Abstract
The biological significance of a known normal and cancer stem cell marker CD133 remains elusive. We now demonstrate that the phosphorylation of tyrosine-828 residue in CD133 C-terminal cytoplasmic domain mediates direct interaction between CD133 and phosphoinositide 3-kinase (PI3K) 85 kDa regulatory subunit (p85), resulting in preferential activation of PI3K/protein kinase B (Akt) pathway in glioma stem cell (GSC) relative to matched nonstem cell. CD133 knockdown potently inhibits the activity of PI3K/Akt pathway with an accompanying reduction in the self-renewal and tumorigenicity of GSC. The inhibitory effects of CD133 knockdown could be completely rescued by expression of WT CD133, but not its p85-binding deficient Y828F mutant. Analysis of glioma samples reveals that CD133 Y828 phosphorylation level is correlated with histopathological grade and overlaps with Akt activation. Our results identify the CD133/PI3K/Akt signaling axis, exploring the fundamental role of CD133 in glioma stem cell behavior.
According to the cancer stem cell (CSC) hypothesis, tumors are formed and maintained by a population of undifferentiated cells that are characterized by their ability for self-renewal and to induce tumorigenesis (1, 2). Critical to CSC research is their prospective identification and isolation from tumor tissue. CD133 (Prominin-1), a 5-transmembrane domain glycoprotein initially identified in humans as a hematopoietic stem cell marker (3, 4), is widely used as a marker of cancer stem cells in brain tumors as well as in colon cancer, hepatoma, and pancreatic cancer (5–9). However, the utility of CD133 in defining cancer stem cells has been questioned following a series of articles. Several groups have reported that CD133− glioblastoma (GBM) cells can form tumors (10–12). The seemingly elusive role of CD133 in defining cancer stem cells in the literature is an outstanding dilemma in cancer research today (13), raising questions regarding the functional significances and the underlying pathways of CD133.
Increasing evidence strongly suggests the functional association of CD133+ cancer stem cell with protein kinase B (Akt) signaling. CD133+ tumor cells derived from hepatoma, colon cancer, and neuroblastoma consistently displayed increased phospho-Akt levels compared with matched CD133− tumor cells (14–16). Indeed, the significance of activating Akt signaling in cancer stem cell is provoked by the known involvement of Akt signaling in normal stem cell biology and tumorigenesis (17–19) and by the dependence of cancer stem cell on Akt signaling. Chemoresistance in CD133+ hepatocarcinoma stem cells may be conferred by activation of Akt (14). In mouse medulloblastoma models, Akt regulates the survival of tumor cells in the perivascular niche bearing stem cell markers (20). Furthermore, Akt inhibition could produce a reduction in the self-renewal and growth of CD133+ cancer stem cell from glioma and colon cancer (16, 21). However, the mechanisms and significances of Akt activation in cancer stem cell remain unknown.
To date, the well-characterized mechanism of Akt activation is activated by the phosphoinositide 3-kinases (PI3Ks) (22, 23). The PI3K/Akt pathway can be activated in a wide spectrum of human cancers through the inactivation of phosphatase and tensin homolog tumor suppressor, the activation of receptor tyrosine kinases, the amplification of Akt family members, or the mutations of the PI3K catalytic subunit (24–26). Nonetheless, the mechanism regulating the PI3K/Akt pathway that is specific in cancer stem cells has not been adequately addressed. In this study, we used CD133+ glioma stem cell model to explore the possibility of CD133 as a component in regulating the PI3K/Akt pathway and to determine the biological consequence of CD133-PI3K interaction.
Results
CD133 Regulates Akt Signaling.
Using techniques described in the Dirks group’s original report first validating CD133 as a glioma stem cell (GSC) cell surface marker (6), we isolated CD133+ and CD133− cells from human glioblastoma samples (T21107, T21109, and T12179; pathological data are shown in Table S1) (Fig. S1A). CD133+ tumor cells showed characteristics consistent with cancer stem cells: namely, neurosphere formation (Fig. S1B), expression of stem cell marker Nestin, and multilineage differentiation with markers for astrocyte (GFAP), neuron (MAP2), or oligodendrocyte (O4) (Fig. S1C). In an in vivo limiting dilution tumor formation assay, CD133+ tumor cells were highly tumorigenic in brains of immunocompromised mice with characteristics of glioblastoma in concordance with previous report (6). CD133− cells rarely formed detectable tumors even when implanted at 1.0 × 105 cells per mouse, except for occasional tumors from CD133− cells derived from T12179 sample at 1.0 × 105 cells (Fig. S1 D–F). Thus, our data confirm that CD133+ subpopulations are enriched for characteristics of glioma stem cells.
