Abstract
Wnt signaling in mouse mammary development and tumorigenesis has been heavily studied and characterized, but its role in human breast cancer remains elusive. Although Wnt inhibitors are in early clinical development, it is unclear whether they will be of therapeutic benefit to breast cancer patients, and subsequently, to which ones. To address this, we generated a panel of Wnt reporting human breast cancer cell lines and identified a previously unrecognized enrichment for the ability to respond to Wnt in the basal B or claudin-low subtype, which has a poor prognosis and no available targeted therapies. By co-injecting Wnt3A expressing human mammary fibroblasts with human breast cancer cell lines into mouse mammary fat pads, we showed that elevated paracrine Wnt signaling was correlated with accelerated tumor growth. Using this heterotypic system and a dual lentiviral reporter system that enables simultaneous real-time measurement of both Wnt-responsive cells and bulk tumor cells, we analyzed the outcome of elevated Wnt signaling in patient-derived xenograft (PDX) models. Interestingly, the PDX models exhibited responses not observed in the cell lines analyzed. Exogenous WNT3A promoted tumor growth in one human epidermal growth factor receptor 2-overexpressing PDX line but inhibited growth in a second PDX line obtained from a patient with triple-negative breast cancer. Tumor suppression was associated with squamous differentiation in the latter. Thus, our work suggests that paracrine Wnt signaling can either fuel or repress the growth of human breast cancers depending on yet to be determined aspects of the molecular pathways they express.
Keywords: tumor stroma, luciferase, Wnt responsive reporter
Wnt signaling plays myriad roles during development and is widely implicated in human diseases. The complexity of Wnt signaling derives from a plethora of ligands (19 Wnts) and receptors [10 Frizzleds, 2 low-density Lipoprotein related receptor protein (Lrp) coreceptors, and 3 receptor tyrosine kinases (RTK): Ror1, Ror2, and Ryk] and deepens with cross-talk between canonical (β-catenin dependent) and noncanonical (β-catenin independent) signaling mechanisms. Canonical Wnt signaling is initiated when a Wnt protein binds to a transmembrane Frizzled receptor and the coreceptor Lrp5/6. This association triggers a cascade of events leading to the stabilization of the intracellular transducer β-catenin, which accumulates, translocates to the nucleus, and serves as a transcriptional coactivator with the T cell factor (Tcf)/lymphoid enhancer-binding factor (Lef) family of transcription factors (1). Wnt was first identified for its ability to induce mammary tumors in mice (2), and Wnt is unique in its ability to transform human mammary epithelial cells when overexpressed alone (3).
Human breast cancers are routinely classified into subtypes in the clinic by immunohistochemical (IHC) analysis of their expression of the estrogen, progesterone, and human epidermal growth factor receptor 2 (Her2) receptors. Low or undetectable expression of these three targetable receptors defines the triple-negative breast cancer (TNBC) subtype, which has the worst prognosis and the fewest treatment options. This heterogeneous group of tumors has been associated with active Wnt signaling by IHC analysis of β-catenin in tumor samples and by expression profiling studies (reviewed in ref. 4), raising the possibility that blocking Wnt signaling could provide a treatment for TNBC.
Part of the controversy surrounding the role of Wnt signaling in human breast cancer stems from the rarity of pathway-activating mutations in human breast tumors (5, 6), as opposed to colon cancer, for which such mutations are common (7). Although mutations are rare, negative regulators, particularly soluble inhibitors such as secreted frizzled related proteins, Dickkopf, and Wnt inhibitory factors, frequently exhibit reduced expression via methylation (reviewed in refs. 8 and 9). This suggests that Wnt activity in vivo may be regulated in a paracrine fashion, consistent with the secreted nature of Wnt ligands. The potential source of Wnt ligands in vivo is likely to be in close proximity to the cancer cells because Wnts diffuse poorly owing to their strong association with membranes and extracellular matrix, their instability at physiological temperatures, and their lack of solubility. Interestingly, Wnt signaling contributes to the pathogenesis of several human breast cancer cell lines (10–13). The effects of pathway activation in trans have not been investigated.
Immortalized cell lines may acquire or be selected for stromal independence upon adaptation to in vitro culture. Therefore, we developed a system to investigate the role of paracrine Wnt signaling in both cell lines and patient-derived xenograft (PDX) models. PDX lines are generated by implanting human tumor tissue orthotopically into immunocompromised mice and maintained by serial transplantation in vivo to bypass in vitro adaptation. We developed a dual lentiviral vector system that enables simultaneous detection and discrimination of bulk tumor cells from those cells with active Wnt signaling to facilitate functional analyses using in vivo imaging. We used these vectors to generate a panel of double-transgenic human breast cancer cell lines and determined that Wnt responsiveness is associated with the subset of TNBCs called “basal B” or “claudin-low.” Transgenic human breast cancer cell lines and PDX models were coinjected with Wnt-secreting human reduction mammary fibroblasts (RMFs) into the mouse mammary fat pad to determine the outcome of activating Wnt signaling in trans. This system enabled real-time analyses of paracrine Wnt signaling in vivo and led to the unexpected finding that Wnt signaling can either promote or inhibit human breast tumorigenesis.
Results
Dual Lentiviral Wnt-Reporter System.
To directly couple Wnt activity to bulk cell population analysis, we developed a dual lentiviral vector system that allows bulk tumor cells and cells with active Wnt signaling to be simultaneously and quantitatively monitored and distinguished.
