Abstract
We have developed a novel human laboratory model to examine two primary aspects of stress-precipitated tobacco relapse: 1) Does stress reduce the ability to resist the first cigarette? 2) Once the first cigarette is initiated, does stress facilitate subsequent smoking? Using a within-subject design, daily smokers (n=37) who were nicotine deprived overnight received a personalized imagery induction (stress or neutral) on two separate days, and then had the option of initiating a tobacco self-administration session or delaying initiation for up to 50 minutes in exchange for three levels of monetary reinforcement. Subsequently, the tobacco self-administration session entailed a 1-hour period in which subjects could choose to smoke using a smoking topography system. Following the stress induction, subjects were less able to resist smoking, smoked more intensely (increased puffs, shorter inter-puff interval, and greater peak puff velocity), and perceived greater satisfaction and reward from smoking. Stress significantly increased hypothalamus-pituitary-adrenal (HPA) axis reactivity, tobacco craving, negative emotion, and physiologic reactivity relative to the neutral condition. Additionally, increased cortisol, ACTH, and tobacco craving were associated with reduced ability to resist smoking following stress. These findings have implications for understanding the impact of stress on smoking relapse and model development to assess smoking lapse behavior.
Keywords: stress, smoking, relapse, HPA-axis, craving, emotion
INTRODUCTION
Currently, there are more than 1 billion cigarette smokers worldwide (WHO, 2010). Tobacco use is responsible for 5.4 million deaths per year and is the most preventable cause of morbidity and mortality in developed nations (WHO, 2010). Nicotine dependence, as with other substance use disorders, is a chronic relapsing condition. Although the majority of smokers are motivated to quit smoking (70%), very few successfully quit on a yearly basis (less than 2%; CDC, 2002).
Several lines of evidence from epidemiological (McKee et al., 2003), animal (Buczek et al., 1999), human laboratory (Rose et al., 1983 Payne et al., 1991; Willner and Jones, 1996), and treatment studies (Baer and Lichtenstein, 1988; Baer et al., 1989) find strong support that stress is a primary mechanism involved in the maintenance of, and relapse to smoking (although see Shiffman et al., 2002). Smokers (35% to 100%) cite stress and associated negative affect states as causal factors in accounts of relapse episodes (Marlett & Gordon, 1980; Shiffman, 1982; Cummings et al., 1985; O’Connell and Martin, 1987; Baer and Lichtenstein, 1988; Swan et al., 1988; Baer et al., 1989; Borland, 1990; Brandon, 1994). Using hand-held computers to collect multiple daily ratings, Shiffman and Waters (2004) prospectively examined the role of stress in the first instance of smoking during a quit attempt (i.e., smoking lapse). They found that rapid increases in negative affect (triggered by a specific stressor such as an argument) were predictive of smoking lapse episodes, and that lapses were typically associated with a convergence of factors (i.e., negative affect, craving, cigarette availability). Additionally, lapse episodes triggered by stress were found to progress more quickly to full-blown relapse (Shiffman et al., 1996).
Given that up to 95% of smokers who experience a lapse return to daily smoking (e.g., Garvey et al., 1992), it is clear that the first occurrence of smoking during a cessation attempt is a critical transition and represents an important target for investigation. However, currently available models examining tobacco-related phenomena have not yet modeled the effect of stress on the ability to resist the first cigarette (see McKee, 2009 for review). To this end, we have developed a paradigm focused on modeling smoking lapse behavior in the laboratory. We have previously developed models demonstrating that nicotine deprivation and alcohol decreased the ability to resist smoking and facilitated subsequent smoking, mirroring what is seen in clinical settings (McKee et al., 2006; 2009).
The current study used a within-subject design to examine the effect of stress on a model of smoking lapse behavior. Daily smokers who were nicotine deprived overnight received a personalized imagery induction (stress or neutral), and then had the option of initiating a tobacco self-administration session or delaying initiation by five-minute increments for up to 50 minutes in exchange for three levels of monetary reinforcement. This delay period modeled their ability to resist smoking. We manipulated the level of monetary reinforcement to determine which level produced the greatest separation across the imagery conditions in the time to resist smoking. Money was provided as an alternative reinforcer in order to provide an incentive for not smoking and to enhance the likelihood that the effects of stress on the relative reinforcing value of tobacco would be detected (see Higgins, 1997; Rodefer et al., 1997). We predicted that stress would decrease the ability to resist smoking, would interact with the level of monetary reinforcement, and would facilitate subsequent smoking.
We also examined hypothalamus-pituitary-adrenal (HPA) axis reactivity (Pomerleau & Pomerleau, 1990; al’Absi et al., 2003, 2005; Back et al., 2008; Childs and de Wit 2009), tobacco craving (Perkins et al., 1992; Erblich et al., 2003; Buchman et al., in press), mood reactivity (Zinser et al., 1992), and physiologic reactivity (Back et al., 2008; Childs and de Wit, 2009) as potential mechanisms contributing to stress-precipitated smoking lapse behavior. Each of these factors has been implicated in the relationship between stress, smoking behavior, and relapse. In particular, we were interested in assessing whether these factors were associated with the decision to start smoking following stress, subsequent ad-lib behavior (including smoking topography), and the reward value of smoking. Stress manipulations have increased smoking rate, puff volume, and puff duration (Rose et al., 1983; Payne et al., 1991), and increased the reported pleasure and arousal from smoking (Zinser et al., 1992) suggesting that stress might sensitize smokers to the reinforcing properties of tobacco (see Koob and Le Moal, 1997; Sinha, 2001).
METHODS AND MATERIALS
Participants
Participants were eligible to enroll in this study if they were 18 to 60 years of age, smoked at least 10 cigarettes per day for the past year, and had urine cotinine levels greater than 150 ng/ml. Subjects were excluded from participation if they met criteria for current (past 6 months) Axis I disorders (excluding nicotine dependence), using illicit drugs, had engaged in treatment for smoking behavior in the past six months, or had medical conditions that would contraindicate smoking behavior. Fifty-five eligible subjects were screened and a total of 37 subjects (15 females, 22 males) completed the study (14 lost interest, 2 were dismissed due to illicit drug use, 2 were dismissed for failing to maintain overnight abstinence on laboratory days). The average age was 37.70 (SD = 10.57). Participants were primarily Caucasian (48.6%) or African American (40.5%), and were primarily high school educated (62.2%; 37.8% college educated). Participants drank alcohol on average 5 times per month (SD = 6.92), and drank an average of 4.55 alcohol units per episode (SD = 5.59). Participants smoked on average 18.43 (SD = 7.96) cigarettes per day, had baseline carbon monoxide (CO) readings of 29.57 ppm (SD = 13.10), and average Fagerstrom Nicotine Dependence Scores (FTND; Heatherton et al., 1991) of 6.05 (SD = 2.28; range 1–10 for measure).
Design
This study is a mixed design that examined the effect of personalized stress imagery compared to neutral imagery on smoking lapse behavior modeled in the laboratory. Personalized imagery (stress vs. neutral) was a within-subject factor and monetary reinforcement ($0.50, $1.00, $1.50) was a fully crossed between-subjects variable. The three levels of monetary reinforcement were selected based on our prior work with the lapse model (McKee, 2009). Subjects were randomly assigned to a monetary condition ($0.50 n=12; $1.00 n=12; $1.50 n=13). Order of personalized imagery was counterbalanced across separate laboratory sessions. The study consisted of an intake session, a script development session, and two laboratory sessions. Subjects were paid $150 per laboratory session with a $50 bonus for completing all scheduled study appointments. Payments were provided by check two to three weeks following study completion.
Procedures
Intake Sessions
All procedures were carried out with the adequate understanding and written informed consent of the participants. The procedures were in accordance with the ethical standards of the Yale School of Medicine Human Investigation Committee. The Structured Clinical Interview for DSM-IV (First et al., 1995) was used to exclude individuals who met diagnostic criteria for current Axis I disorders (except nicotine dependence). Participants completed an assessment developed by the Yale Transdisciplinary Tobacco Use Research Center to assess smoking history.
