Abstract
Plasma lipidomic studies using high performance liquid chromatography and mass spectroscopy offer detailed insights into metabolic processes. Taking the example of the most abundant plasma lipid class (phosphatidylcholines) we used the rich phenotypic and lipidomic data from the ongoing San Antonio Family Heart Study of large extended Mexican American families to assess the variability of association of the plasma phosphatidylcholine species with metabolic syndrome. Using robust statistical analytical methods, our study made two important observations. First, there was a wide variability in the association of phosphatidylcholine species with risk measures of metabolic syndrome. Phosphatidylcholine 40:7 was associated with a low risk while phosphatidylcholines 32:1 and 38:3 were associated with a high risk of metabolic syndrome. Second, all the odd chain phosphatidylcholines were associated with a reduced risk of metabolic syndrome implying that phosphatidylcholines derived from dairy products might be beneficial against metabolic syndrome. Our results demonstrate the value of lipid species-specific information provided by the upcoming array of lipidomic studies and open potential avenues for prevention and control of metabolic syndrome in high prevalence settings.
Keywords: high performance liquid chromatography, mass spectroscopy, phosphatidylcholine, molecular biology
INTRODUCTION
Combination of high performance liquid chromatography with mass spectroscopy has revolutionized our understanding of lipid diversity in various tissues and diseases.[1] This technology makes possible for the first time large scale lipidomic studies promising detailed and fundamental insights into the intricate pathways of lipid metabolism and their perturbations in chronic complex diseases.[2, 3] Arguably, it may be tempting to simplify these associations by combining the lipid species based from a lipid class. However, we posit that such a combination of species into a single lipid class will lead to an oversimplification and overinterpretation of the underlying biological and epidemiological associations. Using the example of the most abundant subclass [4] of phospholipids – phosphatidylcholines – we examine the inter-lipid species variability in association with metabolic syndrome. Here, we demonstrate that consideration of individual lipid species is more informative than summarizing results for the entire class of phosphatidylcholines with respect to their association with metabolic syndrome (MS) in samples from several extended families of Mexican Americans who were enrolled in the San Antonio Family Heart Study (SAFHS).[5]
MATERIALS AND METHODS
The SAFHS is a large study of extended Mexican American families in San Antonio that began in 1991. Details of enrollment procedures, inclusion and exclusion criteria and phenotypic assessment of the study subjects have been extensively described elsewhere.[5, 6] Data and samples used in this study were collected during the first visit of the participants (from 1992 to 1996). Informed consent was obtained from all participants before collection of samples. The Institutional Review Board of the University of Texas Health Sciences Center at San Antonio approved the study. We included data on 1358 individuals from 42 pedigrees. The clinical characteristics of the study subjects are shown in Table 1.
Table 1.
Clinical characteristics of the study subjects
| Characteristic | Value |
|---|---|
| Age [mean (SD)] y | 39.24 (16.74) |
| Females [n (%)] | 859 (63.25) |
| Fasting glucose [mean (SD)] mmol/l | 5.59 (2.46) |
| 2-hour post-glucose load glucose [mean (SD) mmol/l | 7.28 (5.00) |
| Fasting insulin [mean (SD)] µ U/ml | 15.96 (19.32) |
| 2-hour post-glucose load insulin [mean (SD)] µU/ml | 76.05 (75.02) |
| Homeostasis Model of Assessment – Insulin Resistance [mean (SD)] | 4.37 (7.17) |
| Waist circumference [mean (SD)] cm | 94.78 (17.20) |
| Body mass index [mean (SD)] Kg/m2 | 29.27 (6.63) |
| Waist-hip ratio [mean (SD)] | 0.90 (0.09) |
| Systolic blood pressure [mean (SD)] mmHg | 120.50 (18.82) |
