Abstract
High sensation seeking is associated with strong approach behaviors and weak avoidance responses. The present study used functional magnetic resonance imaging (fMRI) to further characterize the neurobiological underpinnings of this behavioral profile using a Go/No-go task. Analysis of brain activation associated with response inhibition (No-go) versus response initiation and execution (Go) revealed the commonly reported right lateral prefrontal, insula, cingulate, and supplementary motor area network. However, right lateral activation was associated with greater No-go than Go responses only in low sensation seekers. High sensation seekers showed no differential activation in these regions but a more pronounced Go compared to No-go response in several other regions that are involved in salience detection (insula), motor initiation (anterior cingulate) and attention (inferior parietal cortex). Temporal analysis of the hemodynamic response for Go and No-go conditions revealed that the stronger response to Go than No-go trials in high sensation seekers occurred in in the earliest time window in the right middle frontal gyrus, right mid-cingulate and right precuneus. In contrast, the greater No-go than Go response in low sensation seekers occurred in the later time window in these same regions. These findings indicate that high sensation seekers more strongly attend to or process Go trials and show delayed or minimal inhibitory responses on No-go trials in regions that low sensation seekers use for response inhibition. Failure to engage such regions for response inhibition may underlie some of the risky and impulsive behaviors observed in high sensation seekers.
Keywords: Functional magnetic resonance imaging, cognitive control, personality
1. Introduction
Many behaviors involve a complex interplay of initiating, executing and inhibiting responses and actions. In the present context, initiation refers to the act of preparing and executing a response to a particular stimulus by engaging the appropriate motor and cognitive systems. Inhibition refers to the act of withholding a response or resisting an urge to act on a particular stimulus. Inhibition and initiation are key components of numerous cognitive behaviors, such as decision making (Rubia et al., 2001), goal-directed behavior (Kenner et al., 2010), cognitive control (Chambers et al., 2007), self-regulation (Hofmann et al., 2009), task switching (Swainson et al., 2003), attentional processing (Garavan & Hester, 2007), and executive function (Sanders et al., 2008). Understanding individual differences in these neurocognitive processes, which are often studied with well-controlled laboratory tasks, is an ongoing inquiry in psychology (Belin et al., 2008; Finn et al., 1999; Zuckerman & Kuhlman, 2000) and neuroscience (Gehring & Willoughby, 2002; Goldstein & Volkow, 2002; Paulus et al., 2003; Schoenbaum et al., 2006).
1.1 Brain Regions Implicated in Response Initiation and Inhibition
Response inhibition and initiation are often studied using variations of the stop signal and Go/No-go (GNG) tasks. In the stop signal task, participants are instructed to respond to cues unless the cue is accompanied by an additional “stop” signal which indicates that the response should be withheld. A Go signal is present (or implied) on every trial, but the stop signals occur only occasionally in order to establish prepotent responding thereby making inhibition on stop trials more difficult. In the GNG task, separate cues are assigned either to Go or No-go signal trials, so the Go signal is not present on every trial. Go signals may typically outnumber No Go signals to establish prepotent Go responding across trials, as in the stop signal task. The GNG task emphasizes response selection processes more than the stop signal task because the Go and No-go signals are present on different trials; nevertheless, response initiation is associated with the Go conditions and response inhibition is associated with the stop or No-go conditions in both tasks.
GNG and stop-signal tasks have been widely used to study the brain regions involved in response inhibition (Aron & Poldrack, 2005; Hester et al., 2004; Simmonds et al., 2008). The network of regions commonly implicated include right lateral frontal cortex (Aron & Poldrack, 2006; Boehler et al., 2010; Chikazoe et al., 2009a; Chikazoe et al., 2009b; Goya-Maldonado et al., 2010; Kenner et al., 2010; Konishi et al., 1998; Konishi et al., 1999; Mostofsky et al., 2003; Rubia et al., 2001; Swainson et al., 2003; Van Gaal et al., 2010; Watanabe et al., 2002; Xue et al., 2008), the anterior cingulate cortex (ACC: Aron & Poldrack, 2006; Dillo et al., 2010; Lutcke & Frahm, 2008; Rubia et al., 2001;), premotor and supplementary motor areas (Chikazoe et al., 2009b; Kenner et al., 2010; Mostofsky et al., 2003; Rubia et al., 2001; Simmonds et al., 2008; Van Gaal et al., 2010; Watanabe et al., 2002; Xue et al., 2008;) and the insula (INS: Aron & Poldrack, 2006; Boehler et al., 2010; Chikazoe et al., 2009b). The major focus of these studies has been on brain regions associated with response inhibition because inhibition failures have been implicated in a wide range of maladaptive behaviors.
Fewer studies have focused on regions involved in response initiation (i.e., preparing and executing Go responses), but distinguishing between response initiation and inhibition networks is important for understanding individual differences in behavioral regulation. For example, do individuals with poor inhibitory control fail to activate the critical No-go circuitry for response inhibition? Or is that same circuitry more strongly engaged for response initiation than inhibition among these individuals? The present study will address these questions with a design in which both response inhibition and initiation are examined.
1.2 Individual Differences in Response Initiation and Inhibition
Certain psychiatric conditions, such as ADHD and addiction, have been linked to problems with behavioral regulation, which can be examined with GNG tasks. For example, children with ADHD or high-impulsive substance-abusing individuals have difficulties inhibiting responses and do not recruit regions typically implicated in response inhibition as strongly as do controls during GNG task performance (Asahi et al., 2004; Booth et al., 2005). Similarly, cocaine addicts who exhibit poor behavioral regulation (see Garavan & Hester, 2007) demonstrate lower levels of prefrontal activation during response inhibition on the GNG task (Hester & Garavan, 2004).
Poor behavioral regulation is also associated with impulsive personality, which is measured in various ways (Joseph et al., in press). There is considerable debate concerning the nature of impulsivity, but many descriptions such as Gray’s model consisting of behavioral approach (BAS) and behavioral inhibition (BIS) systems (Carver & White, 1994), include concepts of approach, sometimes described as sensitivity to reward, sensation seeking or novelty seeking, as well as concepts of behavioral inhibition, described as a strong self-control or high harm avoidance. Impulsivity can be viewed as the product of an overactive approach system and/or an underactive inhibitory system. Sensation seeking is a personality trait characterized by the tendency to seek novel sensations and experiences even if that endeavor involves high levels of risk. Consequently, high sensation seekers (HSS) are more likely to have an overactive approach system and weaker inhibition system than low sensation seekers (LSS; Depue & Collins, 1999; Zuckerman, 1979). For example, sensation seeking is one factor that contributes to reported alcohol consumption in college students (Zuckerman & Kuhlman, 2000).
An overactive approach system coupled with a weaker inhibitory system can be explored by comparing brain activation in HSS and LSS. Joseph and colleagues (2009) identified specific brain activation patterns for LSS and HSS during emotional processing for high and low arousal images. The right anterior insula/inferior frontal gyrus, often associated with autonomic arousal, exhibited greater activation in HSS than LSS for high versus low arousal items. Left ACC, a region implicated in behavioral regulation, was activated more strongly in LSS for high versus low arousal items. Additionally, convergent measures of impulsive sensation seeking, such as urgency and disinhibition, were correlated with activity in regions that were more active during high-arousal conditions.
