Abstract
The blood-brain barrier (BBB), a dynamic and complex barrier formed by endothelial cells, can impede the entry of unwanted substances – pathogens and therapeutic molecules alike – into the central nervous system (CNS) from the blood circulation. Taking into account the fact that CNS-related diseases are the largest and fastest growing unmet medical concern, many potential protein- and nucleic acid-based medicines have been developed for therapeutic purposes. However, due to their poor ability to cross the BBB and the plasma membrane, the above-mentioned bio-macromolecules have limited use in treating neurological diseases. Finding effective, safe, and convenient ways to deliver therapeutic molecules into the CNS is thus urgently required. In recent decades, much effort has been expended in the development of drug delivery technologies, of which cell-penetrating peptides (CPPs) have the most promising potential. The present review covers the latest advances in CPP delivery technology, and provides an update on their use in CNS-targeted drug delivery.
Keywords: Central nervous system, blood-brain barrier, cell-penetrating peptides, drug delivery.
INTRODUCTION
The blood-brain barrier (BBB) is a dynamic and complex barrier that exists in all vertebrate organisms with well-developed central nervous systems (CNSs) and which is interposed between the blood and CNS to protect the brain against invading pathogenic organisms [1, 2]. It is also the most unique and challenging barrier formed by endothelial cells to impede the entry of unwanted substances such as therapeutic molecules into the CNS via the blood circulation [3, 4]. As the number of people who suffer from CNS-related diseases increases, there is an urgent demand for the development of effective therapeutic methods to enable therapeutic molecules to cross the BBB [5].
Despite rapid developments in our understanding of the BBB and great advances in medical technology, many CNS-associated diseases remain beyond the reach of conventional therapies [6]. This predicament is not caused by a lack of candidate therapeutic molecules but by the failure of nearly 98% of small molecules and 100% of large molecules to cross the BBB into the CNS [7, 8]. The only way to overcome this obstacle is to find a suitable delivery system. As the existing delivery methods may conflict with the natural functioning of the BBB, the ongoing effective approaches should be carefully studied with respect to their influence on the overall protective function of BBB. Considering the poor efficiency of the traditional delivery methods, the present review just covers the aggressive research efforts that are being made in this field, paying a close attention to the development of new cell-penetrating peptide-based therapeutic strategies.
THE STRUCTURE AND PHYSIOLOGICAL FUNCTION OF BBB
The BBB, as a continuous impermeable cellular barrier, is primarily composed of non-fenestrated brain endothelial cells characterized by tight junctions (TJs), which dramatically limit the transport of unwanted substances to the CNS [9]. TJs at the BBB are composed of an intricate combination of trans-membrane proteins and cytoplasmic accessory proteins, which are linked to the actin cytoskeleton to form extremely restrictive cell-to-cell connections [1, 3]. The structural properties of TJs, such as the diffusional features enforced by the lipid bilayer and the directional characteristics of the specific transport proteins in the cell membrane make the BBB a continuous cell membrane [4, 6]. Furthermore, the TJs act as a fence in the lateral cell membrane that segregates unwanted substances to either the luminal or abluminal membrane domain and impose restrictions on their free movement from one side of the endothelium to the other [10].
In addition, it is now well accepted that the functional structure of the BBB includes more than just brain microvessel endothelial cells [11], and several other cell types such as adjoining pericytes and astrocytes are the critical determinants that maintain the brain capillary phenotype. These cells combine with the extracellular basal membrane and microglia to form the support system of the BBB [10, 12, 13]. Together with the surrounding neurons, these components form an intact and functional neurovascular unit [14]. Brain endothelium, the principal and most effective part of the BBB, is formed from abundant exchange vessels, such as capillaries and post-capillary venules, with thin walls and wide surface scales. In the human brain, approximately 100 billion capillaries make up a total length of approximately 650 km and a total global surface area 20 m2 of brain capillary endothelium [15].
Therefore, the unique biological characteristics of the BBB owe its low permeability to a variety of important factors, including: (1) very few pinocytotic vesicles [2, 16, 17]; (2) TJs strengthened by the interaction of astrocytes and pericytes with brain endothelia cells [18]; (3) the synergistic inductive functions of certain cells, such as astrocytes, pericytes, perivascular macrophages and neurons [19-22]; (4) the permeability restriction of unwanted agents by ATP-binding cassette (ABC) transporters, insulin receptors, multidrug resistance-associated proteins (MRPs), and transferrin receptors [23-25]; and (5) the lack of lymphatic drainage and the absence of the major histocompatibility complex (MHC) [26]. All these features of the BBB make it a selective barrier, which, in conjunction with the cerebrospinal fluid (CSF), continuously flush therapeutic molecules back to the blood stream [27, 28].
THE THERAPEUTIC MOLECULE DELIVERY TECHNOLOGIES FOR CROSSING THE BBB
Passive Diffusion
Passive diffusion, a concentration-dependent transport method, allows multiple lipid-soluble therapeutic molecules through the BBB without an energy requirement [29, 30]. There is high correlation between the crossing rate of therapeutic molecules and their lipid solubility and molecular weight [31, 32]. Lipid solubility can be determined by the logD octanol/water partition co-efficient at pH 7.4 [33] and only the molecules with a molecular weight less than 400 Da are permeable to the BBB [34]. The co-effect of lipid solubility and molecular weight is believed to be attributable to the functional expression of several ABC transporters that deliver therapeutic molecules across the BBB [35]. However, these are not always the absolute restriction factors for BBB penetration. Currently there are many types of efficient CNS-active drugs in clinical use, such as those drugs that carry a positive charge. Basically, their entry is probably due to the reaction between the positively charged drugs and the negatively charged glycocalyx and phospholipid of the outer leaflet of the BBB [36]. For example, lipophilic compounds such as hydroxyzine and triprolidine can access the CNS by passive diffusion when administered via the nasal cavity [37], and acetylcholinesterase reactivators also appear to undergo passive transport through the BBB [38].
Endogenous Carrier-Mediated Transports
Endogenous carrier-mediated transport, which involves ATP- and transporter-dependent active transport, solute carrier (SLC) transporters, and receptor-mediated endocytosis, is characterized by selectivity and saturability and requires energy expenditure by the cell [30, 39]. For ATP-dependent active transport, breast cancer resistance protein (BCRP), P-glycoprotein (P-gp) and the MRP family are the classical transporters [39]. BCRP is a multidrug resistance protein that mediates apically directed drug transport [40]. Prazosin and cimetidine are two typical substrates of BCRP [41]. P-gp in brain capillary endothelial cells functions as an efflux pump in the physiological state and P-gp-mediated efflux of cyclosporin A is a major reason for its restricted transfer from the blood to the brain [42]. MRP proteins contribute to the cellular efflux of endogenous anionic glutathione or glucuronate conjugates (substrates for MRP1), cyclic nucleotides (substrates for MRP4 and MRP5), and glutathione (co-substrate for MRP1 and MRP4) [43]. Although transporting a variety of substrates across the BBB into the CNS by BCRP, P-gp and MRP has been widely described, low efficiency is still a major problem for these endogenous carrier-mediated transports [39, 44-49].
The substrates for BCRP, which is expressed at the luminal membrane of the BBB, include cytotoxic compounds, sulfated conjugates of therapeutic drugs, and hormones; the overlapping profile and similar localization of those substrates demonstrate that the BCRP has a limited BBB penetration [44]. P-gp, a member of the ABC transporter superfamily, is also expressed at the luminal membrane of the BBB and can positively pump a variety of therapeutic drugs back into systemic circulation [45]. Recent study suggested that the cooperation of P-gp and BCRP can transport substrates from endothelium to blood [46]. The MRP family, comprised of nine members (MRP1-9), is competent efflux pumps that are capable of the delivery of structurally diverse lipophilic anions [47]. Some members of the MRP family seem to be located in either the luminal or the abluminal membrane, or sometimes both [48]. For example, MRP1, MRP4, and MRP5 are clearly localized on the luminal side of brain capillary endothelial cells, MRP4 and MRP5 have also been detected in astrocytes of the subcortical white matter, and MRP5 is present in pyramidal neurons [43]. Together, they may play a role in the chemoresistance of the BBB [49].
Most polar molecules cannot passively diffuse through the BBB and the cells in the BBB express a large number of SLCs [44]. There are 51 families of SLC transporters and they play crucial roles in numerous cellular physiological processes, including importing or exporting nutrients, neurotransmitters and metabolites [50]. Among the members of the SLC family, the organic cation transport (OCT) system (SLC21) and organic anion transport (OAT) system (SLC22) are of particular interest due to their roles in delivering therapeutic molecules across the BBB. Depending on the sub-cellular localization of these transporters at the BBB, endogenous therapeutic molecules can be transported into or pumped out of the brain [39].
