Abstract
Cellular differentiation leading to formation of the bradyzoite tissue cyst stage is the underlying cause of chronic toxoplasmosis. Consequently, mechanisms responsible for controlling development in the Toxoplasma intermediate life cycle have long been sought. Here, we identified 15 Toxoplasma mRNAs induced in early bradyzoite development that encode proteins with apicomplexan AP2 (ApiAP2) DNA binding domains. Of these 15 mRNAs, the AP2IX-9 mRNA demonstrated the largest expression increase during alkaline-induced differentiation. At the protein level, we found that AP2IX-9 was restricted to the early bradyzoite nucleus and is repressed in tachyzoites and in mature bradyzoites from 30-d infected animals. Conditional overexpression of AP2IX-9 significantly reduced tissue cyst formation and conferred alkaline pH-resistant growth, whereas disruption of the AP2IX-9 gene increased tissue cyst formation, indicating AP2IX-9 operates as a repressor of bradyzoite development. Consistent with a role as a repressor, AP2IX-9 specifically inhibited the expression of bradyzoite mRNAs, including the canonical bradyzoite marker, bradyzoite antigen 1 (BAG1). Using protein binding microarrays, we established the AP2 domain of AP2IX-9 binds a CAGTGT DNA sequence motif and is capable of binding cis-regulatory elements controlling the BAG1 and bradyzoite-specific nucleoside triphosphatase (B-NTPase) promoters. The effect of AP2IX-9 on BAG1 expression was direct because this factor inhibits expression of a firefly luciferase reporter under the control of the BAG1 promoter in vivo, and epitope-tagged AP2IX-9 can be immunoprecipitated with the BAG1 promoter in parasite chromatin. Altogether, these results indicate AP2IX-9 restricts Toxoplasma commitment to develop the mature bradyzoite tissue cyst.
Keywords: gene regulation, gene expression, Apicomplexa
The apicomplexan Toxoplasma gondii has an exceptional range of animals that may serve as host for its intermediate life cycle, whereas the definitive life cycle occurs in a feline host (1). Together, oocysts shed by cats into the environment and tissue cysts in food products contribute to rates of human exposure that are estimated at one in three by age 50 in the US (25% for >20 y of age) (2, 3) and nearly 100% by the end of childhood in other parts of the world (4). Toxoplasma infections are thought to be lifelong because of the development of the tissue cyst, which is effectively invisible to the immune system and clinically untreatable. The bradyzoite tissue cyst is an essential part of the life cycle of Toxoplasma, and recrudescence of the tissue cyst leading to chronic cycles of toxoplasmosis is a major cause of mortality in AIDS patients. Experimental data indicates Toxoplasma sporozoite or bradyzoite infections follow a similar developmental course through the tachyzoite stage that is characterized by changes in growth and stage-specific gene expression, which leads ultimately to the mature tissue cyst (5–7). Determining how gene expression is regulated in these critical developmental transitions will be important to unraveling the underlying mechanisms responsible for chronic Toxoplasma infections.
The mechanisms of gene-specific regulation in Apicomplexa have remained elusive. The expression profiles of large portions of the cell cycle transcriptome of Plasmodium falciparum and Toxoplasma asexual stages are progressive with little understanding yet as to how these serial patterns are controlled (8, 9). In Toxoplasma, conversion between developmental stages is also linked to significant changes in gene expression (5). Data mining of genome sequences has recently identified a family of plant-related AP2 domain (DNA binding) containing proteins in the Apicomplexa [apicomplexan AP2 (ApiAP2) proteins] (10). Based on genome sequence annotation, 68 of these factors are found in Toxoplasma and 27 in P. falciparum (8, 11, 12). In Toxoplasma, 24 ApiAP2 factors are cell cycle regulated in tachyzoites (8), whereas 18 are expressed in a cascading fashion during the P. falciparum intraerythrocytic cycle (12). Relatively few ApiAP2 proteins are characterized; however, stage-specific gene activation (13–16) as well as chromatin biology and genome maintenance (17) are functions identified so far. Like their plant counterparts, ApiAP2 factors can bind promoter elements of distinct coregulated gene clusters (12) and, therefore, it is likely these proteins will be major regulators of apicomplexan transcription.
