Abstract
Objective
This study was designed to evaluate the relationship of age, gender, ethnicity and salivary flow rates on dental caries in an adult population using data collected from the Oral Health San Antonio Longitudinal Study of Aging (OH:SALSA).
Background
Saliva is essential to maintain a healthy oral environment and diminished output can result in dental caries. Although gender and age play a role in the quantity of saliva, little is known about the interaction of age, gender and ethnicity on dental caries and salivary flow rates.
Materials and Methods
Data from the 1,147 participants in the OH: SALSA was analyzed. The dependent variables were the number of teeth with untreated coronal caries, number of teeth with root caries, and the number of coronal and root surfaces with untreated caries. The independent variables were stimulated and unstimulated glandular salivary flow rates along with the age, sex, and ethnicity (e.g. European or Mexican ancestry) of the participants.
Results
Coronal caries experience was greater in younger participants while root surface caries experience was greater in the older participants. Coronal caries was lower in the older age groups while the root caries experience increased. Men had a statistically significant (p<0.02) higher experience of root caries than women. Values for unstimulated and stimulated parotid salivary flow rates showed no age difference and remained constant with age, whereas the age differences in the unstimulated and stimulated submandibular/sublingual salivary flow rates were significant. The mean number of teeth with coronal and root caries was higher in Mexican-Americans than in European-Americans.
Conclusions
Over one-fourth of the adults between the ages of 60 and 79 have untreated root caries over one-third having untreated coronal caries. Lower salivary flow rates play a significant role in the both the number of teeth and the number of surfaces developing caries in these adults. Women and individuals of European-American ancestry experience less caries.
Keywords: Caries, Saliva, Aging, Ethnicity
Introduction
In the United States, improvements in oral health care, such as community water fluoridation, advanced technology, and better oral hygiene have resulted in a large proportion of the adult population retaining some or all of their teeth [1, 2]. Despite these improvements, our geriatric population is still at risk for dental caries, periodontitis, benign mucosal lesions, and oral cancer [3, 4]. Dental caries is the most prevalent oral disease in this age group and can lead to tooth loss and eventually edentulous [5]. Being edentulous negatively affects the quality of life is affected; complete denture wearers have reduced masticatory function compared to dentate persons [6]. The number of teeth in participants of the Baltimore Longitudinal Study of Aging, the number of teeth that an individual had was found to be a significant and independent risk indicator for early mortality [7].
Saliva is essential to maintain oral health. Lowered saliva flow can lead to speech dysfunction and difficulty in swallowing as well as mucosal infections and rampant dental caries [8, 9, 10]. Protection against dental caries is dependent on both salivary quantity and composition of the secretions [10, 11] with the former being the strongest [10, 11]; the quantity of saliva is the stronger risk indicator for dental caries [12].
Age-related changes in salivary gland morphology and salivary composition have been reported in healthy individuals. With increasing age, the parenchyma of the salivary glands is gradually replaced by adipose and fibrovascular tissue, and the acini become atrophied reducing salivary volume [13]. Likewise, changes in salivary components (e.g., enzymes or antibodies) have been reported with aging [14-16]. Results from studies looking at the relationship between age and salivary flow rates are mixed: some show an effect [17, 18] while others show no alterations [19, 20].
Saliva is produced in several glands: the major salivary glands (parotid, submandibular and sublingual) and the minor salivary glands located in the oral tissues’ submucosa. In the past, whole saliva (i.e., salvia combined from all glands) has been used when studying the relationship between salivary flow and dental caries. However, for therapeutic tissue targeting, it may be important to identify the role specific glands play in dental caries.
The purpose of this study was to evaluate the role of flow rates from the individual major salivary glands in the development of dental caries taking into consideration age, gender and ethnicity.
Material and Methods
Study Subjects
Residents from San Antonio, Texas, were randomly selected and enrolled in a cross-sectional assessment of the oral health of adults called Oral Health: San Antonio Longitudinal Study of Aging (OH: SALSA). Questionnaires were used to obtain sociodemographic characteristics, general health, ethnicity, and oral and general health behavior data. Age was uniformly distributed. A total of 1,147 subjects were enrolled for dental screening. Participants with fewer than six anterior teeth or fewer than eight posterior teeth were excluded from the study. These exclusion criteria applied to 267 individuals leaving 880 subjects who were divided into the following age groups (36-49 years, 50-59 years, 60-69 years, and 70-79 years). Separate data analysis was conducted for each age group.
Caries Evaluation
Clinical oral evaluations were performed to measure both coronal caries and root caries. Clinical examinations were carried out by dentists and recorders who had previously participated in a training and standardization course developed by the National Institute for Dental and Cranial Research in 1990. Outcome measures were the number of teeth with untreated crown caries, the number of roots with untreated coronal caries, the number of teeth with untreated root caries, the number of coronal surfaces with untreated caries, and the number of root surfaces with untreated caries.
