Abstract
Two direct-acting antivirals (DAAs) against hepatitis C virus (HCV): telaprevir and boceprevir, are now available in combination with peginterferon plus ribavirin for the treatment of chronic hepatitis C infection. Although these drugs are potent inhibitors of HCV replication, they occasionally result in severe adverse events. In the present clinical trials, in their stead, several second-generation DAAs are being investigated. Most of them are being viewed with high expectations, but they also require the combination with peginterferon plus ribavirin. In the near future, we might be using all-oral DAAs and interferon-free regimens for the treatment of HCV-infected patients, and these would be potent inhibitors of HCV and have less adverse events.
Keywords: HCV, Telaprevir, Boceprevir, Sofosbuvir, Daclatasvir
Review
Introduction
Hepatitis C virus (HCV) chronically infects an estimated 170 million people worldwide [1]. HCV infection is one of the major causes of end-stage liver disease and hepatocellular carcinoma (HCC) worldwide [2-4]. Approximately 30% of patients who develop acute hepatitis C recover spontaneously, signaled by improved symptoms, normalized liver-related chemistries, loss of HCV RNA from serum, and the development of HCV antibody [5-7]. In chronic hepatitis C, the progression of liver fibrosis is slow, but steady. It has been reported that the progression rate of liver fibrosis is 0.10-0.13 U/year in untreated patients [8]. Progression of chronic HCV infection is not linear in time, probably because many cofactors are involved in changing the rate of development of fibrosis, cirrhosis, and HCC [6]. Cirrhosis rates become significant after 20 years of HCV infection. About 20-30% of patients could develop a progressive liver disease leading to cirrhosis and HCC [5,7]. HCC develops at about 1-7% per year [5,7]. It has been demonstrated that subjects who achieve sustained virological response (SVR) have a clear advantage at histological and clinical levels compared to those who do not achieve SVR [8-12]. The present standard for the judgment of SVR is undetectability of serum HCV RNA at 24 weeks post-treatment.
Preventive measures against HCV, including vaccine development, are now in progress [13]. But the standard of care (SOC), peginterferon and ribavirin therapy, and new standard of care (NSOC), combination protease inhibitors such as telaprevir or boceprevir with peginterferon plus ribavirin therapy, have been approved for the eradication of HCV in US, Europe, and Japan [14-18]. Even with these advances in antiviral therapies against HCV, SVR rates were ~70% in HCV genotype-1 treated with NSOC and ~80% in HCV genotype-2/3 treated with SOC. Rash also occurs in 56% of patients treated with NSOC, compared to 34% of patients treated with SOC alone. Other adverse events were still present [19], although even interferon is also associated with severe adverse events [20]. When we treat patients infected with HCV in daily clinical practice, it seems important to be aware of the potential treatments of HCV in the near future, as the development of new drugs is always ongoing.
HCV belongs to the flaviviridae family, and HCV genome is a positive-strand ~9.6-kb RNA. HCV has a 5′ untranslated region (5′UTR), a long open reading frame, and a 3′UTR. An internal ribosomal entry site (IRES), containing the 5′UTR and part of the core coding region, forms a stem-loop structure and supports translation initiation of HCV genome in a cap-independent manner [21,22]. HCV genome encodes a single precursor polyprotein that is processed by host signal peptidases and HCV proteases, resulting in structural (core, envelopes E1 and E2, and p7) and nonstructural (NS2, NS3, NS4A, NS4B, NS5A and NS5B) proteins. Direct-acting antivirals (DAAs) against HCV are classified into several categories: 1) HCV NS3/4A protease inhibitors, 2) HCV NS5B polymerase inhibitors, 3) HCV NS5A inhibitors, and others. In the near future, interferon-sparing regimens and treatment with all-oral DAAs will play major roles in treating HCV-infected patients (Figure 1).
Figure 1.
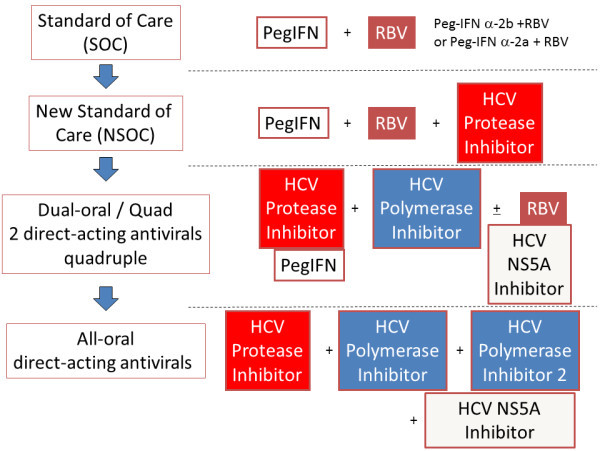
Treatments for chronic hepatitis C in the present and future. PegIFN, peginterferon; RBV, ribavirin.
Standard of care (SOC) treatment for HCV infection
Interferon, combination interferon plus ribavirin, and peginterferon plus ribavirin increased SVR rate from ~5% to ~40-80%, depending on the HCV genotypes [18,23]. Peginterferon plus ribavirin treatment for 48 weeks, the SOC treatment for HCV genotype 1-infected patients, leads to only ~50% SVR in those patients with high viral loads, who were mostly null-responders or relapsers [23-27]. On the other hand, peginterferon plus ribavirin treatment for 24 weeks, the SOC treatment for HCV genotype 2-infected patients, leads to ~80% SVR in those patients (Table 1). Race has been shown to be a factor in the response to therapy for HCV infection [25,27]. A higher homocysteine level is also one of factors predicting a nonresponse to treatment [28]. Because the favorable interleukin 28B (IL28B) associated-single nucleotide polymorphisms (SNPs), leading to better response, exist at substantially greater frequency in European than African populations, they also explain approximately half of the difference in response rates between African-Americans and patients of European ancestry [29]. IL28B SNPs help to improve the treatment outcomes in HCV-patients treated with SOC [29-34]. In future interferon-included regimens, IL28B SNPs may also provide useful information about the treatment response even before treatment.