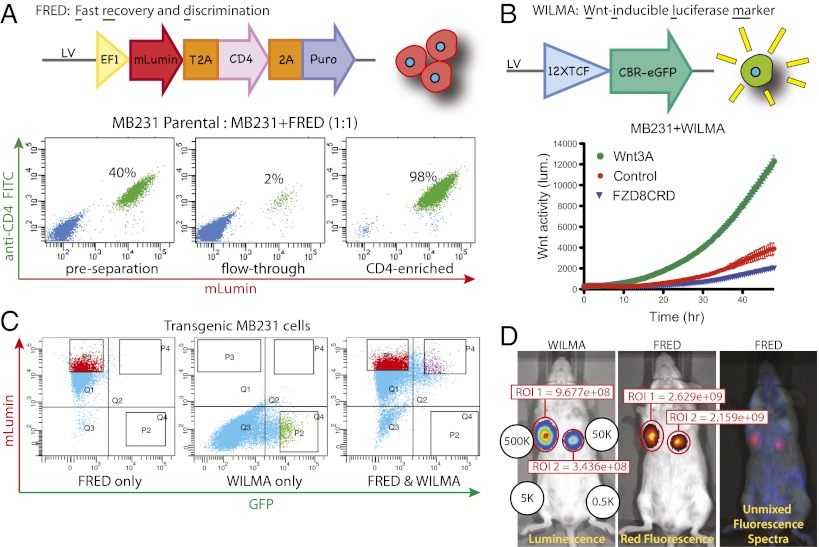
The first lentiviral vector enables fast recovery and discrimination (FRED) of transduced cells (Fig. 1A). A constitutively expressed multicistronic ORF encodes the red fluorescent protein mLumin (14), a truncated CD4 surface protein (to enable magnetic separation), and a Puromycin resistance gene. We evaluated FRED functionality in MDA-MB-231 cells (MB231-F for FRED transduced). Stable MB231-F cells were mixed 1:1 with parental MB231 cells and gently separated using magnetic microbeads coupled to anti-CD4 antibodies (Fig. 1A). This strategy enables 98% efficient separation of FRED transduced from nontransduced cells.
Fig. 1.
Dual lentiviral Wnt reporter system. (A) Schematic of the FRED lentiviral vector (Upper). All FRED-transduced cells express red fluorescence, represented by the cartoon cluster of red cells to the right. (Lower) Magnetic separation of FRED-transduced MB231 cells from parental MB231 cells. Cells were mixed, incubated with magnetic particles coupled to anti-CD4 antibody, subjected to magnetic separation, and then stained with anti-CD4 (FITC). (B) Schematic of the WILMA lentiviral vector (Upper). Only WILMA-transduced cells with active Wnt signaling will be luminescent and green, represented by the cartoon of a luminescent green cell to the right. (Lower) Specificity of the WILMA reporter was determined by a real-time bioluminescence monitoring assay. WILMA-transduced MB231 cells were cocultured with iCHO cells secreting WNT3A (green circles) or Fzd8CRD (blue triangles), or parental iCHO cells (control, red circles), and luminescence was measured every 30 min for 48 h. (C) FACS profiles of transgenic cells. FRED-transduced cells were selected with Puromycin, thus all of the cells express mLumin (Left). To collect WILMA-transduced cells, GFP+ cells were collected 24 h after treatment with the GSK-3 inhibitor XV (Center). Double transgenic cells expressing mLumin and GFP were collected after XV treatment (Right). (D) MB231-FW cells were injected into the third and fourth mammary pads in the quantities indicated. Two weeks later, bioluminescence and fluorescence were measured. Signal intensity within the region of interest is shown in units of Efficiency (luminescence) or Radiance Efficiency (fluorescence).
The second vector encodes a Wnt-inducible luciferase marker (WILMA) (Fig. 1B). A Wnt-responsive promoter element comprising 12 copies of the TCF binding sequence (15) regulates expression of a click-beetle red luciferase/eGFP (CBR-eGFP) fusion protein (16). CBR luciferase emits red-shifted light compared with firefly luciferase and thus suffers less from absorption and scattering through tissue, making it more useful for in vivo imaging. Fusion of CBR to eGFP enables identification of individual Wnt-responsive cells by both flow cytometry and microscopy.
We tested the Wnt-responsiveness of WILMA by transducing MB231, known to be Wnt-responsive (11), with the WILMA lentivirus. MB231-W cells were then exposed to WNT3A or the soluble Wnt inhibitor FZD8CRD produced by inducible CHO (iCHO) cells in coculture (17) (Fig. 1B). As expected, WNT3A induced reporter expression as measured by luciferase activity. The transduced population showed a basal level of luciferase activity that was suppressed by exposure to FZD8CRD, consistent with previous reports that MB231 cells have some endogenous Wnt signaling (11). To recover cells transduced with WILMA, we transiently activated signaling downstream of the receptor by treating the cells with the GSK-3 inhibitor Factor XV. Twenty-four hours after XV treatment, cells with the most intense green and red fluorescence were collected by FACS and expanded in vitro (Fig. 1C).
The suitability of these vectors for in vivo imaging was assessed by injecting double transgenic (MB231-FW) cells into mouse mammary fat pads. Both luminescence and fluorescence were detected after injecting 500,000 or 50,000 cells, but not 5,000 or 500 cells (Fig. 1D). Autofluorescence in the mouse makes fluorescence imaging less sensitive, so we grew six MB231-FW tumors and performed fluorescence imaging ex vivo (in a dish) to determine whether red fluorescence can be used as a surrogate for cell number (Fig. S1). Cell number was strongly correlated with ex vivo red fluorescence of intact tumors (r2 = 0.98).
Basal B (Claudin-Low) Cell Lines Are Wnt-Responsive.
Although many human breast tumors display evidence of Wnt pathway activity, the majority of human breast cancer cell lines do not display constitutively active Wnt signaling (10, 11). We hypothesized that this discrepancy could be due to the in vitro context in which cell lines are grown and that some cell lines may be able to activate Wnt signaling in response to exogenously applied Wnt ligands.
