Abstract
Background
Migraine is one of the most common health problems for children and adolescents. If not successfully treated, it can impact patients and families with significant disability due to loss of school, work and social function. When headaches become frequent, it is essential to try to prevent the headaches. For children and adolescents this is guided by extrapolation from adult studies, a limited number of small studies in children and adolescents and practitioner preference. The aim of the Childhood and Adolescent Migraine Prevention (CHAMP) study is to determine the most effective preventive agent to use in children and adolescents.
Methods
CHAMP is a double-blinded, placebo-controlled, multi-center, comparative-effectiveness study of amitriptyline and topiramate for the prevention of episodic and chronic migraine, designed to mirror real-world practice, sponsored by the US National Institute of Neurological Disorders and Stroke/National Institutes of Health (U01NS076788). The study will recruit 675 subjects between the ages of 8 and 17 years old, inclusive, who have migraine with or without aura or chronic migraine as defined by the International Classification of Headache Disorders, 2nd Edition, with at least 4 headaches in the 28 days prior to randomization. The subjects will be randomized in a 2:2:1 (amitriptyline: topiramate: placebo) ratio. Doses are weight based and will be slowly titrated over an 8 week period to a target dose of 1 mg/kg of amitriptyline and 2 mg/kg of topiramate. The primary outcome will be a 50% reduction in headache frequency between the 28 day baseline and the final 28 days of treatment (weeks 20–24).
Conclusions
The goal of the CHAMP study is to obtain level 1 evidence for the effectiveness of amitriptyline and topiramate in the prevention of migraine in children and adolescents. If this study proves to be positive, it will provide information to the practicing physician as how to best prevent migraine in children and adolescents and subsequently improve the disability and outcomes.
Keywords: Childhood and Adolescent Migraine Prevention, Amitriptyline, Topiramate
INTRODUCTION
Migraines in children and adolescents
Recurrent headaches and migraine are one of the most common health complaints for children and adolescents. Migraine is an episodic pain disorder that can vary from an infrequent occurrence to occurring on a daily basis and become very disabling. Migraine in children and adolescents is similar to adult migraine, although it tends to be of shorter duration and can have an impact on not only the patient, but the entire family as parents and guardians need to cope with the alterations of their lives when their child has a headache. Similarly, the pathophysiology of migraine can be presumed to be similar to adults. In children with the potential of early intervention with effective treatment may impact the disease progression before many of the refractory aspects in adults become established(1).
Effective early treatment should be expected to change this trajectory of morbidity and costs. Yet, research focused on treatment of pediatric migraine remains severely limited. Clinically, prevention medication is an option when migraine is disabling and/or occurring on a regular basis (e.g., 1×/week or more). The goal of prevention therapy is significant reduction of headache frequency and improvement in headache-related disability. However, no prevention medication is FDA-approved for childhood migraine. The vast majority of children who present to their primary care provider with migraine do not receive any prophylactic therapy despite the suffering and disability caused by this disease. Treatment practices vary widely even amongst headache specialists due to the absence of evidence based treatment results from clinical trials. Placebo-controlled clinical trials of prevention medications for pediatric migraine are necessary to establish solid evidence based early treatments.
To address this deficit, the Childhood and Adolescent Migraine Prevention (CHAMP) Study (http://clinicaltrials.gov NCT01581281) was developed by the Cincinnati Children's Hospital Medical Center (Clinical Coordinating Center) and the University of Iowa (Data Coordinating Center). This 3-arm, randomized, double-blinded, parallel group, placebo-controlled trial will test the efficacy and safety of amitriptyline (AMI) and topiramate (TPM) compared to placebo in 675 children and adolescents between age 8 and 17 with migraine at up to 40 research centers across the U.S. This study will attempt to determine which of these two medications is the most efficacious in the prevention of childhood and adolescent migraine in the “real-world” setting.
The specific aims are:
Aim 1: To test if amitriptyline is superior to placebo in reducing headache frequency and headache-related disability;
Aim 2: To test if topiramate is superior to placebo in reducing headache frequency and headache-related disability;
Aim 3: To evaluate the tolerability and side effects for each therapy, and
Aim 4: To compare the efficacy of amitriptyline and topiramate in reducing headache frequency and headache-related disability.
The primary outcome will be the comparison of the rate of a ≥ 50% reduction in headache frequency from the 4 week baseline period to the last 4 weeks of this 24-week trial. Secondary outcomes include the reduction of migraine disability as measured with PedMIDAS (Pediatric MIgraine Disability ASsessment)(2, 3), the absolute reduction in headache and migraine frequency and overall tolerability. The use of these primary and secondary outcome measures will allow a determination of which medication is superior in the prevention of migraine and can most change the outcome for children and adolescents with migraine.
Impact
Recurrent headache and migraine are one of the most common pain complaints in children and adolescents. Up to 10% of younger children(4) and up to 28% of adolescents(5) suffer from recurrent headaches, the majority of which have migraine features(6). Migraine is an episodic disorder that requires both an acute treatment plan to minimize the duration of the individual attacks and a prevention plan that reduces the frequency. Both long duration migraines and frequent migraines can result in lost school, family, and social interactions. Migraine can significantly impact the lives of these families and children. Past population studies have demonstrated that over 130,000 school days are missed every two weeks and 3 million bedridden days occur per month due to pediatric migraine(7). Negative impact of having migraines on overall quality of life is similar to pediatric cancer, heart disease, and rheumatic disease(8). As the migraine frequency increases, this leads to increasing disability both to the patient and the family. This leads to increased health care costs and lost educational and work activities.
Health care costs are 70% higher for a family with a migraineur than a non-migraine affected family, and direct medical costs for children with migraine are reported to be similar to those for adults(9). The majority of children with migraine continue to experience migraines into adulthood(10),] resulting in an annual economic impact in the U.S. of approximately $36 billion due to both direct medical costs and lost productivity(11). Preventing headaches may not only reduce the frequency of the headaches, but also may results in improved effectiveness of acute medication. The majority of children and adolescents with migraine continue to experience migraines into adulthood(10).
BACKGROUND
Why a need for prevention
When migraine becomes frequent prevention medication is indicated. There are no absolute criteria for when this transition occurs, but evidence from adult studies suggests that this should be considered when the average frequency reaches 1 headache per week(12, 13). When this threshold is reached, the likelihood of frequency progression and possible transformation has been demonstrated in large population based studies. As the headache frequency continues to increase there is a proportional increase in the likelihood of the development of chronic migraine and refractory headaches. This is becoming an increasingly impactful health concern(14, 15).
The etiology of this worsening is complex, but may be related to both biological and psychological factors(16). As migraine is a genetically based disease, risk, especially in children, is based on the occurrence of the migraine related genes (genotypic expression)(17–19). As migraines become more frequent, it can be speculated that these genes have increased activity or influence on the pathways involved (phenotypic expression)(20–22). This results in a hypersensitive phenomenon that lowers the threshold for the occurrence of the next migraine. This hypersensitive phenomenon has been supported by the observations that as patients' headaches become more frequent they may have increased photophobia, phonophobia, allodynia and promontory symptoms. The mechanisms of action of preventative medication appear to reverse this hypersensitivity either through multiple re-uptake inhibition (AMI) or alteration of channel function and neurotransmission (TPM).