| Diastolic blood pressure [mean (SD)] mmHg | 70.65 (10.34) |
| Total serum cholesterol [mean (SD)] mg/dl | 189.37 (38.46) |
| Serum triglycerides [mean (SD)] mg/dl | 147.94 (106.97) |
| HDL cholesterol [mean (SD)] mg/dl | 50.29 (12.96) |
| LDL cholesterol [mean (SD] mg/dl | 111.12 (32.96) |
| Subjects with Metabolic syndrome [n (%)] | 565 (41.49) |
Outcomes
The primary goal of this study was to examine the association of the concentration of plasma phosphatidylcholine species with metabolic syndrome. For this, we defined MS in two alternative ways. First, we used the definition recommended by the International Diabetes Federation (IDF)[7] which classifies a person as having metabolic syndrome if there is central obesity (waist circumference >102 cm for Mexican American males and >88 cm for Mexican American females) and any two of the following: serum triglycerides (TG) >150 mg/dl or use of lipid lowering drugs; serum high density lipoprotein (HDL) concentration <40mg/dl for males and <50 mg/dl for females; systolic blood pressure (SBP) >130 mmHg or diastolic blood pressure (DBP) >85 mmHg or use of antihypertensive drugs; and fasting glucose (FG) >100 mg/dl or previously diagnosed type 2 diabetes. Alternatively, we conducted principal components analyses on the following nine traits and used the first principal component as a continuous trait which reflected the risk of metabolic syndrome: FG, fasting insulin (FI), waist circumference (WC), body mass index (BMI), SBP, DBP, total serum cholesterol (TSC), serum TG and serum HDL concentration
Estimation of plasma concentration of phosphatidylcholine species
Plasma levels of phosphatidylcholine species were estimated by a combination of high performance liquid chromatography and mass spectroscopy in the Metabolomics Laboratory, Baker IDI Heart and Diabetes Institute, Melbourne, Australia. Ten µL aliquot of plasma was combined with 200 µL CHCl3/MeOH (2:1) and 15µL of internal standard mix and then briefly vortexed. Samples were mixed (rotary mixer, 10 min), sonicated (water bath, 30 min), kept at room temperature (20 min) and centrifuged (16,000×g, 10 min). The supernatant was dried under a stream of nitrogen at 40°C. Extracted lipids were resuspended in 50µL H2O-saturated BuOH with sonication (10 min), followed by 50µL of 10 mM NH4COOH in MeOH. Mass spectrometric analysis was performed using 1 µL injections of the lipid extracts.
Identification and quantification of lipid species was done by liquid chromatography electrospray ionisation-tandem mass spectrometry using Applied Biosystems 4000 QTRAP machine. Liquid chromatography was performed on a Zorbax C18, 1.8 µm, 50 × 2.1 mm column at 300 µL/min. Solvents A and B consisted of tetrahydrofuran:methanol:water in the ratios (30:20:50) and (75:20:5) respectively, both containing 10 mM NH4COOH. Precursor ion scans and neutral loss scans were used to identify the lipid species. Quantification of individual lipid species was then performed using scheduled multiple-reaction monitoring (MRM) in positive ion mode.[8, 9] Lipid concentrations (in pmols/ml) were calculated by relating the peak area of each species to the peak area of the corresponding internal standard. From these results, we used data on a total 45 phosphatidylcholine species excluding the phosphatidylcholine analogs like modified phosphatidylcholines, ether phosphatidylcholines and lysophosphatidylcholines.
Statistical analysis
Principal components analysis was conducted by deriving eigenvalues and selecting factors with a minimum eigenvalue of 1 and using varimax rotation. Based on this optimum factor solution we generated standardized factor scores and used these scores as a continuous measure of metabolic syndrome. The validity of this measure of metabolic syndrome was assessed using receiver operating characteristic (ROC) curve compared to the IDF definition of metabolic syndrome. Optimum factor score was determined by estimating the distance of each point on the ROC curve from the upper left-hand corner of the ROC plot and then finding the minimum distance.