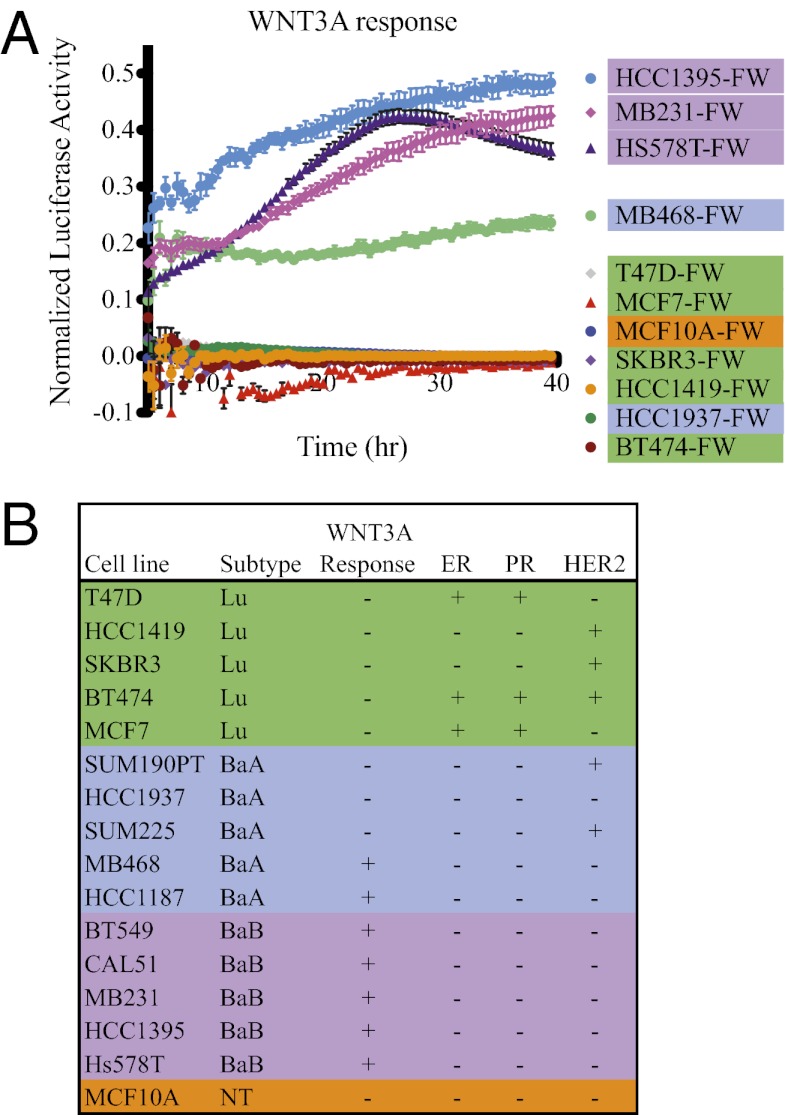
Expression profiling analysis of human breast tumors has enabled categorization into at least five “intrinsic” subtypes: luminal A, luminal B, Her2-enriched, basal-like, and claudin-low (18–20); however, similar analysis of large panels of immortalized breast cancer cell lines identified only three subsets: luminal, basal A, and basal B, with the basal B subset corresponding to claudin-low tumors (21–23). We generated a panel of double transgenic (FW) human cell lines in which each subset was represented, plus the nontransformed human mammary epithelial cell line MCF10A. To confirm successful transduction and intact signaling machinery downstream of β-catenin, potential reporter cell lines were prescreened by treatment with Factor XV (Fig. S2A). Cell lines that failed to induce WILMA under these conditions were excluded from the panel. The cell line panel was then assayed for response to WNT3A by real-time bioluminescence coculture assays with WNT-producing iCHO cells (Fig. 2A and Fig. S2 B and C). Response to WNT3A was normalized to reporter induction by Factor XV, to control for variable copy number of the WILMA transgene. WNT3A induced WILMA expression in 7 of 16 cell lines: MB231, HCC1395, Hs578T, BT549, CAL51, HCC1187, and MB468, which are all TNBC. Interestingly, we found that the ability to respond to exogenously applied Wnt ligands segregated between the subsets, with a response observed in five of five basal B, two of five basal A, and none of five luminal cell lines (Fig. 2B).
Fig. 2.
Wnt responsiveness of breast cancer cell lines. (A) Real-time bioluminescence monitoring assay. Cells were cocultured with parental (control) or WNT3A-producing iCHO cells, and luminescence was measured every 30 min for 40 hours. To adjust for variable transduction efficiency of WILMA, data were normalized by subtracting absorbance in the presence of control from absorbance in the presence of WNT3A, and the remainder was divided by absorbance in the presence of GSK-3 inhibitor XV (because inhibition of GSK-3 activates the reporter regardless of the cell’s ability to respond to Wnt ligands). The cell lines BT549, SUM190PT, and SUM225CWN were analyzed separately and are presented in Fig. S2. (B) Summary of Wnt-responsiveness of the entire cell line panel with indication of estrogen receptor (ER), progesterone receptor (PR), and HER2 status and subset classification.
Stromal-Derived Wnt Activates Signaling in Breast Cancer Cells in Vivo.
