Abstract
The transformation of normal cells to malignant, metastatic tumor cells is a multistep process caused by the sequential acquirement of genetic changes. To identify these changes, we compared the transcriptomes and levels and distribution of proteins in a four-stage cell model of isogenically matched normal, immortalized, transformed, and metastatic human cells, using deep transcriptome sequencing and immunofluorescence microscopy. The data show that ∼6% (n = 1,357) of the human protein-coding genes are differentially expressed across the stages in the model. Interestingly, the majority of these genes are down-regulated, linking malignant transformation to dedifferentiation. The up-regulated genes are mainly components that control cellular proliferation, whereas the down-regulated genes consist of proteins exposed on or secreted from the cell surface. As many of the identified gene products control basic cellular functions that are defective in cancers, the data provide candidates for follow-up studies to investigate their functional roles in tumor formation. When we further compared the expression levels of four of the identified proteins in clinical cancer cohorts, similar differences were observed between benign and cancer cells, as in the cell model. This shows that this comprehensive demonstration of the molecular changes underlying malignant transformation is a relevant model to study the process of tumor formation.
Cancer development is a multistep process where genetic changes are accumulated, thus progressively transforming cells into a cancerous phenotype (1, 2). Over the last decades, numerous investigators have studied the underlying molecular mechanisms for malignant transformation. This has resulted in many models that explain the development of a malignant phenotype of human cells, for instance the “hallmarks of cancer” by Hanahan and Weinberg (3, 4).
With new technologies for deep sequencing, novel opportunities to study the underlying molecular events leading to cancers have emerged. A large number of investigations have analyzed mutations occurring in tumors, such as the analysis of mRNA expression, microRNA expression, and DNA copy number in a large number of tumors in the Cancer Genome Atlas (5). Similarly, the Human Protein Atlas project (6) studies human cancers using a proteome-wide collection of antibodies, resulting in publicly available immunohistochemistry images covering 20 different human cancer types.
An interesting approach to studying the molecular mechanisms underlying cancer is to use an isogenically matched cell model in which normal cells are progressively transformed into malignant cells. There are several studies where genetic elements such as oncogenes have been introduced to different types of cells in an accumulative order as an attempt to mimic the natural steps of transformation (7, 8). One such cell model is the four-stage model based on BJ fibroblasts developed by the Weinberg group, in which primary fibroblast cells were immortalized with telomerase reverse transcriptase (TERT), further transformed with the SV40 large-T antigen, and finally made to metastasize by the introduction of oncogenic H-Ras (RASG12V) (9). We have used this cell-line model for a genome-wide, comprehensive analysis of the molecular mechanisms that underlie malignant transformation and metastasis, using transcriptomics and immunofluorescence-based protein profiling.
Results
Morphological Changes in the Four-Stage Cell Model.
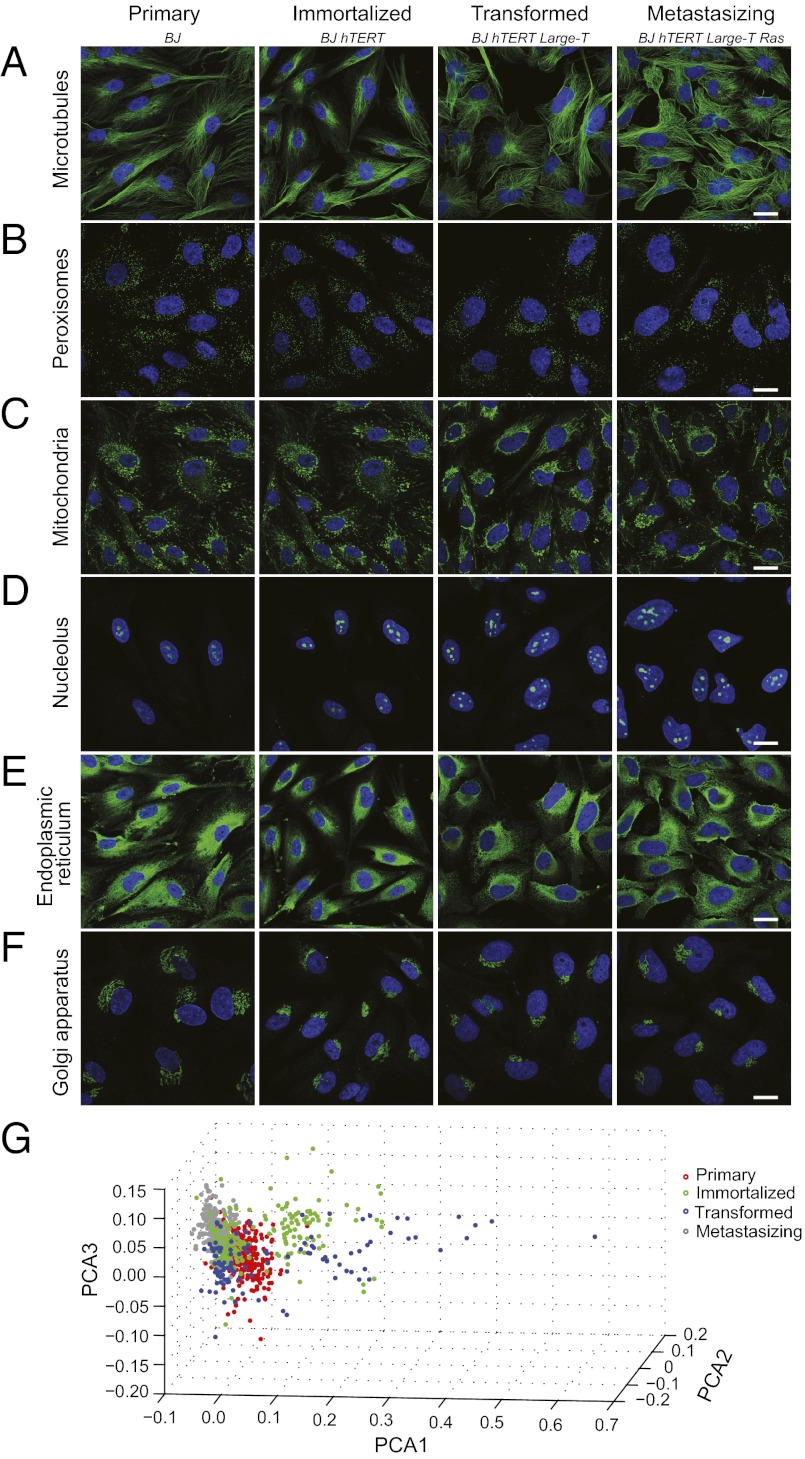
The morphologies of the four cell lines that represent the different stages of malignancy were studied using antibodies targeting different subcellular structures (Fig. 1A). As expected, the overall morphology of the primary cells is more elongated, whereas the cell morphology of the last two stages is irregular and the fibroblast appearance is lost. Whereas some subcellular components, such as microtubules, peroxisomes, and the endoplasmic reticulum, are similar in the four cell lines, others are morphologically altered. For example, the number of nucleoli is increased in the transformed cells, likely reflecting an increase of cellular proliferation. In addition, the morphology of the Golgi apparatus is altered, showing an aggregated perinuclear phenotype in the transformed cells.