Here, we report the characterization and functional validation of an ApiAP2 transcription factor in Toxoplasma. AP2IX-9 is a nuclear-restricted protein expressed transiently during tachyzoite to bradyzoite development. AP2IX-9 specifically binds cis-regulatory elements (CRE) functionally mapped to bradyzoite promoters (18) including the classic bradyzoite marker, small heat shock protein bradyzoite antigen 1 (BAG1) (19). Using gene disruption and conditional expression, we demonstrate AP2IX-9 is the first Apicomplexa transcriptional repressor that operates to regulate developmental gene expression. By preventing bradyzoite gene expression and promoting parasite growth, AP2IX-9 regulates the balance between parasite expansion and forming end-stage bradyzoites required for transmission to the definitive host.
Results
Toxoplasma ApiAP2 Factors Are Induced During Early Bradyzoite Differentiation.
Of the 68 ApiAP2-encoding genes predicted in the Toxoplasma genome (11), there is evidence for expression by microarrays for 44 of these genes (>40th percentile) in the tachyzoite (constitutive and stage specific) (8). To identify ApiAP2 genes regulated during early bradyzoite differentiation in Toxoplasma, we queried developmental transcriptome data representing the three genetic lineages (type I-GT1, type II-ME49, and type III-CTG) that dominate parasite populations of North America and Europe (18). From these microarray data, we classified 15 ApiAP2 mRNAs as increased (9 down-regulated) during in vitro bradyzoite induction in at least one strain (heatmap; SI Appendix, Fig. S1A). This collection of developmentally regulated ApiAP2 genes are predicted to encode a diverse group of proteins ranging in size from 286 to 3,256 amino acids containing one to three ApiAP2 domains (protein maps; SI Appendix, Fig. S1A). Interestingly, more than half of the ApiAP2 genes showing regulated mRNA expression (up and down) in the bradyzoite (14 of 24 total) also had cyclical mRNA profiles in the tachyzoite cell cycle.
One of the largest expression changes of any ApiAP2 mRNA was AP2IX-9, which was consistently increased during early bradyzoite induction in all three Toxoplasma lineages. To verify that the mRNA profile was predictive of AP2IX-9 protein expression, we endogenously tagged the AP2IX-9 locus in a type II Prugniaud strain carrying a disruption of the KU80 gene that enhances gene targeting (strain designated PruQ) (20). PruQ-AP2IX-9HA parasites induced to differentiate by pH 8.2 media were positive for tissue cyst wall formation by Dolichos biflorus agglutinin (DBA) lectin staining, and they coexpressed AP2IX-9HA protein exclusively in the nucleus (Fig. 1) similar to other ApiAP2 factors (8). The number of vacuoles positive for AP2IX-9HA reached 79% by 2 d after shift into pH 8.2 media (%HA positives), and this induction profile was confirmed by Western blot analysis and semiquantitative RT-PCR (SI Appendix, Fig. S1B). By contrast, microarray analysis (see ToxoDB for data summary) indicates AP2IX-9 mRNA is minimally expressed in tachyzoites and in mature bradyzoites in tissue cysts (isolated from 21 d infected mice) (21), indicating AP2IX-9 mRNA expression is transient. AP2IX-9HA protein largely followed the native mRNA profile with minimal expression in tachyzoites of the PruQ-AP2IX-9HA transgenic strain (Fig. 1 and SI Appendix, Fig. S1B), and AP2IX-9HA protein was not detected in mature bradyzoites from PruQ-AP2IX-9HA-infected mice (30 d after infection; SI Appendix, Fig. S1C). Parasites from in vivo tissue cysts are capable of expressing other ApiAP2 proteins, such as the cell cycle factor AP2VI-1HA (SI Appendix, Fig. S1C) (8). Altogether, these results indicate AP2IX-9 is only expressed during early bradyzoite differentiation and, consistent with this conclusion, we found AP2IX-9HA-positive vacuoles decreased after peaking at 2 d in alkaline-shifted cultures (Fig. 1). The decrease in AP2IX-9HA nuclear fluorescence was not associated with a loss of tissue cysts because the number of DBA-positive vacuoles continued to increase throughout the experiment (Fig. 1, % DBA); by 4 d after alkaline shift, nearly half the DBA-positive vacuoles were negative for AP2IX-9HA protein.
Fig. 1.