Table 1.
Standard of care treatment and sustained virological response rates for chronic hepatitis C patients
| References | G | Number of patients | Naïve or re-treatment | Formula of therapy | Duration of treatment (weeks) | SVR rates (%) | Notes |
|---|---|---|---|---|---|---|---|
| [24] |
G1 |
298 |
|
Peginterferon alfa-2a plus ribavirin |
48 |
46 |
|
| 285 |
|
Interferon alfa-2b plus ribavirin |
48 |
36 |
|
||
| 145 |
|
Peginterferon alfa-2a plus placebo |
48 |
21 |
|
||
| [24] |
G2/G3 |
140 |
|
Peginterferon alfa-2a plus ribavirin |
48 |
76 |
|
| 145 |
|
Interferon alfa-2b plus ribavirin |
48 |
61 |
|
||
| 69 |
|
Peginterferon alfa-2a plus placebo |
48 |
45 |
|
||
| [24] |
G4 |
13 |
|
Peginterferon alfa-2a plus ribavirin |
48 |
77 |
|
| 11 |
|
Interferon alfa-2b plus ribavirin |
48 |
36 |
|
||
| 9 |
|
Peginterferon alfa-2a plus placebo |
48 |
22 |
|
||
| [25] |
G1 (98%) |
100 |
|
Peginterferon alfa-2b plus placebo |
48 |
19 |
Blacks |
| 100 |
|
Peginterferon alfa-2a plus ribavirin |
48 |
52 |
Non-Hispanic Whites |
||
| [26] |
G2/3 |
732 |
|
Peginterferon alfa-2a plus ribavirin |
16 |
65 |
|
| 731 |
|
Peginterferon alfa-2a plus ribavirin |
24 |
76 |
|
||
| [27] | G1 | 269 |
naive |
Peginterferon alfa-2a plus ribavirin |
48 |
34 |
Latino |
| 300 | naive | Peginterferon alfa-2a plus ribavirin | 48 | 49 | Non-Latino |
G, genotype; SVR, sustained virological response.
New standard of care (NSOC) treatment for HCV infection
In 2011, telaprevir and boceprevir were the first approved DAAs against HCV. The triple combination therapy of telaprevir or boceprevir plus ribavirin and peginterferon-alfa is the NSOC treatment for chronic HCV genotype 1-infected patients [35-42]. Telaprevir (VX-950) is a potent, selective inhibitor of NS3/4A protease, which is essential for HCV replication [43]. SVR rates among treatment-naïve patients were ~70% in telaprevir-included regimens [35,36,38,40]. The SVR rates among patients with no previous response were 30~40% and those among patients with a previous relapse were 70~75%, both in telaprevir-included regimens [37,39]. SVR rates were significantly higher in telaprevir-included regimens than in SOC among patients who had a previous relapse (83-88% vs. 24%), a partial response (54-59% vs. 15%) and non- response (29-33% vs. 5%) [39,44]. Thus, SVR rates were higher among patients who had previously had relapses than among nonresponders. Response-guided therapy is also useful in the telaprevir-included regimen (Table 2) [40]. Boceprevir (SCH 503034) is a potent ketoamide inhibitor of HCV NS3 serine protease [45]. The addition of boceprevir to SOC results in higher SVR rates in both treatment-naïve and re-treated patients infected with HCV genotype 1 (Table 3) [41,42]. SVR rates were significantly higher in boceprevir-included regimens than in SOC among patients who had a prior relapse (69-75% vs. 29%) or a prior nonresponse (40-52% vs. 7%) [42]. Viewed from the clinical data of first-generation protease inhibitors, telaprevir and boceprevir, these drugs showed potent inhibition of HCV, although they occasionally led to severe adverse events [46,47].
Table 2.
New standard of care treatment with telaprevir for chronic hepatitis C patients
| References | G | Number of patients | Naïve or re-treatment | Formula of therapy, and duration of treatment (weeks) | SVR rates (%) | Notes |
|---|---|---|---|---|---|---|
| [35] |
G1 |
79 |
naive |
T12PR24 |
61 |
PROVE1 Study |
| 79 |
naive |
T12PR48 |
67 |
|||
| 17 |
naive |
T12PR12 |
35 |
|||
| 75 |
naive |
PR48 |
41 |
|||
| [36] |
G1 |
81 |
naive |
T12PR24 |
69 |
PROVE2 Study |
| 82 |
naive |
T12PR12 |
60 |
|||
| 78 |
naive |
T12P12 |
36 |
|||
| 42 |
naive |
PR48 |
46 |
|||
| [37] |
G1 |
115 |
re-treatment |
T12PR24 |
51 |
PROVE3 Study |
| 113 |
re-treatment |
T12PR48 |
53 |
|||
| 111 |
re-treatment |
T24P24 |
24 |
|||
| 114 |
re-treatment |
PR48 |
14 |
|||
| [38] |
G1 |
363 |
naive |
T12PR |
73 |
ADVANCE Study |
| |
|
364 |
naive |
T8PR |
67 |
|
| |
|
49 |
naïve |
PR48 |
44 |
|
| [39] |
G1 |
145 |
re-treatment |
T12PR48 |
93 |
REALIZE Study |
| |
|
141 |
re-treatment |
Lead-in T12 PR48 |
89 |
|
| |
|
68 |
re-treatment |
PR48 |
24 |
|
| [40] |
G1 |
162 |
naïve |
T12PR24 (RGT) |
92 |
ILLUMINATE Study |
| |
|
160 |
naïve |
T12PR48 (RGT) |
88 |
|
| |
|
118 |
naïve |
T12PR48 (non-RGT) |
64 |
|
| 100 | naïve | Discontinued treatment before wk 20 | 23 |
Table 3.
