Abstract
Until recently, reliable markers for adult stem cells have been lacking for many regenerative mammalian tissues. Lgr5 (leucine-rich repeat-containing G-protein coupled receptor 5) has been identified as a marker for adult stem cells in intestine, stomach, and hair follicle; Lgr5-expressing cells give rise to all types of cells in these tissues. Taste epithelium also regenerates constantly, yet the identity of adult taste stem cells remains elusive. In this study, we found that Lgr5 is strongly expressed in cells at the bottom of trench areas at the base of circumvallate and foliate taste papillae and weakly expressed in the basal area of taste buds and that Lgr5-expressing cells in posterior tongue are a subset of K14-positive epithelial cells. Lineage-tracing experiments using an inducible Cre knock-in allele in combination with Rosa26-LacZ and Rosa26-tdTomato reporter strains showed that Lgr5-expressing cells gave rise to taste cells, perigemmal cells, along with self-renewing cells at the bottom of trench areas at the base of circumvallate and foliate papillae. Moreover, using subtype-specific taste markers, we found that Lgr5-expressing cell progeny include all three major types of adult taste cells. Our results indicate that Lgr5 may mark adult taste stem or progenitor cells in the posterior portion of the tongue.
Keywords: Lgr5, Adult taste stem cell, progenitor, Taste bud, Lineage tracing
INTRODUCTION
The detection and identification of sapid chemicals in the oral cavity are largely mediated by taste receptor cells found in taste buds [1]. Taste buds contain ~50-100 elongated taste cells of three morphologically and functionally distinct subtypes: type I, type II, and type III. Taste bud cells constantly self-renew during an organism's life span, with an average turnover time of ~10-16 days [2-4]. Taste bud cell homeostasis in the adult is balanced by cell apoptosis and regeneration of new cells from a presumed stem cell population. However, the identity of adult taste stem cells is unknown. Studies using pulse-chase with 3H-thymidine, BrdU labeling, and lineage tracing with the basal epithelial marker K14 (keratin 14) have demonstrated that cells residing in the basal layer of the papillae are capable of giving rise to cells in taste buds [2, 5]. However, K14 is not specific to the taste epithelium because it also marks surrounding non-taste epithelium. Thus, whether there is a specific subpopulation of K14-positive epithelial cells in the vicinity of the taste buds that represents taste stem cells is an open question.
Recently, Lgr5 (leucine-rich repeat-containing G-protein coupled receptor 5) has been demonstrated to be a marker for adult stem cells in intestine, stomach, and hair follicle [6-8]. Lineage-tracing studies have demonstrated that Lgr5+ cells can produce all lineages of cells in these three tissues [6-8]. Remarkably, isolated single Lgr5+ cells from adult intestine and stomach can form organoids ex vivo, resembling the tissue architecture of the gut or stomach [6, 9]. In intestine, there are also Lgr5-negative stem cells that are positive for Bmi1 or mTert [10, 11]. Bmi1+ stem cells may give rise to Lgr5+ stem cells in intestine [10-13]. However, the exact relationship between these two pools of stem cells has not been settled.
We set out to determine if adult taste stem cells might be related to adult intestinal stem cells based on the known relatedness of taste receptor cells and gut endocrine cells that both express taste signaling proteins [14, 15] and intestinal hormones and transporters [16, 17] and because both have a taste-cell-like “paracrine” appearance [18]. We report here that Lgr5 is expressed in cells at the base of taste papillae of the posterior tongue. Using Lgr5-regulated reporter mice, we found that Lgr5+ cells can produce all three lineages of adult taste bud cells. Our findings suggest that Lgr5 marks adult taste stem/progenitor cells in the posterior tongue.
MATERIALS AND METHODS
Reverse transcription PCR
cDNA from circumvallate (CV) papilla, the surrounding non-taste (NT) epithelial tissue devoid of taste cells, and intestinal tissue (duodenum) was made using Clontech smart cDNA technology. Lgr5 primers for PCR are 5′ gtccacatgctcctgtcctt 3′ (forward) and 5′ agactctccagggtggcagt 3′ (reverse). The amplified fragment (983 bp) was confirmed by DNA sequencing. GAPDH was used as a control reference gene for the amount and quality of cDNA in reverse-transcription PCR (RT-PCR).
Lineage Tracing
Transgenic mice were obtained from Jackson Labs. Lgr5-EGFP-IRES-creERT2 mice [7] were crossed with Rosa26-LacZ [19] or Rosa26-tdTomato [20] mice to generate Lgr5+/-, Rosa26-LacZ+/- and Lgr5+/-, Rosa26-tdTomato+/- progeny. Genotyping used primer sets recommended by Jackson Labs. The expression of GFP further confirmed the genotype. Lineage-tracing experiments used 6- to 16-week-old male mice. For five injections at 1-day intervals (5 days of injections), we followed a procedure described previously (Okubo et al., 2009), with a slight modification of a daily intraperitoneal injection of 0.22 mg/g tamoxifen (Sigma, T5648) which was dissolved in sunflower seed oil (Sigma, S5007). For a single tamoxifen injection (1-day injection), 0.22 mg/g tamoxifen was injected intraperitoneally. At the end of 1-day or 5-day tamoxifen induction, mice were sacrificed after 1 day, 2 weeks, 1 month, 2 months, and 6 months. Lgr5+/-, Rosa26-LacZ+/-mice were given either 1-day or 5-days of tamoxifen injections, whereas all Lgr5+/-, Rosa26-tdTomato+/- mice were given just one tamoxifen injection (Table. S1). All experiments were performed under National Institutes of Health guidelines for the care and use of animals in research and approved by the Institutional Animal Care and Use Committee of the Monell Chemical Senses Center.
β-Galactosidase staining
Immediately after removal, the tongues and duodena from the tamoxifen-induced mice were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 2 h. After extensive washes with PBS, tissues were immersed in 30% sucrose overnight and embedded in OCT. Sections 10 μm thick were stained in X-gal solution (5 mM potassium ferrocyanide, 5 mM potassium ferricyanide, 2 mM MgCl, 0.02% NP-40, 0.1% cholate, 2 mg/ml X-gal) overnight at room temperature and counterstained with nuclear fast red.