If high frequency headaches are sustained, this increased hypersensitivity may result in long-term changes in neuroanatomy, neurophysiology, and psychological response patterns. Neuroimaging studies have demonstrated altered brainstem appearance with iron deposition as the presence of frequent migraine persists over time(23). Clinical and physiological assessments have demonstrated the increased hypersensitivity with lack of normal attenuation whether due to auditory, visual or sensory response patterns(24). As headache frequency persists, the ability of patients to cope with their headaches and daily life stressors is further compromised(25). This is further enhanced by the presence of co-morbid medical and psychological conditions.
Preventative medications may be a key intervention to reverse and prevent this progression. It can reasonably be speculated that the earlier in life this prevention is initiated the overall benefit and potential life-long impact can be reduced.
Why a 3 in 1 approach
The use of a 3 in 1 study design (AMI vs. placebo, TPM vs. placebo, and AMI vs. TPM) yields a unique strength, allowing for the incorporation of an a priori decision making process. Using a three tiered approach for analysis of the results of this trial, the 3 in 1 study design will allow a “winner” to be chosen among the three therapies tested. The first tier is based upon our primary endpoint, >50% reduction rate for headache frequency during one month. This endpoint is the most relevant to current practitioners and is recommended as the key variable for migraine prevention medication trials by the International Headache Society (IHS)(26). It is also the primary outcome that families expect when they bring their children for headache treatment.
The results will yield four possible practice recommendations: (1) topiramate first choice because it is superior to placebo and amitriptyline on primary outcome; (2) amitriptyline first choice because it is superior to placebo and topiramate on primary outcome; (3) both therapies possible first choice because both are superior to placebo but were not different on primary outcome; and (4) neither therapy first choice because neither are superior to placebo.
If scenario (3) occurs, and a recommendation cannot be made based upon the primary endpoint, tier 2 will be utilized - migraine-related disability (i.e., PedMIDAS). If one medication is superior to the other on this variable, it is the “winner.” If not, tier 3 would be utilized - tolerability. If one medication was better tolerated than the other, it would be the “winner.” Problems with tolerability would be defined as having a drop-out rate greater than 35% or a dropout rate that is statistically worse than the other therapy.
If a winner cannot be identified based upon tier 3 criteria, then the outcome of the trial would be that both therapies are efficacious and it would be individualized clinical decisions based upon subject presentation that would guide first choice in practice. Of course, recommendations will also take into account the safety data generated by this novel study.
Why comparative effectiveness
Patient's response to preventative medication can be variable due to a number of factors. Some of this variability is due to differences in study design, including limitations on inclusion/exclusion criteria, age of subjects, frequency of headaches, dosages utilized and outcome measured. These differences restrict the ability to compare medications using similar paradigms. Additionally, if “real-world” aspects of the experience of youth with migraine are not incorporated into the study design, the comparative effectiveness and applicability to change practice is further reduced. Practitioners treating their patients are often asked “which medication is best?” When agents are studied using the same entry and evaluation criteria within a single study this question has a much higher propensity to be answered. A comparative effectiveness study has the greatest potential to resolve this concern and be readily translated into clinical practice.
METHODS
Overall study design
This study is an intent-to-treat, 3-arm, randomized, double-blinded, parallel group, placebo-controlled trial in order to simultaneously assess the impact of two medication therapies (AMI and TPM) for migraine prevention in children and adolescents. A total of 675 children and adolescents between 8 and 17 years old, inclusive, with migraine will be recruited from up to 40 sites across the U.S. Subjects will be randomized in a 2:2:1 fashion to receive either AMI (n=270), TPM (n=270), or placebo (n=135). Key endpoints will be headache frequency, migraine-related disability, tolerability, and safety. Subjects will be recruited over a three year period, with an average of 17 subjects per site. Each subject will be followed for approximately 34 weeks in the various phases of the study, with visits, timelines, and interventions as indicated in the flow chart (Figure 1):
Figure 1.
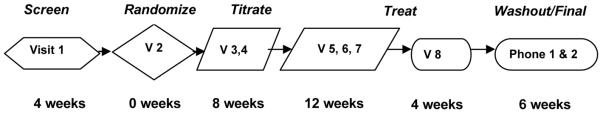
Visit Sequence. Subjects are seen at 8 visits over the duration of the study with additional phone visits during the titration phase and after treatment phase for safety and tolerability assessment. Screening visit includes identification of subjects, validation of their inclusion and exclusion, baseline assessment, and study education. At Randomization visit, inclusion and exclusion is reassessed, safety and baseline assessment validated and calendar reviewed prior to randomization. Titration visits (in person and via phone discussion) will review tolerability of titration, allowing for adjustment as needed (Figure 2) and continued education. During Treatment/Maintenance phase subject will have safety evaluations (visit 5 and 8) and tolerability evaluated (visit 5, 6, 7 and 8) and continuing review of procedures with reinforcement of diary completion (visit 7) before final assessment (visit 8).Washout (phone 1) and final assessment (phone 2 – 28 days after completion of all medication) will assess overall response and tolerability.
Inclusion/Exclusion (Table 1)
Table 1.
Inclusion and Exclusion Criteria
Inclusion Criteria
|
Exclusion Criteria
|
For a study to effectively change practice, the subjects enrolled need to represent the patients seen in typical practice without adding complications due to patients having additional co-morbid medical or psychological factors that would negatively impact the subjects' ability to complete the study, have the appropriate potential to respond, and have generalizability to the typical patient. In order to accomplish this result, careful identification of subjects is needed. The CHAMP study philosophy was to incorporate the subjects most commonly seen in a specialty practice where preventative medication is indicated. This was achieved through the use of fairly broad inclusion criteria.
These inclusion criteria restrict the research subjects to a clearly definable group of pediatric patients with an established diagnosis using standardized international criteria, while broadening the headache frequency range to those patients that would be most likely to benefit from preventative treatment. As noted above, the impact of frequent migraine on a patient's life is one of the major decision factors in determining the necessity of preventative medication. The use of a readily applied, standardized, reliable, and validated disability assessment (PedMIDAS) specific to the pediatric headache population adds to the translatable potential of this study.
While it is important to have a generalizable inclusion of subjects, it is equally important to exclude subjects that are not appropriate for the unified limitations of a clinical research study. The CHAMP study philosophy was to exclude those subjects who have a lower propensity to respond or may be harmed if they participated in the study or would create undue complexity to study completion and generalizability.
These exclusion criteria can be broadly grouped into:
-
1)
those patients who have a diminished potential to respond to the CHAMP protocol (continuous headaches, overusing acute medication, outside the dosage range utilized based on weight, or previous unresponsiveness or adverse reaction to treatment options),
-
2)
those patients who have complicating medical history either due to disease processes (abnormal test results, presence of complicating medical or psychiatric disease, or history of an illness that preclude the use of either of the agents used in the study) or
-
3)
those patients being treated with concurrent medications that may have their own treatment potential for migraine as preventions or due to medications that may interact with the treatment options.
There are also several practical components that would lead to exclusion including the inability to swallow pills or the impression that the subject would likely be noncompliant with the study protocol.