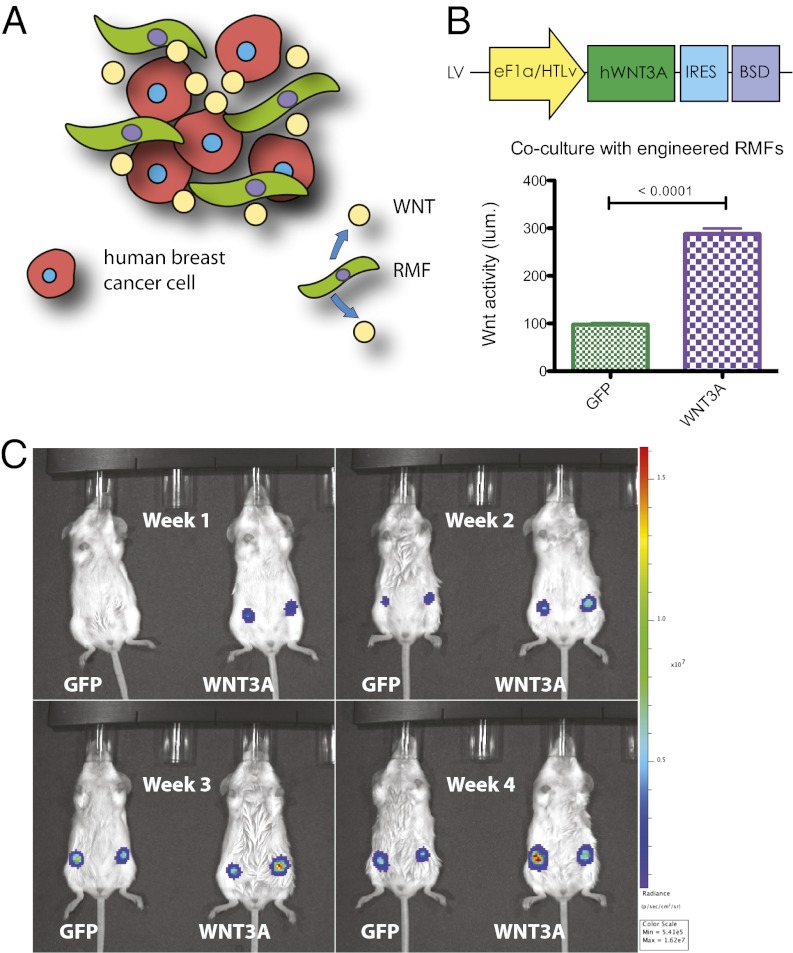
We examined the effects of ectopic paracrine Wnt signaling on human breast cancer growth in vivo. Because the poor stability and insolubility of Wnt proteins make it unlikely that even regular injection of Wnt protein into the tumor site will maintain activated signaling for longer-term experiments, we developed a method to supply Wnts in vivo. We reasoned that a continuous proximal source of Wnt protein could be provided by introducing Wnt-overexpressing cells and better model a natural setting in which WNT ligands are secreted by adjacent stroma (Fig. 3A). We therefore used immortalized human mammary fibroblasts from reduction mammoplasty (RMFs) that we engineered to secrete WNT3A into the tumor microenvironment because the ability of such cells to “condition” mouse mammary fat pads has been established (24). Expression of bioactive WNT3A was confirmed by coculturing transgenic RMF cells with HEK293A Wnt-reporter cells and measuring luciferase activity. RMF-WNT3A induced Wnt reporter expression compared with RMF-GFP (Fig. 3B).
Fig. 3.
Orthotopic heterotypic recombinations. (A) Schematic of orthotopic heterotypic recombination assay. Transgenic human breast cancer cells (red) were coinjected into the mouse mammary fat pad with human reduction mammary fibroblasts (green) that were engineered to overexpress WNT3A. (B) Schematic of the WNT3A expression lentiviral vector (pJG071) (Upper); the control vector (pJG070) has eGFP in the place of WNT3A. Transgenic RMFs were cocultured with HEK293A cells carrying the SUPERTOPFLASH Wnt reporter; luminescence was measured 24 h later (Lower). (C) Orthotopic heterotypic recombination pilot experiment. WNT3A-producing or control (GFP) RMFs were coinjected with MB231-FW cells into the left and right number four mouse mammary fat pads, in duplicate (representative cage is shown).
As a pilot experiment to test the orthotopic heterotypic recombination system in vivo, MB231-FW cells were coinjected into the mammary fat pad with transgenic RMF cells in a ratio of 1:10, and luminescence was measured weekly. Inoculations with RMF-WNT3A cells displayed brighter luminescence than inoculations with RMF-GFP cells (Fig. 3C), suggesting that transgenic RMF cells can nonautonomously modulate Wnt activity in coinjected breast cancer cells.
WNT3A Promotes MB231 and MB468 Tumorigenesis.
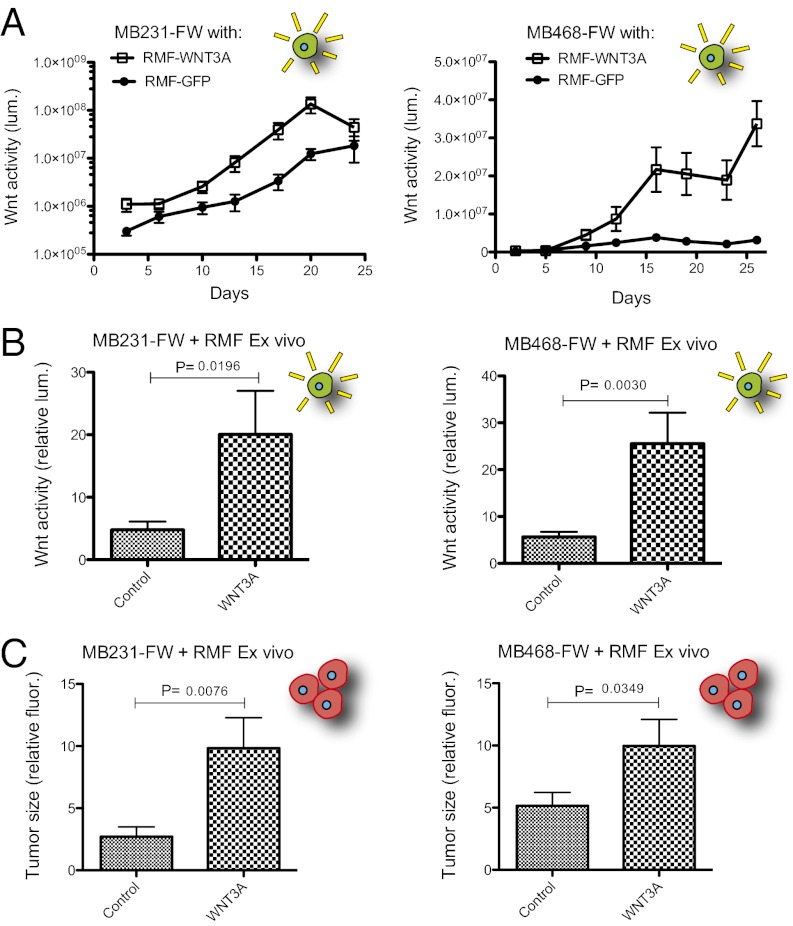
We confirmed that RMF-WNT3A cells can activate Wnt signaling in coinjected breast cancer cells, and we determined whether this activation promotes or inhibits tumor growth by expanding our analysis to a larger cohort of mice. Transgenic RMF cells were coinjected with either MB231-FW or MB468-FW cells, and luminescence was measured twice per week (Fig. 4A). Luminescence was higher in the presence of WNT3A in both cases. The density of RMFs in heterotypic tumors, determined by IHC visualization, severely declines by 4 wk (Fig. S3A). This decline is coincident with the convergence of MB231-FW tumor luminescence in control and WNT3A conditions. MB468-FW, which does not exhibit basal Wnt activity, formed tumors with clear separation of luminescence between control and WNT3A conditions for the duration of the 4-wk period until tumors were harvested. Excised tumors were subjected to luminescence and fluorescence imaging ex vivo (Fig. 4 B and C). RMF-WNT3A tumors displayed increased red fluorescence in addition to higher luminescence, indicating that exposure to WNT3A increased the tumor growth of both MB231-FW and MB468-FW (Fig. 4 B and C).
Fig. 4.
