google Scholar]
- 18.Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Yang S, Lin H, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–350. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:691–701. doi: 10.1038/nrgastro.2010.172. [DOI] [PubMed] [Google Scholar]
- 21.Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 22.Harte AL, da Silva NF, Creely SJ, et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J Inflamm (Lond) 7:15. doi: 10.1186/1476-9255-7-15. 20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldblum SE, Rai U, Tripathi A, et al. The active Zot domain (aa 288–293) increases ZO-1 and myosin 1C serine/threonine phosphorylation, alters interaction between ZO-1 and its binding partners, and induces tight junction disassembly through proteinase activated receptor 2 activation. FASEB J. 2011;25:144–158. doi: 10.1096/fj.10-158972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol. 2012;590:447–458. doi: 10.1113/jphysiol.2011.219691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagnerberger S, Spruss A, Kanuri G, et al. Toll-like receptors 1–9 are elevated in livers with fructose-induced hepatic steatosis. Br J Nutr. 2012;107:1727–1738. doi: 10.1017/S0007114511004983. [DOI] [PubMed] [Google Scholar]
- 26.Spruss A, Kanuri G, Stahl C, Bischoff SC, Bergheim I. Metformin protects against the development of fructose-induced steatosis in mice: role of the intestinal barrier function. Lab Invest. 2012;92:1020–1032. doi: 10.1038/labinvest.2012.75. [DOI] [PubMed] [Google Scholar]
- 27.Volynets V, Machann J, Küper MA, et al. A moderate weight reduction through dietary intervention decreases hepatic fat content in patients with non-alcoholic fatty liver disease (NAFLD): a pilot study. Eur J Nutr. 2012 Apr 28; doi: 10.1007/s00394-012-0355-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumas ME, Barton RH, Toye A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci U S A. 2006;103:12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuchs M. Non-alcoholic Fatty liver disease: the bile Acid-activated farnesoid x receptor as an emerging treatment target. J Lipids. 2012;2012:934396. doi: 10.1155/2012/934396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang PY, Caspi L, Lam CK, et al. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452:1012–1016. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- 32.Cluny NL, Reimer RA, Sharkey KA. Cannabinoid signaling regulates inflammation and energy balance: the importance of the brain-gut axis. Brain Behav Immun. 2012;26:691–698. doi: 10.1016/j.bbi.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Kirpich IA, Solovieva NV, Leikhter SN, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–682. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu L, Baker SS, Gill C, et al. Characterization of the gut microbiome in non-alcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology. 2012 Oct 11; doi: 10.1002/hep.26093. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Huang JS, Barlow SE, Quiros-Tejeira RE, et al. The NASPGHAN Obesity Task Force. Consensus Statement: Childhood Obesity for Pediatric Gastroenterologists. J Pediatr Gastroenterol Nutr. 2012M Aug 16; doi: 10.1097/MPG.0b013e31826d3c62. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Socha P, Horvath A, Vajro P, et al. Pharmacological interventions for nonalcoholic fatty liver disease in adults and in children: a systematic review. J Pediatr Gastroenterol Nutr. 2009;48:587–596. doi: 10.1097/MPG.0b013e31818e04d1. [DOI] [PubMed] [Google Scholar]
- 37.Chalasani N, Younossi Z, Lavine JE, et al. American Gastroenterological Association; American Association for the Study of Liver Diseases; American College of Gastroenterology The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 38.Sajjad A, Mottershead M, Syn WK, et al. Ciprofloxacin suppresses bacterial overgrowth, increases fasting insulin but does not correct low acylated ghrelin concentration in non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;22:291–299. doi: 10.1111/j.1365-2036.2005.02562.x. [DOI] [PubMed] [Google Scholar]
- 39.Million M, Angelakis E, Paul M, et al. Comparative meta-analysis of the effect of Lactobacillus species on weight gain in humans and animals. Microb Pathog. 2012;53:100–108. doi: 10.1016/j.micpath.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Murphy EF, Cotter PD, Hogan A, et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut. 2012 Aug 9; doi: 10.1136/gutjnl-2011-300705. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41.Gøbel RJ, Larsen N, Jakobsen M, et al. Probiotics to obese adolescents, RCT examining the effects on inflammation and metabolic syndrome. J Pediatr Gastroenterol Nutr. 2012;55:673–678. doi: 10.1097/MPG.0b013e318263066c. [DOI] [PubMed] [Google Scholar]
- 42.Ma X, Hua J, Li Z. Probiotics improve high fat diet-induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821–830. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esposito E, Iacono A, Bianco G, et al. Probiotics reduce the inflammatory response induced by a highfat diet in the liver of young rats. J Nutr. 2009;139:905–911. doi: 10.3945/jn.108.101808. [DOI] [PubMed] [Google Scholar]
- 44.Lee HY, Park JH, Seok SH, et al. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet induced obese mice. Biochim Biophys Acta. 2006;1761:736–744. doi: 10.1016/j.bbalip.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Yadav H, Jain S, Sinha PR. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition. 2007;23:62–68. doi: 10.1016/j.nut.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 46.Lirussi F, Mastropasqua E, Orando S, et al. Probiotics for non-alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst Rev. 2007:CD005165. doi: 10.1002/14651858.CD005165.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loguercio C, De Simone T, Federico A, et al. Gut-liver axis: a new point of attack to treat chronic liver damage? Am J Gastroenterol. 2002;97:2144–2146. doi: 10.1111/j.1572-0241.2002.05942.x. [DOI] [PubMed] [Google Scholar]
- 48.Loguercio C, Federico A, Tuccillo C, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540–543. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 49.Aller R, De Luis DA, Izaola O, et al. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci. 2011;15:1090–1095. [PubMed] [Google Scholar]
- 50.Vajro P, Mandato C, Licenziati MR, et al. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2011;52:740–743. doi: 10.1097/MPG.0b013e31821f9b85. [DOI] [PubMed] [Google Scholar]
- 51.Parnell JA, Reimer RA. Prebiotic fiber modulation of the gut microbiota improves risk factors for obesity and the metabolic syndrome. Gut Microbes. 2012;3:29–34. doi: 10.4161/gmic.19246. [DOI] [PMC free article] [PubMed] [Google Scholar]