Although these exclusion criteria have some potential to lessen the “real world” applicability of the study, the practicality of including highly motivated subjects that are without complications, while still being representative of the vast majority of families that seek care, are essential to a study's success. In many cases, the exclusion criteria would also exclude the use of the medications in the real world practice and thus can provide additional guidance to the choice of preventative medication.
Outcome decision
Headache day & episode
Headache day is defined as the presence of any headache within a 24 hour period starting and ending at midnight. Using a time lock to the calendar day allows children and adolescents to easily quantify the presence of headache and has been demonstrated to be one of the most consistent measures for determining the frequency of headache and migraine. It has the added advantage to incorporate headaches that are rapidly and successfully treated before the full characteristics of migraine are expressed. It also eliminates the confusion of possible mixed headache conditions and is the most understandable measure for parents and patients (i.e., they just want their headaches gone). For all of these reasons, headache frequency as defined by a headache day was determined to be the most reproducible and accurate measure to collect, in contrast to the other frequency measures discussed below.
Headache episode is similar to a headache day, but without the anchoring of midnight. This allows for both the occurrence of multiple shorter duration headaches within a single day, as well as headaches that persist longer than a 24 hour period. Given this higher variability in duration, this measure is less robust in determining outcome as a shorter duration headache (i.e., 1–2 hours) and is equated to a headache that may last days. In patients' and families' lives, these two headache types are not equivalent. It is also more focused on the efficacy of the acute treatment. Given the observations that preventative treatment has the potential to alter the efficacy of acute treatment, it is a worthwhile measure to obtain for secondary analysis.
Migraine day & episode
Migraine day is defined as the presence of any headache within a 24 hour period starting and ending at midnight, which reaches a level of headache features and associated symptoms that meet the ICHD-II for migraine without aura or migraine with aura. It is independent of frequency and thus eliminates the need for separation of episodic and chronic migraine. Although this is less meaningful to the patient and family, the improvement in migraine features may be more highly correlated with an improvement in disability, functionality and response to acute medication. This proposed research approach has the added benefit to determine if a particular preventative medication has an impact on individual migraine components. It is limited, however, due to treatment response of acute medications that may prevent the expression of migraine features. This effect lessens to ability and robustness of using migraine day and episode as outcome measures.
The logic of migraine episode analysis is similar to headache episode analysis, with the added feature of quantifying and restricting the analysis to migraine specific features.
Study Procedures
The study is intended to represent a “real world” approach that incorporates acute, preventative and biobehavioral therapy. Throughout the study, the subjects will be instructed on appropriate use and dosing of acute therapy including NSAIDs and triptans as deemed appropriate by the site investigator. Additionally, subjects will be uniformly instructed on the importance of biobehavioral therapy for headache management including the healthy habits of adequate hydration without caffeine, regular exercise, avoidance of skipping meals with a healthy diet and maintaining regular sleep. Additional preventative medication or interventions or suspected preventative measures will not be allowed.
Establishment of a clear baseline for comparison to response is integral to achieving success of the study outcomes. The CHAMP study uses a 4 week minimum period to establish this baseline. This period will assure that the headache frequency is adequate to meet the inclusion criteria, while also serving as an assessment tool to assure the adherence of research subjects to the study protocol.
Randomization
Randomization will be stratified by age (8–12 and 13–17) and number of headaches per month (episodic: 4–14, chronic: 15 or more). A randomization table was generated for each of the strata using a permuted block design with random block sizes.
Titration
Both active agents will be titrated to dose based on weight of the subject. AMI will be titrated to a dose of approximately 1 mg/kg per day. This dose was chosen based on retrospective optimal outcomes of clinical practice as well as expert opinion by many of the site investigators. The dosing of amitriptyline for migraine prevention has varied greatly (10 to 150 mg) without clear guidance to dose response effectiveness or adjustment by subject's weight. In order to address this short-coming, the effectiveness in an open-labeled dose finding study with 1 mg/kg/day was determined to have maximum effectiveness without any additional benefit beyond this dosing(27, 28). This dose has subsequently been used for clinical practice and in the drug + coping skills management of chronic migraine. Feedback from the AHS Pediatric section membership also indicated that this was the typical approach used in current practice. Thus, a dose of 1 mg/kg was chosen up to a maximum dose of 100 mg/day.
TPM will be titrated to approximately 2 mg/kg per day. This dosage was determined by adjusting for weight of standardized doses in adults, averaging adult weight and previous studies with efficacy for TPM in migraine. The recommended adult dosing of topiramate for migraine prophylaxis is 100 mg to 200 mg per day in two equally divided doses. In a dose determining study comparing 50 mg and 100 mg that was a randomized, double-blinded, placebo controlled study of 103 adolescents (age 12–17), the 100 mg daily dose (divided into twice a day dosing) was found to be statistically superior to placebo (median headache frequency reduction in the last 12-weeks of treatment of 72.2% versus 44.4%), while the 50 mg per day (divided twice a day) did not differ statistically from placebo(29). The 50% migraine reduction rate was 83% of subjects for 100 mg/day of topiramate. This study did not adjust for the weight of the adolescent, but used a standardized dose. For the 100 mg group, the average daily dose across titration and maintenance periods was 74 +/− 19 mg/day. In clinical practice the child's weight is often considered when dosing topiramate. Feedback from the AHS Pediatric section membership also indicated that this was the typical approach used in current practice. To be consistent with the approach to dosing of amitriptyline in this trial as well as with clinical practice, a weight-based dosing plan was chosen that also allowed for maximizing the opportunity for participants to achieve a potential full dose of at least 70 mg/day or the equivalent of 2 mg/kg up to a maximum dose of 200 mg/day.
The use of weight based dosing has several distinct advantages, while also adding complications to the study design. Weight-based dosing in pediatrics is common practice, taking into account the variability in size across developmental age, while allowing for a broad applicability of dosing beyond the age and size ranges utilized in the study. It also allows for personalization of dosing in practice and provides a guideline for dosage initiation in practice.
Weight-based dosing is complicated in real world application due to the availability of tablet size. Although an exact weight based dosing using powdered formulation with encapsulation would result in the most accurate determination of dosage, this would not be practical in the real world practice setting. In order to address this issue, the CHAMP study uses existing tablet sizes (AMI – 10 mg, 25 mg, 50 mg, 75 mg and 100 mg; TPM – 50 mg and 100 mg) in combination with pill cutting and over-encapsulation to maintain the blind. Dosage per weight is determined by 10 kg weight ranges. This has the distinct advantage of being directly translatable to clinical practice. In addition, doses are administered in a twice a day manner, with the AMI arm receiving placebo in the morning.
As both the AMI and TPM are best tolerated when titrated slowly, the achievement of this weight based dosing is accomplished over an 8 week period in a blinded fashion. This also emulates the real world and can adjust for practice variation.
In clinical practice, patients may tolerate different titration schedules, based on individual sensitivities. To adjust for this, a blinded modification of strategy was developed (Figure 2). Any time during the titration phase, the individual site investigator can adjust the schedule. Possible options are to reduce the dose to the previous level or to continue the current dose without the scheduled increase. As the subject becomes more tolerant to the treatment, there will be the option to resume the titration schedule. With the exception of only one reduction allowed, the modification of holding and resuming the titration can be done multiple times. To allow for the attainment of the maximally tolerable dose, if there is a dosage adjustment, the subjects will be allowed an additional two weeks to continue the titration.