New standard of care treatment with boceprevir for chronic hepatitis C patients
| References | G | Number of patients | Naïve or re-treatment | Formula of therapy, and duration of treatment (weeks) | SVR rates (%) | Notes |
|---|---|---|---|---|---|---|
| [41] |
G1 |
363 |
naive |
PR48 |
38 |
SPRINT-2 |
| 368 |
naive |
PR4+BocPR24+PR22 |
63 |
|||
| 366 |
naive |
PR4+BocPR44 |
66 |
|||
| [42] | G1 | 80 |
re-treatment |
PR48 |
21 |
HCV RESPOND-2 |
| 162 |
re-treatment |
PR4+BocPR24+PR22 |
59 |
|||
| 161 | re-treatment | PR4+BocPR44 | 66 |
PR48 group received peginterferon alfa-2a and ribavirin for 48 weeks; T12PR12, T12PR24 or T12 PR48 group received telaprevir for 12 weeks and peginterferon alfa-2a and ribavirin for 12, 24 or 48 weeks, respectively; T12P12 group received telaprevir and ribavirin for 12 weeks; T24P24 group received telaprevir and peginterferon alfa-2a for 24 weeks; RGT, response-guided therapy; Boc, boceprevir.
Second-generation HCV NS3/4A inhibitors
Simeprevir (TMC435) is an investigational HCV NS3/4A protease inhibitor administered orally once daily, and it is currently in phase III clinical development [48]. It differs from the first generation protease inhibitors in terms of its once-daily administration. Superior efficacies of simeprevir and peginterferon plus ribavirin were observed compared to those of peginterferon plus ribavirin alone in treatment-naive [49] and previously treated patients (Table 4) [50]. Although anemia and rash, respectively, were notable adverse events in boceprevir and telaprevir, those of simeprevir and peginterferon plus ribavirin did not differ from those of peginterferon plus ribavirin alone in phase II studies [48-50].
Table 4.
New standard of care treatment with other drugs (second-generation DAAs) chronic hepatitis C patients
| References | G | Number of patients | Naïve or re-treatment | Formula of therapy, and duration of treatment (weeks) | SVR rates (%) | Notes |
|---|---|---|---|---|---|---|
| [48,49] |
G1 |
78 |
Naïve |
TMC435 12 W peginterferon/ribavirin RGT |
82.1 |
TMC435 (simeprevir) 75 mg daily |
| 75 |
TMC435 24 W peginterferon/ribavirin RGT |
74.7 |
||||
| 77 |
TMC435 12 W peginterferon/ribavirin RGT |
80.5 |
TMC435 150 mg daily |
|||
| 79 |
TMC435 24 W peginterferon/ribavirin RGT |
86.1 |
||||
| 77 |
Placebo |
64.9 |
|
|||
| [48,50] |
G1 |
27 |
Relapsers |
PR PR48 |
37 |
|
| 79 |
TMC435 100 mg PR48 |
85 |
||||
| 79 |
TMC435 150 mg PR48 |
85 |
||||
| 23 |
Partial responders |
PR PR48 |
9 |
|||
| 68 |
TMC435 100 mg PR48 |
57 |
||||
| 69 |
TMC435 150 mg PR48 |
75 |
||||
| 16 |
Null responders |
PR PR48 |
19 |
|||
| 50 |
TMC435 100 mg PR48 |
46 |
||||
| 51 |
TMC435 150 mg PR48 |
51 |
||||
| [55] |
G1 |
12 |
|
3 mg daclatasvir PR48 |
42 |
Treatment-naïve or less than 4 wks of exposure to ribavirin or interferon-based therapy |
| 12 |
10 mg daclatasvir PR48 |
83 |
||||
| 12 |
60 mg daclatasvir PR48 |
83 |
||||
| 12 | Placebo PR48 | 25 |
MK-5172, a novel P2-P4 quinoxaline macrocyclic peptide, maintained potency across a genetically diverse panel of genotype 1a and 1b sequences from plasma of HCV-infected patients. This drug is to be used in combination with peginterferon plus ribavirin or with other DAAs [51]. Faldaprevir (BI 201335) is an inhibitor of HCV NS3/4A protease and is undergoing phase III clinical trials [52,53].
HCV NS5A and NS5B inhibitors
HCV NS5A inhibitor daclatasvir (BMS-790052) with potent clinical effects has been found in the HCV replicon system [54]. Daclatasvir is a potent NS5A replication complex inhibitor and increases the antiviral potency of peginterferon and ribavirin [55] (Tables 4).
Sofosbuvir (GS-7977/PSI-7977) is a nucleotide inhibitor of HCV NS5B polymerase. Triple therapy including peginterferon plus ribavirin and sofosbuvir cures >90% of patients treated for 12 or 24 weeks regardless of HCV genotype [56]. However, SVR rates of sofosbuvir plus ribavirin were ~76% for interferon-naive patients and ~11% for prior null responders in HCV genotype 1 patients [56,57]. Sofosbuvir plus peginterferon/ribavirin in treatment-naive patients with HCV genotype 1 leads to higher RVR rates and SVR rates than SOC (88-94% vs. 21%; and 56-83% vs. 43%, respectively) [58]. It was also reported that sofosbuvir in combination with low- or full- dose ribavirin for 24 weeks leads to higher efficacy in difficult-to-treat HCV infected genotype 1 patients [59].