Other studies have used fMRI to examine sensation seeking in varied behavioral domains such as reward and novelty processing (Krebs et al., 2009), monetary incentive delay responding (Abler, et al., 2006), risky behaviors (Freeman & Beer, 2010) and gambling (Lemenager et al., 2011). However, to our knowledge, no studies have examined differences in brain activation between HSS and LSS on a GNG task. Therefore, the present study used a GNG task and fMRI to identify brain regions implicated in response inhibition (No-go trials) and response initiation (Go trials) for high and low sensation seekers. The motivation for using an extreme group comparison of high and low sensation seekers was to maximize the likelihood of biological differences between group members (e.g., Bardo et al., 1996).
We used a GNG task that equally emphasized response inhibition and initiation by presenting equal numbers of No-go and Go trials. This design did not confound response inhibition trials with lower frequency of occurrence (see Chikazoe et al., 2009a) or with additional visual stimulation that was not present on Go trials. If individuals with poor inhibitory control fail to activate the critical circuitry for response inhibition, then we expect the predominant outcome to be greater activation in LSS than HSS in the No-go condition as an index of a stronger regulatory system. Conversely, an overactive approach system (in HSS) would predict stronger activation in the Go condition in HSS than LSS.
2. Results
The main contrast of interest was the group comparison of HSS and LSS for the two contrasts, Go > No-go and No-go > Go, as these contrasts isolated regions differentially modulated by sensation seeking status for response inhibition and initiation. Note that the group comparison (LSS > HSS) for the No-go > Go contrast is logically equivalent to the group comparison (HSS > LSS) for the Go > No-go contrast (see Methods). Because of this logical equivalence, the regions isolated by the group comparison of LSS > HSS for the No-go > Go contrast could either be interpreted as greater activation for LSS for No-go or greater activation for HSS for go. This ambiguity necessitated follow-up ANOVAs in regions of interest which allowed us to determine which of the group or condition differences were driving the interaction.
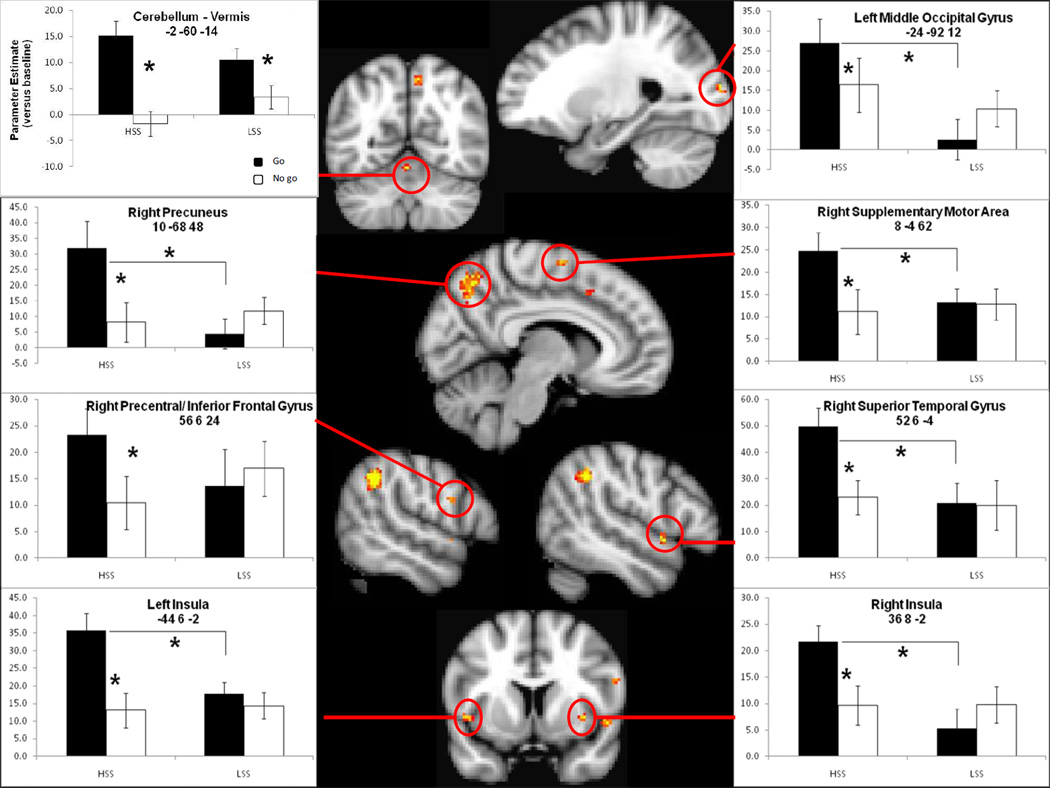
Fourteen ROIs emerged from the group comparison of LSS > HSS for the No-go > Go contrast, as shown in Table 1. As expected, all fourteen regions showed a significant trial type (Go, No-go)×Sensation seeking status (HSS, LSS) interaction according to the follow-up ANOVAs. Four of these regions showed a main effect of trial type in which there was greater signal for response initiation (Go) than response inhibition (No-go): the right superior temporal gyrus, F(1, 38) = 7.82, p = .008, the cerebellar vermis, F(1, 38) = 29.41, p < .001, the right supplemental motor area, F(1, 38) = 10.03, p = .003, and the left INS, F(1, 38) = 16.77, p < .001. Only the left middle occipital gyrus showed a main effect of sensation seeking status, F(1, 38) = 4.37, p = .04, in which HSS recruited this region more strongly than LSS (as seen in Figure 2).
Table 1.
Regions that were more active for Go than No-go and in which HSS showed greater responses than LSS. A minimum cluster size of 10 voxels was required and the threshold was set at z = 2.58, p = .005, uncorrected. MNI = Montreal Neurological Institute.
| MNI | Number | Go v. No Go x HSS v. LSS |
||||
|---|---|---|---|---|---|---|
| Region | x | y | z | BA | of voxels | F(1, 38) |
| p-value | ||||||
| L. Insulaa | −44 | 6 | 2 | 48 | 14 | 9.18 |
| 0.004 | ||||||
| R. Supplementary Motor Areaa | 8 | −4 | 62 | 6 | 16 | 8.81 |
| 0.005 | ||||||
| Cerebellum – vermisa | −2 | −60 | −14 | 17 | 4.91 | |
| 0.033 | ||||||
| R. Superior Temporal Gyrusa | 52 | 6 | −4 | 38 | 23 | 7.00 |
| 0.012 | ||||||
| R. Insula | 36 | 8 | −2 | 48 | 17 | 14.03 |
| 0.001 | ||||||
| R. Precentral/Inferior Frontal Gyrus | 56 | 6 | 24 | 6 | 18 | 5.80 |
| 0.021 | ||||||
| L. Middle Occipital Gyrusb | −24 | −92 | 12 | 18 | 21 | 6.97 |
| 0.012 | ||||||
| R. Precuneus | 10 | −68 | 48 | 7 | 272 | 11.69 |
| 0.002 | ||||||
| L. Anterior Cingulate Cortex | −8 | 22 | 22 | 24 | 16 | 11.43 |
| 0.002 | ||||||
| R. Middle Fronta Gyrus | 38 | 36 | 36 | 46 | 22 | 9.63 |
| 0.004 | ||||||
| R. Inferior Frontal Gyrus | 32 | 18 | 26 | 47 | 22 | 7.18 |
| 0.011 | ||||||
| R. Middle Frontal Gyrus | 26 | 40 | 32 | 46/9 | 30 | 10.62 |
| 0.002 | ||||||
| R. Mid Cingulate | 6 | 16 | 42 | 32 | 46 | 5.48 |
| 0.025 | ||||||
| R. Inferior Parieta Lobe | 56 | −48 | 38 | 40 | 171 | 18.61 |
| <.001 | ||||||
Regions also showing a main effect of trial type (Go v. No go).
