Abstract
Understanding how and why plant communities vary across space has long been a goal of ecology, yet parsing the relative importance of different influences has remained a challenge. Species-specific models are not generalizable, whereas broad plant functional type models lack important detail. Here we consider plant trait patterns at the local scale and ask whether plant chemical traits are more closely linked to environmental gradients or to changes in species composition. We used the visible-to-shortwave infrared (VSWIR) spectrometer of the Carnegie Airborne Observatory to develop maps of four plant chemical traits—leaf nitrogen per mass, leaf carbon per mass, leaf water concentration, and canopy water content—across a diverse Mediterranean-type ecosystem (Jasper Ridge Biological Preserve, CA). For all four traits, plant community alone was the strongest predictor of trait variation (explaining 46–61% of the heterogeneity), whereas environmental gradients accounted for just one fourth of the variation in the traits. This result emphasizes the critical role that species composition plays in mediating nutrient and carbon cycling within and among different communities. Environmental filtering and limits to similarity can act strongly, simultaneously, in a spatially heterogeneous environment, but the local-scale environmental gradients alone cannot account for the variation across this landscape.
Keywords: community ecology, hyperspectral remote sensing, imaging spectroscopy, plant functional traits
Two key goals in ecology are to explain the distribution and diversity of plants in their environment and to understand how nutrients and energy flow through systems. These goals have direct relevance to the world around us: in the setting of climate change, land use intensification, species invasions, and other pressures, understanding how ecosystems function is central to the development of a predictive science of the biosphere (1). Nevertheless, most studies of plant diversity have focused on taxonomic, not functional, diversity (2), whereas ecosystem process models rely on broad plant functional types (PFTs) and assume little variation within, for example, all temperate broadleaved deciduous trees (3). It has been suggested that plant functional traits could serve as an intermediary between species focused models and those relying on PFTs (2). Readily measurable plant traits, like leaf nitrogen content or specific leaf area, map onto the “leaf economics spectrum” (4, 5), which reflects life history strategies and tradeoffs, like leaf life span and nutrient investment. The variation in plant traits across an ecosystem can therefore be treated as a proxy for variation in functional diversity and can help constrain ecosystem process models (6).
A major focus of plant trait studies has been evaluating the importance of environmental filtering to trait distributions. When we measure these relationships, however, we typically find significant but weak correlations between environmental gradients and traits, both globally (e.g., ref. 5) and locally (e.g., ref. 7, 8). Local- or landscape-scale studies are often confounded by their reliance on species-level averages, whereas meta-analyses typically convolve disparate data sets and may ignore within-site environmental heterogeneity (9). Additionally, environmental filtering is only one of a suite of processes operating in community assembly, and other processes like dispersal limitation, disturbances, competition, predation, and drift may be of equal or greater importance (10, 11). These dynamic processes are often thought to be less critical to models operating across large regions and at coarse resolutions. Recent studies argue, however, that disturbances like fire (12), logging (13), and herbivory (14) play important roles even at global scales. The prevalence of these diffuse disturbances, combined with the well-recognized role of plant composition in ecosystem processes (15), suggests the need for assessing the sources of trait variation across large scales while still resolving the contributions of individual organisms to that variation.
Advances in airborne remote sensing can address some of these challenges by facilitating the mapping of plant chemical traits over large areas at fine resolutions (16, 17). These maps allow us to compare the relative importance of environment vs. community composition and to consider traits at the individual, rather than at the species, level. In addition, geostatistical approaches can help parse the relative importance of ecosystem assembly processes (18). Although many of the functional traits typically measured in the field, like leaf longevity, sclerophylly, and wood density, cannot be measured from the air, plant chemical traits express or contribute to many life history strategies. Here, we consider the landscape-scale distribution of four fundamental plant chemical traits: leaf nitrogen per mass (Nmass; expressed as a percentage), leaf carbon per mass (Cmass; expressed as a percentage), leaf water concentration (WL, expressed as a fraction of wet weight), and canopy water content (WC; in millimeters). These and other chemical traits are critical to rates of nutrient cycling and to plant physiology (19). Variation in Nmass expresses differences in photosynthetic capacity (20) and has been used to constrain terrestrial carbon budgets (21), Cmass is a measure of the construction and maintenance cost of leaves and is related to sclerophylly and leaf longevity (22, 23), WL reflects drought stress and sclerophylly (24), and WC is closely tied to total leaf volume and leaf area index (25).
By using a new airborne hyperspectral sensor that is part of the Carnegie Airborne Observatory (CAO) Airborne Taxonomic Mapping System (AToMS) (17), we developed maps of these four foliar traits across a Mediterranean-type landscape (Jasper Ridge Biological Preserve, San Mateo County, CA) that includes a broad range of vegetation types (7, 26). We then used these trait maps to ask how much of the local-scale variation can be linked to environmental gradients and known land-use history, versus how much of the variation can be explained by changes in plant community alone. We also considered the distributions of trait values within mapped plant communities to test whether individual communities are convergent or divergent in trait space. If trends in plant traits are truly generalizable, and environmental filtering and niche specialization are the dominant processes driving diversity at the landscape scale, we would expect to find close relationships between environmental gradients and key traits, as well as reduced variance within communities (27). If variation in traits is more strongly controlled by species identity, we would expect a closer relationship between traits and shifts in plant community and perhaps evidence of limits to similarity (28) within communities. A large amount of residual variation would suggest that other factors like past disturbances, dispersal limitation, and/or neutral processes (11) may be acting to generate functional diversity within and between communities.