Script Development Session
Exposure to stress and relaxing-neutral imagery in the laboratory study used a personalized guided imagery method (see Sinha, 2009). The stress imagery script was developed by having subjects identify and describe in detail a recent stressful experience occurring within the last 6 months that they had personally experienced as ‘most stressful’. ‘Most stressful’ was determined by having subjects rate their perceived stress on a 10-point Likert scale where 1=’not at all stressful’ and 10=’the most stress they recently felt in their life’. Only situations rated as 8 or greater were accepted as appropriate for script development. A neutral script was developed from the subject’s description of a personal neutral-relaxing situation. Scripts were developed by a PhD clinician and then audiotaped for presentation during the laboratory sessions. Each script was approximately 5 min in length.
Laboratory Sessions
Each subject completed two 6.5 hour laboratory sessions (stress vs. neutral imagery) which took place at the Yale Center for Clinical Investigation. The average time between laboratory sessions was 7.38 days (SD = 11.12), with the majority of subjects (89%) completing both sessions within 14 days of each other. Time between sessions was not a significant covariate of the effect of imagery condition on the primary outcome, time to resist smoking (imagery condition × time between sessions, F=.01, p=.94).
Baseline Assessment Period
Laboratory sessions started at 9:00am. See Figure 1 for study timeline. Participants were instructed to smoke a final cigarette at 10:00pm the night before the lab session. Abstinence was confirmed the following morning with a carbon monoxide (CO) reading < 50% of their intake value and later confirmed with nicotine plasma levels (mean=2.49 ng/ml, SE=0.28). An IV cannula was inserted to obtain blood samples throughout the laboratory session. Baseline assessments of breath CO, breath alcohol, plasma cotinine and nicotine levels, urine drug screens, urine pregnancy screen, and vitals were obtained. Additional measures of emotion, tobacco craving, and nicotine withdrawal were obtained. Participants were provided with a standardized lunch at 11:15am to control the time since last food consumption. Subjects also practiced progressive muscle relaxation techniques and completed imagery training during this period where they were asked to visualize and rate everyday scenes (Sheehan, 1967). From 10:00am to 12:30pm, subjects were able to watch TV and read.
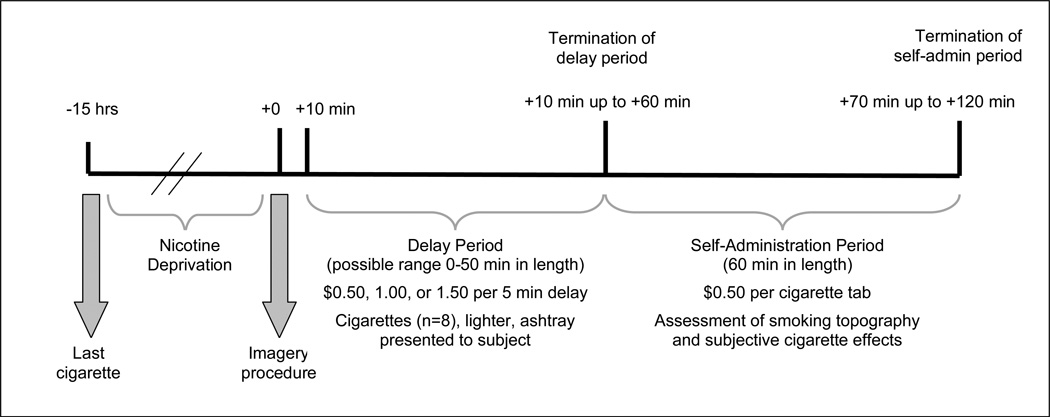
Figure 1.
Timeline of study procedures for laboratory sessions. Note: Assessment of cortisol and ACTH occurred at −30 min, −15 min, +10 min, +20 min, +40 min, and +60 min from the imagery procedure and remained fixed regardless of when the termination of the delay period occurred. Assessments of craving, emotion, physiologic reactivity, and nicotine withdrawal occurred at −15min, +5min, termination of the delay period, and +30min and +60min during the self-administration period. The imagery procedure commenced at 1pm.
Personalized Imagery Procedure
At 12:55pm subjects were instructed to clear their mind of any worrying thoughts and to focus on deep breathing. At 1:00pm they were told “You will soon hear a situation being described to you. Your task is to close your eyes and imagine yourself in the situation being described, ‘as if’ it were happening right now. Allow yourself to become completely involved in the situation, by involving your mind and body in actually doing what is being described. Continue imagining until you are asked to stop.” The subject then listened to the script (stress or neutral) over headphones. Following the script, subjects rated how clearly they were able to imagine the scene on a 140 mm visual analogue scale. Mean vividness ratings were 120.39 (SE = 2.63).
Delay Period
At 1:10pm participants were presented with a tray containing 8 cigarettes of their preferred brand, a lighter, and an ashtray. Participants were instructed that they could commence smoking at any point over the next 50 minutes. However, for each 5-minute block of time that they delayed or ‘resisted’ smoking they would earn $0.50, $1.00, or $1.50 depending on their assigned monetary condition. We recorded the time (in minutes) when subjects announced that they wanted to smoke (range 0–50 minutes).
Smoking Self-Administration Period
The ad-lib smoking session was 60 minutes in length and started once participants decided to end the delay period (or delayed for the full 50 minutes). Participants were provided with 8 cigarettes of their preferred brand. Participants were also provided with a $4 ‘smoking tab’ and were instructed to ‘smoke as little or as much as you wish’ using the smoking topography equipment. Participants were further instructed that for each cigarette they lit, it would cost them $0.50 of their tab. Money earned for delaying smoking and any unused portion of the ‘smoking tab’ was paid to the subjects at the end of each laboratory session. All subjects were discharged at 3:15pm.
Timing of Assessments
Tobacco craving, emotion ratings, physiologic reactivity, and nicotine withdrawal were assessed pre-imagery, post-imagery, end of delay, and +30min and +60min during the ad-lib smoking period. HPA levels (ACTH, cortisol) were assessed −30, −15, +5, +20, +40, +60min post-imagery. Smoking topography (hand-held CRESS, Plowshare Technologies) and subjective cigarette effects were assessed during the ad-lib period.
Measures
The primary measures were length of the delay period (i.e., time to initiate smoking) and the number of cigarettes smoked during the ad-lib period.
Subjective measures
Tobacco craving was assessed with the Tiffany Questionnaire of Smoking Urges-Brief (QSU-Brief; Cox et al., 2001), a 10 item measure used to evaluate urges to smoke in response to positive (Factor 1) or negative (Factor 2) reinforcement (VAS scale, range 1–100). The Differential Emotion Scale (DES), a 30 item self-report questionnaire, was used to assess current emotional state for positive (e.g., happy, joy) and negative (e.g., sadness, anger) emotion states (VAS scale, range 1–100; Izard, 1972). DSM-IV symptoms of nicotine withdrawal were assessed with the 8-item Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1986). Instructions were worded to assess current symptoms of withdrawal (range 0–32). The Cigarette Effects Scale is a 10-item self-report questionnaire which assesses satisfaction, psychological reward, nausea/dizziness, craving relief, and enjoyment of airway sensations associated with smoking (VAS scale, range 1–100; West et al., 1992).
Physiologic measures
A pulse sensor was attached to the subject’s forefinger to obtain a measure of pulse rate. Blood pressure was measured using a Critikon Dynamap.
Smoking topography
A hand held Clinical Research Support System (CreSS from Plowshare Technologies) was used to assess smoking topography. Smoking topography measures of interest included puff frequency, puff volume, puff duration, inter-puff interval, depth of inhalation, and inter-cigarette interval.
Carbon Monoxide (CO) Levels
Carbon monoxide levels were measured using a Vitalograph Breath CO, from Vitalograph, Inc., which is a precision instrument for detecting carbon monoxide in exhaled breath. Carbon monoxide is known to have a half-life of 4 hours. This instrument measures CO in the range of 0–5000 ppm and has no cross-sensitivity to hydrogen or other positrons.