Toxoplasma AP2IX-9 is a nuclear factor induced by alkaline-pH stress. The AP2IX-9 factor was C-terminally tagged with 3xHA via homologous recombination in PruΔku80 strain (designated PruQ). PruQ-AP2IX-9HA parasites grown in human foreskin fibroblast (HFF) cell monolayers were costained with anti-HA antibody (red for AP2IX-9HA expression), biotin-labeled D. biflorus agglutinin (DBA; green for the presence of cyst wall), and DAPI (blue for genomic DNA). After exposure to pH 8.2 media, AP2IX-9HA protein was detected exclusively in the parasite nucleus (copositive for DAPI). Few vacuoles were positive for AP2IX-9HA expression in parasites grown under tachyzoite conditions (12% pH 7.0 media). HA- and DBA-positive vacuoles (%) for each condition are indicated on the right. Note that AP2IX-9HA staining declined after 2 d in both the number of vacuoles positive and the intensity of nuclear fluorescence.
AP2IX-9 Binds cis-Regulatory Elements of the Bradyzoite NTPase Promoter.
To determine the DNA binding motif recognized by AP2IX-9, we expressed the single ApiAP2 domain of AP2IX-9 as a glutathione-S-transferase (GST) fusion protein (GST-IX-9). Affinity-purified GST-IX-9 recombinant protein was assayed by using a protein binding microarray (12), and the top three motifs from this analysis revealed a conserved 6-bp core DNA sequence motif 5′-CAGTGT-3′/3′-GTCACA-5′ (enrichment scores >0.45; SI Appendix, Fig. S2A). GST-IX-9 binding to this motif was validated by using electrophoretic mobility shift assays (EMSAs) (SI Appendix, Fig. S2B; Dataset S1 for all sequence designs). Few functional CRE sequences are known in Toxoplasma; however, the promoter CRE controlling a bradyzoite NTPase isoform (B-NTPase) shares the GT/CA repeats (18) found in the AP2XI-9 binding motif. In EMSAs, GST-IX-9 protein bound the B-nucleoside triphosphatase (NTPase) CRE sequence in a manner consistent with the results of previous promoter mutagenesis (Fig. 2) (18). GST-IX-9 binding to the B-NTPase CRE was effectively competed by a mutant sequence (Fig. 2, mut 4) that retains 51% of promoter function, whereas binding to the B-NTPase probe was not effected by a mutant sequence that fully disrupts promoter function (Fig. 2, mut 3) (18). These results suggest AP2IX-9 could regulate the transcription of B-NTPase and, perhaps, other bradyzoite genes.
Fig. 2.
AP2IX-9 binds functional sequence elements of the bradyzoite NTPase (B-NTPase) promoter. Recombinant GST-AP2IX-9 protein binds DNA (arrow) carrying the B-NTPase CRE in a dose-dependent manner and was inhibited by competition with unlabeled mut 4 DNA but not with mut 3 DNA. The probe and competitive DNA sequences used here duplicate key features of the CRE sequence controlling the B-NTPase promoter (probe) and two mutant promoters (mut 3 and 4) tested for activity in luciferase reporter assays (in vivo*, promoter mutations are underlined; see ref. 18 for original luciferase results). Lane 1, biotin-labeled probe alone; lanes 2–4, 0.1, 0.3, or 0.9 μg of GST-IX-9; lanes 5–7, probe plus 0.3 μg of GST-IX-9 and 50-, 100-, or 300-fold unlabeled mutant 3 competitor DNA; lanes 8–10, probe plus 0.3 μg of GST-IX-9 and 50-, 100-, and 300-fold unlabeled mutant 4 DNA. Black triangles represent increasing amounts of protein or unlabeled competitor DNA fragments.
AP2IX-9 Regulates Bradyzoite Differentiation in Multiple Strains.