L. MOG showed a main effect of sensation seeking status (HSS v LSS)
Figure 2.
Regions that were more active for Go than No-go among HSS. In addition, the cerebellar vermis was more active for Go than No-go among LSS.
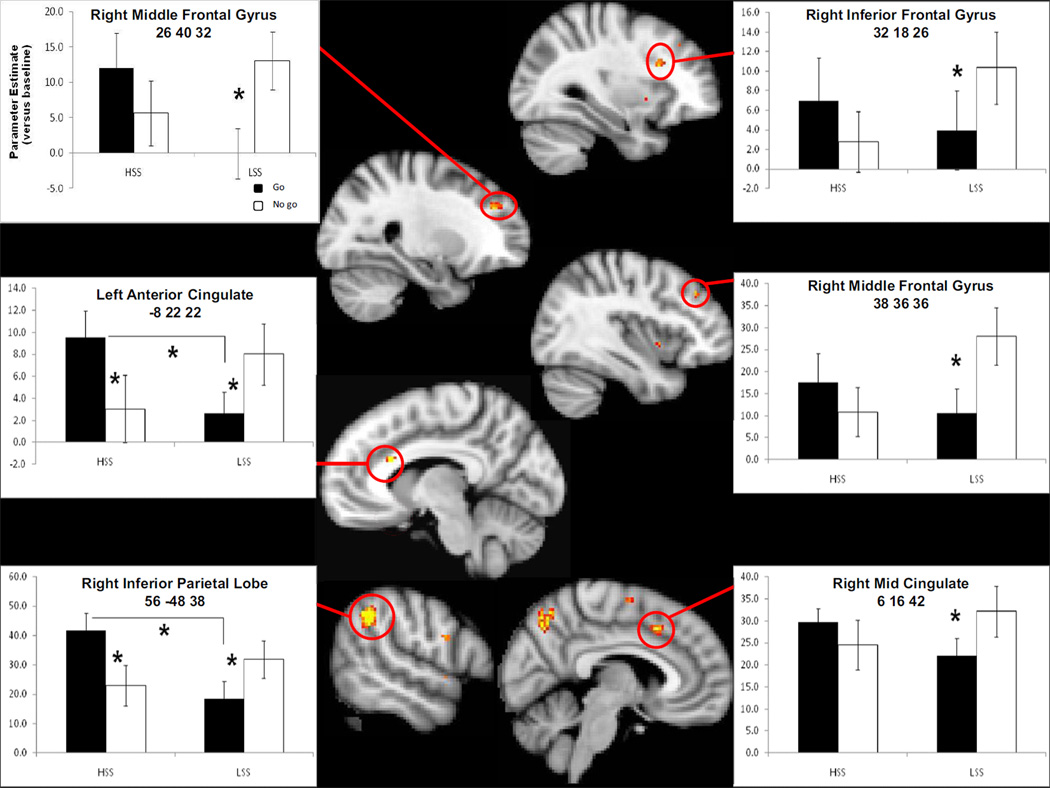
Figures 1 and 2 show activation maps with mean parameter estimates versus baseline by trial type (black bars for Go trials) and sensation seeking status on the x-axis for the fourteen regions in the Table. Figure 1 shows response inhibition regions (more activation for No-go than Go in LSS) whereas Figure 2 shows response initiation regions (more activation for Go than No-go in HSS). Figure 1 also shows two regions (left anterior cingulate cortex, ACC, and right inferior parietal cortex, IPL) in which HSS showed greater recruitment for Go than No-go trials, LSS showed greater recruitment for No-go than Go, and greater Go activation for HSS compared to LSS. None of the fourteen regions showed a greater No-go than Go response for HSS.
Figure 1.
Regions showing greater activation for No-go than Go in LSS. Additionally, the left anterior cingulate and right inferior parietal lobule showed greater Go than No-go activation for HSS compared to LSS.
2.1 Response Inhibition Regions
One set of post hoc follow-up tests analyzed the main effect of sensation seeking for Go and No-go trials separately whereas another set of post-hoc tests analyzed the main effect of trial type within each group separately. Post hoc tests conducted with the Sidak adjustment for multiple comparisons revealed that LSS showed greater signal for No-go compared to Go in the right inferior parietal lobe (p = .02), the right mid cingulate (p = .03), right middle frontal gyrus-BA 46 (p = .004), the right inferior frontal gyrus (p = .03), the right middle frontal gyrus-BA 46/9 (p = .003), and the left ACC (p = .04), as seen in Figure 1. No regions showed significantly more activation for No-go compared to Go trials for HSS. No regions showed a greater response in LSS than HSS for No-go trials. With one exception, these regions showed a significant positive correlation between Go minus No-go difference score and scores on the ZKPQ-SS, a measure of sensation-seeking status distinct from the BSSS (.47 < r < .56, all p’s < .002). The one exception was the right inferior frontal gyrus, which fell short of statistical significance (r = .31, p = .055). The left ACC region also showed a significant positive correlation between Go minus No-go fMRI signal and scores on the UPPS-Urgency scale (Whiteside and Lynam, 2001), a dimension of impulsivity distinct from sensation seeking (r = .32, p = .05).
2.2 Response Initiation Regions
Post hoc tests conducted with the Sidak adjustment for multiple comparisons showed that for Go trials, there was a greater response for HSS than LSS in the right inferior parietal lobule (p = .01) and the left ACC (p = .03), as seen in Figure 1, and in the right precuneus (p = .01), the right superior temporal gyrus (p = .009), the left middle occipital gyrus ( p= .004), the right INS (p = .01) the right supplementary motor area (p = .03), and the left insula (p = .004), as seen in Figure 2. No regions showed significant differences between HSS and LSS for No-go trials. HSS showed greater signal for Go than No-go in the right precuneus (p = .001), the right inferior parietal lobe (p = .001), the right superior temporal gyrus (p < .001), the left middle occipital gyrus (p = .04), the right precentral/inferior frontal gyrus (p = .01), the right INS (p < .001), the vermis (p < .001), the right supplementary motor area (p < .001), the left ACC (p = .01), and the left INS (p < .001). Only the vermis showed greater activation for Go than No-go in LSS (p < .001). All of these regions showed a significant positive correlation between Go minus No-go difference score and scores on the ZKPQ-SS (.39 < r < .60, all p’s < .014). The vermis and right supplementary motor area also showed a significant positive correlation between Go minus No-go fMRI signal and scores on the UPPS-Urgency scale: r = .40, p = .012 for vermis; r = .38, p = .018 for right supplementary motor area.