Results and Discussion
Chemical Diversity.
Our field-derived leaf chemical trait values spanned a large proportion of the reported global range (5), with field-measured Nmass varying from 0.35% to 2.99%, Cmass ranging from 39.8% to 52.2%, and WL ranging from 0.16 to 0.86 (Table S1). As expected, there was a clear correspondence between plant traits and life history strategies. The highest Nmass values were in deciduous trees and the lowest values were among grasses, forbs, and evergreen chaparral species. This pattern is consistent with other studies (29–31), and is likely linked to species differences and to environmental variation. Deciduous trees at Jasper Ridge tend to grow in mesic or wet environments, whereas evergreen shrub species tend to be found in more xeric conditions, where light is abundant but water is limiting. Evergreen plants are able to assimilate carbon for more of the year, however, and so they may accrue similar amounts of carbon annually, meaning that lower rates of photosynthesis and lower Nmass do not necessarily indicate a competitive disadvantage (30). The low Nmass values in grasses and forbs (from 0.52% to 0.74%) are likely a result of grass senescence, given that the sampling and flight took place in early June; a previous study of Nmass in Jasper Ridge grasslands in April found higher concentrations and no difference between serpentine and sandstone substrates (32).
Although the highest Cmass value was in a deciduous willow (Salix lasiolepis), the other high carbon plants at Jasper Ridge were all evergreen chaparral species. The lowest values were again in the grasses and forbs, followed by the “upland” deciduous species, including Juglans californica and Aesculus californica. This gradient in Cmass values has been linked to the cost of individual leaves (22)—when leaves have long life spans, they are worth more investment to the plant than when they only last one season. The high Cmass and Nmass values measured in the Salix species may reflect the fact that these species are isolated to wet, nutrient-rich alluvial areas, where they can afford to make expensive leaves every spring to better compete for light.
The highest WL values were in the aquatic species Typha latifolia and the wetland grasses, but also in three deciduous species. Low WL values were found, again, among grasses, followed by in evergreen woody plants including Quercus agrifolia and Adenostoma fasciculatum. Although we expected WL in June to be a reflection of early drought stress and access to perennial water sources, there was a rare heavy rain event on June 4, 2011 (immediately preceding data collection), so patterns in leaf water content are likely more similar to what would be expected earlier in the year (30).
These field data confirm our understanding of the PFTs found at Jasper Ridge and their similar life history strategies and phenologies. Grasses were low in C, N, and water content, whereas forbs (primarily thistles) were low in C and N but high in water content. Upland deciduous trees were high in N but low in C, whereas evergreen chaparral shrubs were high in C and low in N and water. Evergreen broadleaved trees were average in C and N but low in leaf water. There are several canopy-dominant, woody, N-fixing species at Jasper Ridge. Although these species were not exceptionally high in Nmass (except Cercocarpus betuloides), their trait patterns differed from those of other plants with similar life history strategies (Table S1).
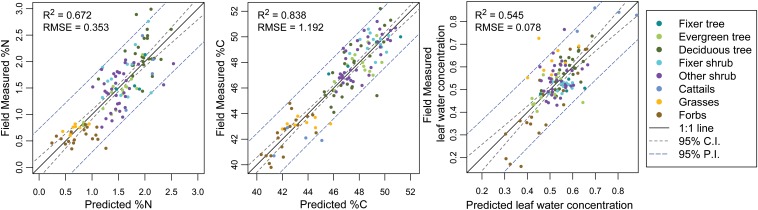
To extend these field data across the landscape, we used partial least squares (PLS) regression and spectral data from the CAO AToMS flight (Fig. 1 and SI Materials and Methods).
Fig. 1.
Observed vs. predicted scatterplots for the three field-calibrated variables. Numbers of latent variables in the PLS regression were selected based on the minimum rms error of prediction by using leave-one-out cross-validation. For N percentage, there are seven latent variables; for C percentage, there are 11; and for leaf water, there are five. PI, prediction interval.
Environmental Filtering and Community Controls on Plant Traits.
To understand the relative importance of environmental gradients, known land use history, plant community, and spatial autocorrelation to the four chemical traits, we used a combined ordinary least squares and simultaneous autoregressive approach on the entire data set as well as subsets of the predictors [ordinary least squares (OLS)–simultaneous autoregressive modeling (SAR); Materials and Methods].