The induction of AP2IX-9 during bradyzoite differentiation suggests this factor may serve a role in promoting parasite development and, by extension, deleting this factor might block development. Several attempts to verify this hypothesis by gene knockout in type II Prugniaud parasites failed despite successful epitope tagging by recombination of the AP2IX-9 locus in this strain (Fig. 1). We also failed to disrupt the AP2IX-9 locus in low-passage isolates of GT1, ME49, and CTG (types I–III, respectively). By contrast, disruption of the AP2IX-9 gene was readily achieved in the PLK clone of type II ME49 (PLKΔap2IX-9) and in the laboratory strain RHΔhxgprtΔuprt developed previously as a model for bradyzoite differentiation by using CO2 starvation (22). These genetic results suggest that AP2IX-9 may be essential to developmentally competent Toxoplasma strains, whereas dispensable in parasites adapted to cell culture (Dataset S1, knockout frequencies). AP2IX-9 is inducible in the parental parasites of both RHΔhxgprtΔuprt and PLK strains, and these strains are able to form tissue cysts, although they have lost the capacity to complete the definitive life cycle in the feline host (23). Therefore, we examined in vitro tissue cyst formation of PLKΔap2IX-9 clones as well as RH strain clones carrying a AP2IX-9 knockout in addition to the deletion of the hypoxanthine-xanthine-guanine phosphoribosyl transferase (HXGPRT) and uracil phosphoribosyl transferase (UPRT) genes (triple knockout strain designated RHΔap2IX-9)(SI Appendix, Fig. S3). Contrary to expectations, under CO2-starvation culture conditions (22), RHΔap2IX-9 clones differentiated much better (49% tissue cysts; SI Appendix, Fig. S3, gray bars) than the double knockout parent (15% cysts in RHΔhxgprtΔuprt strain). Tissue cyst numbers were also increased in PLKΔap2IX-9 clones compared with the PLK parent strain following pH 8.2 induction (SI Appendix, Fig. S3, black bars).
The increased formation of tissue cysts in PLK and RHΔhxgprtΔuprt parasites lacking the AP2IX-9 protein suggested this factor might operate as a repressor rather than an activator of bradyzoite development. To test whether AP2IX-9 is a repressor, a conditional expression allele of AP2XI-9 was engineered in the developmentally competent Prugniaud strain (Pru) by creating a fusion of the FKBP destabilization domain (DD) (24) with the N terminus of AP2IX-9 (DDAP2IX-9; see design SI Appendix, Fig. S4A). In this ectopic expression design, DDAP2IX-9 transcription was controlled by the constitutive α-tubulin promoter and, as expected, steady-state levels of DDAP2IX-9 mRNA were independent of Shield-1 addition or the media pH (SI Appendix, Fig. S4A, mRNA). Importantly, regulation of the DDAP2IX-9 fusion protein was achieved by stabilization of the DD domain by using the small molecule Shield-1 (SI Appendix, Fig. S4 A and B, Western blot and immunofluorescence assay (IFA) analysis) (24). Endogenous posttranscriptional mechanisms also contributed to DDAP2IX-9 protein levels with the highest expression resulting from the combination of pH 8.2 media plus Shield-1, whereas in tachyzoites (pH 7.0 media), DDAP2IX-9 expression was lower because of native degradation that could be partially overcome with Shield-1 (SI Appendix, Fig. S4 A and B).
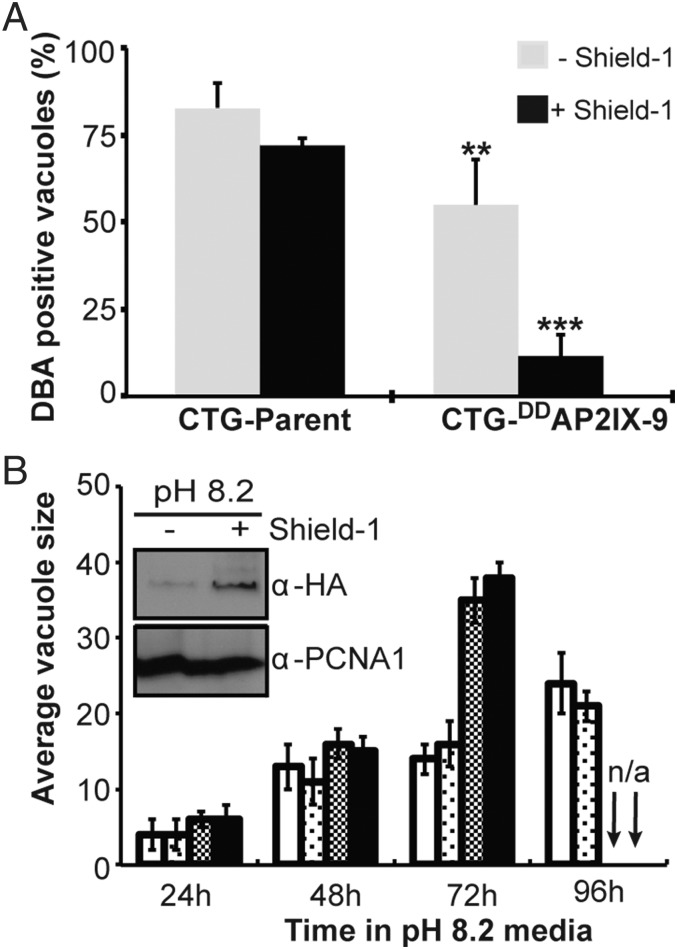
Using this conditional model, we explored how extended stabilization of DDAP2IX-9 under alkaline conditions influenced tissue cyst formation. In the Pru-parental strain, tissue cyst formation in pH 8.2 media was robust and independent of Shield-1 (>75% cysts formed by 72 h; SI Appendix, Fig. S4C). By contrast, Pru-DDAP2IX-9 parasites pretreated with Shield-1 (250 nM Shield-1, 6 h before alkaline shift) produced half the number of tissue cysts (32%) as parent controls (75%) (SI Appendix, Fig. S4C). The addition of Shield-1 at the time of pH 8.2 shift inhibited cyst numbers in Pru-DDAP2IX-9 parasites by 60%. To confirm AP2IX-9 inhibition of tissue cyst formation was not strain specific, we obtained a DDAP2IX-9-expressing clone in a low-passage type III CTG strain (Fig. 3). CTG-DDAP2IX-9 parasites shifted into pH 8.2 media plus Shield-1 were severely restricted in tissue cyst formation (12% tissue cysts; Fig. 3A), and this inhibition was substantially reversed if DDAP2IX-9 levels were lowered by omitting Shield-1. Thus, AP2IX-9 acts to repress bradyzoite differentiation in developmentally competent type II and III strains.