2.3 Hemodynamic Responses
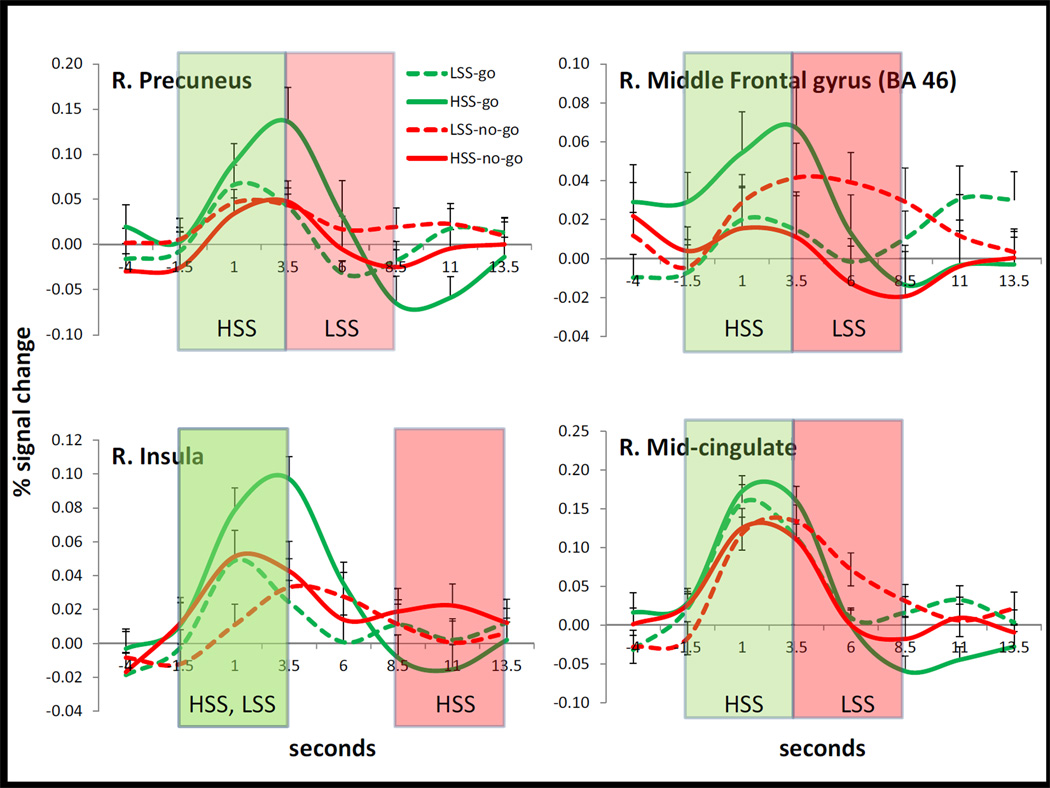
Hemodynamic responses (HDRs) for the ROIs in Table 1 were more closely examined to determine whether the modulation of fMRI signal by sensation seeking status and condition (Go, No-go) was further qualified by the timing of the HDR. To this end, area under the curve (AUC) was calculated within three time windows (time points 2 to 4; time points 4 to 6; time points 6 to 8) separately for each subject and condition in each ROI. The reason for calculating AUC was to reduce the 8 time points to a more manageable and interpretable number of time windows. Mixed ANOVAs (one for each brain region) examined the effect of condition (Go, No-go), time window (early, middle, late) and sensation seeking on AUC. We were most interested in whether the threeway interaction was significant, because that would indicate that HSS and LSS recruit the region differentially for Go and No-go conditions in different time windows. Note that the ANOVAs conducted on PE values (Figures 1 and 2) already indicate which regions were differentially recruited for Go and No-go conditions in HSS and LSS. The present analysis was conducted to examine whether the differential response in the two groups varied over the time course of the hemodynamic response.
Four regions showed this three-way interaction (Figure 3): the right precuneus, F(2, 76) = 4.6, p = .013, the right mid-cingulate, F(2, 76) = 4.0, p = .022, the right middle frontal gyrus (BA 46), F(2, 76) = 4.5, p = .015, and the right insula, F(2, 76) = 4.2, p = .018. Paired t-tests examined the difference between Go and No-go conditions in each time window for each subject group to further characterize this interaction. For HSS, the Go condition yielded greater activation than the No-go condition (all p’s < .041) in the early time window in the right precuneus, the right mid-cingulate, the right middle frontal gyrus (BA 46; green shading in Figure 3). For LSS, the No-go condition yielded greater activation than the Go condition (all p’s < .022) in the middle time window in these same three regions (red shading in Figure 3). The right insula yielded a somewhat different pattern. Both HSS and LSS showed a greater response to Go than No-go in the early time window (p = .037; green shading in Figure 3) but HSS also showed a greater response to No-go than Go conditions in the late time window (p = .043; red shading in Figure 3).
Figure 3.
Hemodynamic responses in four regions that showed a three-way interaction of sensation seeking, condition and time window. The x-axis, “Seconds”, indicates time relative to stimulus onset. The y-axis is percent signal change. Green overlays indicate time windows with a greater response to Go than to No-go trials in the group designated by the text (e.g., HSS, LSS). Red overlays indicate time windows with a greater response to No-go than to Go trials in the group designated by the text. Error bars are standard error of the mean.
3. Discussion
Using a GNG task, this study identified brain regions that were differentially recruited for response inhibition and response initiation among high and low sensation seekers. Regions that were more strongly recruited for No-go than Go conditions were linked to response inhibition and regions that showed a greater response to Go than to No-go conditions were linked to response initiation. While inhibition is often the cognitive process of greater interest in GNG experiments, this study examined both initiation and inhibition because both dimensions are involved in real-world activities (Hofmann et al., 2009; Rubia et al., 2001). Interestingly, response initiation conditions revealed more group differences than response inhibition conditions in the present study. No group differences emerged for No-go trials despite the fact that response inhibition is much more commonly assessed than response initiation in the literature.
We suggest the following working model of differential neural recruitment for response inhibition in HSS and LSS. One component of this model is that HSS may anticipate or attend more to initiation conditions whereas LSS may anticipate or attend more to inhibition conditions. This tuning process may be reflected in regional activation that is associated with salience detection (insula), engagement of motor behaviors (left ACC) and re-orienting attention to salient events (right IPL). The second component of this model is that HSS may have more difficulty switching into inhibition mode (in RIFG, RMFG) leading to a less differentiated response for Go and No-go conditions or a delayed No-go response in regions that are normally associated with response inhibition. This model is elaborated more below.
3.1 High sensation seekers are more sensitive to Go conditions
The majority of regions modulated by sensation seeking status showed greater Go than No-go responses in HSS but no differential response in LSS or a greater No-go than Go response in LSS: right precuneus, right precentral / inferior frontal, bilateral insula, right supplementary motor area, left middle occipital, right superior temporal cortex. Many of these regions have been associated with response inhibition in other studies (Aron & Poldrack, 2006; Boehler et al., 2010; Chikazoe et al., 2009a; Chikazoe et al., 2009b; Goya-Maldonado et al., 2010; Kenner et al., 2010; Konishi et al., 1998; Konishi et al., 1999; Mostofsky et al., 2003; Rubia et al., 2001; Simmonds et al., 2008; Swainson et al., 2003; Van Gaal et al., 2010; Watanabe et al., 2002; Xue et al., 2008). The finding that these regions are associated with initiation in HSS may seem surprising, but few studies have examined modulation of response inhibition regions by individual differences in personality. The typically-reported inhibition response in these regions may only apply to individuals who are low in sensation seeking. Greater activation for Go than No-go conditions in a number of different regions in HSS suggests that approach cues (i.e. the Go signal) may be more salient for this group. Only one region, the cerebellar vermis, showed a greater response in the Go than No-go condition for both subject groups.
An additional facet of sensation seeking, Urgency, was also positively correlated with greater Go compared to No-go signal in three of these regions (vermis, right supplementary motor area and left ACC). These regions are engaged in planning and initiation of motor movements (Ghez & Fahn, 1985; Nachev, Kennard & Husain, 2008). Urgency is defined as rash and impulsive behavior related to emotional dyscontrol (Whiteside & Lynam, 2001). Together these findings suggest that systems involved in the planning and initiation of motor behavior may be more strongly primed for action in HSS, which is consistent with an overactive approach system.