Some therapeutic molecules usually can be delivered across the BBB via the receptor-mediated transcytosis (RMT) system, which is generally a three-step procedure involving receptor-mediated endocytosis at the blood side followed by intracellular movement and exocytosis at the brain side of brain endothelial cells [15]. Several receptors on the BBB, such as the transferrin receptor (TfR), insulin receptor (IR), IGF receptors 1 and 2 (IGFR1 and 2), the low-density lipoprotein receptor (LDLR), the low-density lipoprotein receptor-related proteins 1 and 2 (LRP1 and 2), the scavenger receptors class A type I (SR-AI), class B type I (SR-BI) and diphtheria toxin receptor, have been extensively studied as part of the RMT system [51, 52]. RMT allows large molecules to be transported across the BBB and is thus a useful method for the delivery of peptides, proteins and certain peptidomimetic monoclonal antibodies into the brain [22, 53]. For example, insulin and transferrin are transported from blood to brain by IRs and TfRs, respectively [54].
DELIVERY OF THERAPEUTIC MOLECULES ACROSS THE BBB MEDIATED BY CELL-PENETRATING PEPTIDES
Although multi-disciplinary approaches are now available, they still can not meet the needs of high doses while limiting the risk of major side effects. Thus, molecule delivery systems that cross the BBB constitute a major piece of the therapeutic puzzle, and new efficient therapeutic molecule delivery systems are still urgently required. Recent rapid advances in molecular biology have enabled the development of novel delivery systems that take advantage of our better understanding of the BBB. Compared with the majority of delivery systems that suffer from various limitations when applied in clinical situations to the transport of therapeutic molecules into the CNS, cell-penetrating peptide (CPP)-based delivery systems show a great ability in carrying macromolecules across cellular membranes, combining a low cellular toxicity with high efficiency [55]. Considering their smaller size (up to 30 amino acids in length), cationic and/or amphipathic CPPs have a greater potential to penetrate the BBB than other transport systems, enabling them to be used as very promising tools for therapeutic purposes in CNS-related diseases [56].
The first CPP, trans-activator of transcription (TAT), derived from human immunodeficiency virus-1 (HIV-1), can be efficiently taken up from the surrounding media [55, 56]. Another penetratin, also known as Antp, derived from the third helix of the antennapedia transcription factor of Drosophila melanogaster [57], is, together with TAT, regarded to be the first group of CPPs derived from natural proteins. Since then, the number of CPPs with effective transduction properties has greatly expanded. The second group of CPPs consists of chimeric molecules, such as transportan (TP), which consists of 12 amino acids derived from the neuropeptide galanin fused with a 14 amino acid peptide from the wasp venom mastoparan [58]. The third group of CPPs consists of the synthetic peptide family, of which polyarginines are the best studied [59, 60].
CPPs are of different sizes and amino acid sequences but with one distinct feature, which is capable to deliver various cargo molecules across the plasma membrane,some even can cross BBB to facilitate the delivery of various therapeutic molecules; thus, they act as molecular delivery vehicles (Fig. 1) [60]. A number of CPPs have already shown great ability in improving therapeutic molecule delivery across the BBB to treat CNS diseases [61]. The CPPs used include TAT, Angiopep, penetratin, TP, rabies virus glycoprotein (RVG), prion peptide, and SynB [62-67]. Different CPPs use distinct cellular translocation pathways, which depend on cell types and cargos, even though the delivery mechanisms are still under debate [60]. Generally, there are two types of mechanisms now proposed: direct penetration and endocytosis-mediated entry. Direct penetration of CPPs occurs via an energy-independent cellular process and it is believed that translocation across biological membranes can progress at 4oC and most likely involves a direct electrostatic interaction with negatively charged phospholipids [68]. Recently, a detailed model for direct penetration has been proposed, which involves strong interactions between CPPs and the phosphate groups on both sides of the lipid bilayer, the translocation of charged residues across the hydrophobic core of the membrane and the passive diffusion of these highly charged peptides across the membrane through the formation of aqueous toroidal pores. This mechanism explains how key ingredients, such as the cooperativity between the peptides, the large positive charge, and specifically the guanidinium groups, contribute to uptake [69].
Fig. (1).
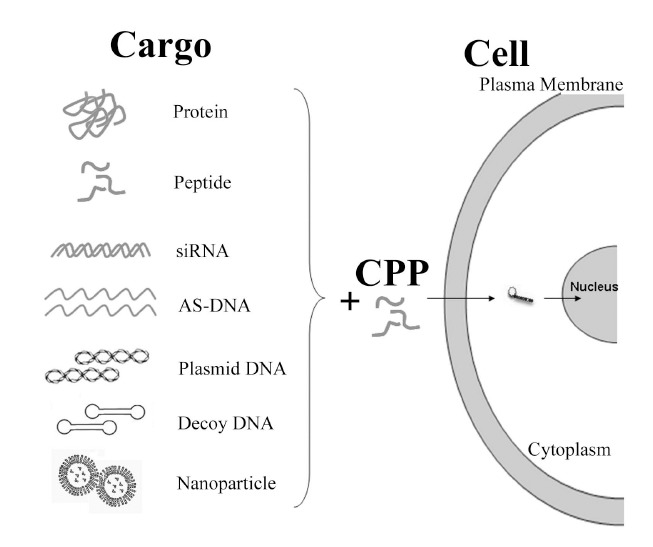
Applications of CPPs in cargo delivery.
Endocytosis-mediated entry is the process of cellular ingestion, where the plasma membrane folds inward to bring substances into the cell. During this process cells absorb substances from outside of the cell by imbibing it with their cell membrane. Study has shown that the cellular entry of penetratin by endocytosis is an energy-dependent process, and that this process is initiated by polyarginines interacting with heparan sulfates that promote endocytosis [70]. Research has also shown that TAT is internalized through a form of endocytosis called macropinocytosis [71]. Another endocytosis-mediated entry is based on the formation of inverted micelles. Inverted micelles are aggregates of colloidal surfactants in which the polar groups are concentrated in the interior and the lipophilic groups extend outward into the solvent. According to this model, a penetratin dimer combines with the negatively charged phospholipids, thus generating an inverted micelle inside of the lipid bilayer. The structure of the inverted micelles permits the peptide to remain in a hydrophilic environment [72, 73].
In summary, CPPs hold great potential as in vitro and in vivo delivery vectors for research and clinical use and they have been successfully used for the delivery of therapeutic molecules such as proteins, peptides, nucleic acids, small molecules and nanoparticles into cells.
Delivery of Proteins and Peptides Across the BBB by CPPs
Therapeutic protein and peptide delivery across the BBB by traditional cargo methods has been challenged by the multiple limitations offered by the complicated physiological surroundings. Although gene therapy is an attractive treatment strategy for many diseases, the therapeutic use of viral vectors in some acute diseases such as acute stroke is not feasible because the delayed increase in active protein levels in patients after transfection result in little benefit. An alternative way to deliver even larger proteins is to use CPPs, a quick, simple and safe delivery system. It occurs in a concentration-dependent fashion and enters into cells through the lipid bilayer component of the cellular membrane. In recent studies, several CPPs have been used for the delivery of biologically active peptides or full-length proteins into the CNS [74] (Tables 1-2). Several studies have shown CPP-mediated delivery of fusion proteins in vitro [74-75]. However, only a few studies have used CPPs as the vehicle to traffic the peptides or proteins across the BBB into the CNS. The first evidence for CPP in vivo delivery was provided by Schwarze et al. [76]. They confirmed that β-galactosidase fused with TAT can be transported into almost all tissues, including the brain, when intraperitoneally injected into mice.
Table 1.