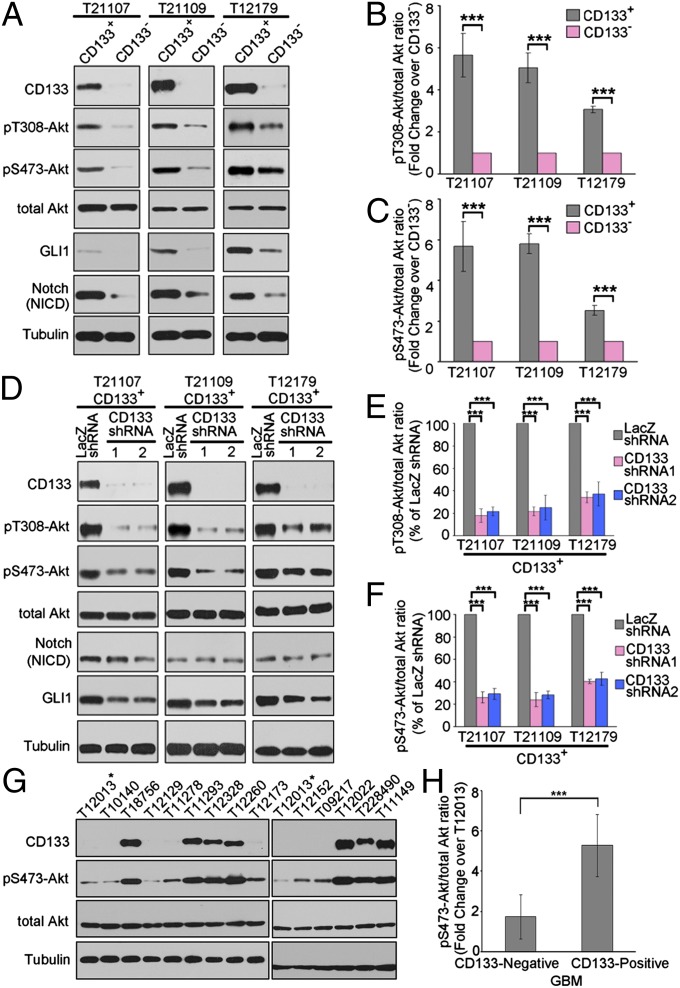
Using this CD133-based selection system, we compared the activity of Akt signaling between CD133+ glioma cells and CD133– glioma cells. Akt achieves its full activity through phosphorylation at both threonine 308 (T308) and serine 473 (S473) (27, 28). Although levels of total Akt proteins were similar between CD133+ and CD133− tumor cells, the phosphorylations of Akt on both S473 and T308 were dramatically up-regulated in CD133+ glioma cells compared with matched CD133− glioma cells (Fig. 1 A–C). Thus, these findings suggest that elevated Akt activity may be a distinctive feature of CD133+ GSC. We next used a lentiviral shRNA-based system to evaluate the requirement for CD133 in Akt activation in CD133+ glioma stem cells. Knockdown of CD133 in CD133+ glioma stem cells obviously reduced the phosphorylation of Akt on both S473 and T308 without changing total Akt levels (Fig. 1 D–F). Consistent with this, Akt signaling was preferentially activated in CD133-positive glioblastoma (GBM) samples compared with CD133-negative GBM samples (Fig. 1 G and H). Thus, these data suggest that CD133 regulates Akt signaling.
Fig. 1.
CD133 knockdown impairs the activity of Akt signaling. (A) The activity of Akt, Notch, and Hedgehog signaling in matched CD133+ and CD133− cells isolated from glioblastoma samples. Whole-cell lysates were analyzed by Western blotting. Tubulin was blotted as a loading control. The figures are presented from three separate experiments. (B and C) The relative densities of pT308-Akt (B) and pS473-Akt (C) to total Akt in A were quantified using densitometry. Values are normalized to that of CD133− cells. Results are expressed as mean ± SD from three separate experiments; ***P < 0.001. (D) The activity of Akt, Notch, and Hedgehog signaling in CD133+ cells with CD133 knockdown. Seventy-two hours after lentivirus infection, whole-cell lysates were analyzed by Western blotting. Tubulin was blotted as a loading control. (D) The figures are presented from three separate experiments. (E and F) The relative densities of pT308-Akt (E) and pS473-Akt (F) to total Akt in D were quantified using densitometry. Values are normalized to that of CD133+ cells infected with beta-galactosidase (LacZ) shRNA lentivirus. Results are expressed as mean ± SD from three separate experiments; ***P < 0.001. (G) Western blot analysis of Akt S473 phosphorylation and CD133 expression levels in GBM samples. The lysates of glioblastoma tissues were analyzed by Western blotting. Tubulin was blotted as a loading control. The figures are presented from three separate experiments. (H) The relative densities of pS473-Akt to total Akt in G were quantified using densitometry. Values are normalized to that of sample T12013 (marked by asterisk). Results are expressed as mean ± SD from three separate experiments; ***P < 0.001.
CD133 Interacts with PI3K Regulatory Subunit P85 Depending on Tyrosine Phosphorylation of Its C-terminal Cytoplasmic Domain.
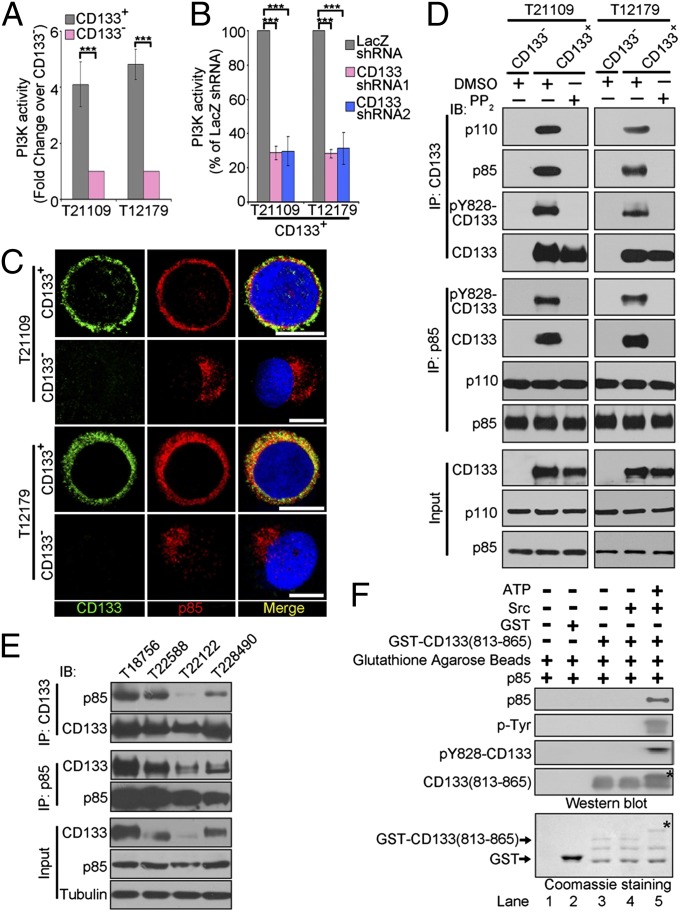
Akt is a well-characterized key downstream effector of the PI3Ks (23). Thus, we suppose that CD133 might regulate Akt signaling through PI3K. Considering that PI3K activates Akt signaling depending on its lipid kinase activity (29), we first compared PI3K activity in CD133+ glioma cells with CD133– glioma cells using PI3K ELISA. CD133+ glioma cells displayed higher level of PI3K activity than matched CD133− glioma cells (Fig. 2A and Fig. S2A). Furthermore, CD133 knockdown attenuated PI3K activity in CD133+ glioma cells (Fig. 2B and Fig S2B). Thus, these results suggest that CD133 regulates PI3K activity.