Saliva Flow Rate Measurements
Saliva was collected from all major salivary glands, and defined as follows: unstimulated (UP) and stimulated (SP) parotid saliva, unstimulated (US) and stimulated (SS) submandibular and sublingual saliva. Twelve hours prior to saliva collection, subjects were not allowed to consume food or beverages, chew gum, smoke, brush their teeth or use mouthwash. Although fasting may reduce salivary flow rates, this protocol was maintained to standardize measurements. All saliva was collected between 7-9 A.M. to maintain diurnal consistency. Unstimulated saliva was collected prior to stimulated saliva. To stimulate saliva, a 2% citrate acid solution was applied on the top dorsal side of the tongue. The sample gathered during the first two minutes was discarded to allow a stable flow of stimulated saliva to be established. Unstimulated and stimulated saliva samples were collected for 5 minutes from each gland source. Flow rate was calculated from the saliva volume over time. Parotid saliva was collected using a specialized Carlson-Crittenden cup placed over Stenson's duct located on the cheek adjacent to the maxillary second molar. A modified mini-suction system was used to collect submandibular/sublingual saliva from the opening of the common duct shared by the submandibular and sublingual glands which is located under the tongue. Stenson's duct was blocked with gauze to prevent parotid saliva from contaminating submandibular/sublingual saliva.
To preserve sample size, an assumption was made that the inability to measure UP, when SP was successfully measured, indicated that the parotid gland had no detectable flow without stimulation and the UP flow rate was defined as “0”. This represented 69.8% of the subjects. Among the 880 subjects with sufficient dentition, only 763 had a complete set of glandular salivary flow data. Therefore, the final sample size was 763 subjects.
Statistical Analysis
The dependent variables were the number of teeth and number of surfaces with untreated coronal and root caries. Independent variables included demographic characteristics including age, sex (Female=1, Male=0) and ethnicity (European ancestry=1, Mexican ancestry=0); and the stimulated and unstimulated flow rates from the major salivary glands. Due to over dispersion evidenced by variances that were consistently larger than means, the count measures (i.e., number of teeth with untreated coronal or root caries and the number of teeth and tooth surfaces with untreated coronal or root caries) were assumed to conform to the negative binomial distribution rather than the Poisson distribution. Generalized linear models based on the negative binomial distribution were performed to predict each caries measure separately with each flow rate separately as a continuous covariate after adjusting for gender, ethnicity, and the gender by ethnicity interaction. Results were considered statistical significance for with β coefficients with p<0.05. If the beta coefficient for a flow rate was less than 0, then the flow rate was assumed to be inversely associated with caries such that higher salivary flow was considered protective against caries. Multiple flow rates were not included as continuous covariates in the generalized linear models because the four flow rates were significantly correlated. A total of 16 generalized linear models were performed for each age group. Since UP measures were imprecise, findings that were significant for UP and non-significant for SP were reported as inconclusive. The statistical analyses were performed using IBM SPSS ® 18.0 software (IBM Inc., Chicago, IL).
Results
Of the 763 subjects included for analysis, 54% were female and 57.7% had a Mexican-American ancestry (Table 1). The number of subjects in each age group was similar except for the 70-79 age group which had fewer participants. For the overall sample, 38.8% had coronal caries and 20.3% had root caries. Root caries experience and the mean number of teeth affected with root caries was higher with increasing age (Table 1). Whereas, the root caries experience doubled (12.7% to 28.7%), the number of affected teeth as well as the number of carious tooth surfaces increased approximately four-fold. This pattern is reversed for coronal caries. The presence of coronal caries was lower (44.4% vs. 37.3%) with increasing age and the number of teeth affected by coronal caries decreased by one-third (1.48 teeth vs. 0.96 teeth). The number of surfaces affected by coronal caries was lower in the 36-49 and 50-59 age group (p<0.002); and was higher in the 50-59 and 60-69 age groups (p<0.04).
Table 1.