Biochemical measures
Four mls of blood were collected at each timepoint to assess plasma ACTH and cortisol. Blood samples were collected in two heparinized tubes for each assay. The tubes were placed on ice immediately after drawing. They were centrifuged at 4°C. Plasma was then aliquoted and stored at −70°C. ACTH was analyzed with a double antibody kit (MPBio) and cortisol with a ‘coat a count’ kit (Siemens). Assays were conducted by the Core Laboratory of the Yale Center for Clinical Investigation. Serum nicotine and cotinine were only collected at the start of the laboratory session to biochemically confirm current nicotine exposure. Cotinine and nicotine were measured by reversed-phase HPLC with UV detection, modified from the literature (Hariharan et al., 1988) to include a micro acid back extraction clean up step which allows for cleaner chromatograms. The lower limit of quantitation for nicotine was set to 4ng/ml and cotinine was set to 25ng/ml. Assays were conducted by Peter Jatlow, Laboratory Medicine, Yale-New Haven Hospital.
Statistical Analysis
Multivariate analyses of variance were used to examine the within-subject effect of imagery condition (stress, neutral) by monetary condition ($0.50, $1.00, $1.50) on the primary outcomes of the length of the delay period and number of cigarettes smoked during the ad-lib period. To examine secondary outcomes, multivariate analyses of variance were used to examine tobacco craving, emotion ratings, physiologic reactivity, and nicotine withdrawal within imagery condition (stress, neutral), and within time (pre-imagery, post-imagery, end of delay, +30min smoking ad-lib, +60min smoking ad-lib). Similar analyses were conducted to examine HPA reactivity with the following timepoints post-imagery [baseline (merged baseline of −30min and −15min), +5, +20, +40, +60min]. As there were baseline differences (not attributable to order effects) in the plasma levels of HPA hormones across imagery conditions, the baseline was used as a covariate. Monetary condition was examined as a potential covariate for the secondary outcomes but did not meet the standard for inclusion (see Tabachnik and Fiddel, 2006). To examine mean smoking topography measures for the first cigarette smoked during the 60 minute ad-lib period, we conducted a multivariate analysis of variance for multiple dependent measures. Given a significant omnibus test (which controls for experiment-wise type 1 error), paired contrasts were used to examine puff number, puff volume (ml), puff duration (s), inter-puff interval (s), and peak puff velocity (ml/s) within imagery conditions. Similar to smoking topography, paired contrasts (within a multivariate analysis) were used to examine subjective cigarette effects following the first cigarette smoked. These analyses were confined to those who smoked during the ad-lib sessions. We also conducted exploratory analysis examining associations between tobacco craving, emotion ratings, physiological and HPA-axis reactivity, smoking topography, smoking-related reward, and time to resist smoking. We found no significant effects of gender on our primary outcome. Additionally, there were no significant main effects of imagery order (F=.00, p=.97) or interactions of order with imagery condition (F=.02, p=.90) on our primary outcome of time to resist smoking.
RESULTS
Modeled-Smoking lapse behavior
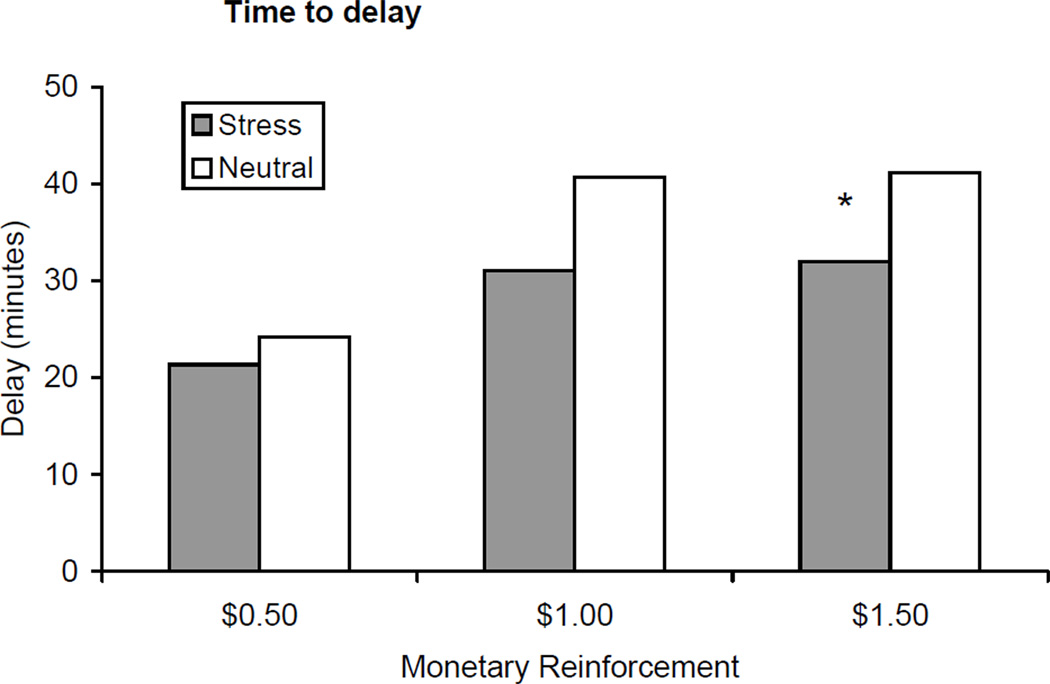
Subjects were less able to resist smoking and terminated their delay period sooner during the stress condition (mean=28.14 min, SE=3.88) compared to the neutral condition (mean=35.33 min, SE=3.07) [F=7.11, p<.01; Cohen d = 0.91; see Figure 2]. The main effect of monetary condition was not significant (p=.16; $0.50 mean=22.75 min; $1.00 mean=35.86 min; $1.50=36.58 min), and did not interact with the imagery condition. Based on our hypothesis, that stress would reduce the time to initiate smoking by monetary condition, we conducted a-priori t-tests across the monetary conditions. There was a significant effect of imagery condition (stress mean=32.00 min; neutral mean=41.15 min) for the $1.50 condition only (p<.05). Additionally, decreased time to initiate smoking was associated with smoking satisfaction (r=−0.50, p<.01).
Figure 2.
Mean delay (+/− SE) to smoking across monetary reinforcement levels and stress vs. neutral conditions. *p < .05 for paired comparisons within timepoint.
Ad-lib smoking
Imagery or monetary condition did not affect the number of cigarettes smoked during the 60 minute ad-lib session (stress mean=1.51 cigarettes, SE=0.18; neutral mean=1.54 cigarettes, SE=0.20). However, there were significant effects of imagery condition on smoking topography for the first cigarette smoked [overall F=3.88, p<.02]. During stress relative to the neutral session, subjects took more puffs (11.83 vs. 9.67), had greater peak puff velocity (41.67 vs. 40.75 ml/sec), and had shorter inter-puff intervals (27.81 vs. 32.93 sec; all p’s for paired contrasts <.01). Additionally, during the stress session compared to the neutral session [overall F=3.03, p<.05], subjects reported greater satisfaction (87.83 vs. 76.33) and reward (64.88 vs. 46.70; all p’s for paired contrasts <.004) from the first cigarette.