Applications of Representative CPPs in vivo
| Applications of Representative CPPs in Proteins Delivery | ||||
| CPPs | Sequences | Cargo | Target | Summary |
| TAT | YGRKKRRQRRR | β-Gal/GDNF/ JNKI1/PSD-95 | Brain | β-Gal fused to TAT resulting in strong β-Gal enzyme activity in mice brain; TAT-JNKI1 administration 3 hours after brain ischemia significantly reduced the infarct volume; TAT-PSD-95 protected cultured neurons from excite-toxicity, and reduced focal ischemic brain damage; TAT-GDNF protected brain neurons from cell death when administered after focal cerebral ischemia [76, 79, 80] |
| TAT-HA | YGRKKRRQRRR-YPYDVPDVA | Bcl-xL | Brain | Administration of TAT-HA-Bcl-xL into mice decreased cerebral infarction in a dose-dependent manner, as determined at 3 d after 90 min of focal ischemia [77] |
| RDP | KSVRTWNEIIPSKGCLRVGGRCHPHVNGGGRRRRRRRRR | BDNF/β-Gal/Luc | Brain | RDP-BDNF showed the neuro-protective properties in mouse experimental stroke including reduction of stroke volume and neural deficit; The brain slices with X-Gal staining were determined the delivery of RDP-β-Gal across the BBB; The time-course relationship of RDP-Luc was studied to confirm the transport efficiency of RDP [81, 82] |
| FGF4 | AAVLLPVLLAAP | SOCS3 | Brain | FGF4-SOCS3 protected mice from lethal effects of staphylococcal enterotoxin B and lipopolysaccharide by reducing production of inflammatory cytokines and hemorrhagic necrosis brain [83] |
| Applications of Representative CPPs in Nucleic Acids Delivery | ||||
| CPPs | Sequences | Cargo | Target | Summary |
| TAT-10H | 5H-YGRKKRRQRRR-5H | Plasmid DNA | Brain | 5H-TAT-5H/DNA complexes improve up to 7000-fold in BBB across efficiency over the original Tat peptide [87] |
| RVG-9R | YTIWMPENPRPGTPCDIFTNSRGKRASNG-9R | GAPDH/BACE1 siRNA | Brain | RVG-9R delivered GAPDH/BACE1 siRNA to neurons, microglia, oligodendrocytes in the brain, resulting in a specific gene knockdown [97] |
| Penetratin | RQIKIWFQNRRMKWKK | APP antisense oligonucleotides | Brain | Penetratin-APP antisense oligonucleotides reduced APP expression and embryonic neural stem cell proliferation in the subventricular zone of the CNS [102] |
| Applications of Representative CPPs in Small-Molecule Drugs Delivery | ||||
| CPPs | Sequences | Cargo | Target | Summary |
| SynB3 | RRLSYSRRRF | Morphine-glucuronide | Brain | Enhanced the brain uptake of morphine-glucuronide in in situ brain perfusion [52] |
| SynB1/SynB3 | RGGRLSYSRRRFSTSTGR/RRLSYSRRRF | Dalargin/Paclitaxel | Brain | Enhanced dalargin in brain uptake, resulting in a significant improvement of anti-nociceptive effect [109] |
| SynB1/SynB5 | RGGRLSYSRRRFSTSTGR/RGGRLAYLRRRWAVLGR | Doxorubicin | Brain | Significantly increase the uptake of doxorubicin into the brain [110] |
| SynB1/D-Penetratin | RGGRLSYSRRRFSTSTGR/RQIKIWFQNRRMKWKK | Doxorubicin | Brain | 20-fold increase of doxorubicin in brain parenchyma by using a capillary depletion method, [111] |
| Angiopep-2 | PFFYGGSGGNRNNYLREEY | Paclitaxel | Brain | Angiopep-2-paclitaxel showed activity in heavily pretreated patients with brain metastases and/or failed prior taxane therapy [115] |
| Angiopep-5 | RFFYGGSRGKRNNFRTEEY | Doxorubicin | Brain | Angiopep-5-doxorubicin exhibited dramatically higher BBB influx rate constants than doxorubicin and pooled within brain parenchymal tissue [113] |
| Applications of Representative CPPs in Nanoparticles Delivery | ||||
| CPPs | Sequences | Cargo | Target | Summary |
| TAT | YGRKKRRQRRR | Qdots | Brain | TAT-Qdot was loading sufficiently high in brain that a gross fluorescent can be visualized using a low power UV lamp [117] |
| TAT-Liposome | YGRKKRRQRRR- Liposome | Coumarin-6 | Brain | TAT-LIP was a promising brain drug delivery system due to its high delivery efficiency across the BBB [121] |
| TAT-cholesterol | YGRKKRRQRRR -cholesterol | G(3)R(6) | Brain | FITC-loaded cholesterol-conjugated G(3)R(6)-TAT can cross BBB, and is a promising antimicrobial agent for treatment of brain infections caused by C. neoformans [120] |
| TAT-PEG-cholesterol | YGRKKRRQRRR-PEG -cholesterol | Ciprofloxacin | Brain | TAT-PEG-cholesterol were effective for delivery of ciprofloxacin across the BBB [119] |
| RVG-BPEI-SS | YTIWMPENPRPGTPCDIFTNSRGKRASNG-BPEI-SS | cy5.5-miR-124a | Brain | The RVG combined with BPEI-SS for neuron-specific targeting in vivo is sufficient to deliver neurogenic microRNA (e.g. cy5.5-miR-124a) into the brain [98] |
| (RXRRBR)2XB | RXRRBRRXRRBRXB | AMO | Brain | Systemic administration of (RXRRBR)2XB-AMO in mice showed efficient uptake in the brain, which can dramatically improve ATM splicing correction efficiency [101] |
| Angiopep-2-PEG | PFFYGGSGGNRNNYLREEY-PEG | Paclitaxel -Alexa488 | Brain | Fluorescent microscopy revealed that Angiopep-2 modified PEG nanoparticle deliver Alexa488 labeled paclitaxel across BBB, and localized in brain endothelial cell monolayers [112] |
| Angiopep-2-O-MWNTs-PEG | PFFYGGSGGNRNNYLREEY-O-MWNTs-PEG | Doxorubicin | Brain | Fluorescence imaging demonstrated that Angiopep-2-O-MWNTs-PEG constituted an ideal dual-targeting drug delivery system to deliver doxorubicin for the treatment of brain tumor [114] |
Table 2.
Recent Applications of Representative CPPs in vitro
| Applications of Representative CPPs in Proteins Delivery | ||||
| CPPs | Sequences | Cargo | Target | Summary |
| TAT | YGRKKRRQRRR | JNKI | Neurons | TAT-JNKI administration 6 hours after oxygen glucose deprivation reduced neurons death at 24 hours [78] |
| Applications of Representative CPPs in Nucleic Acids Delivery | ||||
| Name | Sequences | Cargo | Target | Summary |
| TAT | YGRKKRRQRRR | Plasmid DNA | Various cell lines | Confocal imaging showed that TAT-DNA mediated transfection was 3-fold more efficient than a standard PEI transfection [85] |
| HC-[poly(K)] | QYIKANSKFIGITEL-poly(K) | Plasmid DNA | Neuroblastoma / glioma mouse/rat hybrid cell line | HC-[poly(K)]-DNA, resulting in non-viral gene delivery and marker gene expression in vitro, was dependent on HC and was neuronal cell type specific [86] |
| RVG-9R | YTIWMPENPRPGTPCDIFTNSRGKRASNG-9R | FvE siRNA | Neuronal cells | RVG-9R delivered FvE siRNA to the neuronal cells, resulting in specific gene silencing within the brain, and afforded robust protection against fatal viral encephalitis in mice [66] |
| Penetratin | RQIKIWFQNRRMKWKK | Caspase-3/8/9 siRNA, SOD1-1/2 siRNA | Primary mammalian hippocampal and sympathetic neurons | Penetratin-caspase-3i/8i/9i siRNA can be uptake by primary mammalian hippocampal and sympathetic neurons efficiently; Penetratin-thiol linker-deliver SOD1-1/2 siRNA to neurons simply, efficiently, and without toxicity [99] |
| TP/TP10 | GWTLNSAGYLLGKINLKALAALAKKIL/AGYLLGKINLKALAALAKKIL | NFкB decoy DNA | Various cell lines | Conjugation of TP/TP10-PNA hexamer or nonamer with NFкB decoy DNA can efficiently depress interleukin-1-induced NFкB activation and interleukin-6 gene expression [104] |
| Applications of Representative CPPs in Small-Molecule Drugs Delivery | ||||
| CPPs | Sequences | Cargo | Target | Summary |
| Angiopep-5 | RFFYGGSRGKRNNFRTEEY | Doxorubicin | Brain cancer cells | Angiopep-5-doxorubicin killed brain cancer cell lines in vitro [113] |
| SynB3 | RRLSYSRRRF | Morphine-glucuronide | Brain cancer cells | SynB3 enhanced the brain uptake of morphine-glucuronide through an in vitro BBB model [52] |
| Applications of Representative CPPs in Nanoparticles Delivery | ||||
| CPPs | Sequences | Cargo | Target | Summary |
| TAT-PEG-Cholesterol | YGRKKRRQRRR- PEG-Cholesterol | FITC | Brain astrocytes cells | Confocal laser scanning microscopy reveals that the uptake of FITC-TAT-PEG-Cholesterol by human astrocytes was much higher than that of free FITC [119] |
| PAMAM-PEG- Angiopep-2 | PFFYGGSGGNRNNYLREEY | Plasmid DNA | Brain glial cells | PAMAM-PEG-DNA-Angiopep-2 can be a potential delivery system for gene therapy of glial tumor [124] |
| PEI-TAT | YGRKKRRQRRR-PEI | Plasmid DNA | Primary neurons | PEI-DNA-TAT improves the cellular uptake of gene vectors and enhances the gene transfection efficiency of primary neurons up to 14-fold [90] |
Cao et al. [77] injected a TAT-Bcl-xL fusion protein into the stroke mouse model, and found that the mortality of neuronal cell death was greatly decreased in the area of ischemic damage. TAT-Bcl-xL treatment before and after ischemia can also reduce infarct volume and neurological deficits after prolonged ischemic insults lasting 90 min. Furthermore, reduced cerebral ischemic damage and protection against ischemia in brain injury have also been reported by using TAT-neuroglobin, TAT-islet-brain-1 (IB-1)/JNK-interacting protein-1 (JIP-1), TAT-PSD-95, TAT-NR2B9c peptide and TAT-GDNF [73, 78-80].