Fig. 1.
Morphological analysis of the cell model. (A–F) Confocal images of immunofluorescently stained cells, where the organelle of interest is shown in green and the nucleus is in blue. The images show staining of the following structures (targeted protein): (A) microtubules (TUBA1A), (B) peroxisomes (ABCD3), (C) mitochondria (HSPA9), (D) nucleolus (USP36), (E) endoplasmic reticulum (CALR), and (F) Golgi apparatus (GOLGA5). (Scale bars, 20 μm.) (G) Scatter plot, using the first three principal components and representation of the four cell stages in the model. The principal component analysis (PCA) was run on the top 50 ranked texture and morphological features from images with IF staining of the nucleus, microtubules, and Golgi apparatus.
To objectively evaluate and quantify differences in morphology, we used automated image analysis. Based on 50 features accounting for most of the differences between the cells (Dataset S1), we show that the cells have a morphology that an automated classifier can accurately identify and distinguish between (Fig. 1B). Nonsupervised clustering shows good agreement with BJ cell stage (rho = 0.643). Noteworthy is that the immortalized and transformed cells are more heterogeneous than the primary and metastasizing cells.
Changes in Gene Expression.
The four cell lines were cultivated and mRNA was isolated for RNA sequencing (RNA-seq). The number of genes with detectable transcripts is similar in the different cell types (Fig. 2A), varying between 12,074 and 12,493 using a false discovery rate (FDR) of 1%. Spearman correlations between duplicate samples are high (0.97–0.98, S2) and the genetic elements introduced into the cells were confirmed (see legend to Fig. 2). The bioinformatics analysis identified a total of 1,357 differentially expressed genes: 214 between the primary and immortalized cells, 856 between the immortalized and transformed cells, and merely 31 between the transformed and metastasizing cells (Fig. 2, Fig. S1, and Dataset S2). To identify genes with a gradual change of expression, we compared the primary and metastasizing stages, and identified thereby an additional 381 differentially expressed genes. Altogether, the RNA-seq data show that the primary stage expressed the highest number of protein-coding genes and that the majority (80%) of the differentially expressed genes are down-regulated en route to malignancy.
Fig. 2.
Overall changes in gene expression across the cell model. (A) Schematic of the cell-line model based on accumulative genetic changes, with summarized RNA-seq results shown below the cells. The introduced genetic changes could be validated by the RNA-seq data as follows for TERT (FPKM), large-T (FPKM), and RasG12V (fraction of reads with mutation, in %) in the four cell lines, respectively: primary: 0, 0, 0; immortalized: 128, 1, 0; transformed: 85, 2219, 0; metastasizing: 103, 2685, 30. (B) Graph showing the over- or underrepresentation of annotated subcellular localization for the group of differentially expressed genes compared with all detected genes. The subcellular structures are listed on the y axis and the differences in percentage points between the differentially expressed genes and all detected genes are shown on the x axis (i.e., overrepresented organelles have a positive value).
Gene-set enrichment analysis was performed to identify the types of proteins with an altered pattern of gene expression. Fig. 2B shows an enrichment analysis of subcellular localization [gene ontology (GO) cellular component] of the differentially expressed genes in relation to all detected genes. Here, extracellular proteins and proteins in the plasma membrane are highly enriched, whereas ribosomal and mitochondrial proteins are not. To further distinguish between differences among up- and down-regulated proteins, these groups were analyzed separately in terms of GO biological process. The large group of down-regulated genes (80%) relates to a diverse set of functions, such as extracellular matrix production, cell adhesion, cell migration, growth factor binding, and angiogenesis. A minority (20%) of the differentially expressed genes are up-regulated and highly enriched for functions that control cellular proliferation, such as DNA replication, mitotic spindle, and cell-cycle control (Dataset S3, validated by independent method in Dataset S4).
Molecular Consequences of Cell Immortalization.