Association analyses were conducted using polygenic regression models that predicted each metabolic syndrome trait based on inverse-normalized plasma concentrations of phosphatidylcholine species after accounting for the kinship structure of study subjects. These models implicitly account for the genetic correlations and kinship structures and do not consider the individual subjects as independent unit. The polygenic regression models were of the following form: MSTi = m + ∑ bk aik + gi + ei where, MST is the metabolic syndrome trait of interest; m is the trait mean; a is the covariate vector of dimension k with b as the corresponding regression coefficients; g is the polygenic effect and e is the residual error for an individual indexed by i. The term g was modeled as a random variable based on the coefficients of relationship in the kinship matrix. Models with the MetS trait used a liability threshold approach. All models included adjustments for age, age2, sex, age × sex interaction and age2 × sex interaction. Further, since the outcome defined on the basis of principal components analysis did not account for the concomitant use of drugs, we adjusted these models for the use of lipid lowering, antihypertensive and antidiabetic agents. In addition, to determine the independent association of the lipid species with metabolic syndrome, we included two known biomarkers of metabolic syndrome as covariates in these models: average size of low-density lipoprotein (LDL) particles and the apolipoprotein B concentration. Statistical significance of the association between the lipid species and phenotypic trait was tested by constraining the regression coefficient to zero and comparing the log-likelihoods of the constrained and unconstrained models in a likelihood ratio χ2 test. Statistical analyses were conducted using the SOLAR software package.[10] Statistical significance was tested at a global type I error rate (α) of 0.05.
We used Li and Ji’s modification [11] of Cheverud’s [12] method to account for multiple comparisons of correlated lipid species. This method uses the eigenvalues of a correlation matrix to estimate the effective number of independent tests and adjusts the global type I error rate using a Sidak correction. In our case, we first estimated the phenotypic correlations between pairs of PtdCho species by conducting bivariate trait analyses.[13–15] Phenotypic correlation between two PtdCho species was estimated as where, rP is the phenotypic correlation between ith and jth PtdCho species, h2 is the heritability, rG is the genetic correlation and rE is environmental correlation.[16] We then calculated the eigenvalues of the phenotypic correlation matrix (dimension 45×45) using the symeigen command in Stata and estimated the effective number of independent PtdCho species as where Meff is the effective number of independent tests, λ is the eigenvalue, I is an indicator function and ⌊ ⌋ is a floor function.[11] Next, we used the estimated value of Meff to determine a Sidak correction using the formula 1−(1− α)1/Meff. All p-values less than this corrected value of α were considered statistically significant.
RESULTS
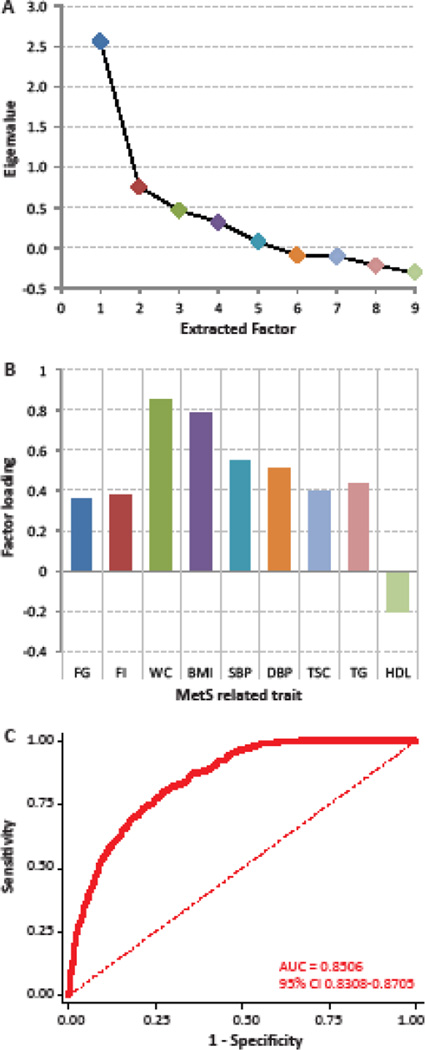
Our results of principal components analysis showed (Figure 1A) that only the first extracted factor had an eigenvalue >1 which explained 73.6% of the total variance. Clinically, HDL levels are negatively associated with MS while the other eight included traits are positively associated. The pattern of factor loadings in this analysis (Figure 1B) exactly followed this expectation, supporting the interpretation of this factor as a measure of metabolic syndrome risk. To validate this measure further we conducted ROC curve analyses (Figure 1C) using the IDF definition of metabolic syndrome as the reference standard and the factor score as the predictor. This measure had an overall accuracy of 85%. The optimum cut-off point was found at a factor score of 0.0974. At this cut-off the sensitivity and specificity of this measure to predict IDF defined metabolic syndrome was 79.57% and 73.27%, respectively. Together, the extracted factor score could serve as an acceptable surrogate of the clinically defined metabolic syndrome with the advantage that it was a normally distributed continuous variable.