HCV drugs in the future
New drugs for the treatment of hepatitis C are now being investigated in phase II and phase III clinical trials (Tables 5 and 6). These include interferon-sparing regimens, which are needed for the treatment of those intolerant to, or medically ineligible for peginterferon plus ribavirin therapy [60,61]. Among treatment-experienced patients with advanced fibrosis or cirrhosis, previous relapsers are likely to respond very well to telaprevir- or boceprevir-based treatment, although advanced liver disease had a greater influence on SVR rates in previous nonresponders [39,42,62,63]. Of importance is interferon-sparing combinations that might potentially be used in all patients who cannot use interferon such as subjects with decompensated cirrhosis or low platelet count. Some DAAs are potent inhibitors independently of HCV genotypes [56,64-66]. The all-oral combination of daclatasvir plus sofosbuvir, with or without ribavirin, leads to higher SVR rates in treatment-naive patients chronically infected with HCV genotypes 1, 2 and 3 [67]. We may select the combination of several drugs according to personal features and/or HCV genotypes. In the near future, all-oral DAAs will treat HCV-infected patients (Figure 1).
Table 5.
New drugs (DAA combinations) for the treatment of hepatitis C in phase II study
| Drug name/category | Drug name/category | Company |
|---|---|---|
| ABT-450r/Protease inhibitor |
ABT-072/Polymerase inhibitor |
Abbott/Enanta |
| ABT-450r/Protease inhibitor |
ABT-267/NS5A inhibitor |
Abbott/Enanta |
| ABT-450r/Protease inhibitor |
ABT-333/Polymerase inhibitor |
Abbott/Enanta |
| Daclatasvir (BMS-79002)/ NS5A inhibitor |
Sofosbuvir (GS-7977)/ Polymerase inhibitor |
Bristol-Myers Squibb/Gilead |
| Daclatasvir (BMS-79002)/ NS5A inhibitor |
Simeprevir (TMC435)/ Protease inhibitor |
Bristol-Myers Squibb/Janssen |
| Danoprevir (RG7227)/ Protease inhibitor |
Setrobuvir (ANA 598)/ Polymerase inhibitor |
Genentech |
| Danoprevir (RG7227)/ Protease inhibitor |
Mericitabine (RG7128)/ Polymerase inhibitor |
Genentech |
| GS-9256 |
Tegobuvir (GS-9190)/ Polymerase inhibitor |
Gilead |
| Incivek (Telaprevir)/ Protease inhibitor |
VX-222/Polymerase inhibitor |
Vertex |
| Mericitabine (RG7128)/ Polymerase inhibitor |
Incivek (Telaprevir)/ Protease inhibitor |
Genentech/Vertex |
| Simeprevir (TMC435)/ Protease inhibitor |
Daclatasvir (BMS-79002)/ NS5A inhibitor |
Janssen/Bristol-Myers Squibb |
| Simeprevir (TMC435)/ Protease inhibitor |
Sofosbuvir (GS-7977)/ Polymerase inhibitor |
Janssen/ Gilead |
| Simeprevir (TMC435)/ Protease inhibitor |
TMC647055/NNI inhibitor/Ritonavir |
Janssen |
| Sofosbuvir (GS-7977)/ Polymerase inhibitor |
GS-5885/ NS5A inhibitor |
Gilead |
| VX-135/Polymerase inhibitor |
GSK2336805/ NS5A inhibitor |
Vertex/GSK |
| VX-135/Polymerase inhibitor | Simeprevir (TMC435)/ Protease inhibitor | Vertex/Janssen |
HCV advocate: http://hcvadvocate.blogspot.ca/p/quick-reference-guide_21.html accessed on 2013/2/24.
Table 6.
New drugs for the treatment of hepatitis C in phase III study (combinations with peginterferon plus ribavirin, or DAA combinations)
|
Combination with peginterferon plus ribavirin | ||
|---|---|---|
| Drug name | Category | Company |
| Daclatasvir (BMS-79002) |
NS5A inhibitor |
Bristol-Myers Squibb |
| Simeprevir (TMC435) |
Protease inhibitor |
Janssen |
| Sofosbuvir (GS-7977) |
Polymerase inhibitor |
Gilead |
|
DAA combinations | ||
|
Drug name/Category |
Drug name/Category |
Company |
| ABT-450r/Protease inhibitor |
ABT-267/NS5A inhibitor and/or ABT-333/Polymerase inhibitor |
Abbott/Enanta |
| Daclatasvir (BMS-79002)/ NS5A inhibitor |
Asunaprevir (BMS-650032)/Protease inhibitor |
Bristol-Myers Squibb |
| Faldaprevir (BI201335)/ Protease inhibitor |
GS-5885/NS5A inhibitor |
Boehringer Ingelheim |
| Sofosbuvir (GS-7977)/ Polymerase inhibitor |
BI207127/Polymerase inhibitor |
Gilead |
| Sofosbuvir (GS-7977)/ Polymerase inhibitor | GS-5885/NS5A inhibitor | Gilead |
HCV advocate: http://hcvadvocate.blogspot.ca/p/quick-reference-guide_21.html accessed on 2013/2/24.