WNT3A promotes tumorigenesis of breast cancer cell lines. Orthotopic heterotypic recombination experiments as in Fig. 3C using MB231-FW (Left) or MB468-FW (Right) cell lines, n = 8 (MB231-FW) or n = 10 (MB468-FW). (A) Bioluminescence was measured twice per week for 3–4 wk. (B) Tumors were harvested at day 27 (MB231-FW) or day 33 (MB468-FW), and luminescence was measured ex vivo. Data were normalized to the lowest value. (C) Ex vivo red fluorescence (mLumin) intensity of the harvested tumors, as in B.
Establishment of Transgenic Primary Tumor Xenograft Lines.
PDX models are emerging as highly relevant systems with great promise to improve the translation of potential breast cancer therapies to the clinic (25). We thus applied these vectors and the heterotypic model system to interrogate Wnt signaling in stably transplantable PDX lines established in our laboratories (details to be presented elsewhere). Transgenic PDX lines are not generated routinely, thus we developed a multigeneration strategy to generate transgenic PDX lines that were serially passaged in mice in vivo such that the tumor cells were never exposed to selective pressures in vitro (Fig. S4A). Luminescence was measured biweekly, and the transgenic FW tumor lines were stable for at least five passages at the time of writing (Fig. S4B). Although the relatively intense signal from the primary tumors prevented detection of micrometastases in vivo, luminescence was seen in resected lungs and lymph nodes ex vivo after collection of the primary tumors (Fig. S4C). These xenograft models were used specifically for in vivo experimentation, because primary breast tumor cells typically undergo limited proliferation in culture.
Variable Effects of WNT3A on Tumorigenesis.
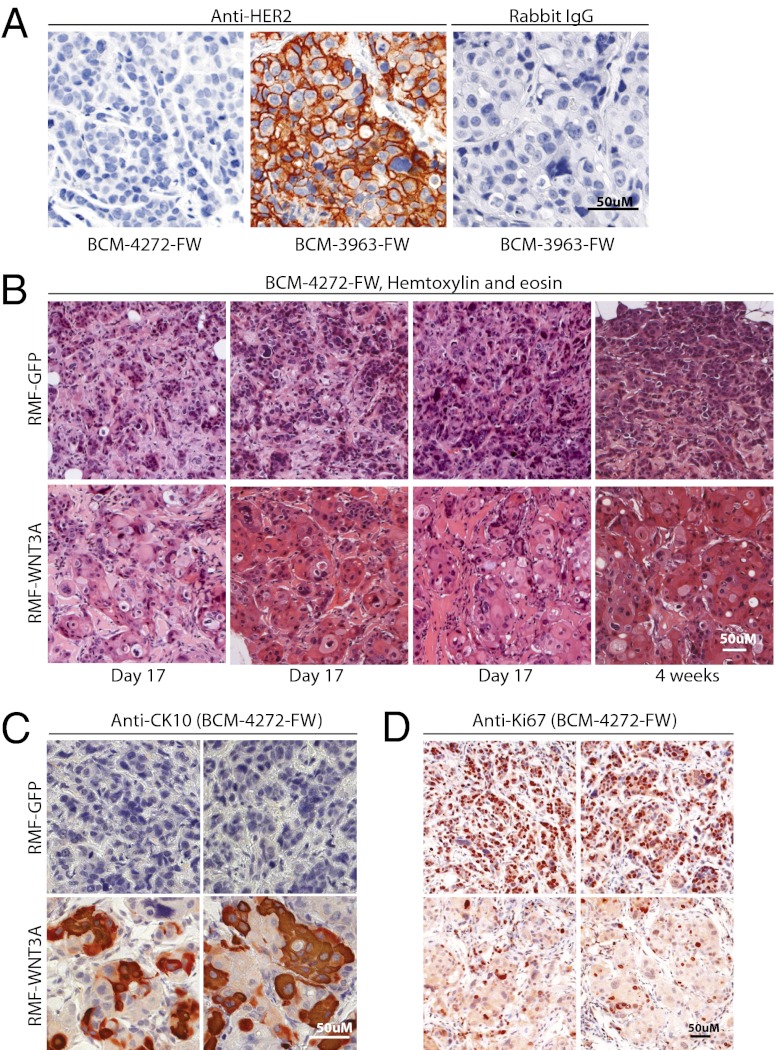
To determine whether the outcome of elevated Wnt signaling in PDX models is the same as that observed in immortalized cell lines, we subjected our transgenic FW tumor lines to the orthotopic heterotypic recombination assay described above. Like MB231-FW and MB468-FW, WNT3A-RMFs reproducibly increased luminescence and fluorescence of BCM-3963-FW, one of our double-transgenic PDX lines, relative to control conditions (Fig. 5 and Fig. S3). Thus, the oncogenic effects of WNT3A in human breast cancer are not limited to immortalized cell lines. Interestingly, BCM-3963-FW was derived from a patient with HER2+ breast cancer, and elevated HER2 expression is stable in the transgenic xenograft line (Fig. 6A). Stimulation of BCM-3963-FW by WNT3A contrasts with our cell line panel where none of five HER+ cell lines were Wnt responsive. Also unexpectedly, WNT3A-RMFs had the opposite effect on a second PDX line, BCM-4272-FW. Here, luminescence was increased, indicating Wnt pathway activation, but red fluorescence was decreased, indicating that tumor growth was inhibited (Fig. 5). This effect was reproducible in two independent experiments with 10 mice in each group (Fig. S3). The BCM-4272-FW tumors grown in the presence of RMF-WNT3A displayed metaplasia (Fig. 6B), with prominent areas of large round cells with abundant pale cytoplasm that resembled squamous cells. These cells stained positive for cytokeratin 10 (Fig. 6C), a marker for squamous differentiation (26). Furthermore, the mean Ki67 proliferating index was significantly lower (P = 0.002) in RMF-WNT3A tumors (26.8% ± 3.2% Ki67 positive cells, n = 3) vs. RMF-GFP tumors (40.6% ± 1.4%, n = 3), consistent with the smaller tumor size. It is not clear whether these effects are direct or indirect; for example, WNT3A could be stimulating secretion of another factor responsible for the observed tumor inhibition. Nevertheless, these results suggest that activated Wnt signaling can either promote or inhibit human breast cancer growth.
Fig. 5.