Fu et al. [81] have developed a vehicle based on a 39-amino acid peptide derived from rabies virus glycoprotein (RDP) to efficiently target β-galactosidase and brain-derived neurotrophic factor (BDNF) to the CNS by systemic administration of RDP-fusion proteins. Furthermore, the RDP-BDNF fusion protein showed neuroprotective properties including a reduction in stroke volume and neural deficits in mouse stroke models [82]. Jo et al. [83] used a CPP composed of a hydrophobic signal sequence derived from fibroblast growth factor 4 (FGF4) to deliver the physiological inhibitor suppressor of cytokine signaling 3 (SOCS3) to the brain. The results implied that replenishing the intracellular stores of conditionally labile SOCS3 with cell-penetrating forms of SOCS3 can effectively suppress the devastating effects of acute inflammation.
Delivery of Nucleic Acids Across the BBB by CPPs
Transfection of cultured cells with nucleic acids (e.g., plasmids, decoy oligodeoxynucleotides, siRNA, and antisense oligonucleotides) are well described, and provide for primary neurons, especially for brain neuronal cells in vivo, a high-yield method that is distinct from that provided by viral vectors. Viral transfection has certain drawbacks, including the complexity of vector preparation, safety concerns, and the generation of immune or inflammatory responses in vivo. Meanwhile, the main problem of non-viral nucleic acids transfection methods is a low efficiency when compared with viral vectors. To overcome these problems, the CPP-nucleic acid conjugate method has been developed, which is proving to be a very powerful tool (Tables 1-2).
As genes can be inserted into the compatible sites of plasmids, and the corresponding complexes can be transfected into living cells, plasmids, which have had a seminal role in the advances of genetic engineering, have been identified as a promising therapeutic approach. A method that uses macro-branched TAT has been proposed for plasmid DNA delivery into various mammalian cell lines and has shown significant transfection capabilities [84]. Furthermore, multimers of TAT have been found to increase the transfection efficiency of plasmid DNA by 6-8 times more than poly-L-arginine or mutant TAT2-M1 and by 390 times compared with standard vectors [85]. Knight et al. [86] reported that when the nontoxic fragment C (HC) of tetanus toxin is covalently bound to polylysine [poly(K)] it can improve the binding of DNA and lead to high transfection efficiency in vitro in N18 RE 105 cells (a neuroblastoma X glioma mouse/rat hybrid cell line) and F98 cell (a glioma cell line).
Lo et al. [87] has observed that the transfection efficiency of DNA in human and rat glioma cell lines was enhanced 7,000-fold when plasmid DNA was mixed with TAT with 10 linked histidine residues (TAT-10H) instead of the original TAT; a further increase in efficiency was found if two cysteine residues were incorporated into the TAT-10H peptide. For gene transfection of CNS cells, CPP-modified nanocarriers, such as TAT-modified liposomes or micelles that allow for intracellular delivery, may be a better way [88]. Researchers always use polyethylene glycol (PEG)/polyethylenimine (PEI)-shielding to improve particle pharmacokinetic behavior, a targeting ligand to facilitate particle-cell recognition and in some case a bioresponsive lipid or pH-triggered polymer to enhance nucleic acid release and intracellular trafficking [89]. A number of groups have observed that a CPP-modified nanocarrier can be an effective system for plasmid delivery. For example, PEI/DNA-TAT improves the cellular uptake of gene vectors and enhances the gene transfection efficiency of primary neurons up to 14-fold [90]. However, CPP-modified nanocarriers require more in vivo studies to support the development of appropriate clinical applications in CNS disease therapy.
Distinct from plasmid DNA delivery, the CPP-mediated transfection of cells with small interfering RNA (siRNA) in vitro, and even in vivo, has been extensively studied [91-93]. RNA interference (RNAi) is a naturally occurring gene silencing process whereby cells degrade complementary mRNA molecules that may be a promising therapeutic approach for many CNS diseases, including brain tumors, neurotrauma, neuropsychiatric diseases, neuromuscular diseases, pain, and infections [94-96]. The BBB is essentially impenetrable to any RNA molecule and part of the reason is that the average mass of any possible siRNA molecule will be approximately 14 kDa. Most animal models and preclinical studies on the delivery of siRNA molecules to the brain are through invasive routes so there is an urgent demand to develop new delivery methods to improve the efficiency of therapeutic siRNA through the systemic route across the BBB.
The rabies virus can enter into neurons because the RVG interacts specifically with the nicotinic acetylcholine receptor on neuronal cells. Kumar et al. [66] showed that RVG-9R can deliver therapeutic siRNA to target neuronal cells in vivo, providing 80% protection against fatal viral encephalitis in mice after three days treatment. Alvarez-Erviti et al. [97] reported that siRNAs were successfully delivered to the mouse brain by systemic injection of RVG-modified exosomes. This study demonstrated that the mRNA and protein levels of beta-amyloid precursor protein (APP) cleaving enzyme 1 (BACE1), a therapeutic target in Alzheimer’s disease, were knocked down by as much as 60% and 62%, respectively, by RNAi in wild-type mice. Hwang et al. [98] also delivered siRNA and microRNA to the mouse brain using an RVG-modified PEI nanocarrier (RVG-PEI-SS) as vehicle.
Other CPPs also can be used as therapeutic siRNA delivery systems. For example, Davidson et al. [99] has found that penetratin can deliver siRNA duplexes to primary mammalian hippocampal and sympathetic neurons in vitro and to the CNS in an in vivo rat model when coupled with siRNA through a thiol-based linkage. This approach caused less toxicity towards the targeted cells in comparison with the liposome-based siRNA approach.
Abundant human genetic diseases caused by mutations can lead to aberrant alternative splicing. Pre-mRNA splicing correction is another promising therapy for these kinds of diseases. Antisense morpholino oligonucleotides (AMOs), a type of neutral single-stranded DNA derivative with a morpholine ring instead of a sugar moiety, complementarily bind to a target point and correct the pre-mRNA splicing by adjusting the splicing localization, thereby providing a potential therapeutic tool for genetic diseases [100, 101]. However, this strategy has been limited by low correction efficiency in vivo and the inability of AMOs to cross the BBB efficiently. Due to their membrane translocation properties, CPPs covalently coupled to an oligonucleotide can increase the delivery of these kinds of molecules, delivering them directly into the cytoplasm and ultimately the nucleus. For example, (RXRRBR)2XB, an arginine-rich CPP (R=L-arginine, X=6-aminohexanoic acid, B=beat-alanine), can dramatically improve ataxia-telangiectasia mutated (ATM) splicing correction efficiency when conjugated with specific AMO [101]. The systemic administration of the FITC-labeled (RXRRBR)2XB-AMO showed efficient uptake by the brain of model mice.
Besides siRNA and splicing correction, CPPs can also be employed to deliver other nucleic acids, such as antisense oligonucleotides, peptide nucleic acids (PNAs), and decoy DNA. Caille et al. [102] have demonstrated that penetratin-APP antisense oligonucleotides reduced APP expression and embryonic neural stem cell proliferation in the subventricular zone (SVZ) of the CNS in adult mice. Decoy DNA, consisting of exogenous double-stranded DNA (dsDNA), was designed to imitate the promoter sequence to inactivate specific transcription factor activity [103], but its poor bioavailability has always hindered its usefulness as a therapeutic nucleic acid. Fisher et al. [104] confirmed that the conjugation of TP/TP10-PNA hexamer or nonamer with NFкB decoy DNA can efficiently depress interleukin-1-induced NFкB activation and interleukin-6 gene expression.
Delivery of Small Molecule Drugs Across the BBB by CPPs
CPPs have also been exploited for improving the transport of small molecules (e.g. chemotherapeutic drugs) across the BBB (Tables 1-2). In addition, TAT-modified micelles have been used to improve the delivery of small molecule drugs, like ciprofloxacin, across the BBB to the brain [105, 106]. SynB peptides and Angiopeps are the most extensively studied vehicles for the delivery of such drugs to the brain [107].
Conjugation of drugs to members of the SynB family of peptides has been shown to increase their activity in the brain in vivo [108]. Rousselle et al. [109] reported that the brain penetration of a variety of poor brain-penetrating drugs, including doxorubicin, benzylpenicillin, paclitaxel and dalargin, was significantly enhanced when the drugs were conjugated to SynB1 or SynB3 and injected intravenously into mice. Drin et al. [110] reported that SynB1 and SynB5 significantly increase the uptake of doxorubicin into the brain. De Boer et al. [52] found that SynB3 enhanced the brain uptake of chemotherapeutic agents both in in situ brain perfusion and in an in vitro BBB/cell model, and further study in a clinical trial showed enhanced transport of morphine-glucuronide to the brain. Furthermore, Rousselle et al. [111] have increased doxorubicin transport into the rat brain by up to 30-fold by covalently coupling the drug to two peptides, SynB1 and penetratin.