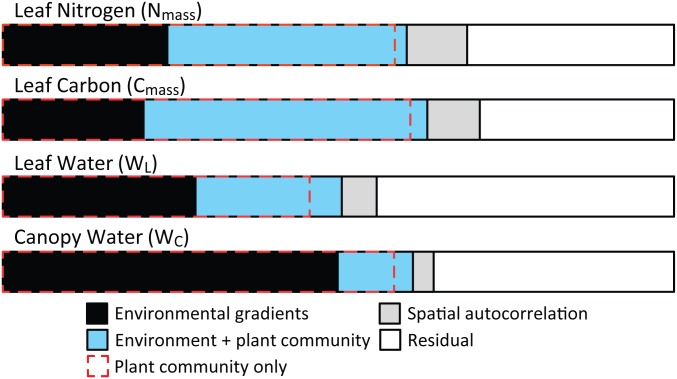
A total of 69% of the variation in Nmass was explained by environmental variation, land use, vegetation community, and spatial autocorrelation (Table 1 and Fig. 2). Spatial autocorrelation explained 9% of this total, with an optimal neighborhood distance of 60 m and a weighting of 1 divided by distance. When considered independent of vegetation community, environmental gradients accounted for only 25% of the variation in Nmass, and most was related to distance from perennial water and land use history (Fig. S1 and Table S2). In contrast, plant community alone accounted for almost all variation independent of spatial autocorrelation: 58.4%. Similar to the field data, Salix-dominated communities and riparian areas were strong predictors of Nmass. The community dominated by the common deciduous shrub Toxicodendron diversilobum was also a strong positive predictor of Nmass, as was the mixed oak (Quercus spp.) class. That environmental gradients did not explain a large portion of the variation in Nmass, despite the link between deciduousness and the riparian areas, highlights a key conclusion of this work: although relationships between the environment and life history strategies may be very strong under certain conditions, they are typically suppressed (or weak) at the scale of entire landscapes.
Table 1.
Coefficients of determination (R2), neighborhood distances, and Moran's I for the different SAR models
| Model | Leaf N (Nmass) | Leaf C (Cmass) | Leaf water (WL) | Canopy water (WC) |
| All environmental gradients*,† | 0.245 | 0.213 | 0.287 | 0.499 |
| Topography† | 0.149 | 0.137 | 0.049 | 0.095 |
| Geology† | 0.002 | 0.002 | 0.001 | 0.002 |
| Land use history† | 0.037 | 0.038 | 0.124 | 0.049 |
| Geographic trends† | 0.084 | 0.076 | 0.155 | 0.185 |
| Plant community*,† | 0.584 | 0.607 | 0.457 | 0.583 |
| Environment plus plant community*,† | 0.602 | 0.633 | 0.505 | 0.611 |
| SAR model (all)*,† | 0.692 | 0.711 | 0.557 | 0.642 |
| Spatial autocorrelation† | 0.090 | 0.078 | 0.052 | 0.031 |
| Neighborhood distance (m) | 60 | 70 | 60 | 50 |
| Moran's I (observed) | 0.001 | 0.002 | 0.006 | 0.005 |
| Moran's I P value | 0.0001 | <0.0001 | <0.0001 | <0.0001 |
Models that also contain the plane view angle variables.
All R2 values are statistically significant (P < 0.001).
Fig. 2.
Stacked bars of R2 values for the four traits and the different models (bar lengths correspond to numbers in Table 1).
Variation in Cmass was similarly well explained by the whole model (71% of variation explained; Table 1 and Fig. 2), with 8% of that variation attributed to spatial autocorrelation and a slightly longer neighborhood distance of 70 m. Again, most of the variation was explained by the vegetation community classification (61%), whereas less than one fourth of the variation could be attributed to environmental gradients. The evergreen chaparral class (dominated by A. fasciculatum) was the strongest predictor of high Cmass values, followed by the Salix class, similar to the field data (Table S3). Cmass was the least well explained by environmental gradients, but the second strongest overall model, suggesting that, of the four traits measured here, Cmass may be the most strongly linked to species composition. Our field data (Table S1) as well as other studies (e.g., refs. 33–35) support this result.
The WL model explained the least landscape-scale variation (56%; Table 1 and Fig. 2), with a small amount of spatial autocorrelation (5.2%). The optimal neighborhood distance was again 60 m. The model had a large geographic trend component (Table 1), linked to distance from water and to the similarity between the east and west ends of the preserve (scaled X-direction squared; X2). Although generally a smaller fraction of the variation in WL was linked to plant communities, some of the strongest positive predictors of WL were the wetland species and the deciduous shrub class, similar to the field data. The difficulty in explaining this trait suggests that it may be much more variable with weather, or that it is tied to environmental gradients that are difficult to measure, like access to ground water or depth to parent material. As WL was the least well predicted of the PLS regression models, it is also possible that this low amount of variation explained is a result of uncertainty in our trait map.
WC was more closely tied to plant communities, but this link was not as strong as in the other models. Environmental gradients alone explained nearly half of the variation in WC, whereas plant communities explained 58% (Table 1 and Fig. 2). Given that WC is closely tied to leaf area index (25), this result suggests that, although leaf traits are more controlled by species differences, whole canopy traits like WC that are related more to growth form are controlled strongly by the environment. Distance to water and summer insolation were the strongest environmental predictors, showing that water limitation and heat stress play large roles in determining the distribution of PFTs (i.e., variation in leaf area index) across the landscape.