Immunohistochemistry
For co-imaging taste cell markers and β-galactosidase or intrinsic tdTomato fluorescence, we used mice that were sacrificed 1-2 months after tamoxifen induction. Tissues were processed and sectioned as above. To detect β-galactosidase, we used a goat anti-β-galactosidase antibody (Biotrend; 1:500) and anti-goat secondary antibody Alexa-Fluo 555 and then treated tissues with the following specific primary antibodies against taste cell markers and secondary antibodies: type I taste cells, rabbit anti-NTPDase2 (nucleoside triphosphate diphosphohydrolase-2; Centre de Recherche du CHUL; 1:500); type II taste cells, guinea pig anti-Trpm5 (transient receptor potential cation channel subfamily M member 5; gift from Dr. Emily Liman; 1:500); and type III taste cells, anti-5-HT (serotonin; Immunostar; 1:100). For serotonin detection, mice were injected with 5-HTP (Sigma) 1 h before being sacrificed to allow detection of 5-HT in type III cells [21]. Species-specific Alexa-Fluo 647-conjugated secondary antibodies were used to visualize specific taste cell markers. For co-labeling of Lgr5-GFP with proliferation biomarkers, a rabbit anti-Ki67 antibody (Novus; 1:100) or a mouse monoclonal antibody against K14 (Developmental Studies Hybridoma Bank; 1:100) was used with paraffin-embedded tissues after antigen retrieval. A chicken anti-GFP antibody (Abcam; 1:500) was used to detect Lgr5-GFP. A goat polyclonal antibody against KCNQ1 (Santa Cruz; 1:500) was used to visualize taste bud cells. Appropriate secondary antibodies were applied accordingly. Secondary antibodies alone were used as negative controls. To visualize the nuclei, 4′,6-diamidino-2-phenylindole (DAPI)-containing mounting medium (Vector Laboratories) was used to cover the tissue sections. All images were acquired by either a Nikon Eclipse E800 microscope or Leica Sp2 confocal microscope. Confocal images were compressed z-stacks, and single optical sections gave rise to the same results as the z-stacks.
RESULTS
Lgr5 is selectively expressed in taste tissue of the posterior tongue
We used reverse-transcription PCR to determine if mRNA of the intestinal stem cell marker Lgr5 is also expressed in adult taste tissue. cDNA was prepared from circumvallate (CV) papilla tissue as well as “non-taste” (NT) lingual epithelium devoid of taste cells. Using exon-spanning primers, an Lgr5 transcript was amplified from cDNA from CV papilla and intestinal tissue but not from NT cDNA (Fig. 1A), indicating that Lgr5 is selectively expressed in taste tissue but not in the surrounding epithelium.
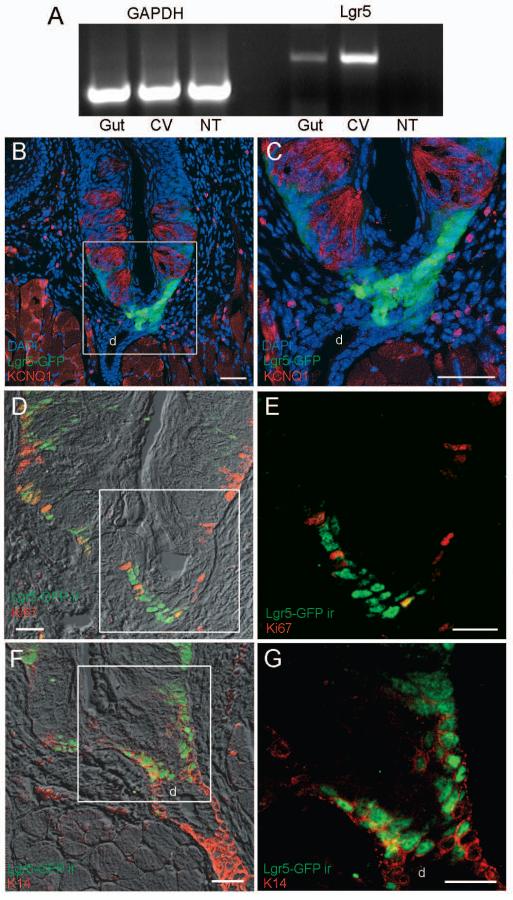
Fig. 1. Lgr5 is expressed in circumvallate papilla.
A) RT-PCR demonstrates Lgr5 expression in gut (positive control) and circumvallate (CV) papilla taste tissue but not in the surrounding non-taste (NT) epithelium. The GAPDH RT-PCR controls confirm equivalent template amounts from gut, CV, and NT cDNA. B-G) Confocal images of Lgr5+ cells in CV papilla. B and C) The Lgr5-GFP transgene was detected by intrinsic fluorescence (green). The strongest GFP signal is at the bottom of the trench area below the CV papilla and adjacent to the opening of the ducts of Von Ebner's glands. A weaker GFP signal is found in cells at the base of the taste buds immediately below and between the mature taste cells (red), which were immunodetected with a KCNQ1 antibody. D and E) Dual immunostaining shows that only some Lgr5+ cells (green, anti-GFP antibody) at the bottom of the CV trench area are also immunoreactive for proliferation marker Ki67 (red, anti-Ki67). F and G) Dual immunostaining shows that all Lgr5+ cells (green, anti-GFP) at the bottom of the CV trench area are also immunoreactive for K14 (red, anti-K14). All images are compressed confocal z-stacks (~5μm thickness). d, duct of Von Ebner's gland. Scale bars: B=80 μm, C=10 μm, and D-G=20μm.
To determine which types of tongue epithelial cells express Lgr5, we used heterozygous mice with one wild-type Lgr5 allele and one allele in which GFP (green fluorescent protein) has been inserted into the Lgr5 gene (a knock-out/knock-in strain) [7]. Thus, GFP serves as a surrogate marker for Lgr5 expression. The fidelity of the Lgr5-GFP knock-in reporter has been validated in multiple tissues [6-8]. Strong GFP signals were detected in cells in the bottom of the trench area below the CV papilla and adjacent to the opening of the ducts of Von Ebner's glands (Fig. 1B,C; Fig. S1), as well as below the foliate papillae and adjacent to the opening of ducts there (Fig. S2). Less intense GFP signals were detected at the base of taste buds of the CV (Fig. 1B,C) and foliate (Fig. S2) papilla immediately below and surrounding the mature taste bud cells marked by expression of the voltage-gated potassium channel KCNQ1 or Trpm5. No GFP signal was detected in other portions of Von Ebner's glands or in tongue epithelium devoid of taste tissue. Furthermore, no GFP signal was detected in the fungiform papillae or soft palate, therefore, we focused on analysis of the Lgr5 expressing cells in posterior tongue.