Craving reactivity
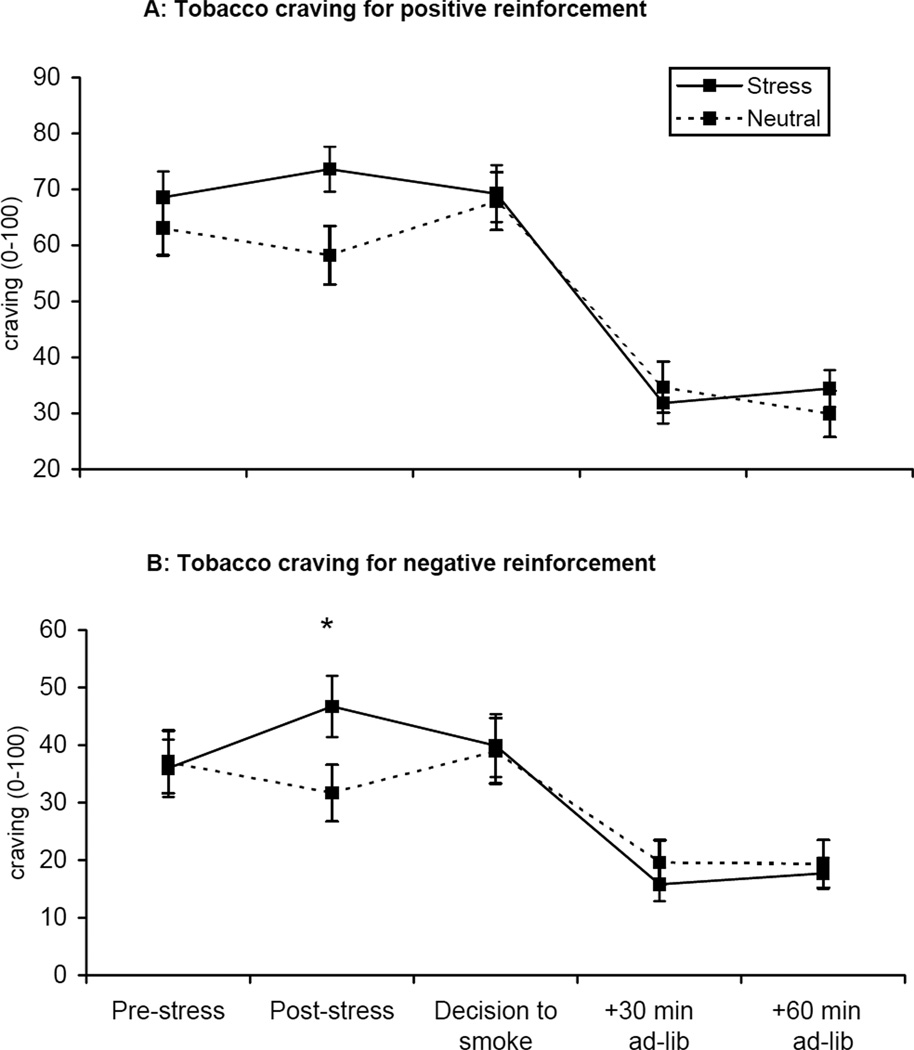
Tobacco craving for positive reinforcement [F=12.17, p<.001] and negative reinforcement [F=19.41, p<.0001; see Figure 3] differed by imagery condition across the laboratory sessions. Craving scores increased following the stress imagery, decreased following the decision to smoke, and continued to decrease during the ad-lib smoking period. Exploratory analysis demonstrated that craving for positive reinforcement, assessed following the stress manipulation, was associated with decreased time to initiate smoking following stress (r=−0.39, p<.03), cortisol levels at the +5min (r=0.38, p<.04) and +20min timepoints (r=0.41, p<.03), ACTH levels at +20min (r=0.40, p<.04), and was also associated with cigarette reward (r=0.51, p<.01).
Figure 3.
Mean tobacco craving for positive reinforcement (A) and for negative reinforcement (B) across time. *p < .05 for paired comparisons within timepoint.
Emotion reactivity
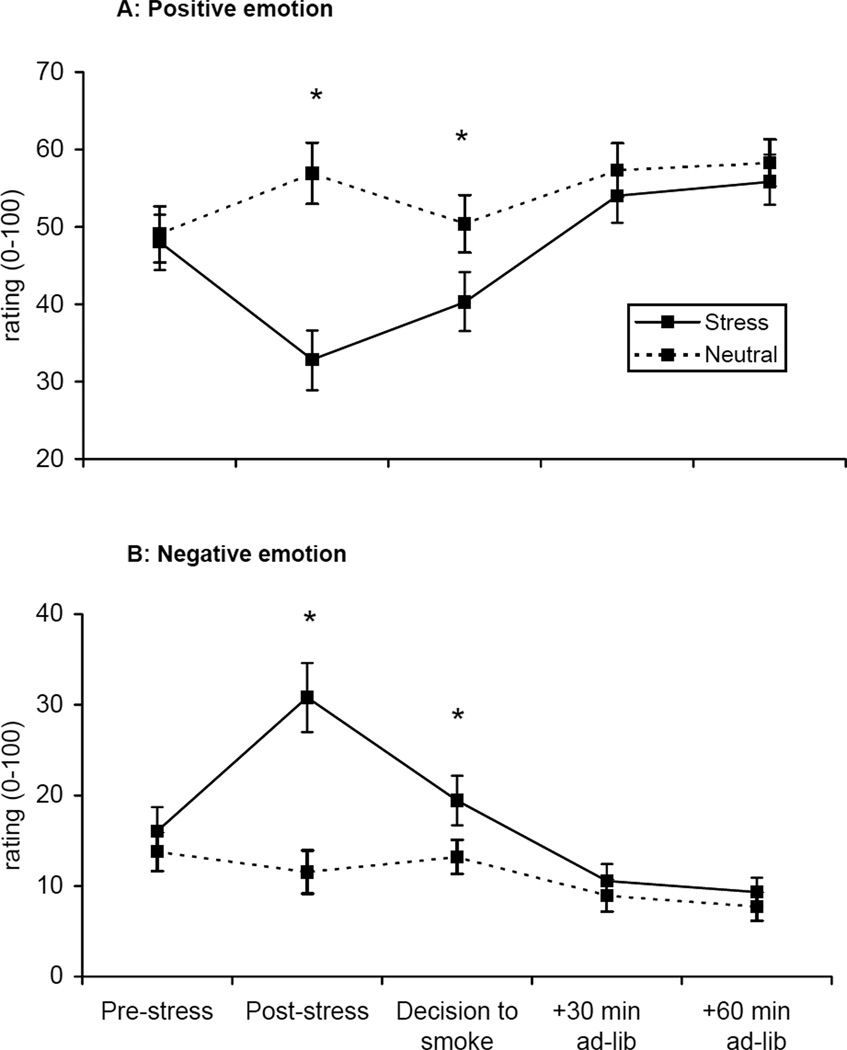
Positive [F=27.58, p<.0001] and negative emotion [F=27.09, p<.0001; see Figure 4] differed by imagery condition across the laboratory session. Positive emotion ratings decreased following the stress imagery, increased following the decision to smoke, and continued to increase during the ad-lib smoking period. The converse pattern was observed for the negative emotion ratings.
Figure 4.
Mean positive (A) and negative (B) emotion across time. *p < .05 for paired comparisons within timepoint.
Physiologic reactivity
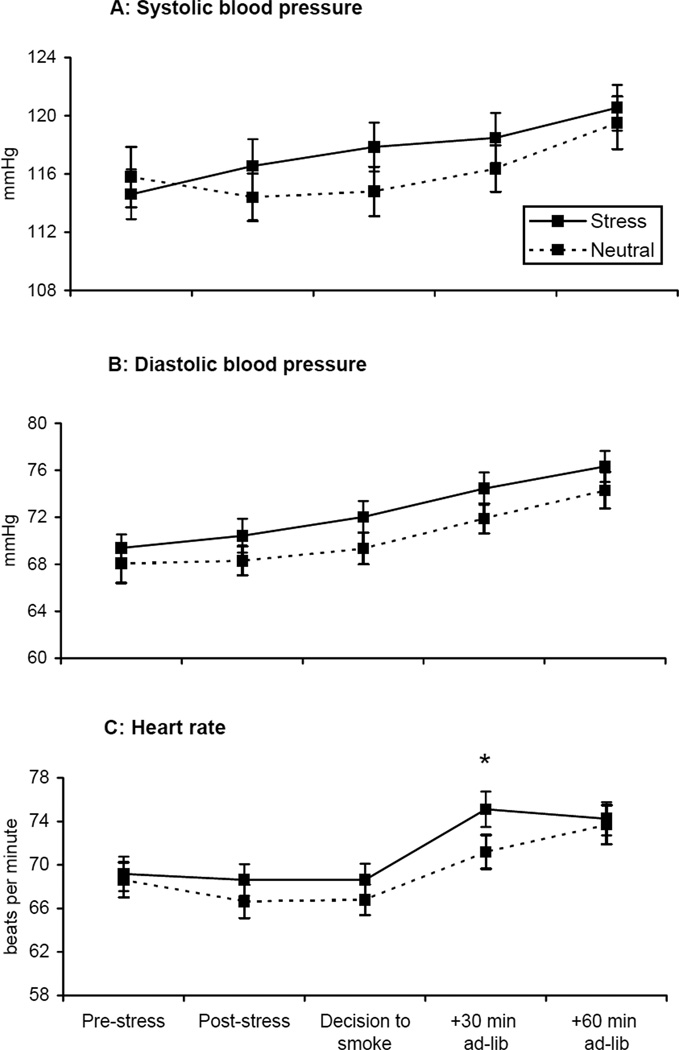
Systolic blood pressure differed by imagery condition across the laboratory session for systolic blood pressure [F=4.72, p<.05; see Figure 5a]. Systolic blood pressure increased following the stress imagery, relative to the neutral imagery condition (within-subject contrast, p<.05). Diastolic blood pressure demonstrated a significant effect of imagery condition [F=5.39, p<.03] and time [F=116.16, p<.0001; see Figure 5b]. Diastolic blood pressure was greater during the stress imagery condition compared to the neutral imagery condition and increased during the laboratory session. Heart rate demonstrated a significant effect of imagery condition [F=4.55, p<.04] and time [F=27.19, p<.0001; see Figure 5c]. Heart rate was greater during the stress imagery condition compared to the neutral imagery condition and increased during the laboratory session.