Considering the individual model coefficient values for the four models together (a subset is shown in Fig. S1; all coefficients are listed in Table S2), there are clearly dominant drivers among the traits. As we expected based on the distribution of field data (Fig. 1) and the summer flight time, grassland vegetation types had lower than otherwise expected values for all four traits. Q. agrifolia–Arbutus menziesii–Umbellularia californica forest had higher values in all but WL in the vegetation-only model and higher values for Cmass and WC in the full model. The A. fasciculatum-dominated vegetation led to notably higher values in Cmass and WL. Of the environmental variables, distance to water was the most consistently important predictor, followed by the X2 geographic trend, meaning that the east and west ends of the site are similar to each other. Although the west end of the preserve is a deciduous Salix-dominated wetland, the east end is dominated by T. diversilobum and A. californica, two deciduous species that are not abundant as canopy-dominant species in many other places in the ecosystem. Land use parcels were also important predictors, especially parcels 1 and 2 (i.e., the eastern edge of the preserve), parcels 5 and 8 (i.e., the west side), and parcel 7 (i.e., the slope on the east side of Searsville Lake). Although we do not know what differences in management took place in these areas, their significance in the models suggests a lasting impact of earlier land use. In all four trait models, the scales of spatial autocorrelation were quite short (50–70 m; Table 1). It is impossible to attribute these distances to a specific driver, but we speculate that these short distances could be driven by patchy recovery patterns in areas that historically may have been burned or grazed.
This study demonstrates that variation in Nmass and Cmass is much more closely tied to changes in vegetation community than to mapped environmental gradients, thereby emphasizing the critical role that species composition plays in mediating nutrient and carbon cycling within and among the different communities. Environmental gradients explained only 25% and 21% of the variation in those traits, respectively, whereas vegetation type explained 58% and 61%. WL was slightly more closely linked to environmental gradients (29%), but noticeably less linked to vegetation type (46%). In contrast, the WC model explained much of the variation in this trait (64%), but a larger fraction could be attributed to environmental gradients (50%). Unlike the other three traits, WC is a whole canopy trait, suggesting that, although leaf traits may be more strongly controlled by community differences, plant form is still linked in large part to environmental gradients.
Similar to our previous work at Jasper Ridge (26), land parcel and presumably land use history played a significant role in predicting spatial distributions of most of the canopy chemical traits, despite the coarseness of the previous ownership map. Differences between parcels explained 4% of the variation in Nmass and Cmass, 12% of the variation in WL, and 5% of the variation in WC (Table 1). The small but significant role of land use in this system, which has been protected for much of the past century, highlights the importance of incorporating information about anthropogenic influences into studies of landscape heterogeneity.
In all, these results show that, at Jasper Ridge, there is not a perfect relationship between plant traits and the environment, and that some traits are more closely tied to environment than others. Although there are clear patterns in the vegetation structure reflected in WC, like dense forest on the north- and east-facing slopes and chaparral on the southwest-facing slope, there are also many exceptions. There are small patches of chaparral on the north-facing slope and stands of trees facing south. The causes of these variations could be unmeasured environmental gradients, like variations in edaphic properties, but they could also be the result of past fires, land use decisions, or fluctuation-dependent processes (9) like a random dispersal event paired with good conditions for plant establishment.
Within-Community Heterogeneity.
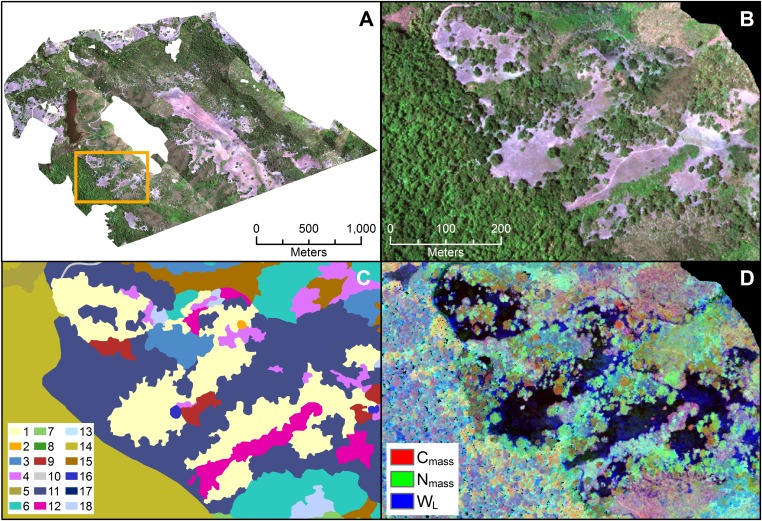
Visual comparison of the plant community map to the CAO AToMS imagery and the trait maps (Fig. 3) reveals the chemical diversity between and within community classes. Although the plant community map explained a large fraction of the variation in these traits (46–61%), the chemical maps show much within-class heterogeneity. Community classification maps are necessarily generalizations, and some of this variation may result from PFT variation (e.g., a deciduous tree in a largely evergreen class), but the large amount of unexplained variation in the trait models suggests that much of this heterogeneity could be caused by variation between and within species, especially within particular plant communities. To test this, we used the field-collected trait data and compared the coefficients of variation (CVs) between species, and then between groups of species corresponding to plant communities (Tables S1 and S3). Although our sample sizes were relatively small, these data can provide some insight into intra- vs. interspecific heterogeneity.
Fig. 3.
(A) True color image of Jasper Ridge shows areas that were masked in white. Yellow box highlights region shown in B–D. (B) Zoomed-in true color map. (C) Vegetation map (legend numbers correspond to communities listed in Table S4; “10” represents developed area). (D) Red/green/blue composite image shows three of the plant traits.