Figure 1.
Principal components analysis of the metabolic syndrome-related traits. (A) Scree plot. (B) Factor loadings on the first extracted factor for each of the nine traits – FG, fasting glucose; FI, fasting insulin; WC, waist circumference; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TSC, total serum cholesterol; TG, serum triglycerides; HDL, serum high density lipoprotein concentration. (C) Receiver-operating characteristic curve for the first factor score as a predictor of clinical metabolic syndrome (based on the International Diabetes Federation definition). AUC, area under the curve; CI, confidence interval
We ran a series of polygenic regression models to test the association of each phosphatidylcholine species with both outcomes. For statistical significance, we needed a corrected type I error rate of 1.92×10−3 which was obtained based on the pair-wise phenotypic correlation structure of PtdCho species that yielded an estimate of the effective number of independent species (Meff) as 26.67. The full correlation matrix and eigenvalues are given in Supplementary Table 1. We observed that 20 PtdCho species (out of 45 quantified) that were significantly associated with at least one MS measure. These results are shown in Table 2 while full results for all the 45 phosphatidylcholines are provided in Supplementary Table 2. Interestingly, all the nine species found significantly associated with the MetS trait were also included in the list of 20 species significantly associated with the Factor1 trait. Of the nine PtdCho species, seven species (with the exception of the PtdCho 40:7 and PtdCho 39:6 species) were positively associated with MetS as well as Factor1 traits. Of these, the associations of lipid species phosphatidylcholine 32:1 and phosphatidylcholine 38:3 with both MetS and Factor1 traits were the most statistically significant.
Table 2.
Statistically significant associations of serum phosphatidylcholine species with metabolic syndrome*
| PtdCho species | Subjects With etS (pmol/ml) |
Subjects Without MetS (pmol/ml) |
b (MetS) |
p (MetS) |
b (Factor1) |
p (Factor1) |
||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | |||||
| PtdCho 30:0 | 2728.5 | 55.1 | 2182.7 | 40.0 | 0.2098 | 1.02×10−5 | 0.0921 | 0.0001 |
| PtdCho 32:0 | 9027.9 | 90.4 | 8310.9 | 58.9 | 0.2031 | 4.39 ×10−6 | 0.1022 | 8.87 ×10−6 |
| PtdCho 32:1 | 25931.9 | 451.1 | 20645.0 | 313.5 | 0.3409 | 1.92 ×10−11 | 0.2066 | 1.05 ×10−16 |
| PtdCho 32:2 | 9820.5 | 107.6 | 8390.6 | 79.5 | 0.2096 | 1.78E ×10−4 | 0.0899 | 0.0019 |
| PtdCho 34:1 | 125628.9 | 915.6 | 117564.8 | 802.4 | 0.1272 | 0.0061 | 0.0845 | 0.0003 |
| PtdCho 34:5 | 65.9 | 2.1 | 50.7 | 1.3 | 0.1186 | 0.0117 | 0.0781 | 0.0013 |
| PtdCho 36:4b | 91234.3 | 1109.7 | 81877.7 | 731.9 | 0.1658 | 0.0003 | 0.0815 | 0.0009 |
| PtdCho 36:5 | 14164.9 | 301.8 | 11469.2 | 197.4 | 0.1876 | 0.0002 | 0.0992 | 0.0001 |
| PtdCho 38:2 | 12164.4 | 159.8 | 11377.7 | 111.9 | −0.0346 | 0.4633 | −0.0790 | 0.0017 |
| PtdCho 38:3 | 54563.3 | 711.9 | 44511.5 | 506.2 | 0.2871 | 1.84 ×10−8 | 0.1574 | 1.16 ×10−9 |
| PtdCho 38:6a | 503.2 | 7.6 | 477.0 | 5.3 | −0.0455 | 0.3161 | −0.0928 | 0.0001 |
| PtdCho 38:7 | 1619.6 | 25.9 | 1507.3 | 18.5 | −0.0668 | 0.1598 | −0.0868 | 0.0006 |
| PtdCho 40:6 | 13436.8 | 188.5 | 11910.5 | 137.7 | 0.1139 | 0.0132 | 0.0969 | 0.0001 |
| PtdCho 40:7 | 4151.1 | 64.8 | 4263.5 | 47.4 | −0.1598 | 0.0003 | −0.1297 | 2.13 ×10−8 |
| PtdCho 33:2 | 3909.6 | 49.1 | 3604.6 | 37.3 | 0.0094 | 0.8422 | −0.0906 | 0.0003 |
| PtdCho 35:2 | 698.5 | 8.7 | 719.9 | 6.1 | −0.1281 | 0.0029 | −0.1357 | 9.48 ×10−10 |
| PtdCho 35:3 | 1811.7 | 23.7 | 1721.2 | 16.8 | −0.0736 | 0.1034 | −0.0914 | 0.0001 |