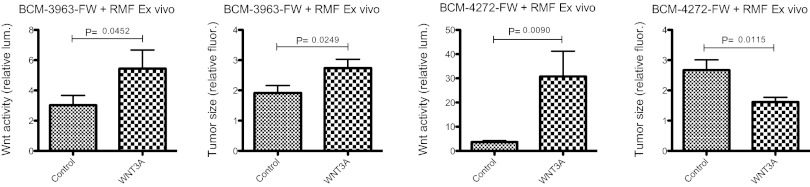
Wnt signaling in PDX lines. Orthotopic heterotypic recombination experiments as in Fig. 4 using the transgenic PDX lines BCM-3963-FW and BCM-4272-FW, n = 10. Tumors were harvested at day 19 (BCM-3963-FW) or day 21 (BCM-4272-FW), and luminescence and fluorescence were measured ex vivo. The entire experiment was repeated with both PDX lines (Fig. S3).
Fig. 6.
Representative histopathology and immunohistochemistry of PDX tumors. (A) Anti-HER2 staining of BCM-4272-FW, (TNBC), and BCM-3963-FW, derived from a HER2-overexpressing breast cancer. Both tumors were from the eighth passage after transduction. Counterstained with Mayer’s hematoxylin for nuclei. (B) H&E staining of BCM-4272-FW coinjected with RMF-GFP (Upper) or RMF-WNT3A (Lower). Eight different tumors are shown, harvested at day 17 or at 4 wk. (C) Anti-CK10 staining of BCM-4272-FW tumors harvested at day 17; four different tumors are shown. (D) Anti-Ki67 staining of BCM-4272-FW tumors harvested at day 17; four different tumors are shown.
Discussion
The dual lentiviral vector system presented here combines a sensitive signaling pathway reporter with cutting edge molecular imaging and magnetic separation modalities. We used this system to generate and characterize multiple state-of-the-art cell line and PDX models of Wnt signaling in breast cancer. Using Wnt signaling as a paradigm, we further showed that it is possible to modulate paracrine signaling pathways in vivo using a relevant cellular source of ligand and to simultaneously monitor the effectiveness of pathway modulation in real time. If used preclinically, these models would enable one to monitor signaling activity in the cells of interest in vivo while simultaneously assessing whether potential therapeutics affect signaling and determining the biological consequences of signaling activation or inhibition.
In our panel of 16 transgenic human breast cancer cell lines, all of the Wnt-responders were triple negative, consistent with literature reports that Wnt-responsiveness correlates with the triple-negative phenotype in human tumors. We improved the resolution of categorizing Wnt-responsiveness by identifying basal B (claudin-low) as the predominant triple-negative subset that is activated by Wnt. We characterized five of five basal B-cell lines as Wnt responsive. The commonality of Wnt responsiveness suggests that this characteristic may be intrinsic to the basal B classification and, by extension, claudin-low tumors. The claudin-low subtype is associated with a core epithelial-to-mesenchymal gene expression signature (27), and Wnt signaling is reported to promote epithelial to mesenchymal transition (EMT) in breast cancer cell lines (28, 29). One explanation for the association we found between the claudin-low subset and Wnt-responsiveness is that Wnt-induced EMT contributes to the EMT signature, and perhaps to the pathology, of claudin-low tumors. Alternatively, claudin-low tumors may originate in mesenchymal-like cells that are naturally Wnt-responsive. Furthermore, because the claudin-low signature is found in both tumors and cell lines (basal B), and basal B-cell lines only rarely display endogenous active Wnt signaling (10, 11, 13), we suggest that the claudin-low signature encodes a latent potential to respond to Wnt signaling. We hypothesize that Wnt pathway activity is superfluous in 2D culture conditions, where nutrients are plentiful and interactions with the microenvironment are relatively simple. Wnt signaling potential may become more relevant when the cells are exposed to stress, such as when grown in suspension in vitro, during engraftment after xenotransplantation, or during metastasis.