Figure 5.
Mean systolic blood pressure (A), diastolic blood pressure (B), and heart rate (C) across time. *p < .05 for paired comparisons within timepoint.
HPA-axis reactivity
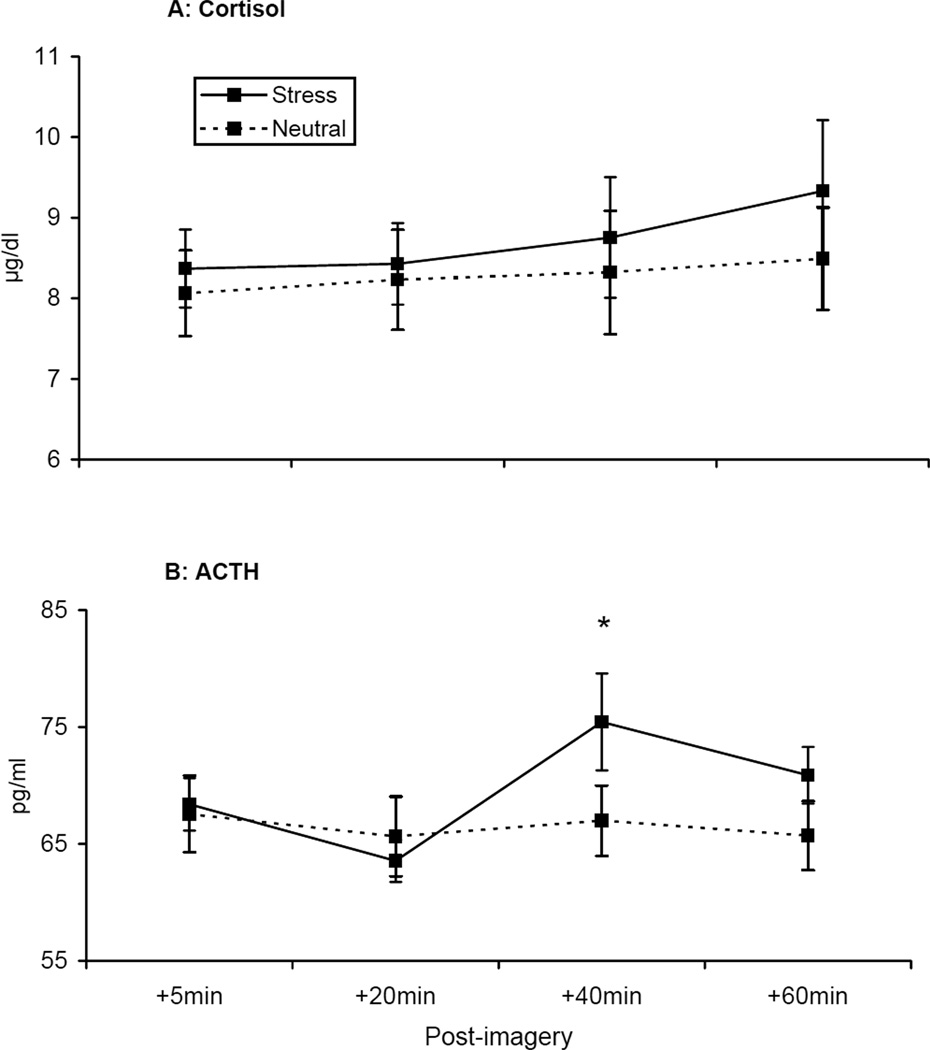
Plasma cortisol levels [F=4.97, p<.04; see Figure 6a] and ACTH levels [F=6.04, p<.03; see Figure 6b] demonstrated a significant interaction of imagery condition by time. For both cortisol and ACTH, there was no significant difference between conditions immediately following imagery, but differences were demonstrated at subsequent timepoints following smoking. For cortisol, within-subject contrasts demonstrated that the change across timepoints was greater (p<.05) in the stress relative to the neutral condition. For ACTH, the change from the +20 to the +40 timepoint was greater (p<.05) in the stress relative to the neutral condition. Exploratory analysis demonstrated that greater cortisol levels (+40min timepoint r=−0.39, p<.03; +60min timepoint r=−0.38, p<.03) and ACTH levels (+40min timepoint r=−0.46, p<.01; +60min timepoint r=−0.41, p<.02) were associated with decreased time to initiate smoking following stress. Additionally, cortisol levels (+40min timepoint r=0.46, p<.02) were associated with smoking satisfaction, and ACTH levels (+5min timepoint r=0.43, p<.03) were associated with smoking reward.
Figure 6.
Mean cortisol (A) and ACTH (B) levels across time. *p < .05 for paired comparisons within timepoint.
Cotinine Levels and Nicotine withdrawal
Plasma cotinine levels did not vary at baseline across the stress (mean=223.95 ng/ml, SE=25.88) and neutral (mean=226.43 ng/ml, SE=22.27) imagery conditions indicating equal nicotine exposure in the 48 hours prior to each of the two laboratory sessions. Nicotine withdrawal did not differ across the imagery condition. Baseline scores were similar across the stress (mean=8.29, SE=1.01) and neutral conditions (mean=7.39, SE=1.12), and did not change following the imagery manipulation. Withdrawal scores decreased significantly during the ad-lib smoking session for both conditions (stress mean=3.83; neutral mean=4.00).
DISCUSSION
Using a novel human laboratory paradigm modeling smoking lapse behavior, the current study demonstrated that personalized stress imagery undermined the ability to resist smoking in a sample of daily smokers who were deprived of nicotine for approximately 15 hours. This effect occurred across the monetary conditions, although there was some evidence that the highest level of reinforcement ($1.50 per 5 minute delay) produced the greatest separation between the imagery conditions. Stress and negative affect are known to decrease self control and executive function, including the ability to delay gratification for rewarding substances (Mischel et al., 1989; Arnsten, 1998; Willner et al., 1998; Muraven and Baumeister, 2000; Tice et al., 2001). We’ve also previously hypothesized that stress promotes addictive behaviors and relapse by increasing craving and decreasing self control for rewarding substances in addicted individuals (Sinha, 2001). The current findings support this previous literature on the behavioral effects on stress and to our knowledge, for the first time elucidates the effects of stress on smoking lapse behavior modeled in the laboratory. These data also support previous studies demonstrating an association between stress and tobacco relapse episodes (Marlett & Gordon, 1980; Borland, 1990; Brandon et al., 1994, Shiffman et al, 1996, 2004; al’Absi et al., 2005).
With regard to the second primary outcome, we found no effect of imagery condition on the number of cigarettes smoked. It is possible that the value of the smoking tab was too high or that the ad-lib period was too brief, producing low levels of smoking which may have masked potential differences. However, we did demonstrate that following stress, subjects smoked their cigarettes more intensely (i.e., increased puffs, greater maximum depth of inhalation, and shorter time in between puffs). These results are consistent with prior findings of Child and de Wit (in press). During a 2-hour ad-lib period, they found no effect of stress on the number of cigarettes smoked but found that the boost in CO readings was greater during the stress condition, suggesting that subjects inhaled more deeply following stress. Additionally, in the current study subjects rated their cigarettes as more satisfying (e.g., taste good) and reinforcing (e.g., calm you down, make you feel less irritable) following stress, compared to the neutral imagery condition, which is consistent with prior findings (Zinser et al., 1992). Finally, the inability to resist smoking following stress was moderately associated with how satisfying cigarettes were rated.