Prevalence of coronal and root caries by age, gender and ethnicity
| Age Group (years) (N) | Sex | Ethnicity* (N) | Coronal caries | Root caries | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % with Caries | Total % with Caries | Mean # affected teeth | Mean # Surfaces | % with Caries | Total % with Caries | Mean # affected teeth | Mean # Surfaces | |||
| 36-49 (205) | Male | MA (53) | 54.7 | 44.4 | 1.48 | 2.25 | 15.1 | 12.7 | 0.22 | 0.36 |
| EA (40) | 42.5 | 12.5 | ||||||||
| Female | MA (77) | 49.4 | 13.0 | |||||||
| EA (35) | 20.0 | 8.6 | ||||||||
| 50-59 (199) | Male | MA (67) | 49.3 | 39.2 | 0.92 | 1.42 | 23.9 | 16.1 | 0.32 | 0.43 |
| EA (31) | 29.0 | 9.7 | ||||||||
| Female | MA (68) | 39.7 | 11.8 | |||||||
| EA (33) | 27.3 | 15.2 | ||||||||
| 60-69 (209) | Male | MA (46) | 54.3 | 34.0 | 1.01 | 2.01 | 32.6 | 25.8 | 0.68 | 1.32 |
| EA (43) | 20.9 | 20.9 | ||||||||
| Female | MA (64) | 40.6 | 29.7 | |||||||
| EA (56) | 19.6 | 19.6 | ||||||||
| 70-79 (150) | Male | MA (33) | 42.4 | 37.3 | 0.96 | 1.93 | 30,3 | 28.7 | 0.89 | 1.65 |
| EA (38) | 34.2 | 44.7 | ||||||||
| Female | MA (32) | 34.4 | 21.9 | |||||||
| EA (47) | 38.3 | 19.1 | ||||||||
MA- Mexican American; EA- European American
Additionally, females had a statistically significant (p<0.02) lower experience of untreated root caries than males. Subjects with European ancestry (EA) had a statistically significant (p<0.001) lower root caries experience than subjects with Mexican ancestry (MA).
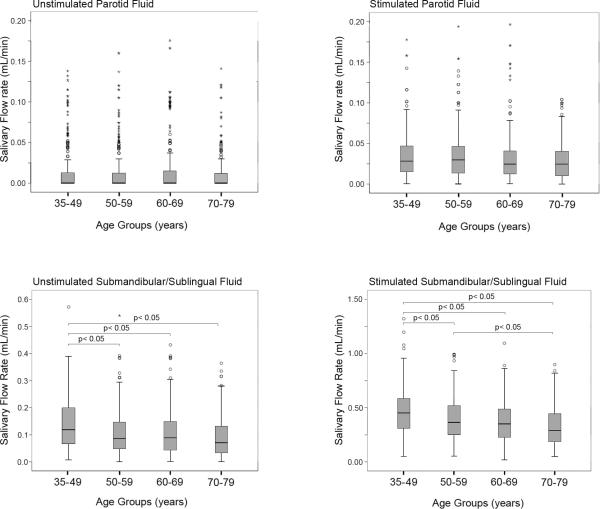
Figure 1 show that both stimulated and unstimulated submandibular/sublingual saliva flow rates decreased with age while stimulated and unstimulated parotid saliva flow rates showed no changes with age. An analysis relating flow rates to caries was undertaken. The results from 16 generalized linear models are shown in Tables 2. For the 36-49 age group, all flow rates (i.e., UP, SP, US, and SS) were found to be inversely associated with both the number of teeth (p<0.02) and number of surfaces (p<0.03) with untreated coronal caries. For untreated root caries, this relationship however, was not seen with the exception of an inverse association between unstimulated parotid flow rate (UP) and the number of untreated carious root surfaces (p<02). For the 50-59 age group, no significant associations between flow rates and caries measures were observed except for stimulated parotid (SP) and the number of untreated carious root surfaces (p<0.02). Within the older age groups, there were more significant relationships observed between flow rates and untreated caries.
Figure 1.
Glandular flow rates within age groups. Flow rates (mL/min) from unstimulated and stimulated parotid and submandibular/sublingual glands were measured from four age groups. O and * represent outliers 1.5x and 3.0x the interquartile range, respectively.
Table 2.
Relationship between Salivary Gland Flow Rates and Caries among Age Groups
| Age Group (years) | Glandular Flow Rate Type* | t-car | r-car | ts-car | rs-car | ||||
|---|---|---|---|---|---|---|---|---|---|
| β | p< | β | p< | β | p< | β | p< | ||
| 36-49 | SP | -1.34 | 0.001 | 0.62 | NS | -1.42 | 0.001 | 0.18 | NS |
| UP | -10.13 | 0.01 | -16.7 | NS | -10.72 | 0.004 | -18.94 | 0.02 | |
| SS | -1.36 | 0.006 | -1.61 | NS | -1.11 | 0.01 | -1.05 | NS | |
| US | -2.32 | 0.02 | -1.36 | NS | -1.96 | 0.03 | -1.09 | NS | |
| 50-59 | SP | 0.21 | NS | -1.22 | NS | 0.28 | NS | -1.52 | 0.015 |
| UP | -3.05 | NS | -0.13 | NS | -1.93 | NS | 0.65 | NS | |
| SS | 0.39 | NS | -0.99 | NS | 1.11 | NS | -0.63 | NS | |
| US | -1.85 | NS | -3.90 | NS | -1.30 | NS | -2.97 | NS | |
| 60-69 | SP | -0.84 | 0.03 | -0.85 | 0.05 | -0.70 | 0.04 | -0.59 | NS |
| UP | -5.62 | NS | -2.45 | NS | -8.34 | 0.01 | -3.71 | NS | |
| SS | -1.69 | 0.004 | -2.50 | 0.001 | -2.23 | 0.001 | -2.88 | 0.001 | |
| US | -1.80 | NS | -3.37 | 0.03 | -3.18 | 0.002 | -3.43 | 0.005 | |
| 70-79 | SP | -0.48 | NS | -0.48 | NS | 0.24 | NS | -0.55 | NS |
| UP | -6.67 | NS | -12.77 | 0.05 | -12.07 | 0.008 | -16.02 | 0.006 | |
| SS | -0.81 | NS | -2.55 | 0.001 | -1.36 | 0.022 | -2.79 | 0.001 | |