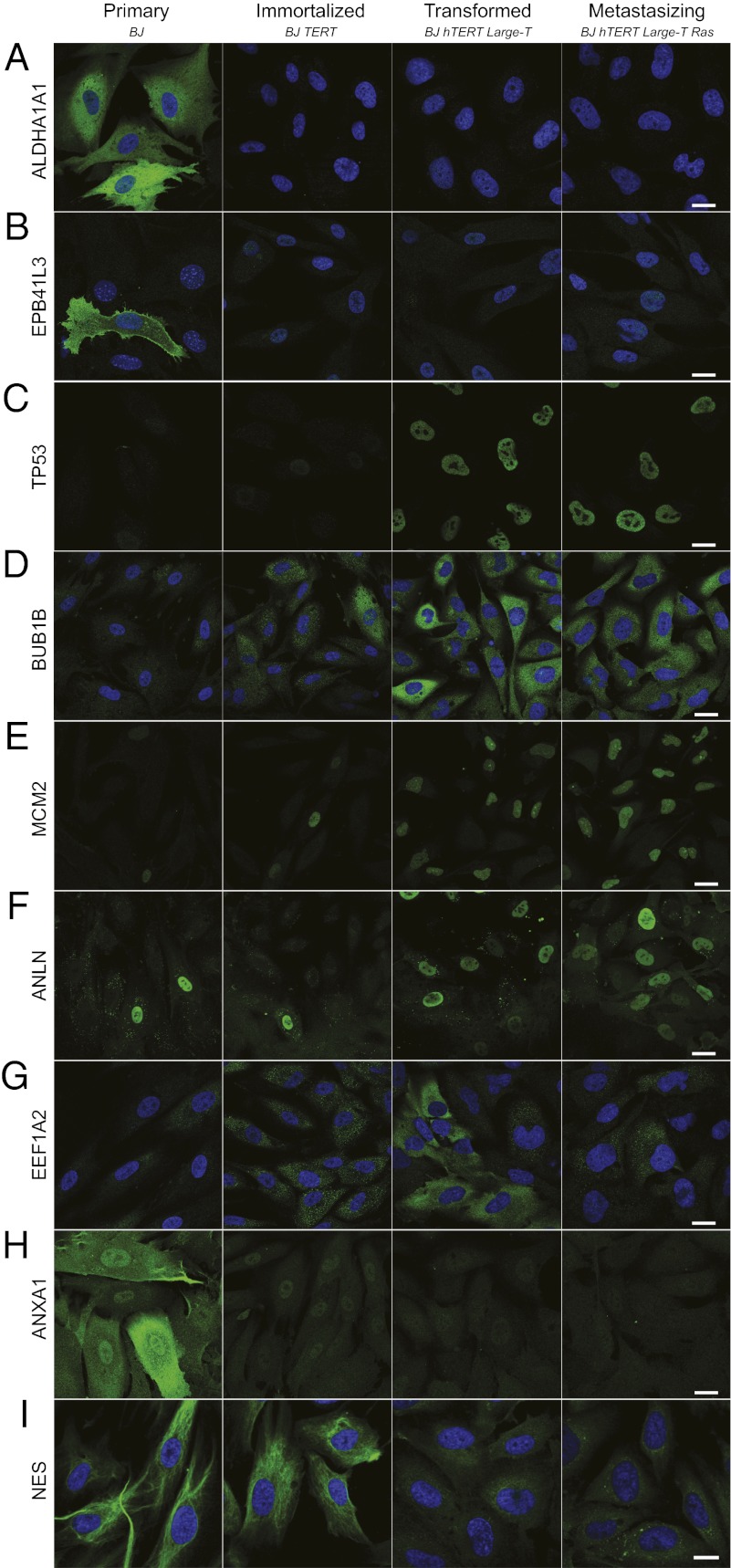
Telomerase reverse transcriptase is the catalytic subunit of the enzyme telomerase and is expressed in most cancers (10). The RNA-seq analysis revealed that immortalization of the fibroblasts by introduction of TERT resulted in 214 differentially expressed genes (Dataset S2). The cytosolic isozymes aldehyde dehydrogenase 1(ALDH1A1) and erythrocyte membrane protein band 4.1-like-3 (EPB41L3) are detected only in primary cells, and are completely absent upon immortalization [ALDH1A1: fragments per kilobase of exon model per million fragments mapped (FPKM) 255 to 0; EPB41L3: FPKM 14 to 0]. Immunofluorescence (IF) analysis shows that whereas both the ALDH1A1 and EPB41L3 proteins show cytoplasmic localization in the primary BJ cells, they are absent in the subsequent stages of the model (Fig. 3 A and B). Beyond alcohol metabolism, the role of ALDH1A1 is not yet understood. There are studies that report ALDH1A1 expression as a favorable prognostic factor in ovarian (11) and pancreatic cancer (12). However, contradicting data also show that positive cells have increased invasive and metastatic capabilities (13) and that ALDH1A1 expression is correlated with poor survival in breast cancer (14).
Fig. 3.
Confocal images of immunofluorescently stained proteins with differential expression. The protein of interest is shown in green and the nucleus is in blue. The images show staining of the following proteins (corresponding FPKM values): (A) ALDH1A1 (256, 0, 0, 0), (B) EPB41L3 (13.8, 0, 0, 0), (C) TP53 (16.6, 33.8, 73.1, 65.2), (D) BUB1B (11.3, 19.1, 41.7, 41.8), (E) MCM2 (34.8, 41.1, 103, 108), (F) ANLN (99.2, 142, 250, 237), (G) EEF1A2 (18.8, 3.74, 154. 29.7), (H) ANXA1 (526, 440, 253, 201), and (I) NES (105, 93.9, 1.85, 4.27). For images D–F, the DAPI channel is available in Fig. S2. (Scale bars, 20 μm.)
Regarding up-regulated genes, epiregulin (EREG), known to be capable of stimulating proliferation of various human cells through the EGF signaling pathway (15), was found to be up-regulated threefold (FPKM 35 to 107) upon immortalization, and subsequently down-regulated 35-fold when the cells were transformed with large-T (FPKM 107 to 3). This suggests that the elevated levels of EREG during the immortalization phase are not required in transformed and metastasizing cells, most likely due to an alternative mechanism for sustained proliferation.
SV40 Transformation Causes Dedifferentiation and Massive Changes in Gene Expression.
The majority of all changes in this cell model occur upon large-T antigen transformation, where 856 genes are differentially expressed (Dataset S2). This broad effect is not surprising, because large-T is expected to mediate its action by inhibition of the p53 and Rb family of tumor suppressors. Large-T binding stabilizes p53, and the transformed cells should thus contain large amounts of functionally inactive p53 (16, 17). Accordingly, our RNA-seq data show a 2.2-fold increase in p53 mRNA levels upon large-T transformation, as also confirmed on the protein level (Fig. 3C).
Upon large-T antigen binding to Rb proteins, their ability to regulate E2Fs is blocked (16). An effective transformation by large-T should therefore give rise to increased levels of E2F transcriptional targets. In agreement with this, we observe that transformation is linked to up-regulated levels (two- to threefold) of transcriptional targets of E2Fs, including the cell-cycle regulators MYBL1, CDC25A, CDC7, and CDCA7, checkpoint proteins such as BUB1B (Fig. 3D), nucleotide synthesis proteins such as DHFR, DNA repair proteins such as FANCG, and DNA replication proteins such as MCM2 (Fig. 3E) and MCM3.