Conclusions
We expect that all-oral DAAs and interferon-free regimens will be applied in the treatment of HCV-infected patients and that they will have more potent efficacy and less adverse events. Further studies are now ongoing.
Competing interests
Dr. Tatsuo Kanda reports receiving lecture fees from Chugai Pharmaceutical, MSD, Ajinomoto, GlaxoSmithKlein and Mitsubishi Tanabe Pharma, and Prof. Osamu Yokosuka received grant support from Chugai Pharmaceutical, Bayer, MSD, Daiichi-Sankyo, and Mitsubishi Tanabe Pharma.
Authors’ contributions
TK drafted the manuscript and all authors read through and made corrections to the manuscript. All authors read and approved the final manuscript.
Contributor Information
Tatsuo Kanda, Email: kanda2t@yahoo.co.jp.
Osamu Yokosuka, Email: yokosukao@faculty.chiba-u.jp.
Masao Omata, Email: momata-tky@umin.ac.jp.
References
- Brownell J, Polyak SJ. Molecular pathways: hepatitis C virus, CXCL10, and the inflammatory road to liver cancer. Clin Cancer Res. 2013. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;2:1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bisceglie AM, Lyra AC, Schwartz M, Reddy RK, Martin P, Gores G, Lok AS, Hussain KB, Gish R, Van Thiel DH, Younossi Z, Tong M, Hassanein T, Balart L, Fleckenstein J, Flamm S, Blei A, Befeler AS. Liver Cancer Network. Hepatitis C-related hepatocellular carcinoma in the United States: influence of ethnic status. Am J Gastroenterol. 2003;2:2060–2063. doi: 10.1111/j.1572-0241.2003.t01-1-07641.x. [DOI] [PubMed] [Google Scholar]
- Kanda T, Yokosuka O, Omata M. Hepatitis C virus and hepatocellular carcinoma. Biology. 2013;2:304–316. doi: 10.3390/biology2010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005;2:383–398. doi: 10.1016/j.cld.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Sarin SK, Kumar M. Natural history of HCV infection. Hepatol Int. 2012;2:684–694. doi: 10.1007/s12072-012-9355-6. [DOI] [PubMed] [Google Scholar]
- Seeff LB. Sustained virologic response: is this equivalent to cure of chronic hepatitis C? Hepatology. 2013;2:438–440. doi: 10.1002/hep.25964. [DOI] [PubMed] [Google Scholar]
- Ryder SD, Irving WL, Jones DA, Neal KR, Underwood JC. Treat Hepatitis C Study Group. Progression of hepatic fibrosis in patients with hepatitis C: a prospective repeat liver biopsy study. Gut. 2004;2:451–455. doi: 10.1136/gut.2003.021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barks RE, Ganne-Carrie E, Fontaine H, Paries J, Grando-Lemaire V, Beaugrand M, Pol S, Trinchet JC. Effect of sustained virological response on long-term clinical outcome in 113 patients with compensated hepatitis C-related cirrhosis treated by interferon alpha and ribavirin. World J Gastroenterol. 2007;2:5648–5653. doi: 10.3748/wjg.v13.i42.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda N, Yuki N, Mochizuki K, Nagaoka T, Yamashiro M, Omura M, Hikiji K, Kato M. Long-term clinical and virological outcomes of chronic hepatitis C after successful interferon therapy. J Med Virol. 2004;2:406–413. doi: 10.1002/jmv.20190. [DOI] [PubMed] [Google Scholar]
- Gentile I, Borgia G. Surrogate endpoints and non-inferiority trials in chronic viral hepatitis. J Hepatol. 2010;2:778. doi: 10.1016/j.jhep.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnu L, Mazzella G, Ascione A, Santantonio T, Piccinino F, Andreone P, Mangia A, Gaeta GB, Persico M, Faqiuoli S, Almasio PL. Italian Association of the Study of the Liver Disease (AISF) Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;2:579–587. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- Ray R. Progress toward development of a hepatitis C vaccine with broad shoulders. Sci Transl Med. 2011;2:94ps33. doi: 10.1126/scitranslmed.3002772. [DOI] [PubMed] [Google Scholar]
- Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;2:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2011;2:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Omata M, Kanda T, Yu ML, Yokosuka O, Lim SG, Jafri W, Tateishi R, Hamid SS, Chuang WL, Chutaputti A, Wei L, Sollano J, Sarin SK, Kao JH, McCaughan GW. APASL consensus statements and management algorithms for hepatitis C virus infection. Hepatol Int. 2012;2:409–435. doi: 10.1007/s12072-012-9342-y. [DOI] [PubMed] [Google Scholar]
- Editors of the Drafting Committee for Hepatitis Management Guidelines: The Japan Society of Hepatology. Guidelines for the management of hepatitis virus infection. Hepatol Res. 2013;2:1–34. doi: 10.1111/hepr.12020. [DOI] [PubMed] [Google Scholar]
- Kanda T, Imazeki F, Yokosuka O. New antiviral therapies for chronic hepatitis C. Hepatol Int. 2010;2:548–561. doi: 10.1007/s12072-010-9193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawitz EJ. Diagnosis and management of telaprevir-associated rash. Gastroenterol Hepatol (N.Y.) 2011;2:469–471. [PMC free article] [PubMed] [Google Scholar]