Angiopeps, a family of Kunitz domain-derived peptides, have also been used in an in vitro BBB model and in vivo studies to deliver drugs to the brain with a high efficiency. Demeule et al. [112] reported that Angiopep-2 can transport Alexa 488 labeled paclitaxel across the BBB to treat brain cancer. ANG1007, the Angiopep-5 conjugated to three doxorubicin molecules, killed cancer cell lines in vitro and crossed the BBB with a dramatically high influx rate [113]. In recent years, much effort has been made to use Angiopeps to deliver drugs or nanoparticles across the BBB to the CNS, showing that Angiopep-mediated targeting is one of the most promising ways to reach the CNS for treatment of brain cancer or brain metastases [114-116].
Delivery of Nanoparticles by CPPs
Nanoparticles, sized between 1 and 100 nanometers, can, in terms of their properties, behave as a whole unit. TAT was recently harnessed by Santra et al. [117] to penetrate the BBB barrier and deliver CdS: Mn/ZnS quantum dots (Qdots) into rat brain tissue. Histological results clearly showed that TAT-Qdots migrated beyond the endothelial cell line of injection to reach the brain parenchyma [118]. Liu et al. [119] provided compelling evidence that TAT facilitates in vitro human brain endothelium cell uptake of nanoparticles self-assembled from TAT-PEG-β-cholesterol and that, more importantly, the nanoparticles with TAT modification were able to cross the BBB and translocate to the nucleus of neurons. Wang et al. [120] studied TAT-cholesterol-G(3)R(6) in a C. neoformansme meningitis rabbit model, which revealed that these nanoparticles crossed the BBB and showed antimicrobial activity against the pathological strains in the brain tissue. Qin et al. [121] found that the majority of TAT-modified liposome (TAT-LIP) accumulated in the brain within 24 h after administration via tail vein injection, even though not all of them were selectively targeted to the brain. These studies hold importance for TAT-mediated transport of nanoparticles into the brain for treatment of brain infections and tracking of nanoparticles in vivo, bringing us a step closer to the development of clinically applicable nanocarriers for the treatment, as well as monitoring, of CNS-related diseases. In addition to TAT, other CPPs also have successfully been employed for drug delivery in brain [122, 123]. For example, Angiopep-2, conjugated to polyamidoamine (PAMAM) via bifunctional PEG and then complexed with the DNA, designated as PAMAM-PEG-Angiopep/DNA nanoparticles, can be a potential delivery system for gene therapy of glial tumor [124] (Table 2).
CONCLUSIONS
An efficient and safe way to treat CNS disorders by systemic drug application is still lacking, despite the considerable effort that has been expended in the development of efficient and reliable drug-carrier systems. CPPs, which are an attractive type of therapeutic molecule delivery vehicle due to their low toxicity and the wide variety of cell types that they are capable of targeting, represent a potentially valuable tool for the intracellular delivery of therapeutic molecules to alter intracellular signaling pathways as well as interfere with intracellular interactions to rebalance a perturbed cellular function and protect neurons in cases of ischemia and neurodegenerative diseases. Preclinical studies demonstrating the successful use of CPPs indicate that CPPs possess great clinical potential for treating various CNS-related diseases by transporting therapeutic molecules across the BBB. Although CPPs have proved to be very useful tools to promote the delivery of therapeutic molecules across the BBB, yet the poor status of potent CPPs available in customized conditions produces a dismal picture for further clinical application. It is armed with this improved knowledge that scientific interest in the field has risen but a lot needs to be done. Hoping drug delivery aimed at the CNS can move into a more passionate phase.
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
We are thankful for the financial support of the Nature Science Foundation of Hubei Province China (No. 2010CDB10705) to CB.L, the Nature Science Foundation of China (No. 81201766) to LL.Z, and the talent initial funding financial support of China Three Gorges University (No. KJ2011B051) to LL.Z.
REFERENCES
- 1.Chen Y, Liu L. Modern methods for delivery of drugs across the blood-brain barrier. Adv. Drug. Deliv. Rev. 2011 doi: 10.1016/j.addr.2011.11.010. doi: 10.1016/j.addr. 2011. 11. 010. [DOI] [PubMed] [Google Scholar]
- 2.Abbott NJ. Dynamics of CNS barriers evolution, differentiation, and modulation. Cell. Mol. Neurobiol. 2005;25:5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cecchell R, Berezowski V, Lundquis S, Culot M, Renfte M, Dehouck MP, Fenart L. Modelling of the blood-brain barrier in drug discovery and development. Nat. Rev. Drug Discov. 2007;6:650–661. doi: 10.1038/nrd2368. [DOI] [PubMed] [Google Scholar]
- 4.Newton HB. Advances in strategies to improve drug delivery to brain tumors. Exp. Rev. Ther. 2006;6:1495–1509. doi: 10.1586/14737175.6.10.1495. [DOI] [PubMed] [Google Scholar]
- 5.Brownlees J, Williams CH. Peptidases, peptides and the mammalian blood-brain barrier. J. Neurochem. 1993;60:1089–1096. doi: 10.1111/j.1471-4159.1993.tb03223.x. [DOI] [PubMed] [Google Scholar]
- 6.Neuwelt E, Abbott NJ, Abrey L, Banks WA, Blakley B, Davis T, Engelhardt B, Grammas P, Nedergaard M, Nutt J, Pardridge W, Rosenberg GA, Smith Q, Drewes LR. Strategies to advance translational research into brain barriers. Lancet Neurol. 2008;7:84 –96. doi: 10.1016/S1474-4422(07)70326-5. [DOI] [PubMed] [Google Scholar]
- 7.Pardridge WM. CNS drug design based on principles of blood-brain barrier transport. J. Neurochem. 1998;70:1781–1792. doi: 10.1046/j.1471-4159.1998.70051781.x. [DOI] [PubMed] [Google Scholar]
- 8.Pardridge WM. Drug targeting to the brain. Pharm. Res. 2007;24:1733–1744. doi: 10.1007/s11095-007-9324-2. [DOI] [PubMed] [Google Scholar]
- 9.Wong HL, Wu XY, Bendayan R. Nanotechnological advances for the delivery of CNS therapeutics. Adv. Drug Deliv. Rev. 2011 doi: 10.1016/j.addr.2011.10.007. doi: 10. 1016/j. addr. 2011. 10. 007. [DOI] [PubMed] [Google Scholar]
- 10.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 11.Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero IA, Couraud PO. Blood-brain barrier-speci?c properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- 12.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 14.Hawkins BT, Egleton RD. Pathophysiology of the blood-brain barrier animal models and methods. Curr. Top. Dev. Biol. 2008;80:277–309. doi: 10.1016/S0070-2153(07)80007-X. [DOI] [PubMed] [Google Scholar]
- 15.Pardridge WM. Blood-brain barrier drug targeting the future of brain drug development. Mol. Interv. 2003;3:105–151. doi: 10.1124/mi.3.2.90. [DOI] [PubMed] [Google Scholar]
- 16.Stewart PA. Endothelial vesicles in the blood-brain barrier are they related to permeability? Cell. Mol. Neurobiol. 2000;20:149–163. doi: 10.1023/A:1007026504843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oldendorf WH, Cornford ME, Brown WJ. The large apparent work capability of the blood-brain barrier a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann. Neurol. 1977;1:409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- 18.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier structural components and function under physiologic and pathologic conditions. J. Neuroimmune. Pharmacol. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 19.Ramsauer M, Kunz J, Krause D, Dermietzel R. Regulation of a blood-brain barrier-speci?c enzyme expressed by cerebral pericytes (pericytic amino peptidase N/pAPN) under cell culture conditions. J. Cereb. Blood Flow Metab. 1998;18:1270–1281. doi: 10.1097/00004647-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Ramsauer M, Krause D, Dermietzel R. Angiogenesis of the blood-brain barrier in vitro and the function of cerebral pericytes. FASEB J. 2002;16:1274–1276. doi: 10.1096/fj.01-0814fje. [DOI] [PubMed] [Google Scholar]