When different time windows of the hemodynamic response were examined, HSS also showed different hemodynamics compared to LSS. In the right precuneus (Figure 3), HSS show a greater Go response in the early time window, whereas LSS show a greater No-go response in the middle time window. HSS never show a No-go response that dominates the Go response. This latter result mirrors the results in the right middle frontal cortex and mid-cingulate. In the right insula, the Go condition induces an early stronger response (compared to No-go) in both groups. However, in HSS, the Go response lasts longer compared to LSS. In addition, HSS show a delayed inhibition response in this region that LSS recruit primarily for initiation.
3.2 High sensation seekers show atypical recruitment of response inhibition network
Lateral prefrontal regions like the inferior and middle frontal cortex are commonly implicated in response inhibition. LSS recruited these regions more strongly on inhibition than initiation trials, but HSS showed no difference between the two conditions. Aron, Robbins & Poldrack (2004) review neuropsychological and neuroimaging evidence that lateral prefrontal regions, especially the right inferior frontal cortex, are a locus for both response inhibition and task-switching. Difficulty in switching into a mode that requires halting a response may underlie the lack of differentiation of Go and No-go responses in HSS. By comparison, LSS show more activation in these inhibitory regions which may reflect successful switching into inhibition mode in order to direct motor systems to terminate an initiated response. The right mid-cingulate showed a similar response profile as the right lateral prefrontal regions. In addition to their involvement in response inhibition reported in other studies (Aron & Poldrack, 2006; Boehler et al., 2010; Chikazoe et al., 2009b; Mostofsky et al., 2003) these frontal and para-limbic regions have also been implicated in top-down behavioral regulation (Cunningham et al., 2005; Matthews et al., 2004; Ochsner et al., 2004). Compared to LSS, HSS do not differentially recruit these regions involved in self-regulation which could contribute to their tendency to engage in risky and potentially problematic behaviors such as drug abuse.
When different time windows of the hemodynamic response were examined, the right mid-cingulate and right middle frontal gyrus (BA 46) showed modulation by sensation seeking status. HSS showed a stronger response to Go than No-go conditions in the early time window and LSS showed a stronger response to No-go than to Go conditions in the middle time window. In addition, in the right middle frontal region, HSS continued to show a stronger response in the middle time window to Go than No-go conditions. This region has been strongly implicated in response inhibition in other studies (Boehler et al., 2010; Mostofsky et al., 2003), but HSS activate this region inappropriately (i.e. to Go conditions) and the inappropriate response persists. In fact, in this region the No-go condition never surpasses the Go condition. In the right mid-cingulate, activation during the No-go trials eventually surpasses Go trials, but only in the later time window.
3.3 Modulation of Attentional and Salience Networks by Sensation Seeking Status
The ACC and insula are important components of the “Salience network” (Menon & Uddin, 2010). According to Menon and Uddin the insula detects both internal and external salient events, and is strongly coupled with the anterior cingulate to “facilitate rapid access to the motor system” (p. 655). Interestingly, in the present study, both the left ACC and insula were modulated by sensation seeking status. The insula showed the greatest response to the Go trials in HSS and this response persisted longer than the Go response in LSS. We suggest that the insula is related to the greater salience of the Go condition and HSS show a more persistent response. The left ACC also showed a greater response to Go than No-go in HSS but this region additionally showed a greater No-go than Go response in LSS. Other studies have also associated the ACC with both initiation (Aron & Poldrack, 2006; Watanabe et al., 2002) and inhibition (Chikazoe et al., 2009b; Dillo et al., 2010; Menon et al., 2001).
Joseph et al. (2009) reported a similar dissociation between the ACC and INS as a function of sensation seeking in the same sample of subjects using an emotional induction task. In that study, HSS showed a strong early response to high arousal items in the right insula, but LSS showed a fairly blunted response to high arousal items in the right insula. A similar pattern emerged in the present study. In addition, in Joseph et al., HSS showed a delayed response to high-arousal items in the left ACC compared to LSS who showed a strong left ACC response in an earlier time window compared to HSS. Joseph et al. interpreted this finding as an overactive approach system in HSS, marked by an early response in the right insula to high arousal items, and a weaker regulatory system in HSS, marked by delayed responding in the left ACC. In the present study, an overactive approach response in HSS was reflected in early and persistent right insula and robust ACC activation for response initiation conditions. A stronger regulatory response in LSS was reflected in greater left ACC activation for response inhibition.
The neurobiological underpinnings of a stronger approach than avoidance system likely include components related to reward processing, novel detection and appetitive responses (Joseph, Lile & Kelly, in press). However, recruitment of these components may depend heavily on the particular cognitive or motivational task used in a study. We suspect that the lack of reward circuitry activation in the present study is likely due to the fact that there were no programmed reinforcement contingencies or feedback based on trial outcome associated with task performance.
The right IPL also showed an interaction of sensation seeking and Go / No-go conditions, with HSS recruiting this region more strongly for Go than No-go and LSS recruiting this region more strongly for No-go than Go conditions. This region is strongly implicated in attention and considered a critical component of a right-lateralized ventral attention network that is involved in re-orienting attention to salient events (Fox et al., 2006). The recruitment of the right IPL in the present study may have depended on the stimulus type that was more relevant for a particular individual. In the present context, we speculate that the Go signal was more salient for HSS and the No-go signal was more salient for LSS. HSS may have more strongly attended or anticipated the Go than the No-go condition or devoted more neural and cognitive resources to that condition, which would be consistent with an overactive approach system that is designed to act on and pursue appetitive goals. In contrast, LSS may have devoted more resources to or more strongly attended the No-go condition, which is consistent with a system designed for regulation or withdrawal. In summary, regions associated with salience detection (right insula), engagement of motor behaviors (left ACC) and re-orienting attention to salient events (right IPL) all showed differential recruitment for Go and No-go conditions by HSS and LSS.
3.4 Limitations of the Present Study
Many studies of response inhibition present fewer No-go than Go trials to induce prepotent responding. The somewhat rarer No-go events become more difficult to inhibit in the context of more frequent Go trials. This manipulation is important for capturing impulsive responding. The goal of the present study was to examine both response initiation and inhibition so the number of trials was necessarily equated for the two conditions to provide sufficient power to examine networks associated with both initiation and inhibition trial conditions. Nevertheless, an interesting future study would be to examine whether inappropriate recruitment of response inhibition regions by HSS (as in the present study) would also emerge if fewer No-go trials were used. We suggest that when the frequency of Go and No-go events is equated, LSS and HSS tune the response inhibition network differently depending on the salience of the two trial conditions. If No-go trials were presented more infrequently, we suspect that inappropriate recruitment of response inhibition regions may be even more pronounced in HSS. However, additional research is needed to test this hypothesis.
3.5 Conclusion
In the present study, individual differences in sensation seeking were more pronounced in Go than No-go trials. This is somewhat surprising given that numerous studies report individual differences in withholding a response during No-go trials. For example, brain regions typically implicated in successful response inhibition are less active in individuals with ADHD (e.g., Booth et al., 2005), high impulsivity (Asahi et al., 2004) or cocaine addiction (e.g., Garavan & Hester, 2007; Hester & Garavan, 2004) versus controls. It is possible that individual differences in No-go activation are more pronounced when comparing clinical versus neurotypical groups than when comparing two neurotypical groups, as in the present study. The present sample was composed of individuals who reported minimal substance abuse, no psychiatric diagnoses, and were medication-free. The main difference between the two groups in the present study was self-reported level of sensation seeking. The novel and interesting finding in the present study was that individual differences in sensation seeking were more pronounced during response initiation than response inhibition trials. We suggest that this reflects greater attention to or salience of Go signals for HSS, which in turn is driven by an overactive approach system. This stronger approach response may be one of the risk factors for problematic behaviors like substance abuse or risky sex, which are associated with higher sensation seeking (Zuckerman & Kuhlman, 2000). Consequently, examining approach behaviors is as pivotal as understanding avoidance and withdrawal behaviors in neurotypical populations.