| PtdCho 37:4 | 352.7 | 5.1 | 354.2 | 3.5 | −0.0471 | 0.2710 | −0.0844 | 0.0002 |
| PtdCho 37:6 | 391.1 | 7.5 | 366.2 | 5.8 | −0.0530 | 0.2542 | −0.0914 | 0.0002 |
| PtdCho 39:6 | 962.8 | 16.9 | 1008.4 | 12.9 | −0.1386 | 0.0016 | −0.1120 | 8.36 ×10−7 |
| Total PtdCho | 1219153.5 | 3345.9 | 1127739.2 | 2376.5 | 0.0079 | 5.8×10−7 | 0.0062 | 3.1×10−11 |
metabolic syndrome was defined in two ways: i) MetS, as a dichotomous trait based on the International Diabetes Federation definition and ii) Factor1, as the first principal component scores derived from the following nine traits – fasting glucose, fasting insulin, waist circumference, body mass index, systolic blood pressure, diastolic blood pressure, total serum cholesterol, serum triglycerides and serum high-density lipoprotein concentration. Statistically significant results using Li and Ji’s method are shown in bold.
PtdCho, phosphatidylcholine; b, regression coefficient; p, significance value
Note: For dichotomous traits (e.g. MetS) SOLAR returns a negative regression coefficient which indicates an increased risk of the dichotomous trait. For consistency of presentation, here we presented the regression coefficients for an inverted MetS trait. Full results for all 45 phosphatidylcholine species are provided in Supplementary Table 2 and they include the goodness-of-fit of each model.
An informative pattern of association of phosphatidylcholine species was observed with the Factor1 trait such that all seven odd chain phosphatidylcholines were negatively associated with this trait whereas the even chain phosphatidylcholines (with the exception of phosphatidylcholine 40:7) were associated with an increased risk of metabolic syndrome. Lastly, we found that most of the plasma phosphatidylcholines that were statistically significantly associated with metabolic syndrome were associated with an increased risk. Indeed, total plasma phosphatidylcholines concentration was also associated with an increased risk of metabolic syndrome (last row Table 2).
DISCUSSION
In this study of Mexican American families, we made two important observations: i) there exists a substantial variability in the association of phosphatidylcholine species with metabolic syndrome and ii) if this variability is ignored and all phosphat idylcholines are considered in toto then, as a class, phosphatidylcholines are associated with an increased risk of metabolic syndrome. Although this latter observation can be partly explained by inclusion of the total serum cholesterol in the definition of MS traits, it is still surprising because phosphatidylcholines are generally considered to be beneficial against a wide variety of obesity-related conditions.[17–19] For example, phosphatidylcholines have been shown to reduce orotic acid-induced fatty-liver by suppressing hepatic lipogenesis in rats [20]; salmon derived omega-3-phosphatidylcholies have been shown to suppress lipogeneic gene expression and enhance lipolytic gene expression [21]; phosphatidylcholines are reduced in women with polycystic ovary syndrome as compared to controls [22]; and phosphatidylcholines are used in ‘injection lipolysis’ with the understanding that they promote lysis of adipose tissue.[23] Concordantly, Walker et al [24] have recently demonstrated the existence of a feedback loop that is primarily driven by phosphatidylcholine levels, involves the SREBP-1 proteins and contributes to metabolic syndrome traits. In this context, the present results imply the existence of a more complex relationship between phosphatidylcholine species and metabolic syndrome than previously appreciated.