| US | -0.24 | NS | -5.81 | 0.004 | -0.20 | NS | -6.90 | 0.001 | |
SP-stimulated parotid; UP- unstimulated parotid; SS- stimulated submandibular/sublingual; US- unstimulated submandibular/sublingual. T-car – number of teeth with coronal caries, r-car – number of teeth with root caries, ts-car – the number of tooth surfaces with coronal caries and rs-car number of tooth surfaces with root caries
In the 60-69 age group, both the SP and stimulated submandibular/sublingual (SS) were significantly inversely associated with the number of teeth with coronal caries; whereas, all saliva flow rates were statistically associated with the number of surfaces with coronal caries (Table 2). The number of teeth with root caries was significantly inversely associated with SP (p<0.05), SS (p<0.001), and unstimulated submandibular/sublingual (US) (p<0.03) flow rates, however, the number of carious root surfaces was inversely associated only with the US (p<0.001) and stimulated and unstimulated SS (p<0.005) flow rates. Within the 70-79 age group, lower salivary flow rates were not associated with the number of teeth with coronal caries while the number of coronal surfaces with untreated caries was inversely associated with both UP (p<0.01) and SS (p<0.22). The number of teeth and number of root surfaces with untreated root caries was inversely associated with UP, SS and US (p<0.05).
The association of age, glandular flow rates, gender and ethnicity on untreated coronal and root caries is summarized in Tables 3 and 4. For the youngest age group (36-49 years old), the main effects of sex and ethnicity were not significant for either the number of teeth or number of tooth surfaces affected by root or coronal caries. However, the interaction between gender and ethnicity was significant for the number of teeth affected by coronal surfaces caries when modeled with UP, US and SS and US, and women SP. Women of European ancestry had fewer coronal surfaces with untreated caries than did women of Mexican ancestry (p<0.03), while there was no significant difference between males of European and Mexican ancestry.
Table 3.
Main effects and interaction for gender and ethnicity within age groups and flow rate types on the number of teeth with coronal or root caries
| Age Group (years) | Caries | Glandular Flow Rate Type* | Main Effect | Gender/Ethnicity Interaction | ||||
|---|---|---|---|---|---|---|---|---|
| Gender | Ethnicity | |||||||
| β | p< | β | p< | β | p< | |||
| 36-49 | Coronal | SP | -0.27 | NS | -0.30 | NS | -0.27 | NS |
| UP | -0.20 | NS | -0.06 | NS | -0.45 | NS | ||
| SS | -0.12 | NS | -0.19 | NS | -0.35 | NS | ||
| US | -0.12 | NS | -0.18 | NS | -0.37 | NS | ||
| 50-59 | SP | -0.64 | 0.01 | -0.84 | 0.01 | 0.68 | NS | |
| UP | -0.66 | 0.01 | -0.80 | 0.02 | 0.67 | NS | ||
| SS | -0.64 | 0.01 | -0.79 | 0.02 | 0.66 | NS | ||
| US | -0.66 | 0.008 | -0.83 | 0.01 | 0.71 | NS | ||
| 60-69 | SP | -0.59 | 0.02 | -1.04 | 0.001 | 0.45 | NS | |
| UP | -0.65 | 0.01 | -1.02 | 0.001 | 0.47 | NS | ||
| SS | -0.63 | 0.02 | -1.04 | 0.001 | 0.44 | NS | ||
| US | -0.69 | 0.01 | -1.10 | 0.001 | 0,55 | NS | ||
| 70-79 | SP | -0.13 | NS | -0.10 | NS | -0.18 | NS | |
| UP | -0.24 | NS | -0.17 | NS | -0.02 | NS | ||
| SS | -0.14 | NS | -0.18 | NS | -0.13 | NS | ||
| US | -0.18 | NS | -0.13 | NS | -0.11 | NS | ||
| 36-49 | Root | SP | -0.34 | NS | -0.02 | NS | -0.06 | NS |
| UP | -0.50 | NS | 0.13 | NS | -0.20 | NS | ||
| SS | -0.42 | NS | -0.15 | NS | 0.09 | NS | ||
| US | -0.45 | NS | -0.12 | NS | 0.04 | NS | ||
| 50-59 | SP | -0.56 | NS | -0.89 | NS | 1.31 | NS | |
| UP | -0.58 | NS | -1.05 | NS | 1.40 | 0.04 | ||
| SS | -0.59 | NS | -1.04 | NS | 1.42 | 0.04 | ||
| US | -0.68 | NS | -1.05 | 0.05 | 1.52 | 0.03 | ||
| 60-69 | SP | -0.50 | NS | -1.16 | 0.001 | 0.26 | NS | |
| UP | -0.55 | 0.05 | -1.10 | 0.001 | 0.25 | NS | ||
| SS | -0.56 | 0.05 | -1.15 | 0.001 | 0.29 | NS | ||
| US | -0.66 | 0.02 | -1.24 | 0.001 | 0.42 | NS | ||
| 70-79 | SP | -0.07 | NS | 0.95 | 0.01 | -0.82 | NS | |
| UP | -0.19 | NS | 0.89 | 0.01 | -0.74 | NS | ||
| SS | 0.00 | NS | 0.92 | 0.01 | -0.91 | NS | ||
| US | -0.20 | NS | 0.91 | 0.01 | -0.85 | NS | ||
Table 4.