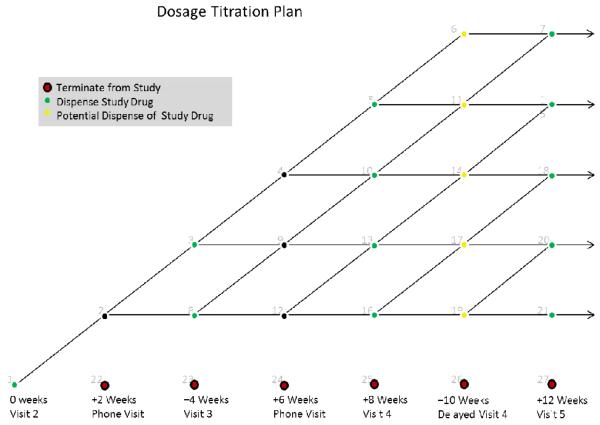
Figure 2.
Titration Plan. Subjects will start titration at randomization visit (visit 2, 0 weeks of medication). At 2 week intervals, subjects will be assessed for tolerability of medication treatment. At each of these nodal points, an assessment is made as to whether to continue increasing the titration, holding the current dose, returning to the previous dose (i.e, dose reduction). All choices are available at each nodal point. However, if 2 reduction are made, subjects will be terminated from the study and proceed to visit 8 assessment. If there is a dosage hold or reduction, subjects will be allowed to have an additional 2 weeks of titration in order to maximize dosing.
Maintenance
Once the subject has reached the end of the titration phase, either achieving the goal dose or the maximum dose within the extended titration window, they will continue this dose for a 16 week maintenance period. It is expected that no dosage adjustments will be made during this maintenance phase. If a subject does develop intolerable side effects and the site investigator feels that a dosage reduction is indicated, the subject will be weaned off of the study medication and enter the end of study analysis.
Patients will continue to be evaluated every 4 weeks during this maintenance phase and be assessed for adherence to medication administration and tolerability and diary completion. During the last 4 weeks of the maintenance stage, the diary will be used for the comparison to the baseline data for the primary outcome measures.
Wean while blinded and transition
At the end of the maintenance phase, the subjects will be weaned off the study medication in a blinded fashion. This will be accomplished over a 2 week period via a dosage reduction every 5 days. At that point, the site investigator with consultation, with the subject and family, will make a determination if continuation of preventative medication is needed. If this determination is made, the subject can be started on any preventative medication, but will not be informed of the study arm they were assigned. This may lead to the potential that the subject will re-start the same medication that they were taking during the study.
STATISTICAL ASPECTS
Statistical Analysis Plan
Primary Hypotheses #1 and #2: Amitriptyline and topiramate will result in an increased percentage of subjects meeting the primary endpoint compared to placebo. Primary Hypothesis #3: Topiramate will result in an increased percentage of subjects meeting the primary endpoint compared to amitriptyline. Note: the opposite is also likely (amitriptyline is superior to topiramate) and
Each of these primary hypotheses will involve a comparison of headache frequency (percentage of days with any headache) between the last 4 weeks of treatment (weeks 20–24) and the 4-week baseline period. Study participants will be allowed to take acute pain medications, including over the counter medications and triptans. For each subject, the primary outcome will involve a determination of whether a 50% or greater reduction in headache frequency was observed during the last 4 weeks of active treatment as compared with the headache frequency during the 4-week baseline period. The only exception will be that any subjects who must be removed from therapy prior to the completion of the 24-week treatment period will be considered `failures' with respect to the primary endpoint. The primary hypothesis will be assessed using a logistic regression model, adjusted for the two stratification variables. For example, the following model will be fit to these data:
where Yi represents the binary variable indicating whether or not the ith subject met the primary outcome requirement of a 50% or greater reduction in headache frequency, X1i is an indicator variable for the baseline age group for the ith subject (=0 if age 8–12, =1 if age 13–17), X2i is an indicator variable for the baseline number of headaches per month for the ith subject (=0 if 4–14, =1 if 15 or more), X3i is an indicator variable for whether the ith subject was randomized to the AMI group, and X4i is an indicator variable for whether the ith subject was randomized to the TPM group. Correspondingly, the three hypotheses of interest will be assessed by performing a hypothesis test of the three pair-wise treatment comparisons of interest:
Each will be tested using a chi-square test at a significance level of 0.017 (= 0.05/3). Due to randomization, it is unlikely that important covariates (such as gender) will be imbalanced in this study. However, should important imbalances occur, we will control for these additional covariates in the logistic regression model.
Major Secondary Hypotheses #1 and #2: Amitriptyline and topiramate will also result in a decrease in absolute migraine disability score (measured by PedMIDAS) compared to placebo.
Major Secondary Hypothesis #3: Topiramate also will result in a decrease in absolute migraine disability score (measured by PedMIDAS) compared to amitriptyline.
Each of these hypotheses will involve a comparison of the change in absolute migraine disability score, as measured by the PedMIDAS. Each subject will have a PedMIDAS score measured at the baseline visit (covering the three months prior to enrollment) and the 24 week visit (the end of the maintenance period – covering the three months from study week 12 to study week 24). The outcome measure for this comparison will be the difference in these two PedMIDAS scores. The mean change from baseline over time for the two groups will be assessed using a linear regression model, adjusted for the baseline PedMIDAS score.
Major Secondary Hypothesis #4: Amitriptyline and topiramate will be well tolerated.
The fourth major secondary hypothesis will involve a comparison of tolerability for the two active treatment groups. Concerns regarding tolerability will be defined as either having a percentage of subjects who complete the entire 24-week treatment period for the two active treatment arms that is significantly lower than the percentage for placebo subjects or significantly less than 65%. To assess tolerability, the percentage of subjects who complete the entire treatment period will be compared across the three groups and the percentage in the active treatments will be examined to see if they are significantly greater than 35%. Both analyses will be conducted using standard chi-square tests.
Major Secondary Hypothesis #5: Amitriptyline and topiramate will not differ from placebo on the occurrence of treatment-emergent serious adverse events.
The fifth major secondary hypothesis will involve a comparison of treatment-related SAE's across the three treatment groups. This will be assessed in two ways. First, the percentage of subjects who experience any treatment-related SAE in each of the three groups will be compared using a chi-square test. Then, the rates of treatment-related SAE's across the three groups will be compared using a Poisson regression model.
Impact of Missing Data
Both primary analyses will follow the intent-to-treat paradigm. As such, it will be critically important to minimize the occurrence of missing data. Obviously, the optimal strategy for dealing with missing data is to make every effort to obtain complete data during the conduct of the study. As described in the companion application, our team will use a variety of methods in order to minimize the percentage of missing data in this trial. Nevertheless, there is likely to be a small percentage of missing data. For subjects who drop out of the study before their 24-week outcome can be obtained, we propose to use a multiple imputation method to impute their outcome. This multiple imputation will be implemented using a model based on migraine frequency at baseline and each intermediate time-point for all subjects with observed data. We will use five separate implementations of this approach, and will average the parameters across all five imputations for the final analysis. This approach will be used for reporting all primary study results. However, in order to test the robustness of our findings, we will employ a number of additional analyses using different approaches for handling the missing data:
1) Use a Kaplan-Meier curve to obtain the estimated percentage of subjects in each group with a ≥ 50% reduction in migraine frequency at 24 weeks. This approach adequately accounts for all censored subjects (assuming censoring is non-informative) and is valid under the assumption that once a child achieves a 50% reduction in migraine frequency, the reduction will be maintained throughout the remainder of the study.