It should be noted that the regression coefficients get scaled based on the range of values of the variable. As can be seen from Table 2, the concentrations of different PtdCho species was highly variable and therefore the regression coefficients cannot be directly compared across species. Similarly, the outcome variables are also measured on very different scales – liability threshold for MetS and the first factor score for the Factor1 trait. Therefore, these regression coefficients cannot be directly compared with one another. Rather, the direction of association and the significance of association need to be collectively considered in conjunction with the mean concentrations to make biologically valid inferences.
Within these constraints, our results also point towards the possibility that odd chain phosphatidylcholines may be specifically beneficial against metabolic syndrome. While the mechanistic and therapeutic implications of this finding are currently unclear, our results closely parallel those of Khaw et al [25] who found that in the subjects prospectively recruited in the EPIC-Norfolk study, the odd chain plasma phospholipid fatty acid concentration was associated with reduced risk of coronary heart disease (CHD) while the even chain plasma phosphatidylcholines generally associated with an increased risk of CHD. Similarly, Meissner et al [26] have reported that there was a 200% increase in odd chain long chain fatty acids associated with the phosphatidylcholine fraction during episodes of metabolic decompensation in patients of propionic academia. It should be noted that the odd chain phosphatidylcholines are generally considered as biomarkers of dairy source.[27] Our results therefore indirectly imply and concur with the view that milk or dairy related phosphatidylcholines may be specifically beneficial against metabolic syndrome. Future studies need to expand on this possibility.
It is also noteworthy that while most of the phosphatidylcholine species containing even chain fatty acids were positively associated with the Factor1 trait, those containing docosohexenoic acid (DHA, 40:7, 38:7, 38:6a) showed a negative association. This finding may relate to the beneficial effects of DHA in relation to inflammation, a feature of MetS etiology. Similarly, the positively MetS-associated phosphatidylcholine species may also be a reflection of the fatty acid composition. For example, PtdCho 32:0 and PtdCho 32:1 both contain the saturated fatty acid – palmitic acid or a combination of palmitic acid and the monounsaturated palmitoleic acid. Both these fatty acids have been linked to adverse health outcomes in relation to MetS.[28, 29] Further work examining total phospholipid fatty acids or the expression of enzymes involved with fatty acid metabolism will be required to understand these relationships.
Nevertheless, our findings have two important implications with regard to plasma lipidomic studies. First, the strength of high dimension lipidomic studies is the information they provide with regards the lipid species. We believe that significant inter-lipid species variability might exist in the association of these species with the outcomes of interest. Recently, Pietiläinen et al [30] have also described a substantial variability in adipose tissue phosphatidylcholines in the context of acquired obesity. Thus, lipidomics approach is likely to be more informative by unraveling the associations at the level of lipid species rather than classes. Second, our study indicates that some phosphatidylcholines (like PtdCho (40:7) and odd chain phosphatidylcholines) may be more favorably associated with metabolic syndrome as compared to other phosphatidylcholines. Therefore our results also provide potential leads to drug development against complex diseases like metabolic syndrome.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by NIH grants R01 DK082610 and R01 DK079169. Data collection for the San Antonio Family Heart Study was supported by NIH grant P01 HL045522. We are grateful to the participants of the San Antonio Family Heart Study for their continued involvement. The development of the analytical methods and software used in this study was supported by NIH grant R37 MH059490. The AT&T Genomics Computing Center supercomputing facilities used for this work were supported in part by a gift from the AT&T Foundation and with support from the National Center for Research Resources Grant Number S10 RR029392. This investigation was conducted in facilities constructed with support from Research Facilities Improvement Program grants C06 RR013556 and C06 RR017515 from the National Center for Research Resources of the National Institutes of Health.