On average, for Nmass, variation within species was equal to 71% of the variation within communities, whereas only 50% and 51% of the variation in Cmass and WL was within individual species. Variation in Nmass is less well constrained within species in this system than are variation in Cmass and WL; however, there are subtle differences between the traits. In Nmass, most of the community-level CVs are higher than nearly all the species-level CVs; however, the coastal scrub and Salix forest communities have very low CVs, suggesting that plants in these communities are experiencing very strong habitat filtering (only a narrow range of trait values can persist in these areas) or are not strongly N-limited and so are not in competition for this nutrient. For Cmass, there is a much larger difference between intraspecific and community-level CVs, suggesting that this trait is controlled at the species level. The only community-level CV that is notably lower than many of the single-species values is A. fasciculatum chaparral, a community made up primarily of evergreen, drought-adapted shrubs, in which we would expect to find “expensive” leaves across species (22). In leaf water fraction, the pattern is less clear, with generally higher within-community CVs but a number of single species with high variation, again highlighting the variability in this trait within and among species.
To consider the variation in traits across the entire landscape, we used the remotely sensed chemical trait maps to evaluate trait distributions within and between plant communities. High variance in a trait within a plant community indicates that the community is capable of supporting a wide range of PFTs, whereas a narrow range suggests that the community is restricted to a subset of types as a result of environmental filtering (36). When we consider the variance of the traits within plant communities (Table S4) at Jasper Ridge, we see that many of the communities have much more narrow trait distributions than the preserve as a whole, but a few have very wide distributions. As expected, the highest variance across all traits is in the savanna-type communities, including the grasslands (which include some shrubs) and the Baccharis pilularis community (a mixture of grass and shrubs). The lowest variances are in the chaparral communities, suggesting strong environmental filtering or examples of arrested succession (37). Although it is tempting to attribute these patterns to environmental gradients alone, and to attribute the lack of fit between the environmental model and the trait maps to missing environmental gradients, Jasper Ridge’s highly variable Mediterranean-type climate suggests that fluctuation-dependent processes (9) may also be also playing a critical role.
Within plant communities, a flat (i.e., platykurtic) distribution of trait values would indicate that competition between individuals is important in the community (7, 28). Here we find, however, generally high kurtosis values, or sharply peaked (i.e., leptokurtic) distributions, within many of the communities (Table S4). Most notably, the three serpentine communities (serpentine grassland, scrubland, and Quercus durata) and the aquatic plant community are consistently the most leptokurtic, whereas annual grassland kurtosis values are low or negative across all four traits. These distributions suggest that environmental filtering is acting very strongly in extreme environments (i.e., serpentine substrate and standing water), and within-community competition is a less important driver in these areas. Overall, far more of the traits and communities were leptokurtic, indicating that limits to similarity are likely not a strong driver of diversity here.
Conclusions and Implications.
When considered independent of species composition, we found that known environmental gradients play a small but important role in determining the distribution of plant functional traits at Jasper Ridge. Within plant communities, plant traits tend to converge, but this can vary from community to community across short distances. This result suggests that in situ studies designed to test the relative importance of ecosystem assembly processes may be highly context-dependent, especially if they focus on a single plant community. Despite its recent land use history, Jasper Ridge has been used in studies of plant distributions for nearly a century (7, 38). Although more remote sites may have less obvious land use histories, recent archaeological evidence (e.g., ref. 39) and the expanding reach of modern humans suggests that no study of current landscape patterns should ignore anthropological impacts.
Here, airborne hyperspectral remote sensing enabled the study of plant functional traits at the landscape scale independent of species composition. These data, coupled with existing site information, allowed us to evaluate relationships among chemical composition, life history strategies, species differences, and environmental filtering, and to identify meaningful differences between these ways of interpreting landscapes. The fine spatial scale of the remotely sensed data and the detailed chemical information in each observation allowed for a close link to be drawn between spectral data and individual plants. Similar approaches could be used to evaluate patterns in slightly coarser data sets like those from the Airborne Visible and IR Imaging Spectrometer (40) (12–20 m). The proposed Hyperspectral IR Imager satellite mission (41) will offer many new opportunities for hyperspectral analysis around the world. Although its coarser resolution (60 m) would miss some of the fine-scale heterogeneity of focus in this study, its repeat, global coverage will add unique dimensions to the current suite of globally mapped plant datasets and will likely yield insights that will revolutionize our understanding of the terrestrial biosphere (42).
Materials and Methods
Site Description.
Jasper Ridge is a 481-ha site along the northeastern foothills of the Santa Cruz Mountains in northern California. The Mediterranean-type climate includes hot, dry summers and average annual precipitation of 652 mm (average from 1975 to 2004), falling mainly between November and April. Elevation ranges from 66 to 207 m above sea level. The site is dominated by a long, flat-topped ridge running northwest to southeast, a reservoir built in 1892, and a near-perennial creek. Despite its small size, Jasper Ridge contains a wide range of California plant species and functional types (7, 26) visible from the air. Needle-leaved evergreens were not considered in this study, and areas where they dominated the canopy were masked from the CAO imagery.
Data Collection.