- 21.Dohgu S, Takata F, Yamauchi A, Nakagawa S, Egawa T, Naito M, Tsuruo T, Sawada Y, Niwa M, Kataoka Y. Brain pericytes contribut e to the induction and up-regulation of blood-brain barrier functions through transforming growth factor-beta production. Brain Res. 2005;1038:208–215. doi: 10.1016/j.brainres.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Pardridge WM. Molecular biology of the blood-brain barrier. Mol. Biotechnol. 2005;30:57–70. doi: 10.1385/MB:30:1:057. [DOI] [PubMed] [Google Scholar]
- 23.Bendayan R, Lee G, Bendayan M. Functional expression and localization of p-glycoprote in at the blood brain barrier. Microsc. Res. Tech. 2002;57:365–380. doi: 10.1002/jemt.10090. [DOI] [PubMed] [Google Scholar]
- 24.Deeken JF, Loscher W. The blood-brain barrier and cancer transporters, treatment, and Trojan horses. Clin. Cancer Res. 2007;13:1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 25.Ronaldson PT, Persidsky Y, Bendayan R. Regulation of ABC membrane transporters in glial cells relevance to the pharmacotherapy of brain HIV-1 Infection. Glia. 2008;56:1711–1735. doi: 10.1002/glia.20725. [DOI] [PubMed] [Google Scholar]
- 26.Wekerle H. Immune protection of the brain-ef?cient and delicate. J. Infect. Dis. 2002;186:140–144. doi: 10.1086/344937. [DOI] [PubMed] [Google Scholar]
- 27.Rip J, Schenk GJ, de Boer AG. Differential receptor-mediated drug targeting to the diseased brain. Expert Opin. Drug Deliv. 2009;6:227–237. doi: 10.1517/17425240902806383. [DOI] [PubMed] [Google Scholar]
- 28.Pathan SA, Iqbal Z, Zaidi SM, Talegaonkar S, Vohra D, Jain GK, Azeem A, Jain N, Lalani JR, Khar RK, Ahmad FJ. CNS drug delivery systems novel approaches. Recent Pat. Drug Deliv. Formul. 2009;3:71–89. doi: 10.2174/187221109787158355. [DOI] [PubMed] [Google Scholar]
- 29.Cohen BE, Bangham AD. Diffusion of small non-electrolytes across liposome membranes. Nature. 1972;236:173–174. doi: 10.1038/236173a0. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Tu M, Kelly RS, Chen C, Smith BJ. Development of a computational approach to predict blood-brain barrier permeability. Drug Metab. Dispos. 2004;32:132–139. doi: 10.1124/dmd.32.1.132. [DOI] [PubMed] [Google Scholar]
- 31.Camenisch G, Alsenz J, van de Waterbeemd H, Folkers G. Estimation of permeability by passive diffusion through Caco-2 cell monolayers using the drugs' lipophilicity and molecular weight. Eur. J. Pharm. Sci. 1998;6:317–324. [PubMed] [Google Scholar]
- 32.van de Waterbeemd H, Camenisch G, Folkers G, Chretien JR, Raevsky OA. Estimation of blood-brain barrier crossing of drugs using molecular size and shape, and H-bonding descriptors. J. Drug Target. 1998;6:151–165. doi: 10.3109/10611869808997889. [DOI] [PubMed] [Google Scholar]
- 33.Clark DE. In silico prediction of blood-brain barrier permeation. Drug Discov. Today. 2003;8:927–933. doi: 10.1016/s1359-6446(03)02827-7. [DOI] [PubMed] [Google Scholar]
- 34.Pardridge WM. Biopharmaceutical drug targeting to the brain. J. Drug Target. 2010;18:157–167. doi: 10.3109/10611860903548354. [DOI] [PubMed] [Google Scholar]
- 35.Kis O Robillard, K Chan GN, Bendayan R. The complexities of antiretroviral drug-drug interactions role of ABC and SLC transporters. Trends Pharmacol. Sci. 2010;31:22–35. doi: 10.1016/j.tips.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Bodor N, Buchwald P. Brain-targeted drug delivery experiences to date. Am. J. Drug Target. 2003;1:13–26. [Google Scholar]
- 37.Kandimalla KK, Donovan MD. Transport of hydroxyzine and triprolidine across bovine olfactory mucosa role of passive diffusion in the direct nose-to-brain uptake of small molecules. Int. J. Pharm. 2005;302:133–144. doi: 10.1016/j.ijpharm.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Karasova JZ, Pohanka M, Musilek K, Zemek F, Kuca K. Passive diffusion of acetylcholinesterase oxime reactivators through the blood-brain barrier Influence of molecular structure. Toxicol. In Vitro. 2012;24:1838–1844. doi: 10.1016/j.tiv.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Gaillard PJ, Visser CC, de Boer AG. Targeted delivery across the blood-brain barrier. Expert Opin. Drug Deliv. 2005;2:299–309. doi: 10.1517/17425247.2.2.299. [DOI] [PubMed] [Google Scholar]
- 40.Jonker JW, Smit JW, Brinkhuis RF, Maliepaard M, Beijnen JH, Schellens JHM, Schinkel AH. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. JNCI . 2000;92:1651–1656. doi: 10.1093/jnci/92.20.1651. [DOI] [PubMed] [Google Scholar]
- 41.Liu YC, Liu HY, Yang HW, Wen T, Shang Y, Liu XD, Xie L, Wang GJ. Impaired expression and function of breast cancer resistance protein (Bcrp) in brain cortex of streptozocin-induced diabetic rats. Biochem. Pharmacol. 2007;74:1766–1772. doi: 10.1016/j.bcp.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 42.Sakata A, Tamai I, Kawazu K, Deguchi Y, Ohnishi T, Saheki A, Tsuji A. In vivi evidence for ATP-dependent and P-glycoprotein-mediated transport of cyclosporin A at the blood-brain barrier. Biochem. Pharmacol. 1994;48:1989–1992. doi: 10.1016/0006-2952(94)90601-7. [DOI] [PubMed] [Google Scholar]
- 43.Nies AT, Jedlitschky G, König J, Herold-Mende C, Steiner HH, Schmitt H-P, Keppler D. Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6) in human brain. Neuroscience. 2004;129:349–360. doi: 10.1016/j.neuroscience.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 44.Huang Y, Sadee W. Membrane transporters and channels in chemoresistance and sensitivity of tumor cells. Cancer Lett. 2006;239:168–82. doi: 10.1016/j.canlet.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 45.Demeule M, Régina A, Jodoin J, Laplante A, Dagenais C, Berthelet F, Moghrabi A, Béliveau R. Drug transport to the brain key roles for the efflux pump P-glycoprotein in the blood-brain barrier. Vas. Pharmacol. 2002;38:339–348. doi: 10.1016/s1537-1891(02)00201-x. [DOI] [PubMed] [Google Scholar]
- 46.Polli JW, Olson KL, Chism JP, John-Williams LS, Yeager RL, Woodard SM, Otto V, Castellino S, Demby VE. An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl) oxy] phenyl}-6-[5-({[2-(methylsulfonyl) ethyl] amino} methyl)-2 -furyl] -4-quinazolinamine. Drug Metab. Dispos. 2009;37:439–442. doi: 10.1124/dmd.108.024646. [DOI] [PubMed] [Google Scholar]
- 47.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 48.Roberts LM, Black DS, Raman C, Woodford K, Zhou M, Haggerty JE, Yan AT, Cwirla SE, Grindstaff KK. Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience. 2008;155:423–438. doi: 10.1016/j.neuroscience.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 49.Loscher W, Potschka H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat. Rev. Neurosci. 2005;6:591–602. doi: 10.1038/nrn1728. [DOI] [PubMed] [Google Scholar]
- 50.Hediger MA, Romero MF, Peng JB, Rolfs A, Takanaga H, Bruford EA. The ABCs of solute carriers physiological, pathological and therapeutic implications of human membrane transport proteins Introduction. Pflugers Arch. 2004;447:465–468. doi: 10.1007/s00424-003-1192-y. [DOI] [PubMed] [Google Scholar]
- 51.Ito K, Suzuki H, Horie T, Sugjyama Y. Apical/basolateral surface expression of drug transporters and its role in vectorial drug transport. Pharm. Res. 2005;22:1559–1577. doi: 10.1007/s11095-005-6810-2. [DOI] [PubMed] [Google Scholar]
- 52.de Boer AG, Gaillard PJ. Drug targeting to the brain. Annu. Rev. Pharmacol. Toxicol. 2007;47:323–355. doi: 10.1146/annurev.pharmtox.47.120505.105237. [DOI] [PubMed] [Google Scholar]
- 53.Pardridge WM. Blood-brain barrier biology and methodology. J. Neurovirol. 1999;5:556–569. doi: 10.3109/13550289909021285. [DOI] [PubMed] [Google Scholar]
- 54.Moos T, Morgan EH. Transferrin and transferrin receptor function in brain barrier systems. Cell Mol. Neurobiol. 2000;20:77 –95. doi: 10.1023/A:1006948027674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- 56.Mäe M, Langel U. Cell-penetrating peptides as vectors for peptide, protein and oligonucleotide delivery. Curr. Opin. Pharmacol. 2006;6:509–514. doi: 10.1016/j.coph.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 57.Vivés E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 58.Pooga M, Hällbrink M, Zorko M, Langel U. Cell penetration by transportan. FASEB J. 1998;12:67–77. doi: 10.1096/fasebj.12.1.67. [DOI] [PubMed] [Google Scholar]