Fig. 2.
CD133 physically interacts with p85. (A) The PI3K activity of CD133+ cells versus CD133− cells derived from glioblastoma specimens were assessed using a PI3 kinase ELISA kit. Values are normalized to that of matched CD133− cells. Results are expressed as mean ± SD from three separate experiments; ***P < 0.001. (B) Targeting CD133 decreases PI3K activity in CD133+ cells. Seventy-two hours after lentivirus infection, the PI3K activity of CD133+ infected with the indicated lentivirus was measured using a PI3 kinase ELISA kit. Values are normalized to that of CD133+ cells treated with LacZ shRNA, which is set as 100%. Results are expressed as mean ± SD from three separate experiments; ***P < 0.001. (C) Colocalization of CD133 and p85 was assessed by immunofluorescence staining of CD133 (green) and p85 (red) in CD133+ and CD133− cells derived from glioblastoma specimens T21109 (Upper) and T12179 (Lower). Nuclei (blue) were counterstained with Hoechst33258. Colocalization of CD133 and p85 is demonstrated by yellow fluorescence. (Scale bars, 10 µm.) (D) Co-IP analysis to determine the interaction between CD133 and p85 in vivo. Lysates of CD133− or CD133+ cells pretreated with DMSO or PP2 (5 µM) for 1 h were subjected to IP using anti-CD133 or anti-p85 antibody, followed by immunoblotting (IB) with anti-CD133, anti-p110, anti-p85, or anti-pY828-CD133 antibodies. Whole-cell lysates were analyzed by IB with anti-CD133, anti-p110, or anti-p85 antibodies as input. (E) Co-IP analysis to determine the interaction between CD133 and p85 in glioblastoma tissues. The lysates of glioblastoma tissues were subjected to immunoprecipitation (IP) using anti-CD133 or anti-p85 antibodies, followed by IB with anti-CD133 or anti-p85 antibodies. (F) In vitro interaction between CD133 and p85. GST or GST-CD133(813-865) (CD133 C-terminal cytoplasmic domain; amino acids 813–865) protein preincubated with or without active Src and ATP in a phosphorylation buffer was incubated with purified p85 protein. The GST pull-down products were blotted with anti-CD133 C-terminal, anti-p85, anti-tyrosine phosphorylation, and anti-pY828-CD133 antibodies (Upper). GST and GST-CD133(813-865) were shown by Coomassie Blue staining (Lower). *Tyr-phosphorylated form of CD133 C-terminal cytoplasmic domain.
The class IA PI3Ks, which are heterodimers composed of p110 catalytic and p85 regulatory subunits, are mostly activated by recruitment to plasma membrane by the interaction between p85 and growth factor receptor tyrosine kinase (23). In accordance with high level of PI3K activity in CD133+ cells, the membrane translocation of p85 and p110 was increased in CD133+ glioma cells compared with CD133– glioma cells (Fig. 2C and Fig. S2 C and D). Further, CD133 knockdown attenuated the membrane translocation of p85 and p110 in CD133+ glioma cells (Fig. 3E). Thus, these data suggest that CD133 promotes PI3K recruitment to plasma membrane.
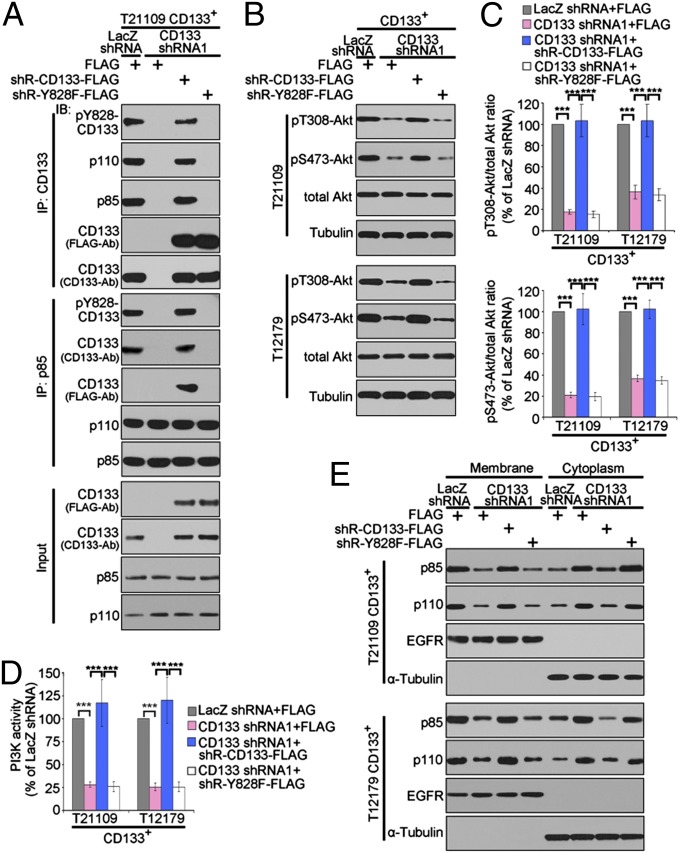
Fig. 3.