As discussed earlier, the fibroblast-like morphology of the cells is lost in this step. In line with this, we observe a down-regulation of fibroblast markers such as α-smooth-muscle actin (ACTA2) and fibroblast activation protein (FAP), as well as many components of the extracellular matrix such as collagens, laminin, and emilin. Altogether, these results indicate that SV40T expression results in a major dedifferentiation of the cells.
Few Genes Show Altered Expression upon Mutant Ras Transformation.
Although transformed and metastasizing cells show similar growth properties in vitro, there is one essential difference: The RasG12V-expressing cell type is capable of forming tumors in mice (9). Despite this, only 30 down-regulated genes and 1 up-regulated gene are identified as a consequence of the Ras mutation (Dataset S2). Interestingly, many of the down-regulated genes are components of the extracellular matrix, and may well be of importance for metastasis and invasion. For instance, laminin α4 chain is down-regulated gradually across the model, almost completely disappearing in metastasizing cells. In addition, carboxypeptidase A4 shows a pronounced down-regulation (FPKM 67 to 1). The cytokine bone morphogenetic protein 4 (BMP4), known to suppress the proliferation of cancer cells and to stimulate cell migration and invasion (18, 19), is also down-regulated. These proteins are interesting targets for further studies aimed at identifying potential markers for metastatic capabilities.
The only up-regulated gene in this step is cytokine granulocyte colony stimulation factor 3 (CSF3). This protein acts in hematopoiesis by controlling the proliferation, differentiation, and function of granulocytes. Previous studies have observed that this protein is up-regulated during cell migration in wound-healing models (20) and that H-Ras–driven increase of CSF3 promotes human breast cell invasion via metalloproteinase 2 (MMP2) (21).
Validation of Differentially Expressed Genes on the Protein Level.
To investigate how well the changes in gene expression on the RNA level are translated to the protein level, we performed IF analysis of a selection of proteins. A number of up-regulated genes were studied, here exemplified by TP53, BUB1B, MCM2, anillin (ANLN), and EEF1A2. These proteins show increased IF staining across the model (Fig. 3 C–G). In many cases, it is not the expression level in each cell that is increased but rather the fraction of cells in the population that express the protein. For instance, MCM2 is only expressed in ∼10% of primary cells, but this fraction gradually increases until all cells in the population express this protein in the metastasizing stage. The same phenomenon is observed for ANLN (Fig. 3F), which has been shown to be overexpressed in many tumor types (22). It is tempting to speculate that this group of up-regulated genes reflects one single hallmark of cancer—increased proliferative signaling. As such, these genes may be potential markers for cancer cell proliferation and poor patient outcome, as already reported for ANLN (23).
We observed a gradual down-regulation of annexin A1 (ANXA1) across the model. The IF analysis reveals an even more pronounced decrease of the protein (Fig. 3H), suggesting additional posttranslational regulation. Interestingly, a strong staining of ANXA1 is observed in the plasma membrane and nucleus of the primary cells, whereas only nuclear staining is observed in the immortalized cells. Loss of plasma membranous ANXA1 has been observed in premalignant lesions of the oral cavity (24), and nuclear localization has been correlated with shorter overall patient survival (25). Thus, we observe a correlation of expression levels and altered subcellular localization of ANXA1 during the early events of cancer development, which highlights that the role of ANXA1 in cancer should be further evaluated.
Fig. 3I shows staining of nestin (NES) in a typical intermediate filament pattern in primary and immortalized cells, whereas the expression is almost completely abolished in the transformed and metastasizing cells. The mechanisms that regulate the expression of NES in solid tumors remain unclear. However, our results do not support earlier suggestions that NES levels correlate with malignant grades and undifferentiated states of tumors (26).
Altogether, a high correlation is observed between differential gene expression on the RNA and protein levels, and information about the spatial distribution of the protein on the single-cell level provides additional information as for EPB41L3, MCM2, ANLN, and ANXA1.
Network Analysis of Proteins Related to Cell-Cycle Progression, Apoptosis, and Cell Migration and Adhesion.
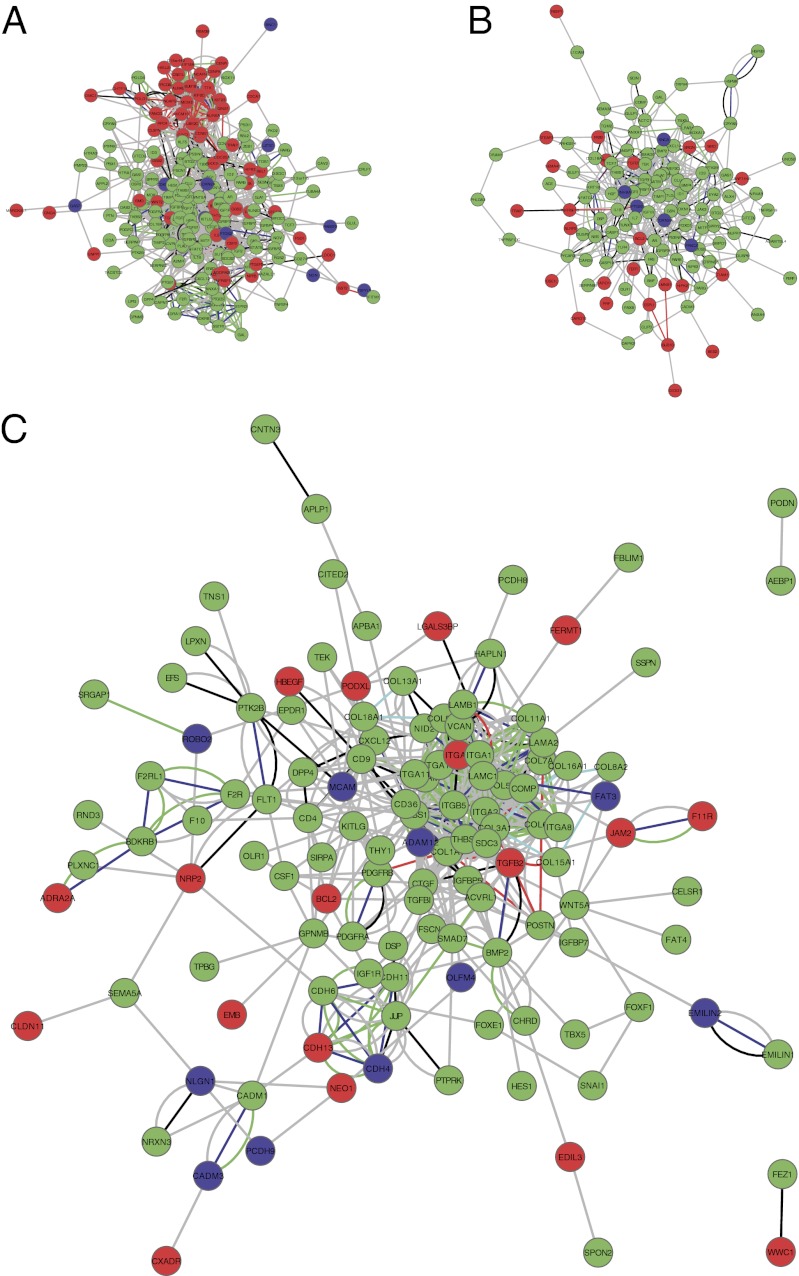
To get a better insight into the molecular mechanisms underlying different hallmarks of cancer in this model, we constructed functional interaction networks for the differentially expressed genes related to the following cellular processes: cell-cycle progression, apoptosis, and cell adhesion and migration (Fig. 4 and Datasets S5 and S6).
