Abstract
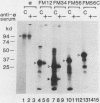
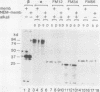
To study the membrane insertion of the Na+,K+-ATPase (EC 3.6.1.37) alpha subunit with six to eight transmembrane segments, mRNAs encoding the entire alpha subunit and its four different domains were prepared and translated in rabbit reticulocyte lysate with rough microsomal membranes. On the basis of the resistance of the membrane-inserted products to alkali extraction and the failure to insert the translation products into N-ethylmaleimide-treated membranes, it is suggested that at least two signal sequences are contained within the four transmembrane segments from the amino terminus of the alpha subunit.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. A., Rose J. K. Structural requirements of a membrane-spanning domain for protein anchoring and cell surface transport. Cell. 1985 Jul;41(3):1007–1015. doi: 10.1016/s0092-8674(85)80081-7. [DOI] [PubMed] [Google Scholar]
- Anderson D. J., Blobel G. Immunoprecipitation of proteins from cell-free translations. Methods Enzymol. 1983;96:111–120. doi: 10.1016/s0076-6879(83)96012-3. [DOI] [PubMed] [Google Scholar]
- Audigier Y., Friedlander M., Blobel G. Multiple topogenic sequences in bovine opsin. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5783–5787. doi: 10.1073/pnas.84.16.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler D. F., Garoff H. Mutants of the membrane-binding region of Semliki Forest virus E2 protein. I. Cell surface transport and fusogenic activity. J Cell Biol. 1986 Mar;102(3):889–901. doi: 10.1083/jcb.102.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler D. F., Melancon P., Garoff H. Mutants of the membrane-binding region of Semliki Forest virus E2 protein. II. Topology and membrane binding. J Cell Biol. 1986 Mar;102(3):902–910. doi: 10.1083/jcb.102.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. G., Boeke J. D., Model P. Fine structure of a membrane anchor domain. J Mol Biol. 1985 Jan 5;181(1):111–121. doi: 10.1016/0022-2836(85)90329-8. [DOI] [PubMed] [Google Scholar]
- Davis N. G., Model P. An artificial anchor domain: hydrophobicity suffices to stop transfer. Cell. 1985 Jun;41(2):607–614. doi: 10.1016/s0092-8674(85)80033-7. [DOI] [PubMed] [Google Scholar]
- Farley R. A., Ochoa G. T., Kudrow A. Location of major antibody binding domains on alpha-subunit of dog kidney Na+-K+-ATPase. Am J Physiol. 1986 Jun;250(6 Pt 1):C896–C906. doi: 10.1152/ajpcell.1986.250.6.C896. [DOI] [PubMed] [Google Scholar]
- Geering K. Biosynthesis, membrane insertion and maturation of Na, K-ATPase. Prog Clin Biol Res. 1988;268B:19–33. [PubMed] [Google Scholar]
- Geering K., Kraehenbuhl J. P., Rossier B. C. Maturation of the catalytic alpha-subunit of Na,K-ATPase during intracellular transport. J Cell Biol. 1987 Dec;105(6 Pt 1):2613–2619. doi: 10.1083/jcb.105.6.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geering K., Meyer D. I., Paccolat M. P., Kraehenbühl J. P., Rossier B. C. Membrane insertion of alpha- and beta-subunits of Na+,K+-ATPase. J Biol Chem. 1985 Apr 25;260(8):5154–5160. [PubMed] [Google Scholar]
- Gilmore R., Blobel G. Translocation of secretory proteins across the microsomal membrane occurs through an environment accessible to aqueous perturbants. Cell. 1985 Sep;42(2):497–505. doi: 10.1016/0092-8674(85)90107-2. [DOI] [PubMed] [Google Scholar]
- Gilmore R., Blobel G., Walter P. Protein translocation across the endoplasmic reticulum. I. Detection in the microsomal membrane of a receptor for the signal recognition particle. J Cell Biol. 1982 Nov;95(2 Pt 1):463–469. doi: 10.1083/jcb.95.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homareda H., Kawakami K., Nagano K., Matsui H. Membrane insertion of alpha and beta subunits of human Na+,K+-ATPase. Prog Clin Biol Res. 1988;268B:77–84. [PubMed] [Google Scholar]
- Hortsch M., Avossa D., Meyer D. I. Characterization of secretory protein translocation: ribosome-membrane interaction in endoplasmic reticulum. J Cell Biol. 1986 Jul;103(1):241–253. doi: 10.1083/jcb.103.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen P. L. Mechanism of the Na+, K+ pump. Protein structure and conformations of the pure (Na+ +K+)-ATPase. Biochim Biophys Acta. 1982 Aug 11;694(1):27–68. doi: 10.1016/0304-4157(82)90013-2. [DOI] [PubMed] [Google Scholar]