2) Assume that all subjects with missing data would not achieve a ≥ 50% reduction in migraine frequency at 24 weeks. This is a conservative approach that should dilute any true differences between the groups if dropout is purely random.
Sample Size Justification
In order to properly determine the required sample size for the proposed trial, four applicable studies were identified. Three of these studies evaluated topiramate versus placebo(29–31) and one study examined amitriptyline in a clinical database(27, 28). Based on this review, there was an average response rate between 45% and 52% for the placebo group. There was generally more variability regarding the percentage of subjects on topiramate who achieve the primary endpoint – the average is 77%, but the effect ranges from 54% to 95% across studies. Finally, the database from the prior study suggests that 70% of subjects taking AMI will achieve the primary endpoint.
For sample size estimation in this trial, it is critically important to keep in mind that this study essentially provides three trials in one. Hence, the chosen sample size must allow adequate power for testing all three primary hypotheses of interest. For the two comparisons with placebo, the results of an NINDS Clinical Research Collaboration (CRC) survey demonstrates that the majority of responders felt that a minimum increase of 20% in the percentage of subjects achieving a 50% reduction in migraine frequency was clinically meaningful, and the threshold needed for the results of a placebo-controlled trial to change practice. Hence, the sample size was chosen to ensure adequate power assuming that 50% of those on placebo versus 70% of those in each of the treatment groups will achieve the primary endpoint. Clearly, smaller differences are important for the head-to-head comparison and it becomes much more difficult to define a minimum clinically meaningful effect (since one could argue that, assuming equal cost and safety profiles, any difference between two active treatments is clinically meaningful). Based on the review above, there is some preliminary evidence that suggests more than 85% of patients in the topiramate group may achieve the primary endpoint. Thus, we have chosen to power the study to detect an absolute difference of 15% or greater in the percentage of subjects achieving the primary endpoint for the AMI vs. TPM comparison.
As stated above, since there are three primary hypotheses, we will apply a Bonferroni correction and plan to test each hypothesis at the 0.017 level. Based on the drop-out rates from two recently published TPM trials using a 100 mg/day dose (Lakshmi: 5%; Lewis: 14%) and our own experience of having a 9% drop-out rate in a single-site AMI trial, we assume a 15% dropout rate. Under these assumptions, enrolling a total of 675 subjects (135 placebo, 270 amitriptyline, and 270 topiramate) provides at least 85% power to detect all treatment/placebo comparisons and 90% power to detect a head-to-head difference of 70% of those on amitriptyline vs. 85% of those on topiramate achieving the primary endpoint.
This demonstrates that the proposed study has adequate power to assess each primary hypothesis of interest. However, since the main objective of the proposed study is to provide a recommendation to clinical investigators regarding the best treatment for children with migraine, the actual “power” of the study may be thought of as making a correct decision regarding a prevention medication using the algorithm described in the companion application. To assess the ability of our trial to meet this goal, we conducted a series of simulation studies. Each simulation consisted of 10,000 replications for each of 64 conditions defined by all possible combinations of plausible values for the true response rates in the three treatment groups: placebo (40%, 45%, 50%, 55%), AMI (50%, 60%, 70%, 80%), and TPM (50%, 70%, 85%, 95%). For each simulated condition, one of four possible decisions was reached (based upon the implementation of our decision algorithm): (1) AMI recommended first-line therapy, (2) TPM recommended first-line therapy, (3) both AMI and TPM are recommended as first-line therapy – use secondary endpoints to decide between the two, and (4) neither can be recommended as first-line therapy. Table 2 summarizes a few key conditions involved in the simulation.
Table 2.
Probability of picking any winner and of picking the correct winner as a function of treatment response rate. Assumes type I error rate of 0.017, sample size of 135 in placebo group and 270 in amitriptyline and topiramate groups.
| Decision | ||||||||
|---|---|---|---|---|---|---|---|---|
| PBO Rate | AMI Rate | TPM Rate | Prob. of Picking Winner | Prob. of Picking “Correct” Winner* | ||||
| AMI Better | TPM Better | Both | None | |||||
| 50% | 50% | 50% | 1% | 1% | 0% | 98% | 3% | 97% |
| 50% | 60% | 70% | 0% | 71% | 20% | 9% | 91% | 91% |
| 50% | 70% | 85% | 0% | 94% | 6% | 0% | 100% | 100% |
• If a single drug is `best', the correct winner is thought to be selected if the final decision is for either that drug or both drugs.
The first row in Table 2 demonstrates that the approach maintains the type I error level at or below the desired level of 0.05. The last two rows demonstrate the advantages of the overall three-in-one approach for selecting a winner. The last row, corresponding to the specific conditions used to justify the same size for the primary hypotheses, demonstrates that the overall approach provides benefits above and beyond what can be achieved with the separate hypotheses. As the table demonstrates, if this set of conditions holds true, our study would have nearly 100% “power” to correctly select topiramate as the appropriate first-line treatment for children with migraine. The second row demonstrates that the combined approach provides a greater likelihood of correctly selecting the winner when the absolute difference between the favorable response rates for amitriptyline and topiramate is only 10%. Under these conditions, our study would have 91% “power” to correctly select topiramate as first-line treatment - a substantial improvement over the 44% power for the individual head-to-head comparison.
The conclusions obtained from the simulations are only strengthened by the fact that this only examines the first tier of the decision algorithm. Assuming that disability and tolerability trends will parallel those of the primary endpoint, this should only increase the overall probability of selecting the correct winner at the end of the trial.
Interim Monitoring Plan
The study will include two interim efficacy assessments that will occur when 225 and 450 subjects have completed their 24-week visit. For these interim analyses, we propose to use the Lan-DeMets alpha spending function approach with O'Brien-Fleming stopping boundaries. Since the Lan-DeMets method allows for analyses at unequal intervals and does not require pre-specifying the time of any interim analyses, the proposed method has the flexibility to adapt should the DSMB request an interim analysis at an alternative time-point or if the DSMB is unavailable at the time of a scheduled interim analysis. Importantly, the placebo arm will be dropped only if both active treatments cross the stopping boundary at one of the pre-specified interim assessments. Importantly, if only one crosses the boundary at the first look, both will be assessed again at the second look and the placebo arm dropped only if both cross the bound at second review.
Due to the complex nature of the study, and the fact that definitive evidence would be required if neither of the active treatments proved superior to placebo, we propose that the trial should only be stopped for futility if both treatments were simultaneously futile (which has an exceptionally low probability). At the time of each interim analysis, we will report the conditional power based on the pre-specified effect of interest. If both conditional power values fall below 20%, we would recommend stopping the trial early for futility.
Assessment tools
Several assessment tools will be used for final analysis. The primary tool will be the study calendar and diary. This will be of a dual nature. All subjects will keep a calendar of their headaches. On the days that they have a headache, they will complete an individual diary page for each headache that occurred during that day. The most severe headache will be tabulated for headache diagnostic criteria assessment as to meeting the ICHD-II for migraine or primary headache. For the primary outcome measure (>50 % reduction in headache frequency defined as headache days), the calendar will serve as the primary source. For the secondary outcomes (migraine days, headache episodes, and migraine episodes) the diaries will augment the calendar details.