Fig. 3.
Overexpression of AP2IX-9 inhibits tissue cyst formation and promotes alkaline-resistant parasite growth. (A) Stimulation of tissue cyst formation in parental type III CTG and CTG-DDAP2IX-9 transgenic parasites was compared 72 h after shift into pH 8.2 media with or without 250 nM Shield-1. The addition of Shield-1 reduced tissue cyst counts ∼fivefold in CTG-DDAP2IX-9 parasites. Statistical significance was evaluated by using the unpaired t test between the parent and the transgenic strain (***P < 0.001; **P < 0.01; n = 5). (B) The effect of stabilizing DDAP2IX-9 with Shield-1 on parasite growth rates was determined by measuring the average vacuole size of CTG parent versus CTG-DDAP2IX-9 parasites. Parental CTG is shown in white bars; no Shield-1 = light dots, plus 250 nM Shield-1 = open bars. CTG-DDAP2IX-9 parasites; no Shield-1, gray bars; plus Shield-1, black bars. Because of host cell lysis the vacuole size for CTG-DDAP2IX-9 parasites was not determined at 96 h (n/a). (Inset) DDAP2IX-9 was induced to detectable levels by pH 8.2 media alone and to higher levels when Shield-1 was added. Anti-PCNA1 antibody staining was included as a loading control.
It is generally accepted that bradyzoite differentiation requires a slowing of parasite growth (25) and, ultimately, this rate change leads to growth arrest in the tissue cyst as mature bradyzoites with nearly 100% haploid DNA content (7). The tight association between bradyzoite development and the parasite cell cycle led us to explore the growth rate of parental CTG and CTG-DDAP2IX-9 parasites. Parental CTG showed the expected slowing of growth in pH 8.2 media, reaching an average vacuole size of ∼22 in 96 h (Fig. 3B), and these populations fully growth arrested before lysing the host cell monolayer. By contrast, CTG-DDAP2IX-9 parasites grew faster in pH 8.2 media than parental controls and, unlike parental CTG cultures, CTG-DDAP2IX-9–infected monolayers were lysed by 96 h (Fig. 3B, arrows). Resistance to the growth inhibition of alkaline media was independent of Shield-1 in CTG-DDAP2IX-9 parasites. The low levels of DDAP2IX-9 in pH 8.2 media (Fig. 3B, Inset) appeared to be sufficient to confer pH-resistant growth on CTG-DDAP2IX-9 parasites, and they also prevented a full reversal of the AP2IX-9 block to tissue cyst formation (Fig. 3A, pH 8.2 minus Shield-1 = 55% cysts compared with >75% cysts in the induced CTG parent). These results suggest AP2IX-9 may act to forestall the growth changes that ultimately lead to the dormant bradyzoite in mature tissue cysts (6, 7). In such a growth promoting role, deletion of AP2IX-9 could cause developmentally competent strains to readily leave the cell cycle, perhaps explaining why knockouts of APIX-9 were not recovered in low-passage strains. To support this idea, we easily disrupted the AP2IX-9 locus in CTG-DDAP2IX-9 parasites (17% of stable transgenics were knockouts), whereas repeated knockout attempts had failed in the developmentally competent CTG parent strain. The phenotype of CTGΔap2IX-9-[DDAP2IX-9] parasites with respect to tissue cyst formation was unchanged from the CTG-DDAP2IX-9 transgenic strains, indicating the DDAP2IX-9 fusion protein had fully replaced the function of the native protein. [Tissue cyst results: CTGΔap2IX-9-[DDAP2IX-9] in pH 8.2 minus Shield-1 produced 74% DBA-positive vacuoles, whereas DBA-positive vacuoles were counted in only 28% of cultures where Shield-1 was added.]