Fig. 4.
Functional interaction networks for differentially expressed genes assigned to the following categories: (A) cell-cycle process, (B) apoptosis, and (C) cell adhesion and migration. Nodes are colored based on the gene expression pattern: down-regulation (green), up-regulation (red), and mixed pattern (blue). Edges are colored based on the source of information: coexpression (red), co-occurrence (light blue), experimental (black), fusion (purple), homology (dark blue), green (knowledge), and gray (text mining).
Fig. 4A shows the network for genes involved in cell-cycle progression. The cluster of up-regulated genes represents the core machinery needed for mitosis, such as Aurora kinases A and B. Other up-regulated centromeric proteins are CENPK and CENP1, members of the kinesin-like protein family (KIF18, KIF23, KIF2C) needed for movement of chromosomes during cell division, and subunits of the condensing complex (NCAPH, NCAPG). Other up-regulated genes are minichromosome maintenance proteins (MCM2 and MCM10) that constitute key components of the prereplication complex. Furthermore, regulators of cell-cycle progression are up-regulated, such as BUB1B involved in the spindle checkpoint, c15orf42 involved in the G2/M checkpoint, and CDC25A needed for progression from G1 to S. This is indeed interesting, as defective chromosomal segregation can cause genetic instability, a condition highly associated with tumorigenesis (27). The cluster of down-regulated genes is functionally more diverse and contains cell-signaling molecules such as interleukins (IL7, IL1B, IL8), growth factors (FGF7, FGF2, PDGFC, PDGFD), and many insulin-like growth factor-binding proteins (IGFBP3, IGFBP4, IGFBP5, IGFBP6, IGFBP7). The involvement of IGFBPs in cancer is not fully understood, but there are reports suggesting that down-regulation of these proteins correlates with cancer progression (28–31).
Fig. 4B shows the network for genes involved in apoptosis. Here, most genes are down-regulated, including Fas, caspase 1, caspase 10, and several annexins, such as ANXA1 and ANXA4. The annexins both have phospholipase A2 inhibitory activity and are considered to be antiapoptotic. Earlier studies have shown conflicting results about ANXA expression in tumors. A study has shown that early loss of ANXA1 in breast carcinomas is maintained in both invasive and metastatic tumors (32), whereas another study showed that ANXA1 inhibits the epithelial-to-mesenchymal transition and abolishes metastasis (33). The results reported here suggest a possible role for down-regulation of ANXA1 in the early events of malignant transformation, already at the stage of immortalization.
Fig. 4C shows the network for genes involved in cell adhesion and migration. Here also, the majority of genes are down-regulated with increased transformation. This group of gene products contains many extracellular matrix components, such as collagens (COL1A1, COL3A1, COL5A1, COL5A3, COL6A3, COL7A1, COL8A2, COL13A1, COL15A1, COL16A1, COL18A1) and the major noncollagenous constituents of basement membranes, laminin (LAMA2, LAMB1, LAMC1) and emilin (EMILIN1, EMILIN2). Another example of a component of the extracellular matrix is versican (VCAN). Interestingly, we observe an initial down-regulation of VCAN upon immortalization (FPKM 3 to 0.3) and a subsequent up-regulation in the metastasizing cells (FPKM 1.4 to 5.4). It has previously been shown that VCAN can promote metastatic processes (34), and our results indicate that VCAN may have a dual role, both early and late in tumorigenesis.
Role of ANXA1, 3-Hydroxybutyrate Dehydrogenase, Type 1, Alanyl Aminopeptidase, and ANLN in Prostate and Colon Tumorigenesis.