In addition to the comparison of baseline vs. the last 4 weeks of maintenance, a continuum assessment will be made through all stages of the study to determine a time course of responsiveness.
Additional outcome measure will include disability changes as assessed by PedMIDAS at the time of randomization compared to the end of maintenance. Side effects and tolerability of study drug based on phone and face to face visit assessment throughout the study will additionally be evaluated. Safety analysis will also include laboratory testing at 3 distinct time-points in the study (screening, end of titration and beginning of final 4 weeks of maintenance). An ECG will also be obtained at the screening visit and at the end of titration to assess for any changes in the QT interval that may be clinically and physiologically significant.
At the final two laboratory assessments, serum levels of the two active medications will be assessed. These will be used for post-study analysis of adherence. In addition, they may provide some degree of guidance of effective serum levels for migraine prevention.
Additional measures to be used in the CHAMP study include the Behavioral Rating Inventory & Executive Function (BRIEF)(32), the Clinical Depression Inventory (CDI)(33), the Functional Disability Inventory (FDI)(34), the Hospital Anxiety and Depression Scale (HADS)(35), Pediatric Quality of Life Inventory (PedsQL)(8, 36–38), the Columbia-Suicide Severity Rating Scale (C-SSRS) Children's Baseline and Children's Since Last Visit(39), and the Headache Impact Test (HIT-6)(40).
Safety Monitoring
All Adverse Events (AEs) and Serious Adverse Events (SAEs), whether uncovered by spontaneous reporting, or the structured AE interview, will be reported as quickly as possible by the clinical site. To help with this process, an innovative Online Adverse Event Reporting system was developed to allow sites to enter data electronically for all adverse events. At the time each AE/SAE is entered into the system, the study investigator will make a determination regarding the relatedness to therapy and severity. All AEs and SAEs are coded using the MedDRA system. For any adverse event meeting the definition of “serious,” the Independent Medical Monitor (IMM) will be notified via email in order to prompt a review of the event for determination of whether the event requires expedited reporting to the FDA. With the assistance of the data coordinators, the Independent Medical Monitor has the option of requesting additional information about any SAE. The IMM will complete a form for each review and this information will be entered into the data entry system.
Safety will be assessed in two ways – both the percentage of subjects who experience any AE and the rate of AEs across the three groups will be compared, by body system, using standard chi-square tests. The additional questions related to whether the AE/SAE is related to treatment, is unanticipated, or is severe will be used to subset these into a series of additional tables. This quarterly review will allow the Independent Medical Monitor to identify any disconcerting discrepancy in the frequency of an AE/SAE between the treatment groups, with particular emphasis placed on the four AEs of most concern to practicing clinicians due to the known side effect profiles of these two treatments: 1) depression, 2) suicidal ideation, 3) fatigue, and 4) slow thinking or problems with attention. Should any such discrepancy arise, or if the IMM feels that an SAE should be shared on an emergency basis with the participating clinical sites, the IMM will notify the NINDS Data Safety Monitoring Board (DSMB).
At the time of each DSMB meeting, the DSMB closed session report will include the same information as the quarterly safety reports to the IMM to allow the DSMB to monitor for the same types of trends. The report will be developed as blinded or unblinded based on the preferences expressed in the DSMB charter. The report will also include a memo from the Independent Medical Monitor to allow the communication of any concerns or findings that may be of interest to the DSMB in their study deliberations. In general, interim safety monitoring will rely on these interim reviews to identify any potential trends of interest. However, due to the increased concern associated with the risk of suicidal ideation, we propose a more stringent guideline to trigger a concern for this variable. We propose that, if the difference in the incidence in suicidal ideation between active treatment and placebo becomes statistically significant at the 0.05 level at any of the interim reviews, the DSMB should consider whether the trial should discontinue the affected treatment arm.
Site Monitoring
This study is a multi-site Phase III clinical trial. Therefore, there is a need for a careful data and safety monitoring plan to ensure the well-being of the youth in this study and the scientific integrity of the project.
The monitoring plan focuses on a risk based monitoring approach incorporating on-site monitoring and central review processes. The objectives of the on-site visit are to: ensure subjects are being consented appropriately and prior to any study procedures, ensure all adverse events are reported in a timely manner, audit data against source documents, verify maintenance of regulatory documents, and verify study drug accountability.
Monitoring begins with the site initiation visit which reinforces training conducted at the initial investigator and study coordinator training meetings in protocol procedures, data entry training, and Good Clinical Practices (GCP). During on-site visits, the monitors verify the protocol is being followed and subjects' rights and safety are being protected. Data review includes informed consents, inclusion/exclusion criteria, endpoint data, adverse events, and protocol deviations. The monitor also reviews the Regulatory files and study drug accountability logs. The initial on-site monitoring visit occurs after three subjects have been randomized in the study. Subsequent visits typically occur annually and are prioritized considering the number of additional subjects randomized and any center issues. Reports are generated to identify centers flagged as outliers in the number of adverse events, data queries, missing data items or missed visits, subject early terminations, and number and type of protocol deviations. These factors as well as central review assessments, and enrollment or study drug accountability issues are considered when prioritizing subsequent visits. Each center will have a study close-out visit confirming completeness of data entry, verifying final study drug accountability, and maintenance of required Regulatory documents.
Central review processes are used to augment on-site monitoring. For CHAMP, these processes include the design of the web-based data entry system with certain features such as logic and range checks, an on-line query system, management of unobtainable data, monitoring of adverse event reporting from submission of forms from sites through the medical monitor evaluation of serious adverse events, and central review of case report forms. All centers are required to submit case report forms on their first randomized subject and selected headache diaries on all randomized subjects for central review. Ten centers are also selected on a quarterly basis to submit randomly selected forms for central review. Centers flagged as having an unacceptable error rate at any point of central review are required to submit additional forms for central monitoring. Central review processes may prompt additional on-site monitoring visits. Site performance based on central review and on-site monitoring are reported to the DSMB for review.
Additional components
Data collection
A variety of programming tools were developed to facilitate the creation of an electronic data capture system. A Data Manager creates a specifications document by inserting item descriptions or requirements related to the Case Report Form (CRF) directly into a specifications template. The specifications documents are detailed and developed after consultation with the Principal Investigators to capture all of the data necessary for the analysis and to monitor safety. These item descriptions include data types (e.g. integer, numeric, date), field types (e.g. text box, radio button, check box), variable names, and mandatory and range requirements. Once the specifications template is complete, software development is automated and applications are written to quickly build a Data Dictionary for each database application to be developed. The web and data entry system for the CHAMP study has been developed, validated and implemented into the Production database environment that will be used by the Clinical Sites in the network.
An advantage of an Internet-based data entry system is that it automatically provides reminders of incomplete data forms and missing values. While a data entry session can be saved at any time, the system defines required fields that must be included before a form is submitted. Each time the study web page is accessed, a list of all incomplete forms appears on the screen. After an incomplete form has been accessed, the user is directed to complete all required fields before the form may be submitted. This process greatly facilitates the timely collection and entry of study data, and greatly reduces problems associated with missing data.