The HPA-axis reactivity, as assessed by ACTH and cortisol, was not greater immediately following the stress imagery. Nicotine is known to activate the HPA axis (Sharp and Beyer, 1986; Pomerleau and Pomerleau, 1990; Matta et al., 1993, 1998), however, chronic nicotine exposure appears to dysregulate the system (Baron et al., 1995; Frederick et al., 1998). During acute nicotine withdrawal, prior work has demonstrated that HPA-axis activation was blunted in response to stress in smokers when compared to non-smokers (al’Absi et al., 2003; Childs and de Wit, 2009). Furthermore, this hyporesponsiveness in the HPA-axis activation has been associated with smoking relapse (al’Absi et al., 2005). However, we demonstrated that during the stress condition but not the neutral condition, the HPA-axis activity increased as subjects commenced to smoke. While ACTH levels increase rapidly, followed by a more gradual increase in cortisol with nicotine exposure (Mello, in press), the current findings suggest that nicotine-associated HPA activation is potentiated in the context of stress. Furthermore, we demonstrated that increased cortisol and ACTH at the latter timepoints were moderately associated with the reduced ability to resist smoking following stress, and with smoking satisfaction and reward respectively. These data support previously suggested notions that the CRF and possibly the HPA-axis play a role in nicotine reinforcement (Sinha, 2001; Goeders, 2002; Koob et al., 2004). The findings are also consistent with the hypothesis that nicotine may be used to restore homeostasis in the HPA system following stress (Munch et al., 1984; McEwen, 2000).
In the current study, cortisol and ACTH levels were associated with smoking satisfaction and reward following stress, similar to findings demonstrated by Mendelson et al. (2002) showing a positive association between the reward value of cocaine and HPA activation. Immediate increases in ACTH were associated with smoking reward, while latter increases in cortisol were associated with smoking satisfaction. These results support the position that stress may contribute to the putative properties of tobacco (Pomerleau and Pomerleau, 1990, 1991; Pauly et al., 1992) and to other substances in general (Koob and Le Moal, 1997; Sinha, 2001).
We demonstrated evidence that tobacco craving for both positive and negative reinforcement was greater following the stress imagery, similar to prior findings (Perkins et al., 1992; Erblich et al., 2003). Additionally, craving for positive reinforcement following stress was negatively associated with the ability to resist smoking, and positively associated with reward from smoking, as well as ACTH and cortisol levels. Buchman et al. (2008) has also demonstrated positive associations between craving and cortisol response following stress. It is possible that craving represents a marker for the incentive to smoke produced by alterations in the CRF-HPA system following exposure to stress.
Consistent with prior work (Back et al., 2008; Childs and de Wit, 2009) stress increased physiologic reactivity in smokers. In the current study, systolic and diastolic blood pressure and heart rate were greater in the stress condition and continued to increase as subjects commenced to smoke. Nicotine withdrawal, combined with an acute stressor has been found to enhance cardiovascular reactivity to stress, compared to stress experienced during non-deprived states (Kirschbaum et al., 1993; Tsuda et al., 1996). Alterations in cardiovascular stress-reactivity have been shown to predict smoking relapse (al’Absi et al., 2005). However, in the current study, measures of physiologic reactivity were not associated with the ability to resist smoking.
As expected, negative emotion increased and positive emotion decreased following stress. Interestingly, emotion ratings started to recover once subjects made the decision to start smoking, but had not yet smoked. It is unknown whether emotion ratings were decaying during the delay period, or whether the decision to smoke precipitated the recovery in emotion suggesting a conditioning process (see Baker et al., 2004). Also worth noting, emotion ratings continued to improve to baseline levels in the case of negative emotion, and to greater than baseline levels in the case of positive emotion as subjects smoked during the ad-lib period. A recent study by Perkins et al. (2008) which manipulated expected and actual nicotine content of cigarettes, found that smoking alleviated negative emotion, craving, and withdrawal following a negative mood induction. However, results demonstrated that the sensory aspects of smoking were more important in alleviating negative affect than nicotine, suggesting that nonpharmacological cues associated with nicotine are sufficient to reinforce smoking behavior. The current study extends these findings by demonstrating that the mere decision to smoke alleviated stress-related increases in negative emotion.
While there is much evidence supporting the notion that stress and negative affect increase the incentive value of smoking (Kassel et al., 2003; Payne et al., 1991; Willner & Jones, 1996), there is much less evidence suggesting that stress and negative affect may actually modulate smoking reinforcement. Current theories of drug use posit that as chronic drug use becomes habitual, the incentive properties which drive use become disconnected from their affective outcomes (Belin et al., 2009). It is possible that stress and negative affect may increase the motivation to smoke, while having no effect on smoking reinforcement. A recent ecological momentary assessment study by Shiffman and colleagues (2009) found that cigarettes smoked during periods of negative affect were rated as less satisfying than cigarettes smoking during periods of positive affect. In light of these findings and others (Shiffman et al., 2002; 2004), the authors conclude that it is unlikely that negative affect and the relief of negative affect play a central role in smoking behavior. However, in the current study we found that stress decreased the latency to smoke, increased the intensity of self-administration, and importantly, increased smoking reinforcement. Additionally, post-stress craving and HPA-axis levels were associated with cigarette reward. While it is possible that stress only influenced craving and latency to smoke, and that stress effects on smoking intensity and reward were a function of differences in craving and latency to smoke, the results support theories which posit that stress and negative affect play a central role in drug use (Baker et al., 2004; Sinha, 2001).
These results also support those who have hypothesized that stress produces state-related changes in brain reward circuits which may sensitize smokers to the reinforcing properties of drugs (see Koob and Le Moal, 1997; Sinha, 2001 for review). Stress co-activates brain stress circuits (corticotropin-releasing factor, ACTH, cortisol) and the putative reward circuitry (mesocorticolimbic dopaminergic system) simultaneously, thereby providing a common neural substrate by which stress may enhance the drug taking experience (Thierry et al., 1976; Dunn 1989; Kalivas & Duffy, 1989; Prasad et al., 1993; Piazza and Le Moal, 1996). Additionally, preclinical work has demonstrated that following acute withdrawal, stress can sensitize or enhance the behavioral or neurochemical response to drugs (Robinson and Berridge, 1993; Kalivas et al., 1998). In the current study, exposure to stress following overnight deprivation appears to have enhanced or sensitized smokers to the rewarding value of cigarettes.
There are several limitations to the current study. First, it should be noted that the sample size was modest and only generalizable to the population studied. However, our sample size was comparable to other laboratory studies modeling the effect of stress in smokers. Second, we failed to find gender differences in our primary outcome, which is inconsistent with other studies documenting differences in stress response in smokers (e.g., al’Absi et al., 2005; Back et al., 2008). However, this is the first investigation which has examined the effect of stress on modeled-smoking lapse behavior. It will be important to replicate this finding to determine whether the effect of stress on the ability to resist smoking remains consistent for both men and women. Third, while we used a well-validated paradigm to induce psychological stress in the laboratory (Sinha, 2009), it would be worthwhile to examine physiological and pharmacological stressors with the lapse paradigm as results may vary across types of stressors (Back et al., 2008). Fourth, subjects were not treatment seeking which has been a criticism of laboratory studies examining various smoking cessation effects (see Perkins et al. 2006). However, money was provided as an alternative reinforcer in order to provide an incentive for not smoking and to enhance the likelihood that the effects of stress on the relative reinforcing value of tobacco would be detected (see Higgins, 1997; Rodefer et al., 1997). Perkins et al. (2006) and others (Stitzer et al., 1986; Gilbert et al., 1999) acknowledge that motivation to abstain can be temporarily raised through the use of monetary reinforcement. Finally, it is unknown whether stress effects are specific to smoking or perhaps attributable to non-specific increases in behavioral responses. While we did not have a specific control condition for non-specific behavioral responses, we also believe that this possibility is less likely. If the stress-precipitated smoking behavior were due to non-specific effects, we would be less likely to see stress effects on smoking reward and biological stress effects on smoking behavior.