Next, we determined if Lgr5-GFP-positive cells (referred to hereafter as “Lgr5+”) are capable of proliferating in taste tissue like Lgr5+ cells in the small and large intestine [7]. Using Ki67 as a cell proliferation marker to identify all actively dividing cells, we observed that some green Lgr5+ cells at the trench below the CV papilla and at the base of CV taste buds also displayed red Ki67 immunoreactivity (yellow-orange cells in Fig. 1D). However, many of the Lgr5+ cells in the trench area were Ki67 negative (green cells in Fig. 1D,E), indicating that most of these Lgr5+ cells are not actively dividing. The non-uniform Ki67 expression in these Lgr5+ cells suggests that there are at least two pools of Lgr5+ cells in taste tissue: Ki67-negative quiescent and Ki67-positive actively cycling cells.
K14 is expressed in basal epidermal cells in taste tissue as well as the surrounding epithelium in the tongue, and K14+ cells have been shown to give rise to the taste bud cells and surrounding keratinocytes in gustatory tissues [5]. If Lgr5 marks adult taste stem or progenitor cells, we would expect Lgr5+ cells to constitute a subset of K14+ epithelial cells. Double immunostaining demonstrated that almost all Lgr5+ cells at the base of the CV papilla also expressed K14 (Fig. 1F,G), and very few Lgr5+ cells appeared to have weak or no K14 immunoreactivity. Interestingly, expression of K14 was stronger in the cells in the trench below the CV papilla than in the basal regions of the taste buds at the base of the CV papilla (Fig. 1F,G), similar to the pattern observed with Lgr5-GFP (Fig. 1B).
Lineage tracing of Lgr5+ cells in CV papilla
By definition, adult stem cells give rise to multiple types of terminally differentiated cells. We used an established lineage-tracing protocol [7] to identify terminally differentiated taste cells derived from Lgr5+ cells. Lgr5-EGFP-IRES-creERT2 mice were crossed with Rosa26-LacZ mice to generate Lgr5+/-, Rosa26-LacZ+/- mice in which tamoxifen-induced Cre generates β-galactosidase activity to mark cells from the Lgr5+ lineage. We examined β-galactosidase+ cells at different time points after tamoxifen induction. Two injection regimens (5 days of injections vs. a single 1-day injection) were used for lineage tracing. Because of the expectation of a low stochastic probability of inducing Cre in cells in taste tissue by tamoxifen, as well as the previous results of Okubo et al. (2009) [5] with K14-creERT tracing studies, we first injected tamoxifen five times during a span of 5 days. Mice were sacrificed at different time points to determine if β-galactosidase activity was present only in those Lgr5+ cells strongly expressing GFP in the trench area and/or weakly expressing GFP at the base of CV papilla taste buds. If β-galactosidase is detected in intragemmal taste bud cells that do not express Lgr5, then the results provide strong evidence that Lgr5+ cells in CV tissue can act as stem cells to give rise to other types of cells.
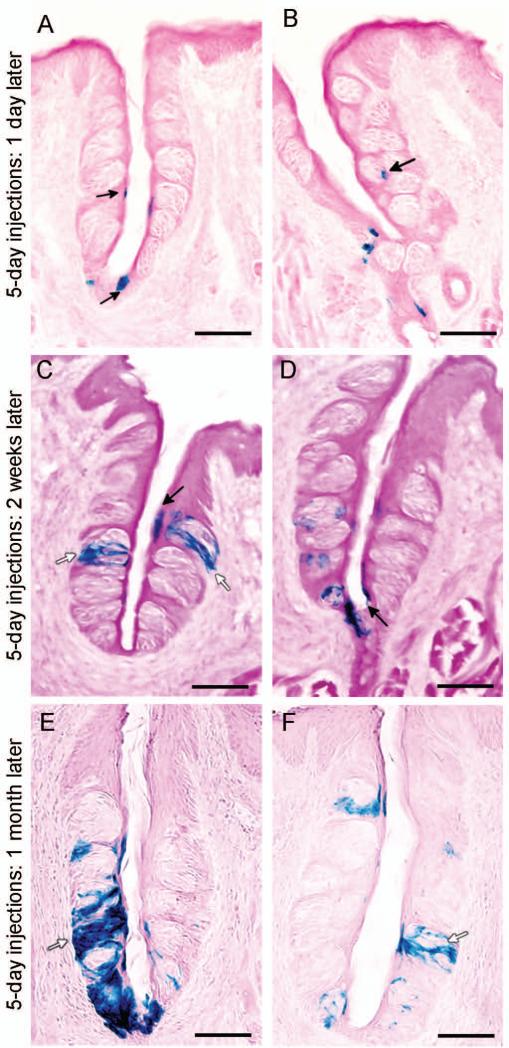
One day after the 5-day tamoxifen induction, β-galactosidase+ cells were readily detected in the CV papilla at the bottom of the trench area and in basal layers and the pore region of taste buds (Fig. 2A,B). Importantly, at this time point we rarely found β-galactosidase+ intragemmal cells (i.e., cells within taste buds; Fig. 2A,B). However, 2 weeks after the five-injection regimen, more β-galactosidase+ cells were found within and surrounding the taste buds, including intragemmal cells with typical taste cell morphology, as well as perigemmal cells (Fig. 2C,D). Thus, Lgr5+ cells can give rise to multiple cell types both within the taste buds and in the immediately surrounding epithelium. One month after tamoxifen induction, we detected even more β-galactosidase+ intragemmal cells within taste buds (Fig. 2E,F). Importantly, cells at the bottom of the trench area and at the base of the taste buds remained β-galactosidase+, indicating that the Lgr5+ cells here are capable of self-renewing. β-Galactosidase+ taste bud cells could be detected even 6 months after the 5-day tamoxifen induction (Fig. S3). In contrast, we never detected any β-galactosidase+ cells within the surrounding NT epithelium or in glandular tissue, indicating that the Lgr5+ cells generate only taste cells and perigemmal cells in taste tissue. No β-galactosidase+ staining was detected in fungiform papillae taste tissue. Duodena were used as positive controls for lineage tracing of Lgr5+ intestinal stem cells (data not shown) [7]. As a negative control, no β-galactosidase+ activity was found in littermates with an Lgr5+/+, Rosa26-LacZ+/- genotype.