Subject retention
As the total study extends over a 5 year period for study sites and over a 24-week period for research subjects, it is essential to retain both the sites and the subjects. To support our recruitment and retention, several marketing tools will be employed: development of print media, and web postings. Print media to support recruitment efforts will include a study introduction letter from the co-investigators, a study flyer, and study tear off pads. The flyer and tear off pads will be made available to study research sites for use in public places, medical practices, and research centers. Web postings will be developed for use on internet sites such as clinicaltrials.gov, the NINDS web site, and other web locations.
Mailings of the study introduction letter and brochure to academic and private pediatric practices may also be employed to ensure that private pediatric neurology practices are aware of the study.
Individual sites may choose to conduct recruitment events to provide information to potential subjects and their parents. Recruitment events may also be conducted in tandem with professional society meetings to raise awareness of the trial among practitioners. These strategies may include the recruitment of additional research sites and investigators if the initial sites under perform in recruitment or the use of specialized medical research websites for promotion of the study.
Acknowledgements
This study was supported by the US National Institute of Neurological Disorders and Stroke (NINDS)/National Institutes of Health (NIH), grant number U01NS376788. This study is registered at ClinicalTrails.gov, Identifier number NCT01581281. We would like to thank the Clinical Coordinating Center, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio and the Data Coordinating Center, Clinical Trials Statistical and Data Management Center, University of Iowa, Iowa City, Iowa, Members of the American Headache Society Pediatric Adolescent Special interest section, Members of the Cincinnati Children's Office for Clinical and Translational Research, and the National Institute of Neurological Disease and Stroke Clinical Research Consortium.
The CHAMP Research Investigators: J. L. Aceves, MD, Scott and White Healthcare, Temple, TX; D. Arun, MD, Saint Louis University, St. Louis, MO; V.L. Atluru, MD, Winthrop University Hospital, Mineola, NY; S. K. Aurora, MD, Stanford Hospitals and Clinics, Palo Alto, CA; N.L. Bennett, MD, Preferred Clinical Research, Pittsburgh, PA; F.R. Berenson MD, Atlanta Headache Specialists, Atlanta, GA; J.L. Bickel, MD, Children's Mercy Hospital, Kansas City, MO; R.J. Bjork, MD, Colorado Springs Neurological Associates, Colorado Springs, CO; H.K. Blume, MD, Seattle Children's Hospital, Seattle, WA; J.M. Cohen, MD, The Headache Institute at Roosevelt Hospital, New York, NY; L.M. Frank, MD, Eastern Virginia Medical School, Norfolk, VA; P.J. Goadsby, MD, University of California-San Francisco Headache Center, San Francisco, CA; H.T. Jacobs, MD, University of Maryland School of Medicine, Baltimore, MD; M.A. Kabbouche, MD, Cincinnati Children's Hospital Medical Center, Cincinnati, OH; S. Kedia, MD, Children's Hospital Colorado, Aurora, CO; A.A. LeBel, MD, Boston Children's Hospital, Waltham, MA; D. Lebron, MD, Texas Children's Hospital, Houston, TX; S.L. Linder, MD, Dallas Pediatric Neurology Associates, Dallas, TX; K.J. Mack, MD, Mayo Clinic, Rochester, MN; H.G. Markley, MD, New England Regional Headache Center, Worcester, MA; J. W. McVige, MD, Dent Neurological Institute, Amherst, NY; H.R. Murali, MD, Marshfield Clinic, Marshfield, WI; A. Pakalnis, MD, Nationwide Children's Hospital, Columbus, OH; E.M. Pearlman, MD, Children's Hospital at Memorial University Medical Center, Savannah, GA; K.R. Ridel, MD, Josephson Wallack Munshower Neurology Research, Indianapolis, IN; A.D. Rothner, MD, The Children's Hospital, The Cleveland Clinic, Cleveland, OH; J.I. Lopez, MD, Renown Neuroscience Institute, University of Nevada, Reno School of Medicine, Reno, Nevada; R. Simmons, MD, Schenectady Neurological Consultants, Schenectady, NY; M. Sowell, MD, University of Louisville Health Sciences Center, Louisville, KY; M. Victorio, MD, Akron Children's Hospital, Akron, OH; P. Winner, MD, Premiere Research Institute, West Palm Beach, FL; M. Yonker, MD, Phoenix Children's Medical Group, Phoenix, AZ.
Footnotes
Conflict of Interest: The authors declare no conflict of interest
References
- 1.Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurol. 2010;9(2):190–204. doi: 10.1016/S1474-4422(09)70303-5. Epub 2010/02/05. [DOI] [PubMed] [Google Scholar]
- 2.Hershey AD, Powers SW, Vockell AL, LeCates S, Kabbouche MA, Maynard MK. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57(11):2034–9. doi: 10.1212/wnl.57.11.2034. [DOI] [PubMed] [Google Scholar]
- 3.Hershey AD, Powers SW, Vockell AL, LeCates SL, Segers A, Kabbouche MA. Development of a patient-based grading scale for PedMIDAS. Cephalalgia. 2004;24(10):844–9. doi: 10.1111/j.1468-2982.2004.00757.x. [DOI] [PubMed] [Google Scholar]
- 4.Abu-Arafeh I, Russell G. Prevalence of headache and migraine in schoolchildren. British Medical Journal. 1994;309:765–9. doi: 10.1136/bmj.309.6957.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Split W, Neuman W. Epidemiology of migraine among students from randomly selected secondary schools in Lodz. Headache. 1999;39:494–501. doi: 10.1046/j.1526-4610.1999.3907494.x. [DOI] [PubMed] [Google Scholar]
- 6.Abu-Arafeh I, Razak S, Sivaraman B, Graham C. Prevalence of headache and migraine in children and adolescents: a systematic review of population-based studies. Dev Med Child Neurol. 2010;52(12):1088–97. doi: 10.1111/j.1469-8749.2010.03793.x. Epub 2010/09/30. [DOI] [PubMed] [Google Scholar]
- 7.Stang PE, Osterhaus JT. Impact of migraine in the United States: Data from the National Health Interview Survey. Headache. 1993;33:29–35. doi: 10.1111/j.1526-4610.1993.hed3301029.x. [DOI] [PubMed] [Google Scholar]
- 8.Powers SW, Patton SR, Hommel KA, Hershey AD. Quality of life in paediatric migraine: Characterization of age-related effects using PedsQL 4.0. Cephalalgia. 2004;24(2):120–7. doi: 10.1111/j.1468-2982.2004.00652.x. [DOI] [PubMed] [Google Scholar]
- 9.Stang PE, Crown WH, Bizier R, Chatterton ML, White R. The family impact and costs of migraine. Am J Manag Care. 2004;10(5):313–20. Epub 2004/05/22. [PubMed] [Google Scholar]
- 10.Bille B. A 40-year follow-up of school children with migraine. Cephalalgia. 1997;17:488–91. doi: 10.1046/j.1468-2982.1997.1704488.x. [DOI] [PubMed] [Google Scholar]
- 11.Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA. 2003;290(18):2443–54. doi: 10.1001/jama.290.18.2443. Epub 2003/11/13. [DOI] [PubMed] [Google Scholar]