Abbreviations
- BMI
Body mass index
- DBP
Diastolic blood pressure
- FG
Fasting glucose
- FI
Fasting insulin
- HDL
High density lipoproteins
- IDF
International Diabetes Federation
- LDL
Low density lipoprotein
- MS
Metabolic syndrome
- ROC
Receiver operating characteristic
- SAFHS
San Antonio Family Heart Study
- SBP
Systolic blood pressure
- TG
Triglycerides
- TSC
Total serum cholesterol
- WC
Waist circumference
Footnotes
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.Meikle PJ, Christopher MJ. Lipidomics is providing new insight into the metabolic syndrome and its sequelae. Curr Opin Lipidol. 2011;22:210–215. doi: 10.1097/MOL.0b013e3283453dbe. [DOI] [PubMed] [Google Scholar]
- 2.Kontush A, Chapman MJ. Lipidomics as a tool for the study of lipoprotein metabolism. Curr Atheroscler Rep. 2010;12:194–201. doi: 10.1007/s11883-010-0100-0. [DOI] [PubMed] [Google Scholar]
- 3.Kotronen A, Velagapudi VR, Yetukuri L, Westerbacka J, Bergholm R, Ekroos K, Makkonen J, Taskinen MR, Oresic M, Yki-Jarvinen H. Serum saturated fatty acids containing triacylglycerols are better markers of insulin resistance than total serum triacylglycerol concentrations. Diabetologia. 2009;52:684–690. doi: 10.1007/s00125-009-1282-2. [DOI] [PubMed] [Google Scholar]
- 4.Koerner TA, Cunningham MT, Zhang DS. The role of membrane lipid in the platelet storage lesion. Blood Cells. 1992;18:481–497. discussion 498–500. [PubMed] [Google Scholar]
- 5.MacCluer JW, Stern MP, Almasy L, Atwood LA, Blangero J, Comuzzie AG, Dyke B, Haffner SM, Henkel RD, Hixson JE, et al. Genetics of atherosclerosis risk factors in Mexican Americans. Nutr Rev. 1999;57:S59–S65. doi: 10.1111/j.1753-4887.1999.tb01790.x. [DOI] [PubMed] [Google Scholar]
- 6.Voruganti VS, Lopez-Alvarenga JC, Nath SD, Rainwater DL, Bauer R, Cole SA, Maccluer JW, Blangero J, Comuzzie AG. Genetics of variation in HOMA-IR and cardiovascular risk factors in Mexican-Americans. J Mol Med (Berl) 2008;86:303–311. doi: 10.1007/s00109-007-0273-3. [DOI] [PubMed] [Google Scholar]
- 7.IDF. The IDF consensus worldwide definition of metabolic syndrome. Brussels, Belgium: IDF Communications; 2006. [Google Scholar]
- 8.Murphy RC, James PF, McAnoy AM, Krank J, Duchoslav E, Barkley RM. Detection of the abundance of diacylglycerol and triacylglycerol molecular species in cells using neutral loss mass spectrometry. Anal Biochem. 2007;366:59–70. doi: 10.1016/j.ab.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smyth I, Hacking DF, Hilton AA, Mukhamedova N, Meikle PJ, Ellis S, Satterley K, Collinge JE, de Graaf CA, Bahlo M, et al. A mouse model of harlequin ichthyosis delineates a key role for Abca12 in lipid homeostasis. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000192. e1000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity (Edinb) 2005;95:221–227. doi: 10.1038/sj.hdy.6800717. [DOI] [PubMed] [Google Scholar]
- 12.Cheverud JM. A simple correction for multiple comparisons in interval mapping genome scans. Heredity (Edinb) 2001;87:52–58. doi: 10.1046/j.1365-2540.2001.00901.x. [DOI] [PubMed] [Google Scholar]
- 13.Melton PE, Rutherford S, Voruganti VS, Goring HH, Laston S, Haack K, Comuzzie AG, Dyer TD, Johnson MP, Kent JW, Jr, et al. Bivariate genetic association of KIAA1797 with heart rate in American Indians: the Strong Heart Family Study. Hum Mol Genet. 2010;19:3662–3671. doi: 10.1093/hmg/ddq274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diego VP, Goring HH, Cole SA, Almasy L, Dyer TD, Blangero J, Duggirala R, Laston S, Wenger C, Cantu T, et al. Fasting insulin and obesity-related phenotypes are linked to chromosome 2p: the Strong Heart Family Study. Diabetes. 2006;55:1874–1878. doi: 10.2337/db05-0668. [DOI] [PubMed] [Google Scholar]