Y828 phosphorylation of CD133 mediates the interaction between CD133 and p85 and is required for CD133 activating Akt signaling. (A) Co-IP analysis to determine the effect of Y828 mutation on the interaction between CD133 and p85. The lysates of CD133+ cells expressing LacZ shRNA, CD133 shRNA1, CD133 shRNA1+shRNA-resistant WT CD133, or CD133 shRNA1+shRNA-resistant Y828F were subjected to immunoprecipitation (IP) using anti-CD133 or anti-p85 antibodies, followed by immunoblotting (IB) with anti-CD133, anti-p110, anti-p85, anti-FLAG, or anti-pY828-CD133 antibodies. shR, shRNA-resistant. (B and C) Western blot analysis of Akt phosphorylation in CD133+ cell expressing LacZ shRNA, CD133 shRNA1, CD133 shRNA1+shRNA-resistant WT CD133, or CD133 shRNA1+shRNA-resistant Y828F. (B) The figures are presented out of three separate experiments. (C) The relative densities of pT308-Akt (Upper) and pS473-Akt (Lower) to total Akt in B were quantified. Results are expressed as mean ± SD from three separate experiments; ***P < 0.001. (D) PI3K activity in CD133+ cells expressing LacZ shRNA, CD133 shRNA1, CD133 shRNA1+shRNA-resistant WT CD133, or CD133 shRNA1+shRNA-resistant Y828F. Results are expressed as mean ± SD from three separate experiments; ***P < 0.001. (E) Membrane and cytoplasmic distribution of p85 and p110 in CD133+ cells expressing LacZ shRNA, CD133 shRNA1, CD133 shRNA1+shRNA-resistant CD133 wt, or CD133 shRNA1+shRNA-resistant Y828F was determined by immunoblotting. EGF receptor (EGFR) was used as the membrane marker, and α-Tubulin was used as the cytosolic marker.
Combined with the fact that CD133 is a 5-transmembrane glycoprotein (3, 4), we hypothesize that CD133 might associate with PI3K regulatory subunit p85. Reciprocal Coimmunoprecipitation (co-IP) assays in CD133+ glioma cells and CD133− glioma cells showed that endogenous p85 regulatory subunit bound to endogenous CD133 (Fig. 2D). Consistent with this, p85 was colocalized with CD133 in the plasma membrane of CD133+ glioma cells (Fig. 2C). To exclude the interaction between CD133 and p85 as a cell culture artifact, we evaluated the interaction between CD133 and p85 in human GBM samples (Table S1). P85 was coprecipitated with CD133 protein in glioblastoma tissues (Fig. 2E), which powerfully confirms the physical interaction between CD133 and p85 in vivo.
P85 frequently binds to certain phosphorylated Tyr residues present in receptor tyrosine kinase (23), whereas CD133 could be phosphorylated on tyrosine-828 and tyrosine-852 in C-terminal cytoplasmic domain by tyrosine kinases Src and Fyn (30). Actually, Src protein was expressed similarly in CD133+ and CD133− tumor cells (Fig. S2E). Thus, a binding assay in vitro was performed to evaluate whether the association of CD133 with p85 depended on the phosphorylation of CD133 C-terminal cytoplasmic domain. After a phosphorylation reaction with or without Src, the C-terminal cytoplasmic domain of CD133 (amino acids 813–865) fused to GST purified from bacteria was used in an in vitro binding assay, along with recombinant p85 (generated from Escherichia coli BL21). Consistent with a previous report (30), the GST-CD133 C-terminal cytoplasmic domain was heavily tyrosine-phosphorylated by Src, resulting in an altered electrophoretic mobility (Fig. 2F, lane 5, indicated by *). The phosphorylated form of CD133 C-terminal cytoplasmic domain directly bound to p85 in vitro (Fig. 2F). Treatment of Src family tyrosine kinase inhibitor PP2 significantly abrogated the interaction between CD133 and p85 in CD133+ glioma cells (Fig. 2D). Together, CD133 interacts with p85 depending on the tyrosine phosphorylation of CD133 C-terminal cytoplasmic domain.
To accurately monitor CD133 phosphorylation, we generated and characterized a rabbit polyclonal antibody against a synthetic phosphopeptide surrounding Y828. This antibody detected a strong band at the same molecular weight as CD133 on CD133 precipitates prepared from CD133− cells expressing WT CD133 or CD133 with a single mutation at Y818, Y819, Y846, or Y852, but not at the Y828F mutant (Fig. S3B). In addition, treatment of cells with Src family kinase inhibitor PP2 abolished the pY828-CD133 signal (Fig. 2D). Incubation of CD133 C-terminal cytoplasmic domain with Src kinase caused a robust pY828 band comigrating with CD133 (Fig. 2F). This series of experiments confirmed specificity of this antibody and further established CD133 as an effector molecule for Src family kinase.
Phosphorylated Y828 Residue in CD133 Binds to P85 and Is Required for Activation of the PI3K/Akt Pathway by CD133.
Amino acid sequence analysis of CD133 revealed five tyrosine residues (Y818, Y819, Y828, Y846, and Y852) in its C-terminal cytoplasmic domain. Y819 conforms to the known p85 consensus-binding motif YXXM (31). Y828 and Y852 are phosphorylated by Src family kinases (30) (Fig. S3A). To determine which tyrosine residue mediates the interaction between CD133 and p85, these five tyrosine residues (Y) were individually mutated to phenylalanine (F) by site-directed mutagenesis techniques. Considering that the absence of CD133 protein expression in CD133− tumor cells provided a low background to evaluate the interaction between p85 and exogenous CD133, we performed reciprocal co-IP assays using lysates from CD133− glioma cells ectopically expressing WT CD133 or its tyrosine mutants. WT CD133 or CD133 with a single mutation at Y818, Y819, Y846, and Y852 equally interacted with p85 in vivo. In contrast, mutation of Y828 to F completely abrogated the ability of CD133 to interact with p85 (Fig. S3B), indicating that Y828 phosphorylation is required for the interaction between CD133 and p85.