- 12.Silberstein SD, Rosenberg J. Multispecialty consensus on diagnosis and treatment of headache. Neurology. 2000;54:1553. doi: 10.1212/wnl.54.8.1553. [DOI] [PubMed] [Google Scholar]
- 13.Lewis D, Ashwal S, Hershey A, Hirtz D, Yonker M, Silberstein S. Practice Parameter: Pharmacological treatment of migraine headache in children and adolescents: Report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63(12):2215–24. doi: 10.1212/01.wnl.0000147332.41993.90. [DOI] [PubMed] [Google Scholar]
- 14.Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache. 2007;47(3):355–63. doi: 10.1111/j.1526-4610.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- 15.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–9. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 16.Svensson DA, Larsson B, Bille B, Lichtenstein P. Genetic and environmental influences on recurrent headaches in eight to nine-year-old twins. Cephalalgia. 1999;19(10):866–72. doi: 10.1046/j.1468-2982.1999.1910866.x. [DOI] [PubMed] [Google Scholar]
- 17.Hershey AD, Powers SW, Kabbouche MA, Burdine D, Glauser TA, Tang Y, et al. Genomic profiles in patients with chronic migraine: responders vs. non-responders. Cephalalgia. 2005;25(10):852–3. [Google Scholar]
- 18.Hershey AD, Burdine D, Kabbouche MA, Powers SW. Genomic expression patterns in medication overuse headaches. Cephalalgia. 2011;31(2):161–71. doi: 10.1177/0333102410373155. Epub 2010/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hershey A, Horn P, Kabbouche M, O'Brien H, Powers S. Genomic expression patterns in menstrual-related migraine in adolescents. Headache. 2012;52(1):68–79. doi: 10.1111/j.1526-4610.2011.02049.x. Epub 2012/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashkenazi A, Sholtzow M, Shaw JW, Burstein R, Young WB. Identifying cutaneous allodynia in chronic migraine using a practical clinical method. Cephalalgia. 2007;27(2):111–7. doi: 10.1111/j.1468-2982.2006.01255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashkenazi A, Silberstein S, Jakubowski M, Burstein R. Improved identification of allodynic migraine patients using a questionnaire. Cephalalgia. 2007;27(4):325–9. doi: 10.1111/j.1468-2982.2007.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123(Pt 8):1703–9. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- 23.Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001;41(7):629–37. doi: 10.1046/j.1526-4610.2001.041007629.x. [DOI] [PubMed] [Google Scholar]
- 24.Goadsby PJ. Migraine pathophysiology. Headache. 2005;45(Suppl 1):S14–24. doi: 10.1111/j.1526-4610.2005.4501003.x. [DOI] [PubMed] [Google Scholar]
- 25.Holroyd KA, O'Donnell FJ, Stensland M, Lipchik GL, Cordingley GE, Carlson BW. Management of chronic tension-type headache with tricyclic antidepressant medication, stress management therapy, and their combination: a randomized controlled trial. JAMA. 2001;285(17):2208–15. doi: 10.1001/jama.285.17.2208. Epub 2001/05/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tfelt-Hansen P, Block G, Dahlof C, Diener HC, Ferrari MD, Goadsby PJ, et al. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia. 2000;20(9):765–86. doi: 10.1046/j.1468-2982.2000.00117.x. Epub 2001/02/13. [DOI] [PubMed] [Google Scholar]
- 27.Hershey AD, Powers SW, Bentti A-L, deGrauw TJ. Standardized dosing of amitriptyline is highly effective in a pediatric headache center population. Headache. 1999;39:357–8. [Google Scholar]
- 28.Hershey AD, Powers SW, Bentti AL, Degrauw TJ. Effectiveness of amitriptyline in the prophylactic management of childhood headaches. Headache. 2000;40(7):539–49. doi: 10.1046/j.1526-4610.2000.00085.x. [DOI] [PubMed] [Google Scholar]
- 29.Lewis D, Winner P, Saper J, Ness S, Polverejan E, Wang S, et al. Randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of topiramate for migraine prevention in pediatric subjects 12 to 17 years of age. Pediatrics. 2009;123(3):924–34. doi: 10.1542/peds.2008-0642. Epub 2009/03/04. [DOI] [PubMed] [Google Scholar]
- 30.Winner P, Pearlman EM, Linder SL, Jordan DM, Fisher AC, Hulihan J. Topiramate for migraine prevention in children: a randomized, double-blind, placebo-controlled trial. Headache. 2005;45(10):1304–12. doi: 10.1111/j.1526-4610.2005.00262.x. [DOI] [PubMed] [Google Scholar]
- 31.Lakshmi CV, Singhi P, Malhi P, Ray M. Topiramate in the prophylaxis of pediatric migraine: a double-blind placebo-controlled trial. J Child Neurol. 2007;22(7):829–35. doi: 10.1177/0883073807304201. Epub 2007/08/24. [DOI] [PubMed] [Google Scholar]
- 32.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior rating inventory of executive function. Child neuropsychology : a journal on normal and abnormal development in childhood and adolescence. 2000;6(3):235–8. doi: 10.1076/chin.6.3.235.3152. Epub 2001/06/23. [DOI] [PubMed] [Google Scholar]
- 33.Kovacs M. Rating scales to assess depression in school-aged children. Acta paedopsychiatrica. 1981;46(5–6):305–15. Epub 1981/02/01. [PubMed] [Google Scholar]
- 34.Walker LS, Greene JW. The functional disability inventory: measuring a neglected dimension of child health status. J Pediatr Psychol. 1991;16(1):39–58. doi: 10.1093/jpepsy/16.1.39. Epub 1991/02/01. [DOI] [PubMed] [Google Scholar]
- 35.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta psychiatrica Scandinavica. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. Epub 1983/06/01. [DOI] [PubMed] [Google Scholar]
- 36.Varni JW, Seid M, Rode CA. The PedsQL: measurement model for the pediatric quality of life inventory. Med Care. 1999;37(2):126–39. doi: 10.1097/00005650-199902000-00003. Epub 1999/02/19. [DOI] [PubMed] [Google Scholar]
- 37.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambulatory pediatrics : the official journal of the Ambulatory Pediatric Association. 2003;3(6):329–41. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. Epub 2003/11/18. [DOI] [PubMed] [Google Scholar]
- 39.Mundt JC, Greist JH, Gelenberg AJ, Katzelnick DJ, Jefferson JW, Modell JG. Feasibility and validation of a computer-automated Columbia-Suicide Severity Rating Scale using interactive voice response technology. Journal of psychiatric research. 2010;44(16):1224–8. doi: 10.1016/j.jpsychires.2010.04.025. Epub 2010/06/18. [DOI] [PubMed] [Google Scholar]
- 40.Kosinski M, Bayliss MS, Bjorner JB, Ware JE, Jr., Garber WH, Batenhorst A, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003;12(8):963–74. doi: 10.1023/a:1026119331193. Epub 2003/12/04. [DOI] [PubMed] [Google Scholar]
- 41.Pearl W. Effects of gender, age, and heart rate on QT intervals in children. Pediatr Cardiol. 1996;17(3):135–6. doi: 10.1007/BF02505201. Epub 1996/05/01. [DOI] [PubMed] [Google Scholar]