Using an orthotopic heterotypic recombination assay we showed that paracrine Wnt signaling exacerbates tumor growth in MB231 and MB468 cell lines. Autocrine Wnt signaling was previously implicated in the tumorigenicity of MDA-MB-231 and other breast cancer cell lines (12, 30). Paracrine signaling is consistent with previous studies showing that overexpression of soluble inhibitors or knockdown of Wnt receptors inhibits xenograft growth.
The heterotypic recombination assay further enabled us to identify two different consequences of paracrine WNT3A signaling on patient-derived xenografts. We report here that elevated Wnt signaling promotes tumor growth in a PDX model (BCM-3963-FW). None of the Her2-overexpressing cell lines we analyzed were responsive to WNT3A by reporter assay, thus it was surprising that WNT3A activated the Wnt reporter and promoted tumor growth in this Her2-overexpressing PDX line. There is a correlation between nuclear β-catenin and Her2 expression in some breast carcinomas, and the Axin2-lacZ reporter is expressed in focal hyperplastic regions in MMTV/neu mice, suggesting that Her2 overexpression and Wnt-responsiveness are not mutually exclusive (31). In contrast, others have reported that nuclear β-catenin and Her2 expression are inversely correlated (32, 33). It was recently reported that development of resistance to trastuzumab (a monoclonal antibody therapeutic against the Her2 receptor) in Her2-overexpressing cell lines is associated with activation of Wnt signaling (34). In agreement with this mechanism of resistance, BCM-3963 was derived from a patient who developed resistance to trastuzumab treatment (www.bcxenograft.org). It is interesting to speculate that antagonism of Wnt signaling might restore trastuzumab sensitivity; however, our current inability to grow PDX lines in culture hinders such mechanistic investigations at this time.
In stark contrast with the consequences of WNT3A signaling in BCM-3963-FW, we observed that this same signal inhibited tumor growth of BCM-4272-FW, which is triple negative. BCM-4272 was derived from a patient with infiltrating ductal carcinoma of no special type. Wnts are known to elicit different effects depending on cellular context. Thus, the opposite effects on tumor growth seen with BCM-4272-FW and BCM-3963-FW may derive from the intersection of Wnt activity with other cellular processes or signaling operating concurrently in those PDX cells. Our observation of Wnt-induced squamous metaplasia in BCM-4272 is congruent with murine studies where overexpression of Wnt1 or activated β-catenin, or mutation of adenomatous polyposis coli also causes squamous metaplasia (35–37). The mammary gland is derived from the ectodermal germ layer, which also gives rise to skin. One could imagine that WNT3A induces squamous differentiation in BCM-4272 by converting the cancer cells to a more primitive state, where they are receptive to skin-specifying cues, possibly related to wounding, present within the xenograft context. This scenario is suggestive of cellular reprogramming, and Wnt signaling promotes reprogramming during induced pluripotent stem cell generation (38). Alternatively, existing tumor heterogeneity or phenotypic plasticity in BCM-4272 may account for WNT3A-induced emergence of cells resembling skin.
Overall, our results support the development of Wnt inhibitors as breast cancer therapeutics, particularly for claudin-low tumors. However, our observation that elevated Wnt signaling can also inhibit tumor growth suggests that patient selection may be essential for the ultimate success of such agents in clinical trials and further suggests that activators of Wnt signaling may even be beneficial to certain patients. Future studies and additional models are needed to enable the development of molecular predictors to prospectively identify individual breast cancers that could be inhibited by Wnt agonists or antagonists. Nevertheless, our system provides a platform to begin to understand and target signaling by Wnt and other paracrine factors in a context relevant to human breast cancer.
Materials and Methods
Orthotopic Heterotypic Recombination Assays.
A total of 40,000 transgenic human breast cancer cells were mixed with 400,000 RMFs in 50 µL of 50:50 matrigel:digestion media and injected into the left and right number four mammary fat pads of 8-wk-old SCID-Beige mice. Mice were imaged twice per week for three weeks, at which point tissues were harvested and imaged ex vivo.
Details on cell culture, lentiviral vector construction, real-time Wnt reporter assay, PDX processing and passaging, lentiviral transduction, imaging, and immunohistochemistry can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank L. Zeng, A. Kraus, and Sally Ganley for technical assistance, M. Clarke and H. Liu for advice on handling patient-derived xenograft lines, R. Shaw for access to imaging instrumentation, R. Moon for the pBAR plasmid, S. Ethier for providing the SUM cell lines, and B. Spike and K. Willert for critically reading the manuscript. Concentrated lentivirus was produced by the Salk GT3 Viral Vector Core Facility. Immunohistochemistry was done by the University of California, San Diego histology core facility. This work was supported by US Department of Defense Breast Cancer Research Program Award W81XWH-09-1-0326 (to J.L.G.), the Susan G. Komen and Breast Cancer Research Foundation (G.M.W.), Salk Cancer Center Support Grant CA014195, National Institutes of Health–National Cancer Institute Grants U54-CA149196 and P50-58183 (to M.T.L.), and by Baylor College of Medicine Cancer Center Support Grant P30 CA125123.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303671110/-/DCSupplemental.