To further verify the significance of Y828 phosphorylation in the interaction between CD133 and p85, we next performed a series of experiments using CD133+ glioma cells coexpressing CD133 shRNA and either shRNA-resistant WT CD133 or shRNA-resistant Y828F mutant. First, reciprocal co-IP assays revealed that p85 was coprecipitated in vivo with endogenous and exogenous CD133 protein, not with exogenous Y828F mutant (Fig. 3A), confirming that Y828 phosphorylation is required for the interaction between CD133 and p85. Second, we evaluated the contribution of Y828 phosphorylation in CD133 activating the PI3K/Akt pathway. Ectopic expression of shRNA-resistant WT CD133, but not that of Y828F mutant, restored the inhibitory effect of CD133 knockdown on the level of Akt phosphorylation (Fig. 3 B and C), on the activity of PI3K (Fig. 3D), and on the membrane translocation of p85 and p110 (Fig. 3E). Together, these data suggest that phosphorylation of Y828 in CD133 is critical for its physical interaction with p85, which activates the PI3K/Akt pathway.
Analysis of the amino acid residues flanking Y828 (Y828DDV) demonstrated that this residue did not match the p85 consensus-binding motif YXXM (31); rather, it closely resembled a number of other nonconsensus p85-binding proteins that lacked the preferred methionine in the Y +3 position (32, 33) (Fig. S3C). Comparative sequence analysis of CD133 in six mammalian species revealed that YDDV sequence was evolutionary conserved (Fig. S3D).
CD133 Maintains the Self-Renewal and Tumor-Initiating Potentials of GSC Depending on Its Y828 Phosphorylation.
We next evaluated the biological function of CD133 activating the PI3K/Akt pathway in glioma stem cell behaviors. We first determined the role of CD133 in self-renewal and tumor-initiating features of GSC. The single-cell neurosphere formation assay and the bulk-culture neurosphere formation assay are the conventional methods of measuring the self-renewal capacity of GSC (6). CD133 knockdown impaired neurosphere formation not only in primary assays (Fig. S4 A–D), but also in secondary passages (Fig. S4E), indicating that CD133 is required for self-renewal of GSC in vitro. Second, we examined the role of CD133 in the tumor-initiating capacity of GSC through an in vivo limiting dilution tumor formation assay. Targeting CD133 significantly reduced the tumor-initiating potential of GSC (Fig. S4 F and G); thus, CD133 is required to maintain the tumorigenic potential of GSC.
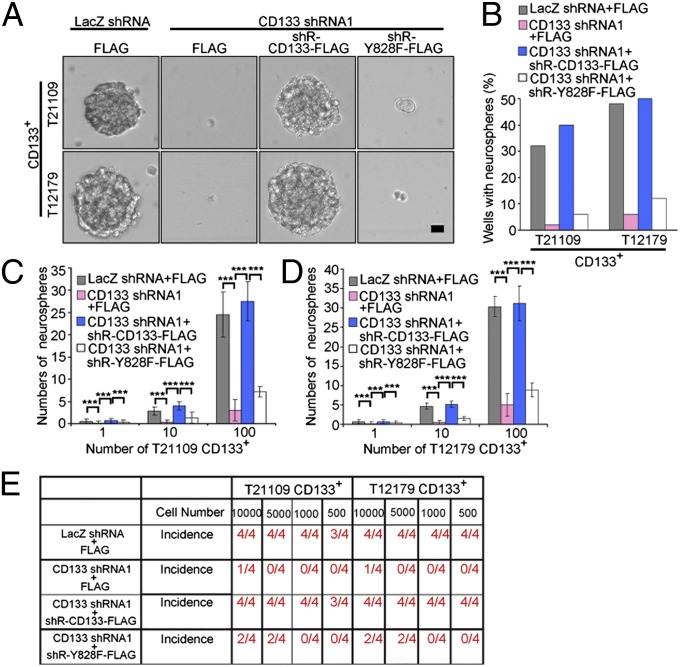
To further determine the significance of CD133 activating the PI3K/Akt pathway, the contribution of Y828 phosphorylation in CD133 maintaining the characteristics of GSC were evaluated. Neurosphere formation assay demonstrated that the inhibitory effect of CD133 knockdown on the self-renewal of GSC was fully rescued by expression of shRNA-resistant WT CD133, but not shRNA-resistant CD133 Y828F mutant (Fig. 4 A–D and Fig. S5A). Furthermore, ectopic expression of WT CD133 but not Y828F mutant fully rescued the inhibitory effect of CD133 depletion on the tumor-initiating capacity of GSC (Fig. 4E). Importantly, CD133 knockdown increased the survival of tumor-bearing animals, which was fully restored by expression of shRNA-resistant WT CD133, but not shRNA-resistant CD133 Y828 mutant (Fig. S5 B and C). Together, these data demonstrate that Y828 phosphorylation is critical for CD133 sustaining GSC self-renewal and tumorigenesis.
Fig. 4.