On June 7, 2011, CAO AToMS was flown over Jasper Ridge, collecting spectral radiance data at a ground-sampling distance (i.e., spatial resolution) of 1.12 m. Each measurement or pixel contains spectral data in 5-nm increments (full width at half maximum) spanning the 380- to 2,510-nm wavelength range (17). Collection details and field and laboratory methods are described in SI Materials and Methods.
Partial Least-Squares Regression.
Continuum-removed spectral data coaligning with the leaf collections were extracted from the imagery. We then used PLS regression to model the three field-measured traits (43) by using the pls package in R (44, 45). The number of latent vectors selected in each PLS analysis was determined by using one-out cross-validation, thereby selecting the number of vectors yielding the lowest rms error of prediction (46). The final PLS equations were then applied across the entire image mosaic (SI Materials and Methods and Fig. S2). A small number of pixels with predicted trait values or normalized difference vegetation index (NDVI) outside of the observed ranges were removed for further analysis (0.12 < NDVI; 0.0 > Nmass > 3.0; 39.0 > C > 53.0; 0.2 > LW > 0.9).
In some ecosystems, studies have shown the NDVI to be correlated with various plant properties (e.g., ref. 47). We tested for such relationships or redundancies between chemical traits by plotting the traits against each other and NDVI and calculating coefficients of determination. We also computed the principal components of the four chemical traits by using a correlation matrix (48) to test whether most of the signal from the imagery was in a single axis of variation or spread among multiple axes of variability (Figs. S3 and S4).
For further modeling, results from the AToMS derived trait maps were then overlaid with the Light Detection and Ranging data and other data, and mean values were calculated for 5 × 5 m pixels.
Environmental Gradients and Land Use History.
To quantify the relationships between the four traits and exogenous factors we gathered as many spatially explicit sources of data as possible. The 51 environmental parameters used in this study can be divided into topographically derived variables, substrate, geographic trends, and land-use history. Maps of a subset of these variables and a description of how they were generated can be found in ref. 26, and details about how the gradients were calculated are provided in SI Materials and Methods.
Mapping Plant Types.
The vegetation community map used in this study was developed by mapping and botany experts at Jasper Ridge and based on field observations and visual interpretation of aerial imagery. Vegetation types follow the methods and types of the California Natural Diversity Database Vegetation Classification and Mapping Program (www.dfg.ca.gov/biogeodata/vegcamp). This map represents the best available site-wide information about species distributions at Jasper Ridge, and it was developed independently of any CAO or AToMS data. For this study, we used information at the “alliance” level, which is the most detailed level of the map. Each polygon has an assigned dominant or set of codominant species, although each community is actually made up of a mixture of many species. We converted each of the 23 vegetation types into a binary layer.
OLS–SAR Modeling.
To quantify the relative importance of the aforementioned environmental and vegetation patterns on the four plant traits while also accounting for spatial autocorrelation, first we used OLS regression to reduce the number of possible predictor variables; we then used SAR. The SAR approach has been successful when used on simulated ecological data, in comparison with other methods (49, 50). We selected optimal neighborhood sizes and weightings to minimize spatial autocorrelation of the error term. This simple, tractable approach, combined with partial regression analysis (18), allows the separation of known environmental gradients, mapped disturbances, unexplained spatial patterns, and remaining uncertainty (51) (SI Materials and Methods and Table S2).
Within-Community Trait Distributions.
For each plant community, we calculated the four traits’ mean, variance, variance as a percentage of the total, and kurtosis (52) from the remotely sensed trait data. To compare intra to interspecific variation we used the field-measured trait values for woody plants and compared the single-species means and CV to community-level CVs. As many of the communities used in the aforementioned analysis were narrowed to one or two species, we used a more general community classification (Table S3) and considered only woody plants and woody plant communities containing three or more measured species.
Supplementary Material
Acknowledgments
We thank D. Knapp and A. Balaji for image processing and data management; T. Hebert and the Jasper Ridge herbarium volunteers for developing and sharing the Jasper Ridge vegetation map; Z. Adham, L. Carranza, M. Colgan, L. Giles, A. Kindel, G. Maltais-Landry, R. Martin, and D. Turner for help in the field and in the laboratory; and P. Vitousek, D. Chadwick, and two anonymous reviewers for helpful comments on the manuscript. This work was supported by a Stanford Interdisciplinary Graduate Fellowship (to K.M.D.) and the Stanford Energy and Environment Affiliates Program (to G.P.A.). The National Center for Atmospheric Research is sponsored by the National Science Foundation. The Carnegie Airborne Observatory is made possible by the Gordon and Betty Moore Foundation, the Grantham Foundation for the Protection of the Environment, Avatar Alliance Foundation, W. M. Keck Foundation, Margaret A. Cargill Foundation, Mary Anne Nyburg Baker and G. Leonard Baker, Jr., and William R. Hearst III.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1215513110/-/DCSupplemental.