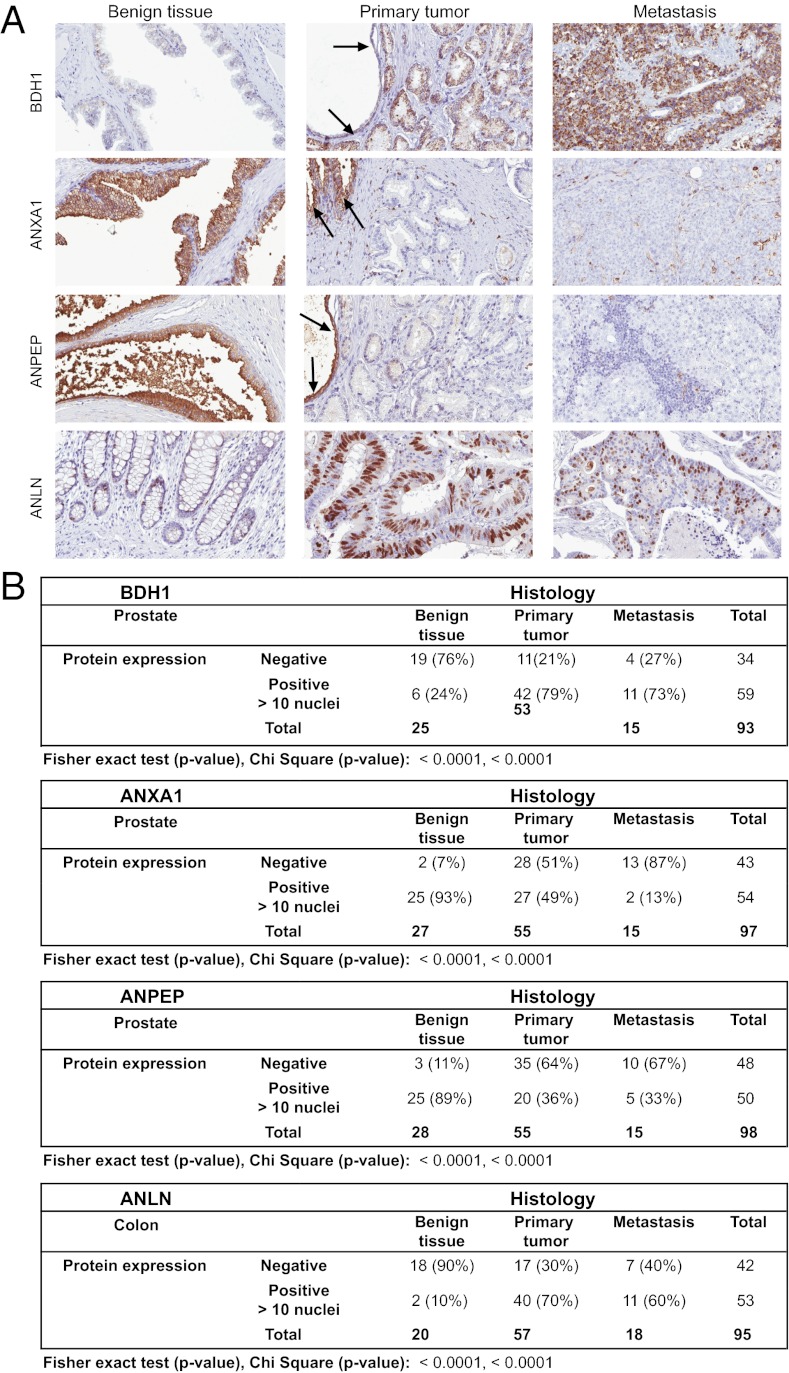
Many genes identified in this study are obvious candidates for follow-up studies of their potential role in cancer development. To determine whether the differentially expressed genes identified in the model are of clinical relevance, we used immunohistochemistry (IHC) to evaluate the expression of a subset of proteins in a clinical prostate cancer cohort including normal prostate, primary prostate cancer of different Gleason grades, and prostate cancer metastasis. Antibodies targeting 10 proteins were tested and 3 of these, 3-hydroxybutyrate dehydrogenase, type 1 (BDH1), ANXA1, and alanyl aminopeptidase (ANPEP), showed differential protein expression when comparing benign, primary cancer, and metastatic cancer (Fig. 5). A fourth target, ANLN, showed only a few positive cell nuclei in the tested prostate tissues and was further tested in a similar colorectal cancer cohort including benign colonic mucosa, primary cancer, and metastases.
Fig. 5.
(A) Examples of immunohistochemically stained sections from benign glands, primary tumor, and metastasis of colon (ANLN) or prostate (BDH1, ANXA1, and ANPEP). The specific protein staining is shown in brown. Arrows mark concomitant normal prostatic glands adjacent to growth of primary prostate cancer. (B) Summarized results from manual assessment of IHC stained prostate tissue biopsies.
The expression of ANLN was observed in the nuclei of normal glandular cells mainly located at the base of colonic crypts. We could observe an increase in the fraction of ANLN-positive nuclei of tumor cell samples compared with control. For this reason, ANLN expression was assessed according to the percentage of positive nuclei, using 10% as the cutoff. With this cutoff, 90% of cores with benign colorectal nuclei were negative, whereas 70% and 60% of cores with primary and metastatic colorectal cancer, respectively, were positive.
The mitochondrial dehydrogenase BDH1 showed a granular cytoplasmic localization in prostatic epithelium. Whereas only 6% of the cores representing normal prostate displayed positive BDH1 protein expression, 79% and 73% of cores with primary prostate cancer and metastasis were BDH1 positive, respectively. ANXA1 displayed cytoplasmic and membranous expression in the prostatic epithelium, and expression was observed in 90% of cores with benign prostatic glands compared with 49% of primary prostate cancer cores and only 13% of cores representing metastasis. ANPEP displayed a similar cytoplasmic protein expression pattern in prostatic epithelium. ANPEP expression was observed in 89% of cores with benign prostatic glands, whereas only 36% and 33% of cores with primary and metastatic prostate cancer were positive, respectively. Automated quantitative IHC image analysis performed on prostate cohorts and manual analysis showed a high concordance with Spearman’s rho correlation coefficients of 0.618, 0.693, and 0.782 for BDH1, ANXA1, and ANPEP, respectively (Fig. S3).
In summary, ANLN and BDH1 showed low expression in normal epithelium compared with the corresponding cancer cells, whereas ANXA1 and ANPEP showed high expression compared with corresponding cancer cells. These results are in accordance with the up- and down-regulation observed for these proteins in the BJ cell model. Both Fisher’s exact and χ2 tests resulted in highly significant P values (<0.0001) for ANLN, BDH1, ANXA1, and ANPEP when comparing benign epithelium and cancer cells (Fig. 5B).