Fig. 2. Lgr5-expressing cells give rise to mature taste cells.
Tamoxifen-induced Lgr5-Cre generates β-galactosidase activity to mark cells from the Lgr5+ lineage. A and B) Representative images of β-galactosidase-stained taste cells (blue) of the Lgr5+ lineage present in the trench at the base of the circumvallate papilla, at the taste pore, and at the basal layer of the taste buds (arrows), one day after 5-days of tamoxifen induction of Lgr5-Cre. C and D) β-Galactosidase-stained taste cells (blue) of the Lgr5+ lineage mark both intragemmal (within the taste bud; white arrows) and perigemmal (surrounding the taste bud; black arrows) cells, two weeks after 5-days of tamoxifen induction of Lgr5-Cre. E and F) β-Galactosidase-stained taste cells (blue) of the Lgr5+ lineage mark many intragemmal taste cells (white arrows), one month after 5-days of tamoxifen induction of Lgr5-Cre. Scale bars: A-F=40 μm.
We also performed a single tamoxifen injection and then examined β-galactosidase expression 1 day later. As expected, the induction efficiency from a single tamoxifen injection was low; however, we could detect a few β-galactosidase+ cells predominantly in the bottom of the trench area and adjacent to the associated duct (Fig. S4), presumably due to the higher expression level of Lgr5 in the bottom of the trench area. Examination of β-galactosidase+ cells from single injections at 2 weeks, 1 month, and 2 months gave patterns of staining similar to those observed with the 5-day injection regimen, albeit with fewer total cells labeled in the CV papilla (Fig. S4) or in the foliate papilla (Fig. S5). Altogether, these data indicate that the Lgr5+ cells give rise to intragemmal and perigemmal cells as well as cells in the pore region.
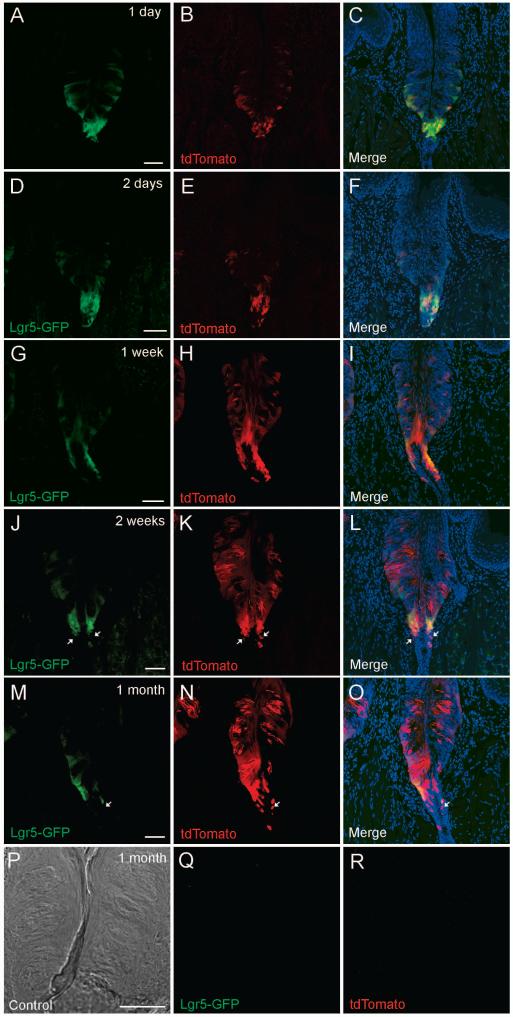
After a long lineage tracing period some cells in the trench area below the CV papilla of Lgr5+/-, Rosa26-LacZ+/- mice are β-galactosidase+ (Fig. 2E & S3). However, due to the architecture of the trench area and the associated duct, we cannot reliably determine if these β-galactosidase+ cells are also Lgr5-EGFP+. To address this question, Lgr5-EGFP-IRES-creERT2 mice were crossed with Rosa26-tdTomato mice to generate Lgr5+/-, Rosa26-tdTomato+/- mice in which tamoxifen-induced Cre generates expression of tdTomato fluorescence protein (red) to mark cells from the Lgr5+ lineage, allowing us to directly visualize (a) Lgr5+ singly positive cells (EGFP green but not induced for tdTomato), (b) Lgr5+/tdTomato+ doubly positive cells (green and red in respective channels and yellow or orange in merged image) and (c) tdTomato+ singly positive cells (red, representing progeny of the Lgr5+ lineage that have been induced to express CRE and tdTomato but no longer express Lgr5+). Our lineage tracing data (1 injection) using Lgr5+/-, Rosa26- tdTomato+/- mice gave similar results to our LacZ tracing data, albeit with much higher induction efficiency and improved resolution. One day or two days after a single tamoxifen injection, we detected induced tdTomato red fluorescent cells among both the strong Lgr5+ cells at the bottom of the trench area and the weak Lgr5+ cells in the base of taste buds, indicating that both subpopulations of Lgr5+ cells can be induced by tamoxifen (Fig. 3A-F). At this short time after induction we rarely detected cells showing tdTomato red fluorescence without also displaying EGFP green fluorescence. One week (Fig. 3G-I) after a single tamoxifen induction, a few intragemmal taste cells showed intrinsic tdTomato red fluorescence. Two weeks (Fig. 3J-L) and one month (Fig. 3M-O) after tamoxifen induction (1 injection), many intragemmal taste cells showed intrinsic tdTomato red fluorescence of varying intensity, but never with EGFP green fluorescence, indicating that these cells are progeny of Lgr5+ cells. Interestingly, many Lgr5+ cells were also tdTomato+ even long after the induction, indicating that they may be capable of self-renewal. At the bottom of the trench area and in the immediately adjacent ducts we observed a few cells 2 weeks or 1 month after induction that were Tdtomato+ but Lgr5- (Fig. 3J-O), suggesting that these cells may be progeny of the neighboring strong Lgr5+ cells at the bottom of the trench area. No green or red fluorescence was found in littermates with an Lgr5+/+, Rosa26-tdTomato+/- genotype 1 month after a single tamoxifen injection (Fig. 3P-R).