Main effects and interaction for gender and ethnicity within age groups and flow rate types on the number of tooth surfaces with coronal and root caries.
| Age Group (years) | Caries | Glandular Flow Rate Type* | Gender/Ethnicity Interaction | |||||
|---|---|---|---|---|---|---|---|---|
| Gender | Ethnicity | |||||||
| β | p< | β | p< | β | p< | |||
| 36-49 | Coronal | SP | -0.14 | NS | 0.05 | NS | -0.67 | NS |
| UP | -0.04 | NS | 0.31 | NS | -0.88 | 0.02 | ||
| SS | 0.06 | NS | 0.16 | NS | -0.78 | 0.03 | ||
| US | 0.06 | NS | 0.15 | NS | -0.79 | 0.03 | ||
| 50-59 | SP | -0.93 | 0.001 | -1.16 | 0.001 | 1.19 | 0.01 | |
| UP | -0.94 | 0.001 | -1.12 | 0.001 | 1.18 | 0.01 | ||
| SS | -0.90 | 0.001 | -1.02 | 0.001 | 1.10 | 0.01 | ||
| US | -0.94 | 0.001 | -1.14 | 0.001 | 1.22 | 0.01 | ||
| 60-69 | SP | -0.58 | 0.02 | -1.18 | 0.001 | 0.45 | NS | |
| UP | -0.71 | 0.002 | -1.19 | 0.001 | 0.51 | NS | ||
| SS | -0.73 | 0.002 | -1.19 | 0.001 | 0.48 | NS | ||
| US | -0.85 | 0.001 | -1.36 | 0.001 | 0.70 | NS | ||
| 70-79 | SP | -0.07 | NS | 0.13 | NS | -0.69 | NS | |
| UP | -0.21 | NS | 0.03 | NS | -0.47 | NS | ||
| SS | -0.01 | NS | -0.06 | NS | -0.67 | NS | ||
| US | -0.09 | NS | 0.11 | NS | -0.65 | NS | ||
| 36-49 | Root | SP | -0.26 | NS | -0.05 | NS | -0.19 | NS |
| UP | -0.34 | NS | 0.24 | NS | -0.48 | NS | ||
| SS | -0.25 | NS | -0.09 | NS | -0.17 | NS | ||
| US | -0.29 | NS | -0.08 | NS | -0.18 | NS | ||
| 50-59 | SP | -1.03 | 0.002 | -1.33 | 0.01 | 1.97 | 0.003 | |
| UP | -1.05 | 0.003 | -1.49 | 0.004 | 2.08 | 0.002 | ||
| SS | -1.06 | 0.001 | -1.51 | 0.004 | 2.11 | 0.001 | ||
| US | -1.12 | 0.001 | -1.51 | 0.004 | 2.20 | 0.001 | ||
| 60-69 | SP | -0.61 | 0.01 | -1.78 | 0.001 | 0.16 | NS | |
| UP | -0.69 | 0.004 | -1.74 | 0.001 | 0.18 | NS | ||
| SS | -0.81 | 0.001 | -1.84 | 0.001 | 0.34 | NS | ||
| US | -0.85 | 0.001 | -1.90 | 0.001 | 0.41 | NS | ||
| 70-79 | SP | -0.27 | NS | 1.04 | 0.001 | -0.87 | NS | |
| UP | -0.43 | NS | 0.98 | 0.001 | -0.79 | NS | ||
| SS | -0.17 | NS | 0.93 | 0.002 | -0.98 | 0.03 | ||
| US | -0.43 | NS | 1.01 | 0.001 | -0.91 | 0.05 | ||
The 50-59 year old group showed more interactions than did the 36-49 age group. For all models predicting untreated coronal and root caries, the main effects of sex and ethnicity were significant. Women and those of European ancestry had significantly fewer teeth and number of tooth surfaces with untreated coronal and root caries than men (p<0.02) and those of Mexican ancestry. Men of European ancestry had fewer surfaces with both untreated coronal and root caries and had significantly less (p< 0.05) teeth affected by root caries than men of Mexican ancestry. In men of European ancestry the mean number of coronal and root surfaces with dental caries was significantly lower (p< 0.02) than for men of Mexican ancestry.