- 59.Rothbard JB, Garlington S, Lin Q, Kirschberg T, Kreider E, McGrane PL, Wender PA, Khavari PA. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat. Med. 2000;6:1253–1257. doi: 10.1038/81359. [DOI] [PubMed] [Google Scholar]
- 60.El-Andaloussi S, Holm T, Langel U. Cell-penetrating peptides: mechanisms and applications. Curr. Pharm Des. 2005;11:3597–3611. doi: 10.2174/138161205774580796. [DOI] [PubMed] [Google Scholar]
- 61.Snyder EL, Dowdy SF. Recent advances in the use of protein transduction domains for the delivery of peptides, proteins and nucleic acids in vivo. Expert. Opin. Drug Deliv. 2005;2:43–51. doi: 10.1517/17425247.2.1.43. [DOI] [PubMed] [Google Scholar]
- 62.Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides from molecular mechanisms to therapeutics. Br. J. Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fonseca SB, Pereira MP, Kelley SO. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv. Drug Deliver. Rev. 2009;61:953–964. doi: 10.1016/j.addr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 64.Gabathuler R. Development of new peptide vectors for the transport of therapeutic across the blood-brain barrier. Ther. Deliver. 2010;1 :571–586. doi: 10.4155/tde.10.35. [DOI] [PubMed] [Google Scholar]
- 65.Gallo G. Making proteins into drugs assisted delivery of proteins and peptides into living neurons. Methods Cell Biol. 2003;71:325–338. doi: 10.1016/s0091-679x(03)01015-x. [DOI] [PubMed] [Google Scholar]
- 66.Kumar P, Wu HQ, McBride JL, Jung K-E, Kim MH, Davidson BL, Lee SK, Shankar P, Manjunath N. Transvascular delivery of small interfering RNA to the central nervous system. Nature. 2007;448:39–43. doi: 10.1038/nature05901. [DOI] [PubMed] [Google Scholar]
- 67.Cai B, Lin Y, Xue XH, Fang L, Wang N, Wu ZY. TAT-mediated delivery of neuroglobin protects against focal cerebral ischemia in mice. Exp. Neurol. 2011;227:224–231. doi: 10.1016/j.expneurol.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 68.Luo D, Saltzman M. Synthetic DNA delivery systems. Nat. Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 69.Herce HD, Garcia AE. Cell penetrating peptides how do they do it? J. Biol. Phys. 2007;33:345–356. doi: 10.1007/s10867-008-9074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lundberg M, Johansson M. Is VP22 nuclear homing an artifact? Nat. Biotechnol. 2001;19:713–771. doi: 10.1038/90741. [DOI] [PubMed] [Google Scholar]
- 71.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell . 1988;55:1189–1119. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 72.Plenat T, Deshayes S, Boichot S, Milhiet P E, Cole R, Heitz F, Grimellec CL. Interaction of primary amphipathic cellpenetrating peptides with phospholipid-supported monolayers. Langmuir. 2004;20:9255–9261. doi: 10.1021/la048622b. [DOI] [PubMed] [Google Scholar]
- 73.Deshayes S, Gerbal-Chaloin S, Morris MC, Aldrian-Herrada G, Charnet P, Divita G, Heitz F. On the mechanism of non-endosomial peptide-mediated cellular delivery of nucleic acids. Biochim. Biophys. Acta. 2004;1667:141–114. doi: 10.1016/j.bbamem.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 74.Theisen DM, Pongratz C, Wiegmann K, Rivero F, Krut O, Krönke M. Targeting of HIV-1 Tat traffic and function by transduction-competent single chain antibodies. Vaccine . 2006;24:3127–3136. doi: 10.1016/j.vaccine.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 75.Cao L, Si J, Wang W, Zhao X, Yuan X, Zhu H, Wu X, Zhu J, Shen G. Intracellular localization and sustained prodrug cell killing activity of TAT-HSVTK fusion protein in hepatocelullar carcinoma cells. Mol. Cells. 2006;21:104–111. [PubMed] [Google Scholar]
- 76.Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 77.Cao G, Pei W, Ge H, Liang Q, Luo Y, Sharp FR, Lu A, Ran R, Graham SH, Chen J. In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J. Neurosci. 2002;22:5423–5431. doi: 10.1523/JNEUROSCI.22-13-05423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hirt L, Badaut J, Thevenet J, Granziera C, Regli L, Maurer F, Bonny C, Bogousslavsky J. D-JNKI1 a cell-penetrating c-Jun-N-terminal kinase inhibitor, protects against cell death in severe cerebral ischemia. Stroke. 2004;35:1738–1743. doi: 10.1161/01.STR.0000131480.03994.b1. [DOI] [PubMed] [Google Scholar]
- 79.Aarts M, Liu YT, Liu LD, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 80.Kilic E, Kilic U, Hermann DM. TAT-GDNF in neurodegeneration and ischemic stroke. CNS Drug Rev. 2005;11:369–378. doi: 10.1111/j.1527-3458.2005.tb00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fu A, Wang Y, Zhan L, Zhou R. Targeted delivery of proteins into the central nervous system mediated by rabies virus glycoprotein-derived peptide. Pharm. Res. 2012;29:1562–1569. doi: 10.1007/s11095-012-0667-y. [DOI] [PubMed] [Google Scholar]
- 82.Xiang L, Zhou R, Fu A, Xu X, Huang Y, Hu C. Targeted delivery of large fusion protein into hippocampal neurons by systemic administration. J. Drug Target. 2011;19:632–636. doi: 10.3109/1061186X.2010.523788. [DOI] [PubMed] [Google Scholar]
- 83.Jo D, Liu D, Yao S, Collins RD, Hawiger J. Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat. Med. 2005;11:892–898. doi: 10.1038/nm1269. [DOI] [PubMed] [Google Scholar]
- 84.Liu ZH, Li MY, Cui DF, Fei J. Macro-branched cell-penetrating peptide design for gene delivery. J. Control Release. 2005;102:699–710. doi: 10.1016/j.jconrel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 85.Rudolph C, Plank C, Lausier J, Schillinger U, Müller RH, Rosenecker J. Oligomers of the arginine-rich motif of the HIV-1 TAT protein are capable of transferring plasmid DNA into cells. J. Biol. Chem. 2003;278:11411–11418. doi: 10.1074/jbc.M211891200. [DOI] [PubMed] [Google Scholar]
- 86.Knight A, Carvajal J, Schneider H, Coutelle C, Chamberlain S, Fairweather N. Non-viral neuronal gene delivery mediated by the HC fragment of tetanus toxin. Eur. J. Biochem. 1999;259:762–769. doi: 10.1046/j.1432-1327.1999.00108.x. [DOI] [PubMed] [Google Scholar]
- 87.Lo SL, Wang S. An endosomolytic Tat peptide produced by incorporation of histidine and cysteine residues as a nonviral vector for DNA transfection. Biomaterials. 2008;29:2408–2414. doi: 10.1016/j.biomaterials.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 88.Sawant RR, Torchilin VP. Intracellular Delivery A Multifunctional and Modular Approach. In: Prokop A, editor. Intracellular delivery. Vol. 1. Springer Science + Business Media Nashville; 2011. pp. 212–213. [Google Scholar]
- 89.Li WJ, Szoke FC. Lipid-based nanoparticles for nucleic acid delivery. Pharm. Res. 2007;24:438–449. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 90.Suk JS, Suh J, Choy K, Lai SK, Hanes J. Gene delivery to differentiated neurotypic cells with RGD and HIV Tat peptide functionalizaed polymeric nanoparticles. Biomaterials. 2006;27:5143–5150. doi: 10.1016/j.biomaterials.2006.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eguchi A, Meade BR, Chang YC, Fredrickson CT, Willert K, Puri N, Dowdy SF. Efficient siRNA delivery into primary cells by a peptide transduction domain-dsRNA binding domain fusion protein. Nat. Biotechnol. 2009;27:567–571. doi: 10.1038/nbt.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meade BR, Dowdy SF. Enhancing the cellular uptake of siRNA duplexes following noncovalent packaging with protein transduction domain peptides. Adv. Drug Deliv. Rev. 2008;60:530–536. doi: 10.1016/j.addr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Said Hassane F, Saleh AF, Abes R, Gait MJ, Lebleu B. Cell penetrating peptides overview and applications to the delivery of oligonucleotides. Cell. Mol. Life Sci. 2010;67:715–726. doi: 10.1007/s00018-009-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lingor P, Bahr M. Targeting neurological disease with RNAi. Mol. Biosyst. 2007;3:773–780. doi: 10.1039/b701169e. [DOI] [PubMed] [Google Scholar]
- 95.Ralph GS, Mazarakis ND, Azzouz M. Therapeutic gene silencing in neurological disorders, using interfering RNA. J. Mol. Med. 2005;83:413–419. doi: 10.1007/s00109-005-0649-1. [DOI] [PubMed] [Google Scholar]