Fig. 3. Lgr5-expressing cells give rise to mature taste cells and cells in the associated ducts.
Tamoxifen-induced Lgr5-Cre generates tdTomato fluorescent protein (red) to mark cells from the Lgr5+ lineage. Representative confocal images of the circumvallate papilla, one day (A-C), two days (D-F), one week (G-I), two weeks (J-L) and one month (M-O) after a single tamoxifen induction of Lgr5-Cre. Images in the left column (A, D, G, J, M) show Lgr5-GFP intrinsic fluorescence (green) at the indicated times. Images in the middle column (B, E, H, K, N) show tdTomato+ (red) cells. The right column (C, F, I, L, O) shows the merged images. Note that increasing numbers of tdTomato+ intragemmal taste cells and perigemmal cells are generated over time after a single tamoxifen induction. At all dates examined after a single tamoxifen induction many Lgr5-GFP+ cells are also tdTomato+ in both the trench area and at the base of the taste buds. A few cells (white arrows) that are tdTomato+ but not Lgr5-GFP+ are present in the ductal area under the trench two weeks or 1 month after a single tamoxifen induction. P-R) A transmitted light image of a circumvallate section (P) of an Lgr5+/+, Rosa26-tdTomato+/- mouse (negative control littermate 1 month after a single tamoxifen induction) shows no EGFP (Q) or tdTomato (R) fluorescence when scanned at the same confocal settings. All images are compressed confocal z-stacks (~8μm thickness). Scale bar: 20 μm.
Lgr5+ cells give rise to multiple types of mature taste bud cells
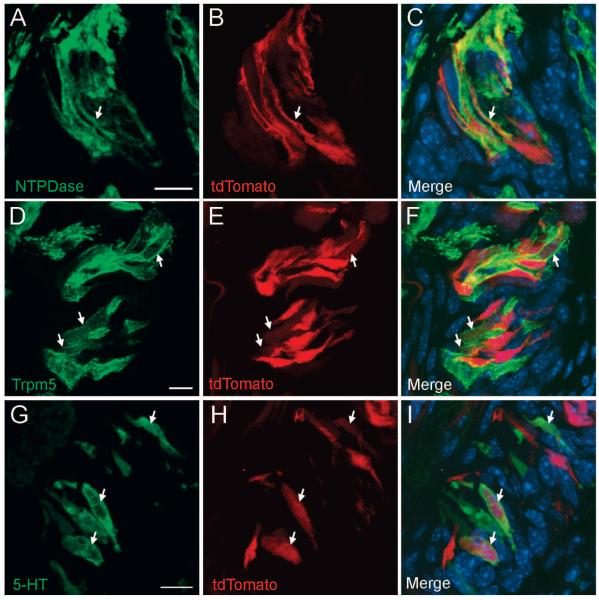
Our data indicate that Lgr5+ cells are the adult stem or progenitor cell source for at least some mature taste cells. A key question is whether the Lgr5+ cells are multipotent stem cells in the parental lineages of all types of mature taste bud cells or unipotent or oligopotent progenitor cells that give rise to one or only a few taste bud lineages. Thus, we determined whether the Lgr5+ cells generated tdTomato+ or β-galactosidase+ taste cells that also express specific markers of all three taste cell subtypes. Type I taste cells are supporting cells that can be marked with NTPDase2 (nucleoside triphosphate diphosphohydrolase-2). Type II taste receptor cells respond to sweet, bitter, and umami tastants and express Trpm5. Type III taste receptor cells respond to sour and salty stimuli and can be marked by their expression of serotonin (5-HT) [1, 22]. At 1 month after tamoxifen induction, we simultaneously visualized intrinsic tdTomato fluorescence and immunostained with individual taste-type-specific antibodies. From this set of experiments, we observed type I (NTPDase2+), type II (Trpm5+), and type III (serotonin+) taste cells that were also tdTomato+ (Fig. 4). Comparable results were obtained using Lgr5+/-, Rosa26-LacZ+/- mice at 1-2 months after tamoxifen induction (Fig. S6). Mature taste cells were generated even 6 months after the 5-day tamoxifen induction (Fig. S7). As noted above Lgr5+ cells give rise also to perigemmal cells, but no cells within the surrounding NT epithelium or in glandular tissue. These results are consistent with Lgr5 marking adult taste stem cells and less consistent with marking more limited progenitor cells.
Fig. 4. Lgr5+ stem/progenitor cells generate all three types of taste bud cells.
Mature taste bud cells marked by Lgr5-Cre-generated tdTomato 1 month after tamoxifen induction were stained with markers for each of the three types of taste cells. A-C) tdTomato (red) is co-expressed in type I taste cells with NTPDase2 (pseudo-colored green). D-F) Lgr5-Cre-generated tdTomato (red) is co-expressed in type II taste receptor cells with Trpm5 (pseudo-colored green). G-I) Lgr5-Cre-generated tdTomato (red) is co-expressed in type III taste receptor cells with serotonin (pseudo-colored green). Arrows show colocalization of tdTomato and taste specific markers. All images are compressed confocal z-stacks (~3 μm thickness). Scale bars: 10 μm.
DISCUSSION
In the present study we found that the stem cell marker Lgr5 is expressed in taste tissue of the posterior tongue where it marks apparent adult taste stem cells that give rise to all three subtypes of mature intragemmal taste cells along with perigemmal cells. Lgr5+ cells of the tongue consist of at least two populations of cells in the circumvallate and foliate papillae regions. The strongly positive Lgr5+ cells sit deep in the trench of the CV and foliate papillae, beneath the taste buds and at the openings of glandular ducts and less intensely positive Lgr5 cells sit at the base of taste buds. It seems plausible that the strong Lgr5+ cells in the trench area may give rise to the weakly positive Lgr5 cells at the base of taste buds, which in turn may give rise to mature taste cells and perigemmal cells. The weakly positive cells may be more constrained progenitor cells whereas the strongly positive cells seem better candidates to be multipotent taste stem cells. We think it less likely that the weakly positive cells give rise to the strongly positive ones or that they comprise two independent populations. Definitively determining the relatedness of these different populations, assessing their multipotency and determining which one(s) are immediate precursors of mature taste cells will require ex vivo organ cultures as have been used for Lgr5+ stems cells of gut.