References
- 1.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- 3.Ayyanan A, et al. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci USA. 2006;103(10):3799–3804. doi: 10.1073/pnas.0600065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Incassati A, Chandramouli A, Eelkema R, Cowin P. Key signaling nodes in mammary gland development and cancer: β-catenin. Breast Cancer Res. 2010;12(6):213. doi: 10.1186/bcr2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jönsson M, Borg A, Nilbert M, Andersson T. Involvement of adenomatous polyposis coli (APC)/beta-catenin signalling in human breast cancer. Eur J Cancer. 2000;36(2):242–248. doi: 10.1016/s0959-8049(99)00276-2. [DOI] [PubMed] [Google Scholar]
- 6.Lin SY, et al. Beta-catenin, a novel prognostic marker for breast cancer: Its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci USA. 2000;97(8):4262–4266. doi: 10.1073/pnas.060025397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howe LR, Brown AM. Wnt signaling and breast cancer. Cancer Biol Ther. 2004;3(1):36–41. doi: 10.4161/cbt.3.1.561. [DOI] [PubMed] [Google Scholar]
- 9.Klarmann GJ, Decker A, Farrar WL. Epigenetic gene silencing in the Wnt pathway in breast cancer. Epigenetics. 2008;3(2):59–63. doi: 10.4161/epi.3.2.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van de Wetering M, et al. Mutant E-cadherin breast cancer cells do not display constitutive Wnt signaling. Cancer Res. 2001;61(1):278–284. [PubMed] [Google Scholar]
- 11.Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6(5):497–506. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda Y, Schlange T, Oakeley EJ, Boulay A, Hynes NE. WNT signaling enhances breast cancer cell motility and blockade of the WNT pathway by sFRP1 suppresses MDA-MB-231 xenograft growth. Breast Cancer Res. 2009;11(3):R32. doi: 10.1186/bcr2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiMeo TA, et al. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 2009;69(13):5364–5373. doi: 10.1158/0008-5472.CAN-08-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu J, et al. A novel far-red bimolecular fluorescence complementation system that allows for efficient visualization of protein interactions under physiological conditions. Biosens Bioelectron. 2009;25(1):234–239. doi: 10.1016/j.bios.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Biechele TL, Moon RT. Assaying beta-catenin/TCF transcription with beta-catenin/TCF transcription-based reporter constructs. Methods Mol Biol. 2008;468:99–110. doi: 10.1007/978-1-59745-249-6_8. [DOI] [PubMed] [Google Scholar]
- 16.Nervi B, et al. Factors affecting human T cell engraftment, trafficking, and associated xenogeneic graft-vs-host disease in NOD/SCID beta2mnull mice. Exp Hematol. 2007;35(12):1823–1838. doi: 10.1016/j.exphem.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green JL, et al. Use of a molecular genetic platform technology to produce human Wnt proteins reveals distinct local and distal signaling abilities. PLoS ONE. 2013;8(3):e58395. doi: 10.1371/journal.pone.0058395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perou CM, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 19.Sørlie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herschkowitz JI, et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. 2007;8(5):R76. doi: 10.1186/gb-2007-8-5-r76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kao J, et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS ONE. 2009;4(7):e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neve RM, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10(6):515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prat A, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12(5):R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuperwasser C, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA. 2004;101(14):4966–4971. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeRose YS, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17(11):1514–1520. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miettinen M. Keratin immunohistochemistry: Update of applications and pitfalls. Pathol Annu. 1993;28(Pt 2):113–143. [PubMed] [Google Scholar]
- 27.Taube JH, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci USA. 2010;107(35):15449–15454. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu ZQ, et al. Canonical Wnt signaling regulates Slug activity and links epithelial-mesenchymal transition with epigenetic Breast Cancer 1, Early Onset (BRCA1) repression. Proc Natl Acad Sci USA. 2012;109(41):16654–16659. doi: 10.1073/pnas.1205822109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yook JI, et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol. 2006;8(12):1398–1406. doi: 10.1038/ncb1508. [DOI] [PubMed] [Google Scholar]
- 30.Schlange T, Matsuda Y, Lienhard S, Huber A, Hynes NE. Autocrine WNT signaling contributes to breast cancer cell proliferation via the canonical WNT pathway and EGFR transactivation. Breast Cancer Res. 2007;9(5):R63. doi: 10.1186/bcr1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalil S, Tan GA, Giri DD, Zhou XK, Howe LR. Activation status of Wnt/ß-catenin signaling in normal and neoplastic breast tissues: Relationship to HER2/neu expression in human and mouse. PLoS ONE. 2012;7(3):e33421. doi: 10.1371/journal.pone.0033421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geyer FC, et al. beta-Catenin pathway activation in breast cancer is associated with triple-negative phenotype but not with CTNNB1 mutation. Mod Pathol. 2011;24(2):209–231. doi: 10.1038/modpathol.2010.205. [DOI] [PubMed] [Google Scholar]
- 33.Khramtsov AI, et al. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176(6):2911–2920. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y, et al. Expression of Wnt3 activates Wnt/beta-catenin pathway and promotes EMT-like phenotype in trastuzumab resistant HER2-overexpressing breast cancer cells. Mol Cancer Res. 2012;10(12):1597–1606. doi: 10.1158/1541-7786.MCR-12-0155-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teulière J, et al. Targeted activation of beta-catenin signaling in basal mammary epithelial cells affects mammary development and leads to hyperplasia. Development. 2005;132(2):267–277. doi: 10.1242/dev.01583. [DOI] [PubMed] [Google Scholar]
- 36.Miyoshi K, et al. Activation of beta -catenin signaling in differentiated mammary secretory cells induces transdifferentiation into epidermis and squamous metaplasias. Proc Natl Acad Sci USA. 2002;99(1):219–224. doi: 10.1073/pnas.012414099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuraguchi M, Ohene-Baah NY, Sonkin D, Bronson RT, Kucherlapati R. Genetic mechanisms in Apc-mediated mammary tumorigenesis. PLoS Genet. 2009;5(2):e1000367. doi: 10.1371/journal.pgen.1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marson A, et al. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3(2):132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.