Within the 60-69 year old group, while all the models were significant for the main effects of sex and ethnicity, the gender/ethnicity interaction effect was not significant. Women and those of European ancestry had less mean number of teeth and number of tooth surfaces affected by coronal or root caries than did men with Mexican ancestry.
In the 70-79 age group, the main effect of sex was not significant for root surfaces in any model whereas ethnicity was significant for root caries experience and the number of root surfaces in women. Those of Mexican ancestry had fewer teeth and tooth surfaces with untreated root caries than did those of European ancestry. Likewise, those individuals with European ancestry had fewer teeth and surfaces with untreated caries than those with Mexican ancestry.
In the 70-79 year old age group, the main effect of ethnicity was significant (p<0.01) for the number of teeth with root caries. The sex by ethnicity interaction was significant only for the SS and US models with untreated carious root surfaces. Women of European ancestry were virtually identical to women of Mexican ancestry; however, number of root surfaces with untreated mean number of teeth with coronal caries was significantly greater (p< 0.02) for those of European ancestry than for those of Mexican ancestry. For the SS and US models predicting root surface caries, the sex by ethnicity interaction was significant (p< 0.05). The root caries mean for women of European ancestry was virtually identical to women of Mexican ancestry while, the mean number of root surfaces that were carious was significantly greater in men of European ancestry when compared to men of Mexican ancestry.
Discussion
Age is an overwhelming factor influencing salivary flow rates. In order to evaluate the effect of salivary flow rate, obtain interactions or differences between gender or, ethnicity on the number of untreated coronal and root caries requires that subjects be stratified by ranges of age as indicated by Mungia, et al. [15]. We showed that salivary flow rates from the parotid gland did not vary significantly with age which is consistent with Baum [21] who also documented no age related reduction in the stimulated secretion from the parotid gland. However, the findings for both unstimulated and stimulated flow did vary significantly. When comparing the youngest age group (36-49 years old) to the other age groups, submandibular/sublingual flow rates, however, was different. Flow rates were significantly lower when comparing the youngest age group (36-49 year old group) to the other older age groups. This result as discussed by Pedersen et. al [22] may be attributed to histologic and physiologic changes altering salivary flow rates throughout the lifetime as one ages. In contrast, Tylenda et al. [20] reported no significant changes in the unstimulated and stimulated flow from the submandibular gland that can be attributed to age. Possible reasons for these disparities could be the distribution of the populations by age and methods used to stimulate the glands in the different studies. Tylenda's study had fewer participants (N= 90) compared to the sample from this study (N= 763). Additionally, the age range was greater (26-to 96-years-old) in Tylenda's study compared to the range (36- to 79-years-old) in this study. The major contributors to unstimulated and stimulated whole saliva flow are the submandibular and parotid glands respectively [23]. This differs from findings reported here where the stimulated parotid flow was lower. However, the submanibular and sublingual flows were comingled during sampling which could account for the difference.
The current study showed that women had lower caries rates than men which is consistent with findings reported by Marques et al. [24] and Papas et. al [25] but differs from a study by Garcia-Cortez et al. [26] showing the reverse. These findings could be the result of studying different age groups and nationalities. Papas et al. [25] included an older European adult population (47-83 years), while the latter studied a population of young adults (18-35 years) living in Mexico.
Caries is a multifactorial disease and other risk factors other than saliva exist includingThe interaction with age, socio-economic status and lack of access to dental care were shown to be risk factors for caries [27]. We find that the experience of coronal caries was lower with age while root caries was higher. In the literature, both coronal and root caries appear to be major problems among the elderly but its incidence have not been well defined [28]. With regards to root caries, the elderly continue to develop high caries rates. Although age is an important factor in determining root caries other factors should be taken into consideration such as diet, periodontal diseases, use of preventive measures such as fluoride and dental visits that can impact the oral health status.
This study compared caries experience within ethnic groups. We found that Mexican-Americans had higher coronal caries incidence than those with European ancestry. A previous study showed that the risk of having untreated cavities in adults 20-50 year old was significantly higher among non-Hispanic blacks and Mexican–Americans than non-Hispanic whites, controlling for age and sex [29]. Although they did not specify the type of untreated caries, that study is consistent with our finding of higher caries experience in Mexican-Americans than in European-Americans, but also found that individuals with European ancestry have significantly (p<0.001) lower root caries rate than those of Mexican ancestry. This finding is also consistent with Trends in Oral Health Status Report (1999-2004) from the U.S. Department of Health and Human Services (2007) [2].