For the current study, we developed a paradigm to evaluate two primary aspects of stress-precipitated smoking lapse behavior; the ability to resist the first cigarette and subsequent smoking. Prior investigations examining stress reactivity in smokers had not yet modeled the ability to resist the first cigarette, which represents an important transition point in a quit attempt as the majority of abstinent smokers (up to 95%) who experience a lapse return to baseline smoking levels (Brandon et al., 1990; Garvey et al., 1992; Kenford et al., 1994). Our smoking lapse model has potential to facilitate translational work in medication development. Modeling the ability to resist smoking is important from a medications development perspective as FDA approval for smoking cessation medications is contingent on demonstrating abstinence effects. Further, the smoking lapse models can be viewed as a logical extension of the cue reactivity paradigm with the flexibility to model the effect of singular or compound cues on smoking lapse behavior. While developing the models requires careful parametric investigations, across our three developed models we see evidence that known precipitants of relapse (i.e., stress, alcohol, nicotine deprivation) facilitated smoking lapse behavior in the laboratory in a similar manner to what is demonstrated clinically (McKee, 2009). We are in the process of validating our smoking-lapse models by screening known medications with proven efficacy for attenuating the effect of the model’s primes on smoking relapse, and by examining whether smoking lapse behavior in the laboratory is predictive of actual quit behavior.
In summary, we demonstrated that stress facilitated smoking lapse behavior in the laboratory, increased HPA activation, craving responses, and physiologic and emotion reactivity. Importantly, this investigation pointed to potential mechanisms involved in stress-precipitated relapse behavior as we were able to model the decision point when smokers were reaching for that first cigarette following a period of abstinence. Results lend support to the hypothesis that stress reactivity, via its effects on multiple subjective (emotion, urges) and neuroendocrine (CRF-HPA-axis responses) can increase the motivation to smoke as well as the putative value of tobacco, and point to a potential mechanism underlying stress-precipitated relapse behavior.
Acknowledgments
Sources of Support : The authors declare that this work was supported by the NIH Roadmap for Medical Research Common Fund through the following grants: RL1DA024857, UL1DE019586, PL1DA024859, and PL1DA024860. Additional NIH grants include R21DA017234, K12DA000167 and T32AA015496. This publication was also made possible by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH roadmap for Medical Research, and by the Department of Mental Health and Addiction Services of the State of Connecticut.
Footnotes
Publisher's Disclaimer: Disclaimers : The authors declare that RS is on the Scientific Advisory Board for Embera Neurotherapeutics and is also a consultant for Glaxo-Smith Kline, Pharmaceuticals.
All other authors declare that they have no conflicts of interest.
REFERENCES
- al’Absi M, Hatsukami D, David GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacol. 2005;181:107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Willmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacol Biochem Behav. 2003;74:401–410. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT. Catecholamine modulation of prefrontal cortical cognitive function. Trends Cognit Sci. 1998;2:436–447. doi: 10.1016/s1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- Back SE, Waldrop AE, Saladin ME, Yeatts SD, Simpson A, McRae AL, et al. Effects of gender and cigarette smoking on reactivity to psychological and pharmacological stress provocation. Psychoneuroendocrinol. 2008;33:560–568. doi: 10.1016/j.psyneuen.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer JS, Lichenstein E. Classification and prediction of smoking relapse episodes: An exploration of individual differences. J Consult Clin Psychol. 1988;56:104–110. doi: 10.1037//0022-006x.56.1.104. [DOI] [PubMed] [Google Scholar]
- Baer JS, Lichtenstein E, Kamarck T, Ransom CC. Prediction of smoking relapse: Analysis of temptations and transgressions after initial cessation. J Consult Clin Psychol. 1989;57:623–627. doi: 10.1037//0022-006x.57.5.623. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Baron JA, Comi RJ, Cryns V, Brinck-Johnsen T, Mercer N G. The effect of cigarette smoking on adrenal cortical hormones. J Pharmacol Exp Ther. 1995;272:151–155. [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: Relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Borland R. Slip-ups and relapse in attempts to quit smoking. Addict Behav. 1990;15:235–245. doi: 10.1016/0306-4603(90)90066-7. [DOI] [PubMed] [Google Scholar]
- Brandon TH. Negative affect as motivation to smoke. Current Direct Psychol Sci. 1994;3:33–37. [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: The process of relapse. Addict Behav. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Buchmann AF, Laucht M, Schmid B, Wiedemann K, Mann K, Zimmermann US. Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers. J Psychopharmacol. doi: 10.1177/0269881108095716. (in press) [DOI] [PubMed] [Google Scholar]
- Buczek J, Lê AD, Wong A, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacol. 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control. Annual smoking-attributable mortality, years of potential life lost, and economic costs United States, 1995–1999. MMWR Morb Mortal Wkly. 2002;51:300–303. [PubMed] [Google Scholar]
- Center for Disease Control. Health Effects of Cigarette Smoking-Fact Sheet, Tobacco Information and Prevention Source (TIPS) 2004 [Google Scholar]
- Childs E, de Wit H. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacol. 2009;203:1–12. doi: 10.1007/s00213-008-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, de Wit H. Effects of acute psychosocial stress on cigarette craving and smoking. Nic Tob Res. doi: 10.1093/ntr/ntp214. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nic Tob Res. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Cummings KM, Jaen CR, Giovino G. Circumstances surrounding relapse in a group of recent exsmokers. Preventive Medicine. 1985;14:195–202. doi: 10.1016/0091-7435(85)90035-0. [DOI] [PubMed] [Google Scholar]
- Dunn A J. Stress-related activation of cerebral dopaminergic systems. Ann NY Acad Sci. 1988;537:188–205. doi: 10.1111/j.1749-6632.1988.tb42106.x. [DOI] [PubMed] [Google Scholar]
- Erblich J, Boyarsky Y, Spring B, Niaura R, Bovbjerg DH. A family history of smoking predicts heightened levels of stress-induced cigarette craving. Addiction. 2003;98:657–664. doi: 10.1046/j.1360-0443.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV, Patient Edition. Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- Frederick SL, Reus VI, Ginsberg D, Hall SM, Munoz RF, Ellman G. Cortisol and response to dexamethasone as predictors of withdrawal distress and abstinence success in smokers. Biol Psychiatry. 1998;43:525–530. doi: 10.1016/S0006-3223(97)00423-X. [DOI] [PubMed] [Google Scholar]
- Garvey A, Bliss R, Hitchcock J, Heinold J, Rosner B. Predictors of smoking relapse among self-quitters: A report from the normative aging study. Addict Behav. 1992;17:367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinol. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Gilbert DF, Crauthers DM, Mooney DK, McClernon FJ, Jensen RA. Effects of monetary contingencies on smoking relapse: influences of trait depression, personality, and habitual nicotine intake. Exp Clin Psychopharmacol. 1999;7:174–181. doi: 10.1037//1064-1297.7.2.174. [DOI] [PubMed] [Google Scholar]
- Hariharan M, Van Noord T, Greden JF. A high performance liquid chromatographic method for routine simultaneous determination of nicotine and cotinine in plasma. Clin Chem. 1988;34:724–729. [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K. The Fagerstrom Test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Brit J Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Chait LD, Griffiths RR. Effects of ethanol on cigarette smoking volunteers without histories of alcoholism. Psychopharmacol. 1984;82:1–5. doi: 10.1007/BF00426371. [DOI] [PubMed] [Google Scholar]
- Higgins ST. The influence of alternative reinforcers on cocaine use and abuse: A brief review. Pharmacol Biochem Behav. 1997;57:419–427. doi: 10.1016/s0091-3057(96)00446-7. [DOI] [PubMed] [Google Scholar]
- Hughes JF, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Izard CE. Patterns of emotions: A new analysis of anxiety and depression. London: Academic Press; 1972. [Google Scholar]