Discussion
Here we describe a comprehensive study of a cell model for cancer malignancy using a combination of deep RNA sequencing and analysis of the corresponding proteins of differentially expressed genes. We demonstrate how the molecular mechanisms underlying the different steps of malignancy as well as the hallmarks of cancer can be scrutinized by a genome-wide approach. The cell model based on human fibroblasts covers normal cells with limited life span to immortalized cells, transformed cells, and cells capable of forming metastasis. It is important to point out that a cell model has limitations mimicking the complex in vivo situation of cancer, including the impact of the tumor microenvironment. However, this cell model allowed us to separate the molecular mechanisms related to the steps of immortalization, transformation, and invasion and metastasis.
The network analysis of the differentially expressed genes provided several insights of general interest. The up-regulated genes are often directly involved in proliferation and cell-cycle control. However, the majority of the differentially expressed genes (80%) are down-regulated. This suggests that the major route toward malignancy involves turning off of genes rather than switching on the expression of novel genes. Interestingly, the down-regulated genes are highly enriched for proteins present on the outside of the cell, either as exposed on the surface of the plasma membrane or secreted. The massive down-regulation of genes, including many typical fibroblast proteins, indicates a gradual dedifferentiation of cells on route to malignancy. In this study, we have performed sequencing of the cellular mRNA and performed a gene-centric data evaluation. A global gene expression encompassing the full transcriptome, including microRNAs and alternatively spliced transcripts, may provide an even more complete view of tumorigenesis. In addition, an extended analysis of protein posttranslational modifications could provide additional insight, especially into the final step of the model, where few genes were differentially expressed.
A classical proteomics strategy based on 2D PAGE analysis and isobaric tag for relative and absolute quantitation (iTRAQ) has earlier been used to study this fibroblast cell-line model and allowed the discovery of 201 differentially expressed proteins (35). Most of the proteins identified by Pütz et al. could be confirmed by our study, although we were, thanks to the higher sensitivity of RNA-seq, able to identify as many as 1,357 differentially expressed genes, corresponding to ∼6% of all human genes. Many of the genes identified in this study are suitable for more in-depth studies to investigate their role in tumorigenesis, for instance ALDH1A1, BMP4, CSF3, VCAN, ANXA1, BDH1, ANPEP, and NES. Some examples of genes possibly involved in the transformation from benign cells to transformed phenotype are listed in Table S1 with examples from each of the proposed hallmarks of cancer.
To conclude, we demonstrate how a combined transcriptomics and protein analysis approach can be used to scrutinize the hallmarks of cancer and define the molecular changes that accompany the immortalization, transformation, and metastatic capabilities of human cells. The identified proteins may serve as promising candidates for markers for the increased cellular proliferation, malignant transformation, invasive growth, or metastatic capacity of tumor cells. We furthermore demonstrate that the altered expression pattern of four proteins identified using the BJ cell model shows similar patterns of deregulation in human tissue samples representing different stages of cancer. Hence, a significant difference in expression was observed between benign epithelium and cancer cells for ANLN in colon and BDH1, ANPEP, and ANXA1 in prostate. This comprehensive demonstration of the molecular changes that underlie malignant transformation and metastasis may serve as a model for studies to understand the complexity of both human cells and tumor development and progression.
Materials and Methods
Cell Cultivation and RNA Sequencing.
Cells were cultivated at 37 °C in a 5% (vol/vol) CO2 environment. RNA was extracted and samples were prepared according to standardized protocols before being sequenced on an Illumina HiSeq 2000.
Immunofluorescence Microscopy and Immunohistochemistry.
Immunofluorescent staining of cells and image acquisition were essentially performed as described in Fagerberg et al. (36). Tissue microarrays were created as previously described with duplicate 1-mm formalin-fixed, paraffin-embedded tissue cores from benign cases as well as cases from primary tumors and metastases for both prostate and colon tissue. Tissue was collected and stored with consent from patients at the Department of Clinical Pathology, Uppsala University Hospital, Uppsala, Sweden. Anonymized tissue samples were used for immunohistochemistry in accordance with Swedish laws and regulations, and with approval from the Regional Ethical Board in Uppsala. Immunohistochemistry and digital slide scanning were performed as previously described (37).
Detailed descriptions of material and methods are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. William C. Hahn (Harvard Medical School) for the BJ cell line and its derivatives; Science for Life Laboratory Stockholm for help with massively parallel sequencing and bioinformatics analysis; and the entire staff of the Human Protein Atlas project and Robert Murphy (Carnegie Mellon University) for providing feature extraction scripts. This work was supported by grants from The Knut and Alice Wallenberg Foundation, the strategic grant to the Science for Life Laboratory, and the Seventh Framework Programme Marie Curie Industry-Academia Partnership and Pathways Program FAST-PATH.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The RNA-seq data reported in this paper have been deposited in the National Center for Biotechnology Information Sequence Read Archive (accession no. SRP019968).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1216436110/-/DCSupplemental.