Conclusions
Dental caries is commonly found in older adults. In this study, over one-fourth of the adults aged between the ages of 60 and 79 have untreated root caries over one-third having untreated coronal caries. Diminished salivary flow rates play a significant role in the both the number of teeth and the number of surfaces developing caries in these adults. Gender in this study salivary flow rate was an important factor in caries experience especially within the 36-49 year old group, and root caries in the 60-69 and 70-79 year old groups. Being a women and having European ancestry are factors against caries, when compared to men and individuals of Mexican ancestry.
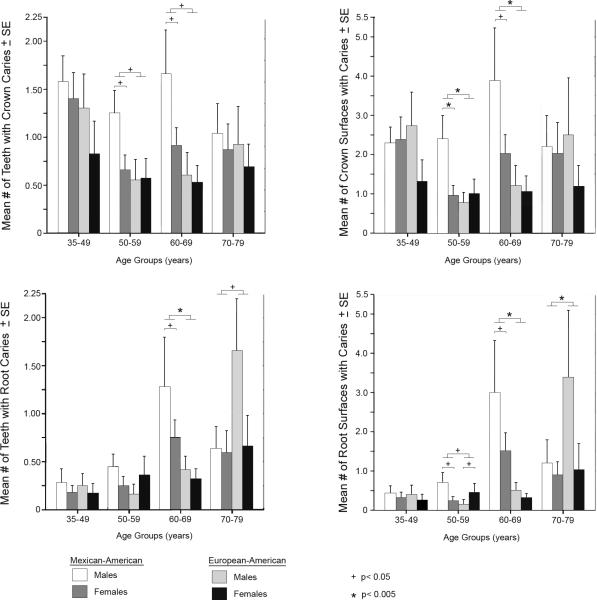
Figure 2.
Interaction for gender and ethnicity within age groups. Sixteen generalized linear models were performed for each age
Acknowledgments
This work was supported by grants from HRSA (D13HP30016 to DPC) and NIH (DE019892 to SC)
References
- 1.Gonsalves WC, Wrightson AS, Henry RG. Common oral conditions in older persons. American Family Physician. 2008 Oct 1;78(7):845–52. [PubMed] [Google Scholar]
- 2.Jeannotte L, Moore MJ. The State of Aging and Health in America 2007. Centers for Disease Control and Prevention; Atlanta, GA: 2007. [Google Scholar]
- 3.Jones JA. Root caries: prevention and chemotherapy. American journal of dentistry. 1995 Dec;8(6):352–7. [PubMed] [Google Scholar]
- 4.Shay K. Root caries in the older patient: significance, prevention, and treatment. Dental clinics of North America. 1997 Oct;41(4):763–93. [PubMed] [Google Scholar]
- 5.Fure S. Ten-year incidence of tooth loss and dental caries in elderly Swedish individuals. Caries research. 2003 Nov-Dec;37(6):462–9. doi: 10.1159/000073401. [DOI] [PubMed] [Google Scholar]
- 6.Allen PF, McMillan AS. A review of the functional and psychosocial outcomes of edentulousness treated with complete replacement dentures. Journal (Canadian Dental Association) 2003 Nov;69(10):662. [PubMed] [Google Scholar]
- 7.Padilha DM, Hilgert JB, Hugo FN, Bos AJ, Ferrucci L. Number of teeth and mortality risk in the Baltimore Longitudinal Study of Aging. The journals of gerontology. 2008 Jul;63(7):739–44. doi: 10.1093/gerona/63.7.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandel ID. Impact of saliva on dental caries. Compendium (Newtown, Pa) 1989;(13):S476–81. [PubMed] [Google Scholar]
- 9.Stookey GK. The effect of saliva on dental caries. Journal of the American Dental Association (1939) 2008 May;139(Suppl):11S–7S. doi: 10.14219/jada.archive.2008.0347. [DOI] [PubMed] [Google Scholar]
- 10.Dodds MW, Johnson DA, Yeh CK. Health benefits of saliva: a review. Journal of dentistry. 2005 Mar;33(3):223–33. doi: 10.1016/j.jdent.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Sreebny LM. Saliva in health and disease: an appraisal and update. International dental journal. 2000 Jun;50(3):140–61. doi: 10.1111/j.1875-595x.2000.tb00554.x. [DOI] [PubMed] [Google Scholar]
- 12.Leone CW, Oppenheim FG. Physical and chemical aspects of saliva as indicators of risk for dental caries in humans. Journal of dental education. 2001 Oct;65(10):1054–62. [PubMed] [Google Scholar]
- 13.Moreira CR, Azevedo LR, Lauris JR, Taga R, Damante JH. Quantitative age-related differences in human sublingual gland. Archives of oral biology. 2006 Nov;51(11):960–6. doi: 10.1016/j.archoralbio.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Fleissig Y, Reichenberg E, Redlich M, Zaks B, Deutsch O, Aframian DJ, et al. Comparative proteomic analysis of human oral fluids according to gender and age. Oral diseases. 2010 Nov;16(8):831–8. doi: 10.1111/j.1601-0825.2010.01696.x. [DOI] [PubMed] [Google Scholar]