Lgr5 is a Wnt target gene; the G protein-coupled receptor Lgr5 has been shown to mark stem cells in multiple regenerative tissues [6-9]. Lgr5 and its homologs interact with R-spondins to augment Wnt signaling [23-25]. Conventional deletion of the Lgr5 gene results in mice with neonatal lethality associated with craniofacial defects [26]. Conditional knockout mice lacking Lgr5 in gut are viable and do not show any severe phenotype [24]. Gut-specific deletion of both Lgr5 and Lgr4 genes results in a severe loss of crypt base columnar cells as well as altered morphology of gut tissue, indicating that these two receptors, presumably acting together, are essential for gut regeneration and maintenance [24].
Taste cells undergo frequent turnover and renewal. It is well established that taste cells are derived from the local epithelium and that basal cells are capable of dividing and can give rise to other cells within taste buds [2, 5, 27, 28]. Two contrasting models have been proposed for taste cell renewal [29]. One model proposes that each taste cell lineage is derived from type-specific progenitor cells; that is, type I progenitor cells give rise to mature type I cells but not other types of taste cells, and so on. Another model proposes that a single progenitor cell gives rise to all types of mature taste cell lineages. Our lineage-tracing data show that all three subtypes of taste bud cells are derived from Lgr5+ cells. However, it remains to be determined if a single Lgr5+ cell can give rise to all three types of taste cells or if there are different subpopulations of Lgr5+ cells that serve as progenitors of each type of taste cell. Ex vivo organ cultures as have been used for Lgr5+ stems cells of gut may also help in this regard [9].
A similar lineage-tracing approach using K14-creERT mice demonstrated that K14+ epithelial cells produce intragemmal taste cells [5]. However, it has not been determined if K14+ cells give rise to all three major subtypes of taste cells. In addition, K14 is a general epithelial marker not specific to the taste tissue, so it is challenging to precisely localize any long-term stem or progenitor cells for taste tissue from K14+ cells. Nevertheless, it has been proposed that K14+ cells in the basal region but outside of the taste bud are candidate adult taste stem/progenitor cells. Our study revealed that the Lgr5+ cells at the bottom of the CV and foliate papillae and at the base of taste buds that are candidates for adult taste stem cells or progenitor cells of the posterior tongue also express K14. However, there are also strongly positive K14+ cells deeper in the ducts below the posterior taste papillae that do not express Lgr5.
Unlike intestinal Lgr5+ stem cells, the niche for maintaining Lgr5+ taste stem/progenitor cells appears to be neuronally dependent. In nerve section experiments, CV taste buds disappear in the first few weeks following denervation but regenerate following nerve regrowth [30]. One hypothesis is that taste stem/progenitor cells require signals from the innervating nerves to give rise to newborn cells in the taste buds and maintain tissue homeostasis. An alternative explanation is that Lgr5+ stem/progenitor cells give rise to fate-committed cells that are nerve dependent and require neuronal signals to develop into terminally differentiated taste cells. Using a similar lineage-tracing approach in combination with nerve section may distinguish between these two possibilities.
In the small intestine of mice Bmi1 labels another population of stem cells distinct from the Lgr5+ stem cells [11, 12]. Given the similarity between taste and gut tissues it is plausible that Bmi1 is expressed also in adult taste stem cells. However, our preliminary analysis of taste tissue from mice carrying Bmi1-Cre [11] and the Rosa26-LacZ allele after tamoxifen injection indicated that Bmi1 does not mark adult taste stem cells (data not shown). Thus, in contrast to its role in small instestine, Bmi1 does not appear to mark adult taste stem cells.
Wnt signaling plays a major role in fungiform taste papillae formation prenatally [31, 32], and this Wnt activity appears to be regionally specific during embryonic development. Genetic deletion of Lef1 (lymphoid enhancer binding factor 1) left mice with a greatly reduced number of fungiform papillae but relatively intact CV papilla [31]. Furthermore, our findings that Lgr5+ cells are candidate taste stem/progenitor cells in the posterior tongue but not in the anterior tongue indicates that developmental regulation of taste tissue in the anterior and posterior parts of tongue differs in this regard also. This is consistent with studies indicating that the posterior third of the tongue is derived from endoderm, whereas the anterior two-thirds of the tongue is derived from stomadeal ectoderm [33]. However, it is also plausible that our inability to detect Lgr5-GFP cells in the anterior portion of the tongue could be due to weak expression of Lgr5-GFP beyond detection in the fungiform papillae or regional silencing of the knockin allele in this particular Lgr5-EGFP strain [34]. It remains to be determined whether Lgr5 or other biomarkers identify adult taste stem cells in the taste buds of the fungiform papillae, which also undergo continual turnover.
Supplementary Material
Supplemental Fig. S1. Lgr5 expression in circumvallate papilla.
Confocal images showing that the strongest-staining Lgr5-GFP cells (green) are adjacent to the opening of the duct of Von Ebner's gland below the circumvallate papilla. A weaker Lgr5-GFP signal is found in the basal cells or perigemmal cells at the base or just outside of the taste buds. Type II taste receptor cells were identified by anti-Trpm5 immunoreactivity (red). DAPI (blue) staining was used to visualize nuclei. d, duct of Von Ebner's gland. The square in (A) corresponds to the area shown at higher magnification in (B). Scale bars: a=80 μm; b=40 μm.
Supplemental Fig. S2. Lgr5 expression in foliate papillae.
(A) Transmitted light image of foliate papillae. (B) Corresponding confocal image showing Lgr5-GFP cells (green) most strongly expressed in the trench area below the foliate papillae. A weaker Lgr5-GFP signal is found in the basal cells or perigemmal cells at the base or just outside of the taste buds. Type II taste receptor cells were identified by anti-Trpm5 immunoreactivity (red). d, duct of Von Ebner's gland. Scale bars: 40 μm.
Supplemental Fig. S3. Lgr5-expressing cells continue to generate mature taste bud cells 6 months after induction.