- Kaiser C. A., Preuss D., Grisafi P., Botstein D. Many random sequences functionally replace the secretion signal sequence of yeast invertase. Science. 1987 Jan 16;235(4786):312–317. doi: 10.1126/science.3541205. [DOI] [PubMed] [Google Scholar]
- Kawakami K., Nagano K. The transmembrane segment of the human Na,K-ATPase beta-subunit acts as the membrane incorporation signal. J Biochem. 1988 Jan;103(1):54–60. doi: 10.1093/oxfordjournals.jbchem.a122239. [DOI] [PubMed] [Google Scholar]
- Kawakami K., Noguchi S., Noda M., Takahashi H., Ohta T., Kawamura M., Nojima H., Nagano K., Hirose T., Inayama S. Primary structure of the alpha-subunit of Torpedo californica (Na+ + K+)ATPase deduced from cDNA sequence. Nature. 1985 Aug 22;316(6030):733–736. doi: 10.1038/316733a0. [DOI] [PubMed] [Google Scholar]
- Kawakami K., Nojima H., Ohta T., Nagano K. Molecular cloning and sequence analysis of human Na,K-ATPase beta-subunit. Nucleic Acids Res. 1986 Apr 11;14(7):2833–2844. doi: 10.1093/nar/14.7.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K., Ohta T., Nojima H., Nagano K. Primary structure of the alpha-subunit of human Na,K-ATPase deduced from cDNA sequence. J Biochem. 1986 Aug;100(2):389–397. doi: 10.1093/oxfordjournals.jbchem.a121726. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morimoto T., Arpin M., Gaetani S. Use of proteases for the study of membrane insertion. Methods Enzymol. 1983;96:121–150. doi: 10.1016/s0076-6879(83)96013-5. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., DeFoor P., Fleischer S., Blobel G. Co-translational membrane integration of calcium pump protein without signal sequence cleavage. Nature. 1981 Jul 2;292(5818):87–88. doi: 10.1038/292087a0. [DOI] [PubMed] [Google Scholar]
- Mueckler M., Lodish H. F. The human glucose transporter can insert posttranslationally into microsomes. Cell. 1986 Feb 28;44(4):629–637. doi: 10.1016/0092-8674(86)90272-2. [DOI] [PubMed] [Google Scholar]
- Noguchi S., Noda M., Takahashi H., Kawakami K., Ohta T., Nagano K., Hirose T., Inayama S., Kawamura M., Numa S. Primary structure of the beta-subunit of Torpedo californica (Na+ + K+)-ATPase deduced from the cDNA sequence. FEBS Lett. 1986 Feb 17;196(2):315–320. doi: 10.1016/0014-5793(86)80270-8. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Modyanov N. N., Broude N. E., Petrukhin K. E., Grishin A. V., Arzamazova N. M., Aldanova N. A., Monastyrskaya G. S., Sverdlov E. D. Pig kidney Na+,K+-ATPase. Primary structure and spatial organization. FEBS Lett. 1986 Jun 9;201(2):237–245. doi: 10.1016/0014-5793(86)80616-0. [DOI] [PubMed] [Google Scholar]
- Robinson J. D., Flashner M. S. The (Na+ + K+)-activated ATPase. Enzymatic and transport properties. Biochim Biophys Acta. 1979 Aug 17;549(2):145–176. doi: 10.1016/0304-4173(79)90013-2. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Lane L. K., Lingrel J. B. Amino-acid sequence of the beta-subunit of the (Na+ + K+)ATPase deduced from a cDNA. Nature. 1986 May 22;321(6068):429–431. doi: 10.1038/321429a0. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Schwartz A., Lingrel J. B. Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature. 1985 Aug 22;316(6030):691–695. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- Sivasubramanian N., Nayak D. P. Mutational analysis of the signal-anchor domain of influenza virus neuraminidase. Proc Natl Acad Sci U S A. 1987 Jan;84(1):1–5. doi: 10.1073/pnas.84.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess M., Handschin C. Deletion analysis of the internal signal-anchor domain of the human asialoglycoprotein receptor H1. EMBO J. 1987 Sep;6(9):2683–2691. doi: 10.1002/j.1460-2075.1987.tb02560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Preparation of microsomal membranes for cotranslational protein translocation. Methods Enzymol. 1983;96:84–93. doi: 10.1016/s0076-6879(83)96010-x. [DOI] [PubMed] [Google Scholar]
- Wessels H. P., Spiess M. Insertion of a multispanning membrane protein occurs sequentially and requires only one signal sequence. Cell. 1988 Oct 7;55(1):61–70. doi: 10.1016/0092-8674(88)90009-8. [DOI] [PubMed] [Google Scholar]
- Zamofing D., Rossier B. C., Geering K. Structural organization of alpha-subunit from purified and microsomal toad kidney (Na+ + K+)-ATPase as assessed by controlled trypsinolysis. Biochim Biophys Acta. 1987 Nov 13;904(2):381–391. doi: 10.1016/0005-2736(87)90388-9. [DOI] [PubMed] [Google Scholar]