- 15.Mungia R, Cano SM, Johnson DA, Dang H, Brown JP. Interaction of age and specific saliva component output on caries. Aging clinical and experimental research. 2008 Dec;20(6):503–8. doi: 10.1007/BF03324876. [DOI] [PubMed] [Google Scholar]
- 16.Preza D, Thiede B, Olsen I, Grinde B. The proteome of the human parotid gland secretion in elderly with and without root caries. Acta odontologica Scandinavica. 2009;67(3):161–9. doi: 10.1080/00016350902751545. [DOI] [PubMed] [Google Scholar]
- 17.Nagler RM, Hershkovich O. Age-related changes in unstimulated salivary function and composition and its relations to medications and oral sensorial complaints. Aging clinical and experimental research. 2005 Oct;17(5):358–66. doi: 10.1007/BF03324623. [DOI] [PubMed] [Google Scholar]
- 18.Yeh CK, Johnson DA, Dodds MW. Impact of aging on human salivary gland function: a community-based study. Aging (Milan, Italy) 1998 Oct;10(5):421–8. doi: 10.1007/BF03339889. [DOI] [PubMed] [Google Scholar]
- 19.Osterberg T, Birkhed D, Johansson C, Svanborg A. Longitudinal study of stimulated whole saliva in an elderly population. Scandinavian journal of dental research. 1992 Dec;100(6):340–5. doi: 10.1111/j.1600-0722.1992.tb01084.x. [DOI] [PubMed] [Google Scholar]
- 20.Tylenda CA, Ship JA, Fox PC, Baum BJ. Evaluation of submandibular salivary flow rate in different age groups. Journal of dental research. 1988 Sep;67(9):1225–8. doi: 10.1177/00220345880670091501. [DOI] [PubMed] [Google Scholar]
- 21.Baum BJ. Evaluation of stimulated parotid saliva flow rate in different age groups. Journal of dental research. 1981 Jul;60(7):1292–6. doi: 10.1177/00220345810600070101. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen W, Schubert M, Izutsu K, Mersai T, Truelove E. Age-dependent decreases in human submandibular gland flow rates as measured under resting and post-stimulation conditions. Journal of dental research. 1985 May;64(5):822–5. doi: 10.1177/00220345850640050801. [DOI] [PubMed] [Google Scholar]
- 23.Navazesh M, Kumar SK. Measuring salivary flow: challenges and opportunities. Journal of the American Dental Association (1939) 2008 May;139(Suppl):35S–40S. doi: 10.14219/jada.archive.2008.0353. [DOI] [PubMed] [Google Scholar]
- 24.Marques MD, Bjertness E, Eriksen HM. Caries prevalence of young adults in Oslo, Norway, and Porto, Portugal. A comparative analysis. Acta odontologica Scandinavica. 1994 Apr;52(2):111–5. doi: 10.3109/00016359409029064. [DOI] [PubMed] [Google Scholar]
- 25.Papas A, Joshi A, Palmer C, Giunta J, Dwyer J. Relationship of diet to root caries. American Journal of Clinical Nutrition. 1995;61(Suppl):423S–9S. doi: 10.1093/ajcn/61.2.423S. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Cortes JO, Medina-Solis CE, Loyola-Rodriguez JP, Mejia-Cruz JA, Medina-Cerda E, Patino-Marin N, et al. Dental caries’ experience, prevalence and severity in Mexican adolescents and young adults. Revista de salud publica (Bogota, Colombia) 2009 Jan-Feb;11(1):82–91. doi: 10.1590/s0124-00642009000100009. [DOI] [PubMed] [Google Scholar]
- 27.Dominguez-Rojas V, Astasio-Arbiza P, Ortega-Molina P, Gordillo-Florencio E, Garcia-Nunez JA, Bascones-Martinez A. Analysis of several risks factors involved in dental caries through multiple logistic regression. International dental journal. 1993 Apr;43(2):149–56. [PubMed] [Google Scholar]
- 28.Meyerowitz C. Geriatric dentistry and prevention: research and public policy (reaction paper). Advances in dental research. 1991 Dec;5:74–7. doi: 10.1177/08959374910050011201. [DOI] [PubMed] [Google Scholar]
- 29.Reid BC, Hyman JJ, Macek MD. Race/ethnicity and untreated dental caries: the impact of material and behavioral factors. Community dentistry and oral epidemiology. 2004 Oct;32(5):329–36. doi: 10.1111/j.1600-0528.2004.00165.x. [DOI] [PubMed] [Google Scholar]