References
- 1.Moorcroft PR. How close are we to a predictive science of the biosphere? Trends Ecol Evol. 2006;21(7):400–407. doi: 10.1016/j.tree.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 2.McGill BJ, Enquist BJ, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends Ecol Evol. 2006;21(4):178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Bonan GB, Levis S, Kergoat L, Oleson KW. Landscapes as patches of plant funtional types: An integrating concept for climate and ecosystem models. Global Biogeochem Cycles. 2002;16(2):1–18. [Google Scholar]
- 4.Reich PB, Walters MB, Ellsworth DS. From tropics to tundra: Global convergence in plant functioning. Proc Natl Acad Sci USA. 1997;94(25):13730–13734. doi: 10.1073/pnas.94.25.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wright IJ, et al. The worldwide leaf economics spectrum. Nature. 2004;428(6985):821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 6.Wang YP, et al. Correlations among leaf traits provide a significant constraint on the estimate of global gross primary production. Geophys Res Lett. 2012;39:L19405. [Google Scholar]
- 7.Cornwell WK, Ackerly DD. Community assembly and shifts in plant trait distributions across an environmental gradient in coastal California. Ecol Monogr. 2009;79:109–126. [Google Scholar]
- 8.Lebrija-Trejos E, Pérez-García EA, Meave JA, Bongers F, Poorter L. Functional traits and environmental filtering drive community assembly in a species-rich tropical system. Ecology. 2010;91(2):386–398. doi: 10.1890/08-1449.1. [DOI] [PubMed] [Google Scholar]
- 9.Chesson P. Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst. 2000;31:343–366. [Google Scholar]
- 10.Clements FE. Plant Succession: An Analysis of the Development of Vegetation. Washington, DC: Carnegie Institution; 1916. Publication 242. [Google Scholar]
- 11.Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton: Princeton Univ Press; 2001. [DOI] [PubMed] [Google Scholar]
- 12.Staver AC, Archibald S, Levin SA. The global extent and determinants of savanna and forest as alternative biome states. Science. 2011;334(6053):230–232. doi: 10.1126/science.1210465. [DOI] [PubMed] [Google Scholar]
- 13.Asner GP, Loarie SR, Heyder U. Combined effects of climate and land-use change on the future of humid tropical forests. Conservation Letters. 2010;3:395–403. [Google Scholar]
- 14.Doughty CE, Wolf A, Field CB. Biophysical feedbacks between the Pleistocene megafauna extinction and climate: The first human-induced global warming? Geophys Res Lett. 2010;37(15):L15703. [Google Scholar]
- 15.Chapin FS, 3rd, et al. Consequences of changing biodiversity. Nature. 2000;405(6783):234–242. doi: 10.1038/35012241. [DOI] [PubMed] [Google Scholar]
- 16.Ustin SL, Gamon JA. Remote sensing of plant functional types. New Phytol. 2010;186(4):795–816. doi: 10.1111/j.1469-8137.2010.03284.x. [DOI] [PubMed] [Google Scholar]
- 17.Asner GP, et al. Carnegie Airborne Observatory-2: Increasing science data dimensionality via high-fidelity multi-sensor fusion. Remote Sens Environ. 2012;124:454–465. [Google Scholar]
- 18.Legendre P, Legendre L. Numerical Ecology. 2nd Ed. Amsterdam: Elsevier; 1998. [Google Scholar]
- 19.Chapin FS, III, Bloom AJ, Field CB, Waring RH. Plant responses to multiple environmental factors. Bioscience. 1987;37(1):49–57. [Google Scholar]
- 20.Field CB, Merino J, Mooney HA. Compromises between water-use efficiency and nitrogen-use efficiency in five species of California evergreens. Oecologia. 1983;60:384–389. doi: 10.1007/BF00376856. [DOI] [PubMed] [Google Scholar]
- 21.Smith M-L, et al. Direct estimation of aboveground forest productivity through hyperspectral remote sensing of canopy chemistry. Ecol Appl. 2002;12(5):1286–1302. [Google Scholar]
- 22.Bloom AJ, Chapin FS, III, Mooney HA. Resource limitation in plants – an economic analogy. Annu Rev Ecol Syst. 1985;16:363–392. [Google Scholar]
- 23.Merino J, Field CB, Mooney HA. Construction and maintenance costs of Mediterranean-climate evergreen and deciduous leaves; I. Growth and CO2 exchange analysis. Oecologia. 1982;53:208–213. doi: 10.1007/BF00545665. [DOI] [PubMed] [Google Scholar]
- 24.Sobrado MA. Aspects of tissue water relations and seasonal changes of leaf water potential components of evergreen and deciduous species coexisting in tropical dry forests. Oecologia. 1986;68:413–416. doi: 10.1007/BF01036748. [DOI] [PubMed] [Google Scholar]
- 25.Asner GP, Nepstad D, Cardinot G, Ray D. Drought stress and carbon uptake in an Amazon forest measured with spaceborne imaging spectroscopy. Proc Natl Acad Sci USA. 2004;101(16):6039–6044. doi: 10.1073/pnas.0400168101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahlin KM, Asner GP, Field CB. Environmental filtering and land-use history drive patterns in biomass accumulation in a Mediterranean-type landscape. Ecol Appl. 2012;22(1):104–118. doi: 10.1890/11-1401.1. [DOI] [PubMed] [Google Scholar]