AP2IX-9 Represses Major Elements of the Bradyzoite Transcriptome.
To identify the genes potentially controlled by AP2IX-9, duplicate total RNA samples from parental CTG and CTG-DDAP2IX-9 parasites induced by pH 8.2 media plus Shield-1 were isolated and analyzed on a custom Toxoplasma Affymetrix GeneChip (26). A total of 116 mRNAs were altered (5% FDR) in this comparison (full gene lists in Dataset S1) with the largest changes resulting from the down-regulation of transcripts in CTG-DDAP2IX-9 parasites including many known bradyzoite mRNAs [e.g., BAG1, lactate dehydrogenase 2 (LDH2), enolase 1 (ENO1), and B-NTPase; Fig. 4A and Dataset S1]. The inhibition of BAG1 and LDH2 mRNA induction in CTG-DDAP2IX-9 parasites was verified by semiquantitative RT-PCR analysis (SI Appendix, Fig. S5A). Several lines of evidence indicated AP2IX-9 was directly responsible for bradyzoite transcriptional repression. First, the AP2IX-9 binding motif (CAGTGT) was enriched in the promoters of the genes showing the highest levels of AP2IX-9 repression (18 of 35 promoters have ≥3 CAGTGT repeats, Fig. 4A, red boxes). Particularly for BAG1, we found 14 repeats of a CAGTG motif (5/6 bp of the AP2IX-9 motif) in the 2-kbp 5′-flanking sequence upstream of the BAG1 translational start that includes the mapped BAG1 CRE (repeats and CRE sites are marked in Fig. 4D diagram). Given the multiple copies of the AP2IX-9 motif in the BAG1 promoter, it was not surprising this transcript was the most down-regulated in the microarray analysis (Fig. 4A). Second, the direct regulation of BAG1 by AP2IX-9 is supported by repression of luciferase activity in a transgenic clone where the BAG1 promoter controls the firefly luciferase gene (PruIC2; Fig. 4B) (18). As expected, the introduction and stabilization of DDAP2IX-9 expression by Shield-1 in the PruIC2 strain strongly inhibited luciferase induction under alkaline stress (Fig. 4B), and this repression was partially reversed by omitting Shield-1 (Fig. 4B). Finally, the direct interaction of AP2IX-9 with the BAG1 promoter was supported by GST-IX-9 binding to the BAG1 CRE (18) in EMSAs and chromatin immunoprecipitation followed by quantitative (qPCR) of DDAP2IX-9–bound genomic DNA showed specific enrichment across the BAG1 promoter (Fig. 4D and SI Appendix, Fig. S5B). Altogether, these results support the view that ApiAP2 factors may have greater flexibility in DNA binding than plant AP2 factors (9, 11). AP2IX-9 was able to bind the B-NTPase functional CRE (Fig. 2) through the GT/CA repeat feature found in the APIX-9 binding motif (CAGTGT), and with the BAG1 promoter CRE (Fig. 4), binding appears to be through the shared CAGT/ACTG feature.
Fig. 4.