(A and B) β-Galactosidase+ cells from the Lgr5+ lineage are found in circumvallate papilla taste bud cells as well as in perigemmal cells (white arrows) 6 months after 5-days of tamoxifen induction of Lgr5-Cre. In addition, β-galactosidase+ cells are found in the trench area at the opening of the Von Ebner's gland (black arrow). (C) 6 months after 5 days of tamoxifen induction many β-galactosidase-stained mucosal cells of the Lgr5+ lineage are present in crypts and villi of the small intestine. d, duct of Von Ebner's gland. Scale bars: 40 μm.
Supplemental Fig. S4. Lgr5-expressing cells generate mature taste cells after a single tamoxifen injection.
(A, B) One day after 1-day single tamoxifen injection, a small number of β-galactosidase-stained taste cells (blue) of the Lgr5+ lineage are present only at the bottom of the trench area (black arrows). β-Galactosidase+ cells from the Lgr5+ lineage are found in circumvallate papilla taste bud cells (white arrows) as well as in perigemmal cells (arrowheads) 2 weeks (C, D) and 1 month (E, F) after a single tamoxifen injection. Scale bars: 20 μm.
Supplemental Fig. S5. Lgr5-expressing cells generate mature taste cells in the foliate papillae 1 month after a single tamoxifen injection.
Tamoxifen-induced Lgr5-Cre generates β-galactosidase activity to mark cells from the Lgr5+ lineage. Intragemmal and perigemmal β-galactosidase+ cells (blue) are found in foliate papillae taste buds (arrows). A and B are two representative sections. Scale bars: 20 μm.
Supplemental Fig. S6. Lgr5+ stem cells generate all three types of taste cells.
Mature taste bud cells marked by Lgr5-Cre-generated β-galactosidase 1-2 months after tamoxifen induction were double stained with anti-β-galactosidase and markers for each of the three types of taste cells. A-D) β-Galactosidase immunoreactivity (green) is co-expressed in type I taste cells with NTPDase2 (red), two months after a single tamoxifen induction. E-H) Lgr5-Cre-generated β-galactosidase immunoreactivity (green) is co-expressed in type II taste receptor cells with Trpm5 (red), 2 months after 5-days of tamoxifen injections. I-L) Lgr5-Cre-generated β-galactosidase immunoreactivity (green) is co-expressed in type III taste receptor cells with serotonin (red), six weeks after a single tamoxifen injection. All sections are compressed Z-stacks (4-10 μm thickness). Scale bars: A, E, and I=20 μm; B-D, F-H, and J-L=8 μm.
Supplemental Fig. S7. Mature taste cells generated from Lgr5-expressing cells 6 months after induction.
Lgr5-Cre-generated β-galactosidase immunoreactivity (green) generated 6 months after induction by 5-days of tamoxifen is co-expressed with Trpm5 (red) in type II taste receptor cells. Asterisks indicate colocalization of β-galactosidase and Trpm5. (A and B) show two representative dual-stained sections. Scale bars: 20 μm.
Acknowledgements
The authors thank Drs. Hong Wang, Ichiro Matsumoto, and Gary K. Beauchamp for critically reading the manuscript. This work was supported by NIH grants DC0101842 (P.J.), DK081421 (R.F.M.), and DC003055 (R.F.M.) and a grant from the Commonwealth of Pennsylvania Department of Health (P.J.); imaging was performed at the Monell Histology and Cellular Localization Core, which is supported in part by funding from NIH-NIDCD core grant 1P30DC011735-01. The antibody against Trpm5 was a gift from Dr. Emily Liman.
Footnotes
Author Contributions: K.K.Y.: data collection and analysis; Y.L.: data collection and analysis; K.M.R: data collection; K.I.: provision of study material; R.F.M: conception and design, manuscript writing; P.J.: conception and design, data collection and analysis, manuscript writing.
Competing Interests: Authors declare no conflict of interest.
REFERENCES
- 1.Bachmanov AA, Beauchamp GK. Taste receptor genes. Annu Rev Nutr. 2007;27:389–414. doi: 10.1146/annurev.nutr.26.061505.111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beidler LM, Smallman RL. Renewal of cells within taste buds. J Cell Biol. 1965;27:263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohn ZJ, Kim A, Huang L, et al. Lipopolysaccharide-induced inflammation attenuates taste progenitor cell proliferation and shortens the life span of taste bud cells. BMC Neurosci. 2010;11:72. doi: 10.1186/1471-2202-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamamichi R, Asano-Miyoshi M, Emori Y. Taste bud contains both short-lived and long-lived cell populations. Neuroscience. 2006;141:2129–2138. doi: 10.1016/j.neuroscience.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 5.Okubo T, Clark C, Hogan BL. Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells. 2009;27:442–450. doi: 10.1634/stemcells.2008-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 8.Jaks V, Barker N, Kasper M, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 9.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery RK, Carlone DL, Richmond CA, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian H, Biehs B, Warming S, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan KS, Chia LA, Li X, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109:466–471. doi: 10.1073/pnas.1118857109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104:15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shin YK, Martin B, Golden E, et al. Modulation of taste sensitivity by GLP-1 signaling. J Neurochem. 2008;106:455–463. doi: 10.1111/j.1471-4159.2008.05397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yee KK, Sukumaran SK, Kotha R, et al. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci U S A. 2011;108:5431–5436. doi: 10.1073/pnas.1100495108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujita T. Taste cells in the gut and on the tongue. Their common, paraneuronal features. Physiol Behav. 1991;49:883–885. doi: 10.1016/0031-9384(91)90198-w. [DOI] [PubMed] [Google Scholar]
- 19.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 20.Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim DJ, Roper SD. Localization of serotonin in taste buds: a comparative study in four vertebrates. J Comp Neurol. 1995;353:364–370. doi: 10.1002/cne.903530304. [DOI] [PubMed] [Google Scholar]
- 22.Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carmon KS, Gong X, Lin Q, et al. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2011;108:11452–11457. doi: 10.1073/pnas.1106083108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Lau W, Barker N, Low TY, et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature. 2011;476:293–297. doi: 10.1038/nature10337. [DOI] [PubMed] [Google Scholar]