- 27.Sterck F, Markesteijn L, Schieving F, Poorter L. Functional traits determine trade-offs and niches in a tropical forest community. Proc Natl Acad Sci USA. 2011;108(51):20627–20632. doi: 10.1073/pnas.1106950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacArthur R, Levins R. The limiting similarity, convergence, and divergence of coexisting species. Am Nat. 1967;101:377–385. [Google Scholar]
- 29.Field CB, Mooney HA. The photosynthesis-nitrogen relationship in wild plants. In: Givnish T, editor. On the Economy of Plant Form and Function. Cambridge, UK: Cambridge Univ Press; 1986. pp. 25–55. [Google Scholar]
- 30.Hollinger DY. Leaf and simulated whole-canopy photosynthesis in two co-occurring tree species. Ecology. 1992;73(1):1–14. [Google Scholar]
- 31.Reich PB, Kloeppel BD, Ellsworth DS, Walters MB. Different photosynthesis-nitrogen relations in deciduous hardwood and evergreen coniferous tree species. Oecologia. 1995;104:24–30. doi: 10.1007/BF00365558. [DOI] [PubMed] [Google Scholar]
- 32.Hungate BA, Chapin FS, III, Zhong H, Holland EA, Field CB. Stimulation of grassland nitrogen cycling under carbon dioxide enrichment. Oecologia. 1997;109:149–153. doi: 10.1007/s004420050069. [DOI] [PubMed] [Google Scholar]
- 33.Kazakou E, Vile D, Shipley B, Gallet C, Garnier E. Co-variations in litter decomposition, leaf traits, and plant growth in species from a Mediterranean old-field succession. Funct Ecol. 2006;20(1):21–30. [Google Scholar]
- 34.Fyllas NM, et al. Basin-wide variations in foliar properties of Amazonian forest: Phylogeny, soils, and climate. Biogeosciences. 2009;6(11):2677–2708. [Google Scholar]
- 35.Asner GP, Martin RE. Canopy phylogenetic, chemical and spectral assembly in a lowland Amazonian forest. New Phytol. 2011;189(4):999–1012. doi: 10.1111/j.1469-8137.2010.03549.x. [DOI] [PubMed] [Google Scholar]
- 36.Keddy PA. Assembly and response rules: 2 goals for predictive community ecology. J Veg Sci. 1992;3:157–164. [Google Scholar]
- 37.Putz FE, Canham CD. Mechanisms of arrested succession in shrublands: Root and shoot competition between shrubs and tree seedlings. For Ecol Manage. 1992;49:267–275. [Google Scholar]
- 38.Cooper WS. The Broad-Sclerophyll Vegetation of California: An Ecological Study of the Chaparral And its Related Communities. Washington, DC: Carnegie Institution; 1922. Publication 319. [Google Scholar]
- 39.McKey D, et al. Pre-Columbian agricultural landscapes, ecosystem engineers, and self-organized patchiness in Amazonia. Proc Natl Acad Sci USA. 2010;107(17):7823–7828. doi: 10.1073/pnas.0908925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green RO, et al. Imaging spectroscopy and the airborne/visible infrared imaging spectrometer (AVIRIS) Remote Sens Environ. 1998;65(3):227–248. [Google Scholar]
- 41.Green RO, Asner GP, Ungar SG, Knox RG. NASA mission to measure global plant physiology and functional types. Proceedings of the 2008 IEEE Aerospace Conference (IEEE, NY) 2008 pp. 1–7. [Google Scholar]
- 42.Schimel D, Asner GP, Moorcroft P. Observing changing ecological diversity in the Anthropocene. Front Ecol Environ. 2013 doi: 10.1890/120111. [DOI] [Google Scholar]
- 43.Martin ME, Plourde LC, Ollinger SV, Smith M-L, McNeil BE. A generalizable method for remote sensing of canopy nitrogen across a wide range of forest ecosystems. Remote Sens Environ. 2008;112(9):3511–3519. [Google Scholar]
- 44.Mevick B-H, Wehrens R. The pls package: Principal component and partial least squares regression in R. J Stat Softw. 2007;18(2):1–24. [Google Scholar]
- 45.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 46.Feilhauer H, Asner GP, Martin RE, Schmidtlein S. Brigthness-normalized partial least squares regression for hyperspectral data. J Quant Spectrosc Ra. 2010;111:1947–1957. [Google Scholar]
- 47.Gamon JA. Relationships between NDVI, canopy structure, and photosynthesis in three Californian vegetation types. Ecol Appl. 1995;5(1):28–41. [Google Scholar]
- 48.Venables WN, Ripley BD. Modern Applied Statistics with S. New York: Springer; 2002. [Google Scholar]
- 49.Beale CM, Lennon JJ, Yearsley JM, Brewer MJ, Elston DA. Regression analysis of spatial data. Ecol Lett. 2010;13(2):246–264. doi: 10.1111/j.1461-0248.2009.01422.x. [DOI] [PubMed] [Google Scholar]
- 50.Dormann CF. Effects of incorporating spatial autocorrelation into the analysis of species distribution data. Glob Ecol Biogeogr. 2007;16:129–138. [Google Scholar]
- 51.Lichstein JW, Simons TR, Shriner SA, Franzreb KE. Spatial autocorrelation and autoregressive models in ecology. Ecol Monogr. 2002;72:445–463. [Google Scholar]
- 52.Dimitriadou E, Hornik K, Leisch F, Meyer D, Weingessel W. 2011. Misc functions of the Department of Statistics (e1071), R package version 1.6 (Technische Universität Wien, Vienna)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.