4. Experimental Procedure
4.1 Participants
Forty adults (20 men) aged 18-25 years participated in this study. Based on their Brief Sensation-Seeking Scale scores (Hoyle et al., 2002), participants in the top quartile based on population norms (Harrington et al., 2003; cutoff score for men and women = 35) were placed in the High Sensation Seeking group (HSS: 10 men; Mean age = 20.2 years, SD = 1.5) and those in the bottom quartile (cutoff score for men = 27, women = 25) were placed in the Low Sensation-Seeking group (LSS: 10 men; Mean age = 21.3 years, SD = 2.5). All participants gave informed consent and were financially compensated for participating. All procedures were approved by the University of Kentucky Medical Institutional Review Board.
Prior to scanning, participants were screened to ensure MRI safety. Exclusion criteria included left handedness, pregnancy, diagnosed learning disabilities, neurological disorders, previous head injury, claustrophobia, major psychiatric disorders, and metal in the body. Urine samples were collected to test for drug use (cocaine, marijuana, amphetamines, benzodiazepines, opioids etc.) and pregnancy during the medical screening and prior to the data collection session. No participants showed evidence of drug use or pregnancy. Participants also completed several additional measures: The Big-Five Inventory (John & Srivastava, 1999), the Urgency, Premeditation, Perseverance, and Sensation Seeking Impulsivity Scale (Whiteside & Lynam, 2001), the Eysenck Personality Inventory (Eysenck & Eysenck, 1975), the Zuckerman Sensation-Seeking Scale (Form V; Zuckerman, 1994), and the Zuckerman-Kuhlman Personality Questionnaire (Zuckerman et al., 1993), the Michigan Alcoholism Screening Test – short form (MAST; Selzer et al., 1975), Beck Depression Inventory (BDI; Beck & Beck, 1972) and a 17-item drug use questionnaire derived from the Addiction Severity Index (McLellan et al., 1992) in which they indicated the last time they used the following substances (never, more than a month ago, or during the past month): coffee or tea, smoked tobacco, smokeless tobacco, nicotine gum or patch, beer, wine, wine coolers, hard liquor, marijuana, inhalants, cocaine, nonprescription narcotics, prescription narcotics, uppers, downers, relaxants, and hallucinogens. If they reported drug use in the past month, they also indicated how many days during the past month that they had used the drug. Independent samples t-tests were conducted to determine whether the HSS and LSS groups were different in terms of age, years of education, MAST, BDI and drug use. The only measure that showed a significant group difference was number of beers consumed in the past 30 days. LSS consumed more beer (M = 4.4, SD = 3.9) than HSS (M = 1.7, SD = 2.5), t(38) = −2.65, p = .012.
4.2 Go/No-go Task
Each participant completed one fMRI event-related run of the GNG task (adapted from Fillmore et al., 2005) consisting of 50 No-go trials, 50 Go trials, and 100 rest trials. Participants first saw a cue (X or O). After a delay of 100, 200, or 300 ms a No-go or Go signal (red or green box, respectively) surrounded the cue for 1000 ms. Both No-go and Go signals were used in the present study to control for the amount of visual information presented in No-go and Go conditions. The cue and signal were then followed by a fixation crosshair for 1200-1400 ms such that each trial lasted 2500 ms. Trial order was randomized according to the scripts for stimulus timing simulation in AFNI’s (Analysis of Functional Neuro-Images) software package (http://afni.nimh.nih.gov). Participants were instructed to press the button under one index finger when presented with the “X” cue and press the button under their other index finger when presented with the “O” cue unless they saw the No-go signal (red box), which indicated they were to make no response at all. Importantly, the instructions mentioned nothing about the Go cue. The assignment of stimulus responses (X or O) to index fingers (left or right hand) was counterbalanced across participants. E-Prime software (Psychology Software Tools, Pittsburgh, PA) was used to display the stimuli and record responses. E-Prime was synchronized with trigger pulses from the scanner such that each individual trial was triggered by the scanner, and participants made button press responses using a fiber-optic response pad (MRA Inc., Washington, PA). Due to a technical error, behavioral button-press responses were not recorded, and we were not able to analyze accuracy or response times.
4.3 Data Acquisition
All participants were scanned using a Siemen’s 3T Trio MRI system equipped for echoplanar imaging (TR=2.5 s, TE=30 ms, flip angle = 81°, 40 axial slices: matrix = 64 × 64, voxel size = 3.5mm3, FOV=224 × 224mm2). High-resolution anatomical structural images were acquired with a T1-weighted MP-RAGE (magnetization-prepared rapid gradient echo) sequence (192 sagittal slices: matrix = 224 × 256, slice thickness = 1mm, no gap, voxel size = 1mm3, FOV=224 × 256mm2).
4.4 fMRI Data Analysis
Using FMRIB’s FSL package (http://www.fmrib.ox.ac.uk/fsl), images in each participant’s time series were motion corrected with the MCFLIRT module. Images in the time series were spatially smoothed with a 3D Gaussian kernel (FWHM = 7 mm3), and temporally high-pass filtered (cutoff = 80s). For the individual subject analysis, FILM (FSL’s Improved Linear Model) was used to estimate the hemodynamic parameters for different explanatory variables (EVs) and to generate statistical contrast maps of interest within each subject. The two EVs for the GNG signal task modeled Go (response initiation) trials and No-go (response inhibition) trials as single impulses which were convolved with a double-gamma hemodynamic response function. The two contrasts of interest were Go > No-go to identify regions in which activation was greater for initiation compared to inhibition and No-go > Go to identify regions in which activation was greater for inhibition compared to initiation. Contrast maps for each subject were normalized into common stereotaxic space (MNI) before mixed-effects group analyses using FMRIB’s FLAME module were performed. The main contrast of interest was the group comparison of HSS and LSS for the two contrasts, Go > No-go and No-go > Go as these contrasts isolated regions differentially modulated by sensation seeking status for response inhibition and initiation. Note that the group comparison (LSS > HSS) for the No-go > Go contrast is logically equivalent to the group comparison (HSS > LSS) for the Go > No-go contrast. In fact, these two contrasts produced nearly identical clusters that differed only slightly in size (average difference in cluster size across the 14 regions was 2.1 voxels, with eight of the regions showing a difference of 0 or 1 voxel) and only slightly in MNI coordinates for the center of the cluster (average difference in MNI-x: .71 mm, MNI-y: .57 mm, MNI-z: .86 mm). Because of this logical equivalence, the regions isolated by the group comparison of LSS > HSS for the No-go > Go contrast could either be interpreted as greater activation for LSS for No-go or greater activation for HSS for go. This ambiguity necessitated the follow-up ANOVAs in regions of interest (below) which allowed us to determine which of the group or condition differences were driving the activation.