Y828 phosphorylation is required for CD133 maintaining glioma stem cell self-renewal and tumorigenesis. (A and B) Single-cell neurosphere formation assay of CD133+ cells expressing LacZ shRNA, CD133 shRNA1, CD133 shRNA1+shRNA-resistant WT CD133, or CD133 shRNA1+shRNA-resistant Y828F. (A) Representative images of neurospheres are shown. (B) The percentage of neurosphere-containing wells in each group. (Scale bar, 10 µm.) (C and D) A total of 1, 10, or 100 CD133+ cells isolated from T21109 (C) or T12179 (D) samples infected with the indicated lentivirus were cultured in 96-well plates. After 10 d, the number of neurosphere was counted. Results are expressed as mean ± SD from three separate experiments; ***P < 0.001. (E) WT CD133, not Y828F mutant, rescues the effect of CD133 knockdown on the tumor-initiating capacity of CD133+ cells. An intracranial limiting dilution tumor formation assay (using 10,000, 5,000, 1,000, and 500 cells per mouse) was performed using CD133+ cells infected with the indicated lentivirus. The table displays the number of mice developing tumors.
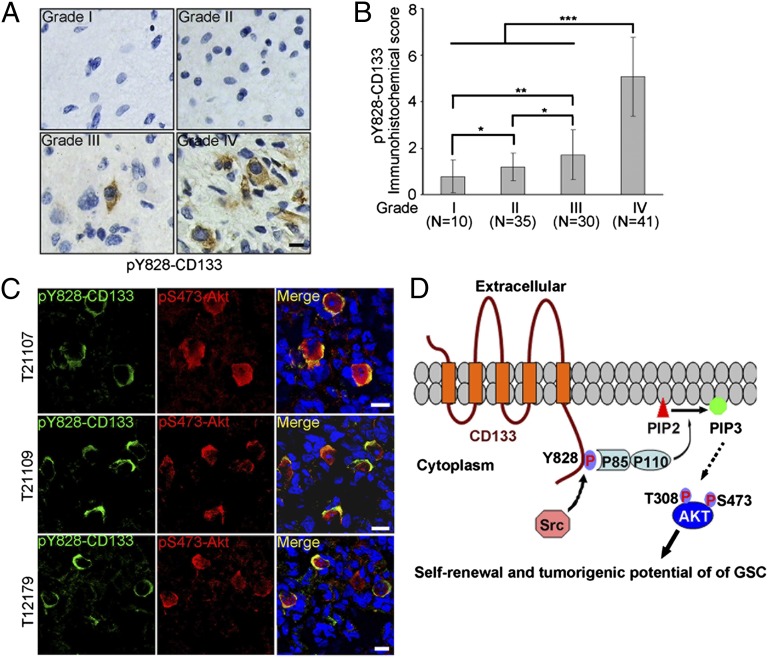
In Human Glioma, Y828 Phosphorylation Level of CD133 Is Correlated with Glioma Grade and Overlaps with Active Akt.
Src family kinases are activated in the vast majority of GBM (34, 35). We hypothesize that Y828 phosphorylation of CD133 could be elevated during malignant progression of human brain tumors. To test this, we performed immunohistochemical (IHC) staining on paraffin-embedded section from human glioma of different grades using the phospho-CD133 (Y828) antibody characterized previously. We first tested the specificity of this antibody in IHC staining. The antibody detected pY828 signals in a grade IV GBM. On adjacent sections, the signal was blocked by the immunizing phosphopeptide but not the unphosphorylated peptide (Fig. S6), indicating that the antibody specifically recognizes pY828-CD133 in tumor sections.
As shown in Fig. 5A, human grade I-II gliomas showed negative-to-lower levels of pY828-CD133 and grade III glioma showed a moderate level of pY828-CD133. In contrast, grade IV glioma displayed a high level of pY828-CD133. Statistical analysis for the level of pY828-CD133 in a panel of gliomas samples showed a significant correlation between pY828-CD133 levels and glioma grades (Fig. 5B).
Fig. 5.
Level of pY828-CD133 in human glioma specimens is correlated with tumor grade and overlaps with active Akt. (A and B) Expression of pY828-CD133 was examined by IHC staining in 116 gliomas samples of different grades. (A) Representative microphotographs of immunohistochemical staining of pY828-CD133 in glioma grade I-IV tissues. (B) The scores for quantitative staining of pY828-CD133 in the tissue sections were determined according to a total score (range, 0–8) that was obtained by combining the score of the percentage of positive cells and the score of the staining intensity. Values are mean ± SD. The level of Y828 phosphorylation of CD133 in glioma with different grade was analyzed (Student t test, two-tailed, *P < 0.05, **P < 0.01, ***P < 0.001). (Scale bar, 10 µm.) (C) Immunofluorescence staining of frozen sections from primary GBM samples reveals coexpression of pY828-CD133 and pS473-Akt. (Scale bar, 10 µm.) (D) Hypothetical model of CD133/PI3K/Akt signaling axis regulating glioma stem cell behaviors.
Considering our finding that CD133 activates the PI3K/Akt pathway depending on Y828 phosphorylation, we predicted a significant association between pY828-CD133 and pS473-AKT expression in GBM samples. To examine this possibility, double-immunofluorescence studies on frozen sections of human GBM were performed using pY828-CD133 and pS473-Akt antibodies. Most tumor cells that expressed pY828-CD133 obviously highly coexpressed pS473-Akt (Fig. 5C), further supporting the role of CD133 Y828 phosphorylation in the activation of Akt in human glioma.
Discussion
In this study, we present evidence that the phosphorylated Y828 residue in CD133 cytoplasmic tail binds to PI3K regulatory subunit p85, resulting in the activation of PI3K/Akt pathway, subsequently promoting the self-renewal and tumor formation of glioma stem cells (Fig. 5D). The identification of the CD133/PI3K/Akt signaling axis supposes that CD133 is a “functional” marker of glioma-initiating cells, providing theoretical support for the utility of CD133 in defining glioma stem cell.