- Tosone G, Borgia G, Gentile I, Cerini R, Conte MC, Orlando R, Piazza M. A case of pegylated interferon alpha-related diabetic ketoacidosis: can this complication be avoided? Acta Diabetol. 2007;2:167–169. doi: 10.1007/s00592-007-0259-1. [DOI] [PubMed] [Google Scholar]
- Honda M, Beard MR, Ping LH, Lemon SM. A phylogenetically conserved stem-loop structure at the 5′ border of the internal ribosome entry site of hepatitis C virus is required for cap-independent viral translation. J Virol. 1999;2:1165–1174. doi: 10.1128/jvi.73.2.1165-1174.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda T, Steele R, Ray R, Ray RB. Small interfering RNA targeted to hepatitis C virus 5′ nontranslated region exerts potent antiviral effect. J Virol. 2007;2:669–676. doi: 10.1128/JVI.01496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poordad F, Dieterich D. Treating hepatitis C: current standard of care and emerging direct-acting antiviral agents. J Viral Hepat. 2012;2:449–464. doi: 10.1111/j.1365-2893.2012.01617.x. [DOI] [PubMed] [Google Scholar]
- Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, Haussinger D, Diago M, Carosi G, Dhumeaux D, Caraxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;2:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- Muir AJ, Bornstein JD, Killenberg PG. Atlantic Coast Hepatitis Treatment Group. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med. 2004;2:2265–2271. doi: 10.1056/NEJMoa032502. [DOI] [PubMed] [Google Scholar]
- Shiffman ML, Suter F, Bacon BR, Nelson D, Harley H, Sola R, Shafran SD, Barange K, Lin A, Soman A, Zeuzem S. ACCELERATE Investigators. Peginterferon alfa-2a and ribavirin for 16 or 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2007;2:124–134. doi: 10.1056/NEJMoa066403. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Torres M, Jeffers LJ, Sheikh MY, Rossaro L, Ankoma-Sey V, Hamzeh FM, Martin P. Latino Study Group. Peginterferon alfa-2a and ribavirin in Latino and non-Latino whites with hepatitis C. N Engl J Med. 2009;2:257–267. doi: 10.1056/NEJMoa0805062. [DOI] [PubMed] [Google Scholar]
- Borgia G, Gentile I, Fortunato G, Borrelli F, Borelli S, de Caterina M, Di Taranto MD, Simone M, Borgia F, Viola C, Reynaud L, Cerini R, Sacchetti L. Homocysteine levels and sustained virological response to pegylated-interferon alpha2b plus ribavirin therapy for chronic hepatitis C: a prospective study. Liver Int. 2009;2:248–252. doi: 10.1111/j.1478-3231.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, Sulkowski M, McHutchison JG, Goldstein DB. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;2:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nishida N, Sugiyama M, Kuroki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Ito Y, Mita E, Tanaka E, Mochida S, Murawaki Y, Honda M, Sakai A, Hiasa Y, Nishiguchi S, Koike A, Sakaida I, Imamura M, Ito K, Yano K, Masaki N, Sugauchi F, Izumi N, Tokunaga K, Mizokami M. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;2:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E, Riordan S, Sheridan D, Smedile A, Fragomeli V, Muller T, Bahlo M, Stewart GJ, Booth DR, George J. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;2:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- Nakamoto S, Kanda T, Imazeki F, Wu S, Arai M, Fujiwara K, Yokosuka O. Simple assay based on restriction fragment length polymorphism associated with IL28B in chronic hepatitis C patients. Scand J Gastroenterol. 2011;2:955–961. doi: 10.3109/00365521.2011.574731. [DOI] [PubMed] [Google Scholar]
- Miyamura T, Kanda T, Nakamoto S, Wu S, Fujiwara K, Imazeki F, Yokosuka O. Hepatic STAT1-nuclear translocation and interleukin 28B polymorphisms predict treatment outcomes in hepatitis C virus genotype 1-infected patients. PloS One. 2011;2:e28617. doi: 10.1371/journal.pone.0028617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura T, Kanda T, Nakamoto S, Wu S, Jiang X, Arai M, Fujiwara K, Imazeki F, Yokosuka O. Roles of ITPA and IL28B genotypes in chronic hepatitis C patients treated with peginterferon plus ribavirin. Viruses. 2012;2:1264–1278. doi: 10.3390/v4081264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, McNair L, Alam J, Muir AJ. PROVE1 Study Team. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;2:1827–1838. doi: 10.1056/NEJMoa0806104. [DOI] [PubMed] [Google Scholar]
- Hezode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, Bronowicki JP, Bourliere M, Gharakhanian S, Bengtsson L, McNair L, George S, Kieffer T, Kwong A, Kauffman RS, Alam J, Pawlotsky JM, Zeuzem S. PROVE2 Study Team. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;2:1839–1850. doi: 10.1056/NEJMoa0807650. [DOI] [PubMed] [Google Scholar]
- McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, Heathcote EJ, Zeuzem S, Reesink HW, Garg J, Bsharat M, George S, Kauffman RS, Adda N, Di Bisceglie AM. PROVE3 Study Team. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;2:1292–1303. doi: 10.1056/NEJMoa0908014. [DOI] [PubMed] [Google Scholar]
- Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Flisiak R, George J, Rizzetto M, Shouval D, Sola R, Terg RA, Yoshida EM, Adda N, Bengtsson L, Sankoh AJ, Kieffer TL, George S, Kauffman RS, Zeuzem S. ADVANCE Study Team. Telaprevir for previously untreated chronic HCV infection. N Engl J Med. 2011;2:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A, Ferenci P, Nevens F, Mullhaupt B, Pockros P, Terg R, Shouval D, van Hoek B, Weiland O, Van Heeswijk R, De Meyer S, Luo D, Boogaerts G, Polo R, Picchio G, Beumont M. REALIZE Study Team. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;2:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M, Sankoh AJ, Adda N, Kauffman RS, George S, Wright CI, Poordad F. ILLUMINATE Study Team. Response-guided teraprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;2:1014–1024. doi: 10.1056/NEJMoa1014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N, DiNubile MJ, Sniukiene V, Brass CA, Albrecht JK, Bronowicki JP. SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;2:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N, Burroughs M, Brass CA, Albrecht JK, Esteban R. HCV RESPOND-2 Investigators. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;2:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Kwong AD, Perni RB. Discovery and development of VX-950, a novel, covalent, and reversible inhibitor of hepatitis C virus NS3.4A serine protease. Infect Disord Drug Targets. 2006;2:3–16. doi: 10.2174/187152606776056706. [DOI] [PubMed] [Google Scholar]
- Gentile I, Viola C, Borgia F, Castaldo G, Borgia G. Telaprevir: a promising protease inhibitor for the treatment of hepatitis C virus infection. Curr Med Chem. 2009;2:1115–1121. doi: 10.2174/092986709787581789. [DOI] [PubMed] [Google Scholar]
- Bogen SL, Pan W, Ruan S, Nair LG, Arasappan A, Bennett F, Chen KX, Jao E, Venkatraman S, Liu R, Cheng KC, Guo Z, Tong X, Saksena AK, Girijavallabhan V, Njoroge FG. Toward the back-up of boceprevir (SCH 503034): discovery of new extended P4-capped ketoamide inhibitors of hepatitis C virus NS3 serine protease with improved potency and pharmacokinetic profiles. J Med Chem. 2009;2:3679–3688. doi: 10.1021/jm801632a. [DOI] [PubMed] [Google Scholar]
- Chopra A, Klein PL, Drinnan T, Lee SS. How to optimize HCV therapy in genotype 1 patients: management of side-effects. Liver Int. 2013;2(Suppl 1):30–34. doi: 10.1111/liv.12080. [DOI] [PubMed] [Google Scholar]
- Kumada H, Toyoda J, Okanoue T, Chayama K, Tsubouchi H, Hayashi N. Telaprevir with peginterferon and ribavirin for treatment-naive patients chronically infected with HCV of genotype 1 in Japan. J Hepatol. 2012;2:78–84. doi: 10.1016/j.jhep.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Tanwar S, Trembling PM, Dusheiko GM. TMC435 for the treatment of chronic hepatitis C. Expert Opin Investig Drugs. 2012;2:1193–1209. doi: 10.1517/13543784.2012.690392. [DOI] [PubMed] [Google Scholar]
- Aerssens J, Fanning F, Scholliers A, Lenz O, Peeters M, De Smedt G, Fried M. Impact of IL28B genotype and pretreatment serum IP-10 in treatment-naive genotype-1 HCV patients treated with TMC435 in combination with peginterferon a-2A and ribavirin in PILLAR Study. J Hepatol. 2011;2(Suppl 1):S5–S6. [Google Scholar]
- Zeuzem S, Berg T, Gane E, Ferenci P, Foster GR, Fried MW, Hezode C, Hirschfield GM, Jacobson I, Nikitin I, Pockros P, Poordad F, Lenz O, Peeters M, Sekar V, De Smedt G, Beumont-Mauviel M. TMC435 in HCV genotype 1 patients who have failed previous pegylated interferon/ribavirin treatment: final SVR24 results of the ASPIRE TRIAL. J Hepatol. 2012;2(Suppl 2):S1–S2. [Google Scholar]
- Summa V, Ludmerer SW, McCauley JA, Fandozzi C, Burlein C, Claudio G, Coleman PJ, Dimuzio JM, Ferrara M, Di Filippo M, Gates AT, Graham DJ, Harper S, Hazuda DJ, McHale C, Monteaqudo E, Pucci V, Rowley M, Rudd MT, Soriano A, Stahlhut MW, Vacca JP, Olsen DB, Liverton NJ, Carroll SS. MK-5172, a selective inhibitor of hepatitis C virus NS3/4a protease with broad activity across genotypes and resistant variants. Antimicrob Agents Chemother. 2012;2:4161–4167. doi: 10.1128/AAC.00324-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuzem S, Asselah T, Angus P, Zarski JP, Larrey D, Mullhaupt B, Gane E, Schuchmann M, Lohse A, Pol S, Bronowicki JP, Roberts S, Arasteh K, Zoulim F, Heim M, Stern JO, Kukolj G, Nehmiz G, Haefner C, Boecher WO. Efficacy of the protease inhibitor BI 201335, polymerase inhibitor BI 207127, and ribavirin in patients with chronic HCV infection. Gastroenterology. 2011;2:2047–2055. doi: 10.1053/j.gastro.2011.08.051. [DOI] [PubMed] [Google Scholar]
- Cooper C. Hepatitis C treatment highlights from the 2011 American Association for the Study of Liver Disease Meeting. Clin Infect Dis. 2012;2:418–425. doi: 10.1093/cid/cis375. [DOI] [PubMed] [Google Scholar]