4.5 Region of Interest Analysis
Regions of interest (ROI) were defined by a minimum cluster size of 10 contiguous voxels (Xiong et al., 1995) and all clusters were thresholded at z = 2.58, p < .005, two-tailed. Parameter estimate (PE) values from the Go > Baseline and No-go > Baseline contrasts were extracted for each subject in each ROI to ensure PE values for the more complex contrasts [e.g. HSS (Go > No-go) > LSS (Go > No-go)] represented activation rather than deactivation for appropriate interpretation. Mixed ANOVAs using SPSS (IBM: Somers, NY) were conducted on PE values from each ROI to confirm and quantify the sensitivity of each ROI to sensation seeking status (HSS, LSS) and trial type (Go, No-go). We also extracted hemodynamic responses in each ROI (using selective averaging; http://sites.la.utexas.edu/poldracklab/software) to identify whether interactions with sensation seeking and trial type occurred in earlier or later windows of the hemodynamic response given prior findings suggesting group differences in peaks of the HDR (Joseph et al., 2009). Selective averaging yielded 8 time points for each condition for each subject and each region. The time points reflected average percent signal change starting 4 sec prior to stimulus presentation up to 16 seconds following stimulus presentation in increments of 2.5 s.
4.6 Correlations with Personality Measures
To address whether the BSSS effectively characterized high and low SS, we used ZKPQ-SS as a convergent measure of sensation seeking. ZKPQ-SS was normally distributed within our sample. Normality was defined as a skewness value less than 2 times the standard error. We conducted correlations between ZKPQ-SS and Go minus No-go signal difference score based on data used in the ANOVAs. The purpose was to show that a convergent measure of SS (a strategy used in Joseph et al. 2009) was comparably related to fMRI response.
An exploratory analysis was also conducted to determine whether other facets of SS were associated with fMRI signal in each ROI. We focused on three different facets of sensation seeking: Form V Disinhibition, Form V – Boredom Susceptibility, and UPPS – Urgency, all of which were normally distributed within our sample. We explored only three measures given concerns with conducting multiple tests. Partial correlations between scores on each of these three variables and the Go – No-go difference score (from the data used in the ANOVAs) were conducted, while controlling for ZKPQ-SS scores. The purpose of this analysis was to explore other facets of sensation seeking that might be related to the differential Go/No-go response in different brain regions. We report those correlations that were significant at an uncorrected level of .05 given that we regarded this analysis as exploratory.
Highlights.
fMRI activation was examined in a go/no-go task in high and low sensation seekers (SS)
Low SS activate expected response inhibition regions for no-go trials
High SS activate some response inhibition regions for go trials
High SS show a stronger go than no-go response in early time window of BOLD response
Stronger go response in response inhibition regions may underlie high-SS impulsivity
Acknowledgements
This research was sponsored by the National Institutes of Health (P50 DA005312, R01 HD052724, P20 RR015592). We thank Kathryn Bylica, Jamie Furstenberg, and Dane Jensen for assistance with data collection and analysis and Faraday Davies and Shalika Whig for help with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abler B, Walter H, Erk S, Kammerer H, Spitzer M. Prediction error as a linear function of reward probably is coded in human nucleus accumbens. NeuroImage. 2006;31:790–795. doi: 10.1016/j.neuroimage.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: Relevance for genetic research in ADHD. Biol Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Aron AR, Poldrack RA. Cortical and Subcortical Contributions to Stop Signal Response Inhibition: Role of the Subthalamic Nucleus. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Asahi S, Okamoto Y, Okada G, Yamawaki S, Yokota N. Negative correlation between right prefrontal activity during response inhibition and impulsiveness: A fMRI study. Eur Arch Psy Clin N. 2004;254:245–251. doi: 10.1007/s00406-004-0488-z. [DOI] [PubMed] [Google Scholar]
- 6.Bardo MT, Donohew RL, Harrington NG. Psychobiology of novelty seeking and drug seeking behavior. Behav Brain Res. 1996;77:23–43. doi: 10.1016/0166-4328(95)00203-0. [DOI] [PubMed] [Google Scholar]
- 7.Beck AT, Beck RW. Screening depressed patients in family practice: A rapid technique. Postgrad Med. 1972;52:81–85. doi: 10.1080/00325481.1972.11713319. [DOI] [PubMed] [Google Scholar]
- 8.Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boehler CN, Appelbaum LG, Krebs RM, Hopf JM, Woldorff MG. Pinning down response inhibition in the brain--conjunction analyses of the Stop-signal task. Neuroimage. 2010;52:1621–1632. doi: 10.1016/j.neuroimage.2010.04.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Booth JR, Burman D, Meyer J, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. Larger deficits in brain networks selective for response inhibition than for visual selective attention in ADHD. J Child Psychol Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- 11.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- 12.Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S, Miyashita Y, Konishi S. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cereb Cortex. 2009a;19:146–152. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- 13.Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S. Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J Neurosci. 2009b;29:15870–15877. doi: 10.1523/JNEUROSCI.3645-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chambers CD, Bellgrove MA, Gould IC, English T, Garavan H, McNaught E, Kamke M, Mattingley JB. Dissociable mechanisms of cognitive control in prefrontal and premotor cortex. J Neurophysiol. 2007;98(66):3638–3647. doi: 10.1152/jn.00685.2007. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham WA, Raye CL, Johnson MK. Neural correlates of evaluation associated with promotion and prevention regulatory focus. Cogn Affect Behav Neurosci. 2005;5:202–211. doi: 10.3758/cabn.5.2.202. [DOI] [PubMed] [Google Scholar]
- 16.Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci. 1999;22:491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- 17.Dillo W, Göke A, Prox-Vagedes V, Szycik GR, Roy M, Donnerstag F, Emrich HM, Ohlmeier MD. Neuronal correlates of ADHD in adults with evidence for compensation strategies: A functional MRI study with a Go/No-Go paradigm. Ger Med Sci. 2010;8:1–8. doi: 10.3205/000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire. London: Hodder & Stoughton; 1975. [Google Scholar]
- 19.Fillmore MT, Marczinski CA, Bowman AM. Acute tolerance to alcohol effects on inhibitory and activational mechanisms of behavioral control. J Stud Alcohol. 2005;66:663–672. doi: 10.15288/jsa.2005.66.663. [DOI] [PubMed] [Google Scholar]
- 20.Finn PR, Justus A, Mazas C, Steinmetz JE. Working memory, executive processes and the effects of alcohol on Go/No-Go learning: Testing a model of behavioral regulation and impulsivity. Psychopharmacology. 1999;146:465–472. doi: 10.1007/pl00005492. [DOI] [PubMed] [Google Scholar]
- 21.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freeman HD, Beer JS. Frontal lobe activation mediates the relation between sensation seeking and cortisol increases. J Pers. 2010;78:1497–1528. doi: 10.1111/j.1467-6494.2010.00659.x. [DOI] [PubMed] [Google Scholar]
- 23.Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- 24.Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- 25.Ghez C, Fahn S. "The cerebellum". In: Kandel ER, Schwartz JH, editors. Principles of Neural Science. 2nd edition. New York: Elsevier; 1985. pp. 502–522. [Google Scholar]