References
- 1.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 2.Land H, Parada LF, Weinberg RA. Cellular oncogenes and multistep carcinogenesis. Science. 1983;222(4625):771–778. doi: 10.1126/science.6356358. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uhlen M, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010;28(12):1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 7.MacKenzie KL, et al. Multiple stages of malignant transformation of human endothelial cells modelled by co-expression of telomerase reverse transcriptase, SV40 T antigen and oncogenic N-ras. Oncogene. 2002;21(27):4200–4211. doi: 10.1038/sj.onc.1205425. [DOI] [PubMed] [Google Scholar]
- 8.Land H, Parada LF, Weinberg RA. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- 9.Hahn WC, et al. Creation of human tumour cells with defined genetic elements. Nature. 1999;400(6743):464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 10.Kim NW, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 11.Chang B, et al. ALDH1 expression correlates with favorable prognosis in ovarian cancers. Mod Pathol. 2009;22(6):817–823. doi: 10.1038/modpathol.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahlert C, et al. Low expression of aldehyde dehydrogenase 1A1 (ALDH1A1) is a prognostic marker for poor survival in pancreatic cancer. BMC Cancer. 2011;11:275. doi: 10.1186/1471-2407-11-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charafe-Jauffret E, et al. Aldehyde dehydrogenase 1-positive cancer stem cells mediate metastasis and poor clinical outcome in inflammatory breast cancer. Clin Cancer Res. 2010;16(1):45–55. doi: 10.1158/1078-0432.CCR-09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khoury T, et al. Aldehyde dehydrogenase 1A1 expression in breast cancer is associated with stage, triple negativity, and outcome to neoadjuvant chemotherapy. Mod Pathol. 2012;25(3):388–397. doi: 10.1038/modpathol.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toyoda H, Komurasaki T, Uchida D, Morimoto S. Distribution of mRNA for human epiregulin, a differentially expressed member of the epidermal growth factor family. Biochem J. 1997;326(Pt 1):69–75. doi: 10.1042/bj3260069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahuja D, Sáenz-Robles MT, Pipas JM. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24(52):7729–7745. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- 17.Rozan LM, El-Deiry WS. p53 downstream target genes and tumor suppression: A classical view in evolution. Cell Death Differ. 2007;14(1):3–9. doi: 10.1038/sj.cdd.4402058. [DOI] [PubMed] [Google Scholar]
- 18.Ketolainen JM, Alarmo EL, Tuominen VJ, Kallioniemi A. Parallel inhibition of cell growth and induction of cell migration and invasion in breast cancer cells by bone morphogenetic protein 4. Breast Cancer Res Treat. 2010;124(2):377–386. doi: 10.1007/s10549-010-0808-0. [DOI] [PubMed] [Google Scholar]
- 19.Farnsworth RH, et al. A role for bone morphogenetic protein-4 in lymph node vascular remodeling and primary tumor growth. Cancer Res. 2011;71(20):6547–6557. doi: 10.1158/0008-5472.CAN-11-0200. [DOI] [PubMed] [Google Scholar]
- 20.Madhyastha H, Madhyastha R, Nakajima Y, Omura S, Maruyama M. Regulation of growth factors-associated cell migration by C-phycocyanin scaffold in dermal wound healing. Clin Exp Pharmacol Physiol. 2012;39(1):13–19. doi: 10.1111/j.1440-1681.2011.05627.x. [DOI] [PubMed] [Google Scholar]
- 21.Park S, Kim ES, Noh DY, Hwang KT, Moon A. H-Ras-specific upregulation of granulocyte colony-stimulating factor promotes human breast cell invasion via matrix metalloproteinase-2. Cytokine. 2011;55(1):126–133. doi: 10.1016/j.cyto.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Hall PA, et al. The septin-binding protein anillin is overexpressed in diverse human tumors. Clin Cancer Res. 2005;11(19 Pt 1):6780–6786. doi: 10.1158/1078-0432.CCR-05-0997. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki C, et al. ANLN plays a critical role in human lung carcinogenesis through the activation of RHOA and by involvement in the phosphoinositide 3-kinase/AKT pathway. Cancer Res. 2005;65(24):11314–11325. doi: 10.1158/0008-5472.CAN-05-1507. [DOI] [PubMed] [Google Scholar]
- 24.Nomura H, et al. Down-regulation of plasma membranous Annexin A1 protein expression in premalignant and malignant lesions of the oral cavity: Correlation with epithelial differentiation. J Cancer Res Clin Oncol. 2009;135(7):943–949. doi: 10.1007/s00432-008-0530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin CY, et al. Nuclear localization of annexin A1 is a prognostic factor in oral squamous cell carcinoma. J Surg Oncol. 2008;97(6):544–550. doi: 10.1002/jso.20992. [DOI] [PubMed] [Google Scholar]
- 26.Krupkova O, Jr, Loja T, Zambo I, Veselska R. Nestin expression in human tumors and tumor cell lines. Neoplasma. 2010;57(4):291–298. doi: 10.4149/neo_2010_04_291. [DOI] [PubMed] [Google Scholar]
- 27.Nowak MA, et al. The role of chromosomal instability in tumor initiation. Proc Natl Acad Sci USA. 2002;99(25):16226–16231. doi: 10.1073/pnas.202617399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maki RG. Small is beautiful: Insulin-like growth factors and their role in growth, development, and cancer. J Clin Oncol. 2010;28(33):4985–4995. doi: 10.1200/JCO.2009.27.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su Y, et al. Insulin-like growth factor binding protein 5 suppresses tumor growth and metastasis of human osteosarcoma. Oncogene. 2011;30(37):3907–3917. doi: 10.1038/onc.2011.97. [DOI] [PubMed] [Google Scholar]
- 30.Vizioli MG, et al. IGFBP7: An oncosuppressor gene in thyroid carcinogenesis. Oncogene. 2010;29(26):3835–3844. doi: 10.1038/onc.2010.136. [DOI] [PubMed] [Google Scholar]
- 31.Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell. 2008;132(3):363–374. doi: 10.1016/j.cell.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Y, et al. Loss of annexin A1 expression in breast cancer progression. Appl Immunohistochem Mol Morphol. 2008;16(6):530–534. doi: 10.1097/PAI.0b013e31817432c3. [DOI] [PubMed] [Google Scholar]
- 33.Maschler S, et al. Annexin A1 attenuates EMT and metastatic potential in breast cancer. EMBO Mol Med. 2010;2(10):401–414. doi: 10.1002/emmm.201000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao D, Vahdat LT, Wong S, Chang JC, Mittal V. Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Cancer Res. 2012;72(19):4883–4889. doi: 10.1158/0008-5472.CAN-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pütz SM, Vogiatzi F, Stiewe T, Sickmann A. Malignant transformation in a defined genetic background: Proteome changes displayed by 2D-PAGE. Mol Cancer. 2010;9:254. doi: 10.1186/1476-4598-9-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fagerberg L, et al. Mapping the subcellular protein distribution in three human cell lines. J Proteome Res. 2011;10(8):3766–3777. doi: 10.1021/pr200379a. [DOI] [PubMed] [Google Scholar]
- 37.Kampf C, Olsson I, Ryberg U, Sjostedt E, Ponten F. Production of tissue microarrays, immunohistochemistry staining and digitalization within the Human Protein Atlas. J Vis Exp. 2012 doi: 10.3791/3620. (63):pii 3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.