- 96.Mathupala SP. Delivery of small-interfering RNA (siRNA) to the brain. Expert Opin. Ther. Pat. 2009;19:137–140. doi: 10.1517/13543770802680195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alvarez-Erviti L, Seow Y, Yin HF, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 98.Hwang DW, Son S, Jang J, Youn H, Lee S, Lee D, Lee Y-S, Jeong JM, Kim WJ, Lee DS. A brain-targeted rabies virus glycoprotein-disulfide linked PEI nanocarrier for delivery of neurogenic microRNA. Biomaterials. 2011;32:4968–4975. doi: 10.1016/j.biomaterials.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 99.Davidson TJ, Harel S, Arboleda VA, Prunell GF, Shelanski ML, Greene LA, Tory CM. Highly efficient small interfering RNA delivery to primary mammalian neurons induces MicroRNA-like effects before mRNA degradation. J. Neurosci. 2004;24:10040–10046. doi: 10.1523/JNEUROSCI.3643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Corey DR, Abrams JM. Morpholino antisense oligonucleotides tolls for investigating vertebrate development. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-5-reviews1015. reviews 1015.1-1015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Du LT, Kayali R, Bertoni C, Fike F, Hu HL, Iversen PL, Gatti RA. Arginine-rich cell-penetrating peptide dramatically enhances AMO-mediated ATM aberrant splicing correction and enables delivery to brain and cerebellum. Hum. Mol. Genet. 2011;20:3151–3160. doi: 10.1093/hmg/ddr217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Caille I, Allinquant B, Dupont E, Bouillot C, Langer A, Muller U, Prochiantz A. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development. 2004;131:2173–2181. doi: 10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- 103.Morishita R, Gibbons GH, Horiuchi M, Ellison KE, Nakama M, Zhang L, Kaneda Y, Ogihara T, Dzau VJ. A gene therapy strategy using a transcription factor decoy of the E2F binding site inhibits smooth muscle proliferation in vivo. Proc. Natl. Acad. Sci. U.S.A. 1995;92:5855–5859. doi: 10.1073/pnas.92.13.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fisher L, Soomets U, Cortes TV, Chilton L, Jiang Y, Langel , Iverfeldt K. Cellular delivery of a double-stranded oligonucleotide NF?B decoy by hybridization to complementary PNA linked to a cell-penetrating peptide. Gene Ther. 2004;11:1264–1272. doi: 10.1038/sj.gt.3302291. [DOI] [PubMed] [Google Scholar]
- 105.Liu L, Guo K, Lu J, Venkatraman SS, Luo D, Moochhala S, Yang YY. Biologically active core/shell nano-particles self-assembled from cholesterol-terminated PEG-TAT for drug delivery across the blood-brain barrier. Biomaterials. 2008;29:1509–1517. doi: 10.1016/j.biomaterials.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 106.Kanazawa T, Taki H, Tanaka K, Takashima Y, Okada H. Cell-penetrating peptide-modi?ed block copolymer micelles promote direct brain delivery via intranasal administration. Pharm. Res. 2011;28:2130–2139. doi: 10.1007/s11095-011-0440-7. [DOI] [PubMed] [Google Scholar]
- 107.Orthmann A, Fichtner I, Zeisig R. Improving the transport of chemotherapeutic drugs across the blood-brain barrier. Expert Rev. Clin. Pharmacol. 2011;4:477–490. doi: 10.1586/ecp.11.26. [DOI] [PubMed] [Google Scholar]
- 108.Adenot M, Merida P, Lahana R. Applications of a blood-brain barrier technology platform to predict CNS penetration of various chemotherapeutic agents.2. Cationic peptide vectors for brain delivery. Pharmacology. 2007;53l:73–76. doi: 10.1159/000098422. [DOI] [PubMed] [Google Scholar]
- 109.Rousselle C, Clair P, Smirnova M, Kolesnikov Y, Pasternak GW, Gac-Breton S, Rees AR, Scherrmann JM, Temsamani J. Improved brain uptake and pharmacological activity of dalargin using a peptide-vector-mediated strategy. J. Pharmacol. Exp. Ther. 2003;306:371–376. doi: 10.1124/jpet.102.048520. [DOI] [PubMed] [Google Scholar]
- 110.Drin G, Cottin S, Blanc E, Rees AR, Temsamani J. Studies on the intern alisation mechanism of cationic cell-penetrating peptides. J. Biol. Chem. 2003;278:31192–31201. doi: 10.1074/jbc.M303938200. [DOI] [PubMed] [Google Scholar]
- 111.Rousselle C, Clair P, Lefauconnier J M, Kaczorek M, Scherrmann J M, Temsamani J. New advances in the transport of doxorubicin through the blood-brain barrier by a peptide vector-mediated strategy. Mol. Pharmacol. 2000;57:679–686. doi: 10.1124/mol.57.4.679. [DOI] [PubMed] [Google Scholar]
- 112.Demeule M, Currie JC, Bertrand Y, Ché C, Nguyen T, Régina A, Gabathuler R, Castaigne JP, Béliveau R. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector Angiopep-2. J. Neurochem. 2008;106:1534–1544. doi: 10.1111/j.1471-4159.2008.05492.x. [DOI] [PubMed] [Google Scholar]
- 113.Ché C, Yang G, Thiot C, Lacoste MC, Currie JC, Demeule M, Régina A, Béliveau R, Castaigne JP. New angiopep-modified doxorubin (ANG 1007) and etoposide (ANG 1009) chemotherapeutics with increased brain penetration. Pharmacol. 2010;53:2814–2824. doi: 10.1021/jm9016637. [DOI] [PubMed] [Google Scholar]
- 114.Ren J, Shen S, Wang D, Xi Z, Guo L, Pang Z, Qian Y, Sun X, Jiang X. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials. 2012;33:3324–3333. doi: 10.1016/j.biomaterials.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 115.Kurzrock R, Gabrail N, Chandhasin C, Moulder S, Smith C, Brenner A, Sankhala K, Mita A, Elian K, Bouchard D, Sarantopoulos J. Safety, pharmacokinetics, and activity of GRN1005, a novel conjugate of angiopep-2, a peptide facilitating brain penetration, and paclitaxel, in patients with advanced solid tumors. Mol. Cancer Ther. 2012;11:308–316. doi: 10.1158/1535-7163.MCT-11-0566. [DOI] [PubMed] [Google Scholar]
- 116.Bertrand Y, Currie JC, Poirier J, Demeule M, Abulrob A, Fatehi D, Stanimirovic D, Sartelet H, Castaigne JP, Béliveau R. Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1. Br. J. Cancer. 2011;105:1697–1707. doi: 10.1038/bjc.2011.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Santra S, Yang H, Stanley JT, Holloway PH, Moudgil BM, Walter G, Mericle RA. Rapid and effective labeling of brain tissue using TAT-conjugated CdSMn/ZnS quantum dots. Chem. Commun. (Camb.) 2005:3144–3146. doi: 10.1039/b503234b. [DOI] [PubMed] [Google Scholar]
- 118.Santra S, Yang H, Holloway PH, Stanley JT, Mericle RA. Synthesis of water-dispersible fluorescent, radio-opaque, and paramagnetic CdSMn/ZnS quantum dots a multifunctional probe for bioimaging. J. Am. Chem. Soc. 2005;127:1656–1657. doi: 10.1021/ja0464140. [DOI] [PubMed] [Google Scholar]
- 119.Liu L, Venkatraman SS, Yang YY, Guo K, Lu J, He B, Moochhala S, Kan L. Polymeric micelles anchored with TAT for delivery of antibiotics across the blood-brain barrier. Biopolymers. 2008;90:617–623. doi: 10.1002/bip.20998. [DOI] [PubMed] [Google Scholar]
- 120.Wang H, Xu K, Liu L, Tan JP, Chen Y, Li Y, Fan W, Wei Z, Sheng J, Yang YY, Li L. The efficacy of self-assembled cationic antimicrobial peptide nanoparticles against Cryptococcus neoformans for the treatment of meningitis. Biomaterials. 2010;31:2874–2881. doi: 10.1016/j.biomaterials.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 121.Qin Y, Chen H, Yuan W, Kuai R, Zhang Q, Xie F, Zhang L, Zhang Z, Liu J, He Q. Liposome formulated with TAT-modified cholesterol for enhancing the brain delivery. Int. J. Pharm. 2011;420:304–312. doi: 10.1016/j.ijpharm.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 122.Tosi G, Costantino L, Rivasi F, Ruozi B, Leo E, Vergoni AV, Tacchi R, Bertolini A, Vandelli MA, Forni F. Targeting the central nervous system in vivo experiments with peptide-derivatized nanoparticles loaded with loperamide and rhodamine-123. J. Control. Release. 2007;122:1–9. doi: 10.1016/j.jconrel.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 123.Vergoni AV, Tosi G, Tacchi R, Vandelli MA, Bertolini A, Costantino L. Nano-particles as drug delivery agents speci?c for CNS in vivo biodistribution. Nano-medicine. 2009;5:369–377. doi: 10.1016/j.nano.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 124.Ke WL, Shao K, Huang RQ, Han L, Liu Y, Li JF, Kuang YY, Ye LY, Lou JN, Jiang C. Gene delivery targeted to the brain using an Angiopep-conjugated plyethyleneglycol-modified polyamidoamine dendrimer. Biomaterials . 2010;30:6976–6985. doi: 10.1016/j.biomaterials.2009.08.049. [DOI] [PubMed] [Google Scholar]