- 26.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiat. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goya-Maldonado R, Walther S, Simon J, Stippich C, Weisbrod M, Kaiser S. Motor impulsivity and the ventrolateral prefrontal cortex. Psychiat Res: Neuroim. 2010;183:89–91. doi: 10.1016/j.pscychresns.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Harrington NG, Lane DR, Donohew L, Zimmerman RS, Norling GR, An J, Cheah WH, McClure L, Buckingham T, Garofalo E, Bevins CC. Persuasive strategies for effective anti-drug messages. Commun Monogr. 2003;70:16–30. [Google Scholar]
- 29.Hester R, Fassbender C, Garavan H. Individual differences in error processing: A review and reanalysis of three event-related fMRI studies using the Go/NoGo task. Cerebral Cortex. 2004;14:986–994. doi: 10.1093/cercor/bhh059. [DOI] [PubMed] [Google Scholar]
- 30.Hester R, Garavan H. Executive dysfunction in cocaine addiction: Evidence for discordant frontal, cingulate and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofmann W, Friese M, Strack F. Impulse and self-control from a dual-systems perspective. Perspect Psychol Sci. 2009;4:162–176. doi: 10.1111/j.1745-6924.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- 32.Hoyle RH, Stephenson MT, Palmgreen P, Lorch EP, Donohew RL. Reliability and validity of a brief measure of sensation seeking. Pers Indiv Differ. 2002;32:401–414. [Google Scholar]
- 33.John OP, Srivastava S. The Big Five Trait taxonomy: History, measurement, and theoretical perspectives. In: Pervin LA, John Oliver P., editors. Handbook of personality: Theory and research. 2nd ed. New York: Guilford Press; 1999. pp. 102–139. [Google Scholar]
- 34.Joseph JE, Kelly TH, Lile JA. The neurobiological basis of personality risk for addiction. In: Bates M, Miller P, editors. Encyclopedia of Addictive Behaviors. Elsevier; in press. [Google Scholar]
- 35.Joseph JE, Liu X, Jiang J, Lynam D, Kelly TH. Neural correlates of emotional reactivity in sensation seeking. Psychol Sci. 2009;20:215–223. doi: 10.1111/j.1467-9280.2009.02283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenner NM, Mumford JA, Hommer RE, Skup M, Leibenluft E, Poldrack RA. Inhibitory motor control in response stopping and response switching. J Neurosci. 2010;30:8512–8518. doi: 10.1523/JNEUROSCI.1096-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by eventrelated functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- 38.Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y. No-go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur J Neurosci. 1998;10:1209–1213. doi: 10.1046/j.1460-9568.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- 39.Krebs RM, Schott BH, Duzel E. Personality traits are differentially associated with patterns of reward and novelty processing in the human substantia nigra/ventral tegmental area. Biol Psychol. 2009;65:103–110. doi: 10.1016/j.biopsych.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 40.Lemenager T, Richter A, Reinhard I, Gelbke J, Beckmann B, Heinrich M, Kniest A, Mann K, Hermann D. Impaired decision making in opiate addiction correlates with anxiety and self-directedness but not substance use parameters. J Addict Med. 2011;5:203–213. doi: 10.1097/ADM.0b013e31820b3e3d. [DOI] [PubMed] [Google Scholar]
- 41.Lutcke H, Frahm J. Lateralized anterior cingulate function during error processing and conflict monitoring as revealed by high-resolution fMRI. Cerebral Cortex. 2008;18:508–515. doi: 10.1093/cercor/bhm090. [DOI] [PubMed] [Google Scholar]
- 42.Matthews SC, Paulus MP, Simmons AN, Nelesen RA, Dimsdale JE. Functional subdivisions within anterior cingulate cortex and their relationship to autonomic nervous system function. NeuroImage. 2004;2:1151–1156. doi: 10.1016/j.neuroimage.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 43.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grisson G, Pettinati H, Argeriou M. The fifth edition of the addiction severity index: cautions, additions and normative data. J Subst Abuse Treat. 1992;9:461–480. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 44.Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a go/no-go response inhibition task. Hum Brain Mapp. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Struct Func. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mostofsky SH, Schafer JGB, Abrams MT, Goldberg MC, Flower AA, Boyce A, Courtney SM, Calhoun VD, Kraut MA, Denckla MB, Pekar JJ. fMRI evidence that the neural basis of response inhibition is task-dependent. Cogn Brain Res. 2003;17:419–430. doi: 10.1016/s0926-6410(03)00144-7. [DOI] [PubMed] [Google Scholar]
- 47.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nature Rev: Neurosci. 2008;9:856–867. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- 48.Ochsner KN, Ray RD, Robertson ER, Cooper JC, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: Neural Systems Supporting the Cognitive Down- and Up-regulation of Negative Emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 49.Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- 50.Rubia K, Russell T, Overmeyer S, Brammer MJ, Bullmore ET, Sharma T, Simmons A, Williams S, Giampietro V, Andrew CM, Taylor E. Mapping motor inhibition: Conjunctive brain activations across different versions of Go/No-Go and stop tasks. Neuroimage. 2001;13:250–261. doi: 10.1006/nimg.2000.0685. [DOI] [PubMed] [Google Scholar]
- 51.Sanders J, Johnson KA, Garavan H, Gill M, Gallagher L. A review of neuropsychological and neuroimaging research in autistic spectrum disorders: Attention, inhibition and cognitive flexibility. Res Autism Spectr Disord. 2008;2:1–16. [Google Scholar]
- 52.Schoenbaum G, Roesch MR, Stalnaker TA. Orbitofrontal cortex, decision-making and drug addiction. Trends Neurosci. 2006;29:116–124. doi: 10.1016/j.tins.2005.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alc. 1975;36:117–126. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- 54.Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–232. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swainson R, Cunnington R, Jackson GM, Rorden C, Peters AM, Morris PG, Jackson SR. Cognitive control mechanisms revealed by ERP and fMRI: Evidence from repeated task-set switching. J Cogn Neurosci. 2003;15:785–799. doi: 10.1162/089892903322370717. [DOI] [PubMed] [Google Scholar]
- 56.Van Gaal S, Ridderinkhof KR, Scholte HS, Lamme VA. Unconscious activation of the prefrontal no-go network. J Neurosci. 2010;30:4143–4150. doi: 10.1523/JNEUROSCI.2992-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watanabe J, Sugiura M, Sato K, Sato Y, Maeda Y, Matsue Y, Fukuda H, Kawashima R. The human prefrontal and parietal association cortices are involved in NO-GO performances: an event-related fMRI study. NeuroImage. 2002;17:1207–1216. doi: 10.1006/nimg.2002.1198. [DOI] [PubMed] [Google Scholar]
- 58.Whiteside SP, Lynam DR. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Pers Indiv Differ. 2001;30:669–689. [Google Scholar]
- 59.Xiong J, Gao J, Lancaster JL, Fox PT. Clustered pixels analysis for functional MRI activation studies of the human brain. Hum Brain Mapp. 1995;3:287–301. [Google Scholar]
- 60.Xue G, Aron AR, Poldrack RA. Common neural substrates for inhibition of spoken and manual responses. Cerebral Cortex. 2008;18:1923–1932. doi: 10.1093/cercor/bhm220. [DOI] [PubMed] [Google Scholar]
- 61.Zuckerman M. Sensation Seeking. Hillsdale, NJ: Lawrence Erlbaum Association; 1979. [Google Scholar]
- 62.Zuckerman M. Behavioral expressions and biosocial bases of sensation seeking. Cambridge, England: Cambridge University Press; 1994. [Google Scholar]
- 63.Zuckerman M, Kuhlman DM. Personality and risk-taking: Common biosocial factors. J Pers. 2000;68:999–1029. doi: 10.1111/1467-6494.00124. [DOI] [PubMed] [Google Scholar]
- 64.Zuckerman M, Kuhlman DM, Joireman J, Teta P, Kraft M. A comparison of three structural models for personality: The big three, the big five, and the alternative five. J Pers Soc Psychol. 1993;65:757–768. [Google Scholar]