- Gao M, Nettles RE, Belema M, Snyder LB, Nguyen VN, Fridell RA, Serrano-Wu MH, Langley DR, Sun JH, O’Boyle DR 2nd, Lemm JA, Wang C, Knipe JO, Chien C, Colonno RJ, Grasela DM, Meanwell NA, Hamann LG. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;2:96–100. doi: 10.1038/nature08960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol S, Ghalib RH, Rustgi VH, Martorell C, Everson GT, Tatum HA, Hezode C, Lim JK, Bronowicki JP, Abrams GA, Brau N, Morris DW, Thuluvath PJ, Reindollar RW, Yin PD, Diva U, Hindes R, McPhee F, Hemandez D, Wind-Rotolo M, Hughes EA, Schnittman S. Daclatasvir for previously untreated chronic hepatitis C genotype-1 infection: a randomised, parallel-group, double-blind, placebo-controlled, dose-finding, phase 2a trial. Lancet Infect Dis. 2012;2:671–677. doi: 10.1016/S1473-3099(12)70138-X. [DOI] [PubMed] [Google Scholar]
- Barreiro P, Vispo E, Poveda E, Fernandez-Montero JV, Soriano V. Hepatitis C therapy: Highlights from the 2012 annual meeting of the European association for the study of the liver. Clin Infect Dis. 2013;2:560–566. doi: 10.1093/cid/cis915. [DOI] [PubMed] [Google Scholar]
- Gane EJ, Stedman CA, Hyland RH, Sorensen RD, Symonds WT, Hindes R, Berrey MM. Once daily sofosbuvir (GS-7977) plus ribavirin in patients with HCV genotypes 1, 2, and 3: the ELECTRON trial. Hepatology. 2013;2(suppl):306A. [Google Scholar]
- Rodriguez-Torres M, Lawitz E, Kowdley KV, Nelson DR, Dejesus E, McHutchison JG, Cornpropst MT, Mader M, Albanis E, Jiang D, Hebner CM, Symonds WT, Berry MM, Lalezari J. Sofosbuvir (GS-7977) plus peginterferon/ribavirin in treatment-naive patients with HCV genotype 1: A randomized, 28-day, dose-ranging trial. J Hepatol. 2012. [Epub ahead of print] [DOI] [PubMed]
- Osinusi A, Heytens L, Lee Y, Bon D, Shivakumar B, Nelson A, Meissner EG, Kohli A, Barrett L, Proschan M, Silk R, Kwan R, Herrmann E, Sneller M, Teferi G, Talwani R, Symonds WT, Polis MA, Mausur H, McHuchison JG, Fauci AS, Kottilil S. High efficacy of GS-7977 in combination with low or full dose ribavirin for 24 weeks in difficult to treat HCV infected genotype 1 patients: interim analysis from SPARE trial. Boston, Massachusetts: Program and abstracts of 63rd Annual Meeting of the American Association for the Study of Liver Diseases; 2012. Abstract LB-4. [Google Scholar]
- Karino Y, Toyoda J, Ikeda K, Suzuki F, Chayama K, Kawakami Y, Ishikawa H, Watanabe H, Hemandez D, Yu F, McPhee F, Kumada H. Characterization of virologic escape in hepatitis C virus genotype 1b patients treated with the direct-acting antivirals daclatasvir and asunaprevir. J Hepatol. 2012. [Epub ahead of print] [DOI] [PubMed]
- Suzuki Y, Ikeda K, Suzuki F, Toyoda J, Karino Y, Chayama K, Kawakami Y, Ishikawa H, Watanabe H, Hu W, Eley T, McPhee F, Hughes E, Kumada H. Dual oral therapy with daclatasvir and asunaprevir for patients with HCV genotype 1b infection and limited treatment options. J Hepatol. 2012. [Epub ahead of print] [DOI] [PubMed]
- Muir AJ, Poordad FF, McHutchison JG, Shiffman ML, Berg T, Ferenci P, Heathcote EJ, Pawlotsky JM, Zeuzem S, Reesink HW, Dusheiko G, Martin EC, George S, Kauffman RS, Adda N. Retreatment with telaprevir combination therapy in hepatitis C patients with well-characterized prior treatment response. Hepatology. 2011;2:1538–1546. doi: 10.1002/hep.24549. [DOI] [PubMed] [Google Scholar]
- Jacobson IM, Pawlotsky JM, Afdhal NH, Dusheiko GM, Forns X, Jensen DM, Poordad F, Schulz J. A practical guide for the use of boceprevir and telaprevir for the treatment of hepatitis C. J Viral Hepat. 2012;2(Suppl 2):1–26. doi: 10.1111/j.1365-2893.2012.01590.x. [DOI] [PubMed] [Google Scholar]
- Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, Reindollar R, Rustgi V, McPhee F, Wind-Rotolo M, Persson A, Zhu K, Dimitrova DI, Eley T, Guo T, Grasela DM, Pasquinelli C. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;2:216–224. doi: 10.1056/NEJMoa1104430. [DOI] [PubMed] [Google Scholar]
- Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, Hindes RG, Berrey MM. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med. 2013;2:34–44. doi: 10.1056/NEJMoa1208953. [DOI] [PubMed] [Google Scholar]
- Herbst DA, Jr, Reddy KR. Sofosbuvir, a nucleotide polymerase inhibitor, for the treatment of chronic hepatitis C virus infection. Expert Opin Investig Drugs. 2013. [Epub ahead of print] [DOI] [PubMed]
- Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson IM, Lawitz E, Lok AS, Hinestrosa F, Thuluvath PJ, Schwartz H, Nelson DR, Eley T, Wind-Rotolo M, Huang SP, Gao M, McPhee F, Sherman D, Hindes R, Symonds WT, Pasquinelli C, Grasela DM. High rate of sustained virologic response with the all-oral combination of daclatasvir (NS5A inhibitor) plus sofosbuvir (nucleotide NS5B inhibitor), with or without ribavirin, in treatment-naive patients chronically infected with HCV genotype 1, 2, or 3. Boston, Massachusetts: Program and abstracts of 63rd Annual Meeting of the American Association for the Study of Liver Diseases; 2012. Abstract LB-2. [Google Scholar]