The interaction between CD133 and PI3K regulatory unit p85 depends on CD133 Y828 phosphorylation. Although Y828 mutation abrogates the ability of CD133 to activate the PI3K/Akt pathway, we were somewhat surprised to observe that ectopic expression of Y828 mutant slightly rescued the inhibitory effect of CD133 knockdown on the self-renewal and tumorigenesis of glioma stem cells. These data suggest the presence of other Y828 phosphorylation–independent mechanisms for CD133 maintaining glioma stem cell behaviors. This phenomenon might be explained partly by the potential role of CD133 in plasma membrane organization. CD133 binds to plasma membrane cholesterol and concentrates selectively in plasma membrane protrusions (36–38), suggesting that CD133 might be important in maintaining an appropriate lipid composition within the plasma membrane. Treatment with the cholesterol-sequestering drug methyl-β-cyclodextrin attenuates the self-renewal of ES and hematopoietic stem cells (39, 40). Thus, the potential role of CD133 association with cholesterol-based lipid microdomains in stem cell self-renewal deserves particular attention; however, deletion of CD133 C-terminal cytoplasmic domain does not impair its concentration in microvillus (41). It seems, therefore, that CD133 might contribute to stem cell self-renewal partly through organizing plasma membrane topology in a C-terminal domain–independent manner.
CD133 is phosphorylated on cytoplasmic tyrosine-828 by Src family kinases (30). The Src family kinases are nonreceptor tyrosine kinases that are activated rapidly on the engagement of multiple cell surface receptors and modulate several cellular processes, including proliferation, adhesion, migration, and differentiation (42, 43). Analyses of tumor biopsies have indicated that Src family kinases are consistently found to activate in glioblastoma (34, 35). Given that CD133 expression has not been consistently shown to be associated with glioma grade (44), the association of Src family kinases activation with glioma progression may partly explain why the level of phospho-Y828-CD133 is associated with glioma grade.
In summary, our results uncover CD133 as an upstream activator of PI3K and, most importantly, as a crucial trigger of self-renewal and tumorigenesis of glioma stem cells. These findings not only provide an improved understanding of the molecular mechanisms underlying tumorigenesis of glioma stem cells, but also suggest an additional target for therapeutic intervention.
Materials and Methods
Detailed methods are described in SI Materials and Methods.
Isolation of CD133+ and CD133− Cells.
CD133+ cells were isolated from primary surgical GBM biopsy specimens in accordance with protocols approved by the Fudan University Institutional Review Broads. CD133+ and CD133− cells were separated through magnetic cell sorting with a CD133 Cell Isolation Kit (Miltenyi Biotec) as previously described (6, 45).
Tumor Formation Assay.
Intracranial transplantation of tumor cells into 6- to 8-wk-old nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice was performed as previously described (6, 45), in accordance with a Fudan University Institutional Animal Care and Use Committee–approved protocol concurrent with national regulatory standards. Mice were maintained up to 180 d or until the development of neurologic signs that significantly inhibited their quality of life (e.g., ataxia, lethargy, seizures, inability to feed).
Assay for Monitoring PI3K Activity.
P85 was immunoprecipitated from equal protein cell lysates with rabbit polyclonal anti-p85 antibody (Millipore), and immunoprecipitated PI3K activity was evaluated with a PI3 kinase ELISA kit (Echelon Biosciences, K-1000s) according to the manufacturer’s protocol and previous descriptions (46, 47).
Statistical Analysis.
In general, significance was tested by unpaired two-tailed Student t test using GraphPad InStat 5.0 software. For in vivo studies, Kaplan-Meier curves and log-rank analysis were performed. The significance of pY828-CD133 expression differences in glioma samples was determined by using Student t test (two-tailed). P values < 0.05 were considered statistically significant. Results are expressed as the mean ± SD.
Supplementary Material
Acknowledgments
We thank Prof. Yalin Huang (Fudan University) for technical assistance on confocal analysis, data analyst Qi Li for technical assistance on data analysis, Jing Qian and Guoping Zhang (Fudan University) for technical assistance on FCS analysis, Prof. Maurizio Pesce for providing plasmid of pRRLSIN.cPPT.hPGK-GFP.WPRE, and Dr. Bernd Giebel and Dr. Denis Corbeil for providing CD133 plasmid. This work was supported by Program for National Natural Scientific Foundation of China Grants 81172059, 30930025, 81272435, and 81001125; National Basic Research Program of China Grant 2013CB910500; New Century Excellent Talents in University Grant NCET-08-0128; Shanghai Municipal Health Bureau Grant XYQ2011047; Shandong Science and Technology Key Program Project Grant 2010GSF10203; and Shanghai Leading Academic Discipline Project Grant B110.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1217002110/-/DCSupplemental.