- 15.Almasy L, Dyer TD, Blangero J. Bivariate quantitative trait linkage analysis: pleiotropy versus co-incident linkages. Genet Epidemiol. 1997;14:953–958. doi: 10.1002/(SICI)1098-2272(1997)14:6<953::AID-GEPI65>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 16.Rainwater DL, Comuzzie AG, VandeBerg JL, Mahaney MC, Blangero J. Serum leptin levels are independently correlated with two measures of HDL. Atherosclerosis. 1997;132:237–243. doi: 10.1016/s0021-9150(97)00104-4. [DOI] [PubMed] [Google Scholar]
- 17.Ng DS, Saw NM. The role of HDL and its modulators in the development of diabetes. Curr Opin Lipidol. 2012;23:167–168. doi: 10.1097/MOL.0b013e3283518614. [DOI] [PubMed] [Google Scholar]
- 18.Gaby AR. Nutritional approaches to prevention and treatment of gallstones. Altern Med Rev. 2009;14:258–267. [PubMed] [Google Scholar]
- 19.Nash DT. Cardiovascular risk beyond LDL-C levels. Other lipids are performers in cholesterol story. Postgrad Med. 2004;116:11–15. doi: 10.3810/pgm.2004.09.1584. [DOI] [PubMed] [Google Scholar]
- 20.Yanagita T, Nagao K. Functional lipids and the prevention of the metabolic syndrome. Asia Pac J Clin Nutr. 2008;17(Suppl 1):189–191. [PubMed] [Google Scholar]
- 21.Shirouchi B, Nagao K, Inoue N, Ohkubo T, Hibino H, Yanagita T. Effect of dietary omega 3 phosphatidylcholine on obesity-related disorders in obese Otsuka Long-Evans Tokushima fatty rats. J Agric Food Chem. 2007;55:7170–7176. doi: 10.1021/jf071225x. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Y, Fu L, Li R, Wang LN, Yang Y, Liu NN, Zhang CM, Wang Y, Liu P, Tu BB, et al. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: plasma metabolomics analysis. BMC Med. 2012;10:153. doi: 10.1186/1741-7015-10-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan D, Rubin JP, Golitz L, Badylak S, Kesel L, Freund J. Refinement of technique in injection lipolysis based on scientific studies and clinical evaluation. Clin Plast Surg. 2009;36:195–209. doi: 10.1016/j.cps.2008.11.001. v-vi; discussion 211–193. [DOI] [PubMed] [Google Scholar]
- 24.Walker AK, Jacobs RL, Watts JL, Rottiers V, Jiang K, Finnegan DM, Shioda T, Hansen M, Yang F, Niebergall LJ, et al. A conserved SREBP-1/phosphatidylcholine feedback circuit regulates lipogenesis in metazoans. Cell. 2011;147:840–852. doi: 10.1016/j.cell.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khaw KT, Friesen MD, Riboli E, Luben R, Wareham N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: the EPIC-Norfolk prospective study. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001255. e1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meissner T, Leichsenring M, Mayatepek E. Odd-numbered long-chain fatty acids in erythrocyte phospholipids as long-term follow-up parameter in propionic acidemia. Clin Chem Lab Med. 2004;42:1005–1008. doi: 10.1515/CCLM.2004.203. [DOI] [PubMed] [Google Scholar]
- 27.Wolk A, Furuheim M, Vessby B. Fatty acid composition of adipose tissue and serum lipids are valid biological markers of dairy fat intake in men. J Nutr. 2001;131:828–833. doi: 10.1093/jn/131.3.828. [DOI] [PubMed] [Google Scholar]
- 28.Kroger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, Doring F, Joost HG, Boeing H, Schulze MB. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr. 2011;93:127–142. doi: 10.3945/ajcn.110.005447. [DOI] [PubMed] [Google Scholar]
- 29.Kim OY, Lim HH, Lee MJ, Kim JY, Lee JH. Association of fatty acid composition in serum phospholipids with metabolic syndrome and arterial stiffness. Nutr Metab Cardiovasc Dis. 2011 doi: 10.1016/j.numecd.2011.06.006. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 30.Pietilainen KH, Rog T, Seppanen-Laakso T, Virtue S, Gopalacharyulu P, Tang J, Rodriguez-Cuenca S, Maciejewski A, Naukkarinen J, Ruskeepaa AL, et al. Association of lipidome remodeling in the adipocyte membrane with acquired obesity in humans. PLoS Biol. 2011;9 doi: 10.1371/journal.pbio.1000623. e1000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.