- Kalivas PW, Duffy P. Similar effects of daily cocaine and stress on mesocorticolimbic dopamine neurotransmission in the rat. Biol Psychiatry. 1989;25:913–928. doi: 10.1016/0006-3223(89)90271-0. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Pierce RC, Cornish J, Sorg BA. A role for sensitization in craving and relapse in cocaine addiction. J. Psychopharmacol. 1998;12:49–53. doi: 10.1177/026988119801200107. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: Correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DW, Smith SS, Wetter D, Baker TB. Predicting smoking cessation. Who will quit with and without the nicotine patch. Journal of the American Medical Association. 1994;27:589–594. doi: 10.1001/jama.271.8.589. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Strasburger CJ, Langkrär J. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmacol Biochem Behav. 1993;44:527–531. doi: 10.1016/0091-3057(93)90162-m. [DOI] [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, et al. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Determinants of relapse: Implications for the maintenance of behavior change. In: Davidson PO, Davidson SM, editors. Behavioral medicine: Changing health lifestyles. New York: Brunner/Mazel; 1980. pp. 410–452. [Google Scholar]
- Matta SG, Foster CA, Sharp BM. Nicotine stimulates the expression of cFos protein in the paraventricular nucleus and brainstem catecholaminergic regions. Endocrinol. 1993;132:2149–2156. doi: 10.1210/endo.132.5.8386611. [DOI] [PubMed] [Google Scholar]
- Matta SG, Fu Y, Valentine JD, Sharp BM. Response of the hypothalamo-pituitary-adrenal axis to nicotine. Psychoneuroendocrinol. 1998;23:103–113. doi: 10.1016/s0306-4530(97)00079-6. [DOI] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McKee SA. Developing human laboratory models of smoking lapse behavior for medication screening. Addict Biol. 2009;14:99–107. doi: 10.1111/j.1369-1600.2008.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Maciejewski PK, Falba T, Mazure CM. Sex differences in the effects of stressful life events on changes in smoking status. Addiction. 2003;98:847–855. doi: 10.1046/j.1360-0443.2003.00408.x. [DOI] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacol. 2006;189:201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK. Hormones, nicotine, and cocaine: clinical studies. Hormones and Behavior. doi: 10.1016/j.yhbeh.2009.10.003. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Mutschler N, Halpern J. Temporal concordance of cocaine effects on mood states and neuroendocrine hormones. Psychoneuroendocrinol. 2002;27:71–82. doi: 10.1016/s0306-4530(01)00036-1. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Rodriguez ML. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Munch A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:24–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psych Bull. 2000;126:247–259. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- Norregaard J, Tonnesen P, Petersen L. Predictors and reasons for relapse in smoking cessation with nicotine and placebo patches. Preventive Medicine. 1993;22:261–271. doi: 10.1006/pmed.1993.1021. [DOI] [PubMed] [Google Scholar]
- O’Connell KA, Martin EJ. Highly tempting situations associated with abstinence, temporary lapse, and relapse among participants in smoking cessation programs. J Consult Clin Psychol. 1987;55:367–371. doi: 10.1037//0022-006x.55.3.367. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Grun EU, Collins AC. Tolerance to nicotine following chronic treatment by injections: a potential role for corticosterone. Psychopharmacol. 1992;108:33–39. doi: 10.1007/BF02245282. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Schare ML, Levis DJ, Colleti G. Exposure to smoking-relevant cues: effects on desire to smoke and topographical components of smoking behavior. Addict Behav. 1991;16:467–479. doi: 10.1016/0306-4603(91)90054-l. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Ciccocioppo M, Conklin CA, Milanak ME, Grottenthaler A, Sayette MA. Mood influences on acute smoking responses are independent of nicotine intake and dose expectancy. J Abnorm Psychol. 2008;117:79–93. doi: 10.1037/0021-843X.117.1.79. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Grobe JE, Stiller RL, Fonte C, Goettler JE. Nasal spray nicotine replacement suppresses cigarette smoking desire and behavior. Clin Pharmacol Ther. 1992;52:627–634. doi: 10.1038/clpt.1992.201. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: a proposal for new methodologies. Psychopharmacol. 2006;184:628–636. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. Pathophysiological basis of vulnerability to drug abuse: Role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Ann Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS. Cortisol responses to a psychological stressor and/or nicotine. Pharmacol Biochem Behav. 1990;36:211–213. doi: 10.1016/0091-3057(90)90153-9. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Pomerleau CS. Research on stress and smoking: progress and problems. Addiction. 1991;86:599–603. doi: 10.1111/j.1360-0443.1991.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Prasad BM, Sorg BA, Ulibarri C, Kalivas PW. Sensitization to stress and psychostimulants: Involvement of dopamine transmission versus the HPA Axis. Ann N Y Acad Sci. 1993;771:617–625. doi: 10.1111/j.1749-6632.1995.tb44714.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Mattox AJ, Thompson SS, Carroll ME. Effects of buprenorphine and an alternative nondrug reinforcer, alone and in combination on smoked cocaine self-administration in monkeys. Drug Alc Depend. 1997;45:21–29. doi: 10.1016/s0376-8716(97)01341-0. [DOI] [PubMed] [Google Scholar]
- Rose JE, Ananda S, Jarvik ME. Cigarette smoking during anxiety-provoking and monotonous tasks. Addict Behav. 1983;8:353–359. doi: 10.1016/0306-4603(83)90035-7. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Beyer H S. Rapid desensitization of the acute stimulatory effects of nicotine on rat plasma adrenocorticotropin and prolactin. J Pharmacol Exp Ther. 1986;238:486–491. [PubMed] [Google Scholar]
- Sheehan PW. Reliability of a short test of imagery. Percept and Motor Skills. 1967;25:744. doi: 10.2466/pms.1967.25.3.744. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Relapse following smoking cessation: a situational analysis. J Consult Clin Psychol. 1982;50:71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Gwaltney CJ, Balabanis MH, Liu KS, Paty JA, Kassel JD, et al. Immediate antecedents of cigarette smoking: An analysis from ecological momentary assessment. J Abnorm Psychol. 2002;111:531–545. doi: 10.1037//0021-843x.111.4.531. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Kirchner TR. Cigarette-by-cigarette satisfaction during ad libitum smoking. J of Abnorm Psychol. 2009;118:348–359. doi: 10.1037/a0015620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gwaltney CJ, Dang Q. Immediate antecedents of cigarette smoking: An analysis of unrestricted smoking patterns. J of Abnorm Psychol. 2004;113:166–171. doi: 10.1037/0021-843X.113.1.166. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: Within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–379. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking lapses: A prospective analysis. J Consult Clin Psychol. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase the risk of drug abuse and relapse? Psychopharmacol. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: Implications for addiction treatment development. Addiction Biol. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzer ML, Rand CS, Below GE, Mead AM. Contingent payment procedures for smoking reduction and cessation. J Appl Behav Anal. 1986;19:197–202. doi: 10.1901/jaba.1986.19-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, Denk CE, Parker SD, Carmelli D, Furze CT, Rosenman RH. Risk factors for late relapse in male and female ex-smokers. Addict Behav. 1988;13:253–266. doi: 10.1016/0306-4603(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5th Edition. Boston: Allyn & Bacon; 2006. [Google Scholar]
- Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of mesocortical DA system by stress. Nature. 1976;263:242–244. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- Tice DM, Bratslavsky E, Baumeister RF. Emotional distress regulation takes precedence over impulse control: if you feel**bad**do it! Person Soc Psychol. 2001;80:53–67. [PubMed] [Google Scholar]
- Tsuda A, Steptoe A, West R, Fieldman G, Kirschbaum C. Cigarette smoking and psychophysiological stress responsiveness: Effects of recent smoking and temporary abstinence. Psychopharmacol. 1996;126:226–233. doi: 10.1007/BF02246452. [DOI] [PubMed] [Google Scholar]
- West EC, Levin ED, Rose J D. Smoking while wearing the nicotine patch; is smoking satisfying or harmful. Clin Res. 1992;40:871A. [Google Scholar]
- Willner P, Jones C. Effects of mood manipulation on subjective and behavioural measures of cigarette craving. Behav Pharmacol. 1996;7:355–363. doi: 10.1097/00008877-199608000-00007. [DOI] [PubMed] [Google Scholar]
- Willner P, Benton D, Brown E, Cheeta S, Davies G, et al. “Depression” increases “craving” for sweet rewards in animal and human models of depression and craving. Psychopharmacol. 1998;36:272–283. doi: 10.1007/s002130050566. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Tobacco Free Initiative. [accessed Feb 16, 2010];2010 www.who.int/tobacco/mpower/tobacco_facts/en/index.html.
- Zinser MC, Baker TB, Sherman JE, Cannon DS. Relations between self-reported affect and drug urges and cravings in continuing and withdrawing smokers. J Abnorm Psychol. 1992;101:617–629. doi: 10.1037//0021-843x.101.4.617. [DOI] [PubMed] [Google Scholar]