References
- 1.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 2.Dirks PB. Brain tumour stem cells: The undercurrents of human brain cancer and their relationship to neural stem cells. Philos Trans R Soc Lond B Biol Sci. 2008;363(1489):139–152. doi: 10.1098/rstb.2006.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin AH, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90(12):5002–5012. [PubMed] [Google Scholar]
- 4.Miraglia S, et al. A novel five-transmembrane hematopoietic stem cell antigen: Isolation, characterization, and molecular cloning. Blood. 1997;90(12):5013–5021. [PubMed] [Google Scholar]
- 5.Hermann PC, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1(3):313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 8.Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 9.Ma S, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132(7):2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 10.Beier D, et al. CD133(+) and CD133(-) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67(9):4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
- 11.Joo KM, et al. Clinical and biological implications of CD133-positive and CD133-negative cells in glioblastomas. Lab Invest. 2008;88(8):808–815. doi: 10.1038/labinvest.2008.57. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, et al. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int J Cancer. 2008;122(4):761–768. doi: 10.1002/ijc.23130. [DOI] [PubMed] [Google Scholar]
- 13.Bidlingmaier S, Zhu X, Liu B. The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. J Mol Med (Berl) 2008;86(9):1025–1032. doi: 10.1007/s00109-008-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27(12):1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 15.Sartelet H, et al. CD133 expression is associated with poor outcome in neuroblastoma via chemoresistance mediated by the AKT pathway. Histopathology. 2012;60(7):1144–1155. doi: 10.1111/j.1365-2559.2012.04191.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang YK, et al. Activation of Akt and MAPK pathways enhances the tumorigenicity of CD133+ primary colon cancer cells. Carcinogenesis. 2010;31(8):1376–1380. doi: 10.1093/carcin/bgq120. [DOI] [PubMed] [Google Scholar]
- 17.Holland EC, et al. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25(1):55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 18.Uhrbom L, et al. Ink4a-Arf loss cooperates with KRas activation in astrocytes and neural progenitors to generate glioblastomas of various morphologies depending on activated Akt. Cancer Res. 2002;62(19):5551–5558. [PubMed] [Google Scholar]
- 19.Hambardzumyan D, Squatrito M, Carbajal E, Holland EC. Glioma formation, cancer stem cells, and akt signaling. Stem Cell Rev. 2008;4(3):203–210. doi: 10.1007/s12015-008-9021-5. [DOI] [PubMed] [Google Scholar]
- 20.Hambardzumyan D, et al. PI3K pathway regulates survival of cancer stem cells residing in the perivascular niche following radiation in medulloblastoma in vivo. Genes Dev. 2008;22(4):436–448. doi: 10.1101/gad.1627008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eyler CE, et al. Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells. 2008;26(12):3027–3036. doi: 10.1634/stemcells.2007-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 24.Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons DW, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaiswal BS, et al. Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell. 2009;16(6):463–474. doi: 10.1016/j.ccr.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stokoe D, et al. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277(5325):567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 28.Alessi DR, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7(4):261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 29.Hirsch E, Costa C, Ciraolo E. Phosphoinositide 3-kinases as a common platform for multi-hormone signaling. J Endocrinol. 2007;194(2):243–256. doi: 10.1677/JOE-07-0097. [DOI] [PubMed] [Google Scholar]
- 30.Boivin D, et al. The stem cell marker CD133 (prominin-1) is phosphorylated on cytoplasmic tyrosine-828 and tyrosine-852 by Src and Fyn tyrosine kinases. Biochemistry. 2009;48(18):3998–4007. doi: 10.1021/bi900159d. [DOI] [PubMed] [Google Scholar]
- 31.Songyang Z, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 32.Kontos CD, et al. Tyrosine 1101 of Tie2 is the major site of association of p85 and is required for activation of phosphatidylinositol 3-kinase and Akt. Mol Cell Biol. 1998;18(7):4131–4140. doi: 10.1128/mcb.18.7.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ponzetto C, et al. A novel recognition motif for phosphatidylinositol 3-kinase binding mediates its association with the hepatocyte growth factor/scatter factor receptor. Mol Cell Biol. 1993;13(8):4600–4608. doi: 10.1128/mcb.13.8.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du J, et al. Bead-based profiling of tyrosine kinase phosphorylation identifies SRC as a potential target for glioblastoma therapy. Nat Biotechnol. 2009;27(1):77–83. doi: 10.1038/nbt.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu KV, et al. Fyn and SRC are effectors of oncogenic epidermal growth factor receptor signaling in glioblastoma patients. Cancer Res. 2009;69(17):6889–6898. doi: 10.1158/0008-5472.CAN-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Röper K, Corbeil D, Huttner WB. Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat Cell Biol. 2000;2(9):582–592. doi: 10.1038/35023524. [DOI] [PubMed] [Google Scholar]
- 37.Corbeil D, et al. The human AC133 hematopoietic stem cell antigen is also expressed in epithelial cells and targeted to plasma membrane protrusions. J Biol Chem. 2000;275(8):5512–5520. doi: 10.1074/jbc.275.8.5512. [DOI] [PubMed] [Google Scholar]
- 38.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci USA. 1997;94(23):12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee MY, Ryu JM, Lee SH, Park JH, Han HJ. Lipid rafts play an important role for maintenance of embryonic stem cell self-renewal. J Lipid Res. 2010;51(8):2082–2089. doi: 10.1194/jlr.M001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamazaki S, et al. Cytokine signals modulated via lipid rafts mimic niche signals and induce hibernation in hematopoietic stem cells. EMBO J. 2006;25(15):3515–3523. doi: 10.1038/sj.emboj.7601236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corbeil D, Röper K, Hannah MJ, Hellwig A, Huttner WB. Selective localization of the polytopic membrane protein prominin in microvilli of epithelial cells—a combination of apical sorting and retention in plasma membrane protrusions. J Cell Sci. 1999;112(Pt 7):1023–1033. doi: 10.1242/jcs.112.7.1023. [DOI] [PubMed] [Google Scholar]
- 42.Summy JM, Gallick GE. Treatment for advanced tumors: SRC reclaims center stage. Clin Cancer Res. 2006;12(5):1398–1401. doi: 10.1158/1078-0432.CCR-05-2692. [DOI] [PubMed] [Google Scholar]
- 43.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4(6):470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 44.Christensen K, Schrøder HD, Kristensen BW. CD133 identifies perivascular niches in grade II-IV astrocytomas. J Neurooncol. 2008;90(2):157–170. doi: 10.1007/s11060-008-9648-8. [DOI] [PubMed] [Google Scholar]
- 45.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 46.Popa-Nita O, et al. Crystal-induced neutrophil activation. IX. Syk-dependent activation of class Ia phosphatidylinositol 3-kinase. J Leukoc Biol. 2007;82(3):763–773. doi: 10.1189/jlb.0307174. [DOI] [PubMed] [Google Scholar]
- 47.Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009;29(8):2011–2022. doi: 10.1128/MCB.01472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.