- 25.Glinka A, Dolde C, Kirsch N, et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/beta-catenin and Wnt/PCP signalling. EMBO Rep. 2011;12:1055–1061. doi: 10.1038/embor.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morita H, Mazerbourg S, Bouley DM, et al. Neonatal lethality of LGR5 null mice is associated with ankyloglossia and gastrointestinal distension. Mol Cell Biol. 2004;24:9736–9743. doi: 10.1128/MCB.24.22.9736-9743.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barlow LA, Northcutt RG. Embryonic origin of amphibian taste buds. Dev Biol. 1995;169:273–285. doi: 10.1006/dbio.1995.1143. [DOI] [PubMed] [Google Scholar]
- 28.Stone LM, Finger TE, Tam PP, et al. Taste receptor cells arise from local epithelium, not neurogenic ectoderm. Proc Natl Acad Sci U S A. 1995;92:1916–1920. doi: 10.1073/pnas.92.6.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone LM, Tan SS, Tam PP, et al. Analysis of cell lineage relationships in taste buds. J Neurosci. 2002;22:4522–4529. doi: 10.1523/JNEUROSCI.22-11-04522.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miura H, Barlow LA. Taste bud regeneration and the search for taste progenitor cells. Arch Ital Biol. 2010;148:107–118. [PMC free article] [PubMed] [Google Scholar]
- 31.Iwatsuki K, Liu HX, Gronder A, et al. Wnt signaling interacts with Shh to regulate taste papilla development. Proc Natl Acad Sci U S A. 2007;104:2253–2258. doi: 10.1073/pnas.0607399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu F, Thirumangalathu S, Gallant NM, et al. Wnt-beta-catenin signaling initiates taste papilla development. Nat Genet. 2007;39:106–112. doi: 10.1038/ng1932. [DOI] [PubMed] [Google Scholar]
- 33.Rothova M, Thompson H, Lickert H, et al. Lineage tracing of the endoderm during oral development. Dev Dyn. 2012;241:1183–1191. doi: 10.1002/dvdy.23804. [DOI] [PubMed] [Google Scholar]
- 34.Barker N, van Oudenaarden A, Clevers H. Identifying the stem cell of the intestinal crypt: strategies and pitfalls. Cell Stem Cell. 2012;11:452–460. doi: 10.1016/j.stem.2012.09.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig. S1. Lgr5 expression in circumvallate papilla.
Confocal images showing that the strongest-staining Lgr5-GFP cells (green) are adjacent to the opening of the duct of Von Ebner's gland below the circumvallate papilla. A weaker Lgr5-GFP signal is found in the basal cells or perigemmal cells at the base or just outside of the taste buds. Type II taste receptor cells were identified by anti-Trpm5 immunoreactivity (red). DAPI (blue) staining was used to visualize nuclei. d, duct of Von Ebner's gland. The square in (A) corresponds to the area shown at higher magnification in (B). Scale bars: a=80 μm; b=40 μm.
Supplemental Fig. S2. Lgr5 expression in foliate papillae.
(A) Transmitted light image of foliate papillae. (B) Corresponding confocal image showing Lgr5-GFP cells (green) most strongly expressed in the trench area below the foliate papillae. A weaker Lgr5-GFP signal is found in the basal cells or perigemmal cells at the base or just outside of the taste buds. Type II taste receptor cells were identified by anti-Trpm5 immunoreactivity (red). d, duct of Von Ebner's gland. Scale bars: 40 μm.
Supplemental Fig. S3. Lgr5-expressing cells continue to generate mature taste bud cells 6 months after induction.
(A and B) β-Galactosidase+ cells from the Lgr5+ lineage are found in circumvallate papilla taste bud cells as well as in perigemmal cells (white arrows) 6 months after 5-days of tamoxifen induction of Lgr5-Cre. In addition, β-galactosidase+ cells are found in the trench area at the opening of the Von Ebner's gland (black arrow). (C) 6 months after 5 days of tamoxifen induction many β-galactosidase-stained mucosal cells of the Lgr5+ lineage are present in crypts and villi of the small intestine. d, duct of Von Ebner's gland. Scale bars: 40 μm.
Supplemental Fig. S4. Lgr5-expressing cells generate mature taste cells after a single tamoxifen injection.
(A, B) One day after 1-day single tamoxifen injection, a small number of β-galactosidase-stained taste cells (blue) of the Lgr5+ lineage are present only at the bottom of the trench area (black arrows). β-Galactosidase+ cells from the Lgr5+ lineage are found in circumvallate papilla taste bud cells (white arrows) as well as in perigemmal cells (arrowheads) 2 weeks (C, D) and 1 month (E, F) after a single tamoxifen injection. Scale bars: 20 μm.
Supplemental Fig. S5. Lgr5-expressing cells generate mature taste cells in the foliate papillae 1 month after a single tamoxifen injection.
Tamoxifen-induced Lgr5-Cre generates β-galactosidase activity to mark cells from the Lgr5+ lineage. Intragemmal and perigemmal β-galactosidase+ cells (blue) are found in foliate papillae taste buds (arrows). A and B are two representative sections. Scale bars: 20 μm.
Supplemental Fig. S6. Lgr5+ stem cells generate all three types of taste cells.
Mature taste bud cells marked by Lgr5-Cre-generated β-galactosidase 1-2 months after tamoxifen induction were double stained with anti-β-galactosidase and markers for each of the three types of taste cells. A-D) β-Galactosidase immunoreactivity (green) is co-expressed in type I taste cells with NTPDase2 (red), two months after a single tamoxifen induction. E-H) Lgr5-Cre-generated β-galactosidase immunoreactivity (green) is co-expressed in type II taste receptor cells with Trpm5 (red), 2 months after 5-days of tamoxifen injections. I-L) Lgr5-Cre-generated β-galactosidase immunoreactivity (green) is co-expressed in type III taste receptor cells with serotonin (red), six weeks after a single tamoxifen injection. All sections are compressed Z-stacks (4-10 μm thickness). Scale bars: A, E, and I=20 μm; B-D, F-H, and J-L=8 μm.
Supplemental Fig. S7. Mature taste cells generated from Lgr5-expressing cells 6 months after induction.
Lgr5-Cre-generated β-galactosidase immunoreactivity (green) generated 6 months after induction by 5-days of tamoxifen is co-expressed with Trpm5 (red) in type II taste receptor cells. Asterisks indicate colocalization of β-galactosidase and Trpm5. (A and B) show two representative dual-stained sections. Scale bars: 20 μm.