AP2IX-9 regulates key features of the bradyzoite genetic program. (A) Nearly three dozen mRNAs (35) are significantly down-regulated (fold change shown) in CTG-DDAP2IX-9 parasites compared with CTG parental controls under pH 8.2 media plus Shield-1 induction conditions. BAG1 mRNA was the most repressed along with other known bradyzoite mRNAs: LDH2, #4 mRNA; ENO1, #5; SAG2D, #8; SAG4.2, #23; BK1, #24; B-NTPase, #29 (see Dataset S1 for full gene list). Colored boxes indicate the number of AP2IX-9 motifs present in each promoter (2 kbp upstream of ATG start). (B) DDAP2IX-9 expression represses induction of a firefly luciferase transgene controlled by the BAG1 promoter (−1,195-bp region) (18). Luciferase activity in whole-cell lysates from PruIC2 parent and PruIC2-DDAP2IX-9 parasites was determined at 72 h after shift to pH 8.2 media ±200 nM Shield-1 and compared with the appropriate pH 7.0 controls (data presented as the ratio pH 8.2/7.0, see SI Appendix, Methods for average light unit values). Gray bars, PruIC2-DDAP2IX-9 parasites; black bars, PruIC2 parent (***P < 0.001) (C) An EMSA was performed with 0, 70, 200, and 600 ng (lanes 1–4) purified GST-IX-9 and 20 fmol of 59-bp 5′-biotin-labeled probe containing the BAG1 CRE sequence (5′-TACTGG-3′/3′ATGACC-5′) (18). A large complex (arrow) above the two probe-associated bands was competed with an unlabeled probe (300×, lane 5). See Dataset S1 for all oligonucleotide sequences. (D) DDAP2IX-9 occupies the BAG1 promoter in parasite chromatin. Specific binding was observed in all six target regions (1–6) tiled across the BAG1 promoter (−950 bp flanking the ATG indicated by arrow in diagram) with the central three regions showing the highest enrichment (see SI Appendix, Fig. S5 for full experimental details). Ten repeat motifs matching 5 of 6 bases in the AP2IX-9 DNA binding motif are indicated by black triangles above the diagram, whereas the BAG1 promoter CRE previously mapped by luciferase assay lies within region 4 and is marked by a triangle below the diagram (18).
Discussion
In this study, we show a member of the large Toxoplasma ApiAP2 protein family (AP2IX-9) regulates parasite development. Disruption of the AP2IX-9 gene promotes formation of the Toxoplasma tissue cyst, whereas overexpression of this factor inhibits this developmental pathway along with major features of the bradyzoite transcriptome. We demonstrate AP2IX-9 is a nuclear protein induced in response to alkaline stress in the early bradyzoite, whereas it is minimally expressed in tachyzoites and end-stage bradyzoites in murine brain tissue cysts. AP2IX-9 binds DNA in a sequence-specific manner, and the AP2IX-9 binding motif is repeated in many bradyzoite promoters where direct binding of this factor to the BAG1 promoter in chromatin was established. ApiAP2 factors have roles in activating developmental gene expression (13–16), and there is evidence an ApiAP2 factor may assist the silencing of var gene expression in P. falciparum (17). The AP2IX-9 factor is the first ApiAP2 transcriptional repressor to be characterized in the Apicomplexa, and this mechanism begins to explain how the early steps in tachyzoite to bradyzoite development are regulated in Toxoplasma. The extent of transcriptional repressors among the ApiAP2 family is unknown; however, this type of mechanism is commonly associated with related AP2 factors of plants. Multiple AP2 family members in plants are repressors of stage-specific gene regulation, especially in flowering (27) and stress responses (28).
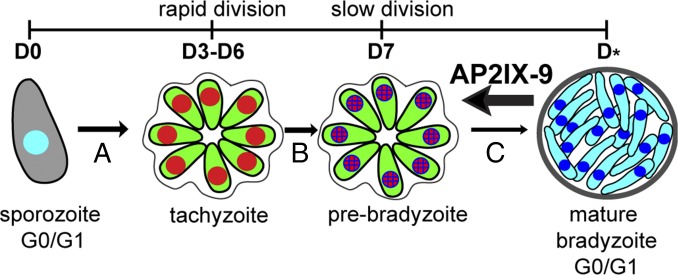
In its natural life cycle, Toxoplasma is a heteroxenous parasite. Its sexual reproductive cycle is exclusive in the felid host with a second clonal reproductive phase that is possible in virtually any endothermic animal. The traditional developmental course widely assumed to unfold in the intermediate host involves three sequential stage switches (e.g., sporozoite to tachyzoite to bradyzoite), although this scheme is likely oversimplified (see model in Fig. 5). The developmental and cell cycle transitions of the intermediate life cycle are accessible in host cells infected with sporozoites or bradyzoites (6, 7), where these end stages first differentiate into the tachyzoite stage (<2 d) followed by limited replication of the emergent tachyzoite (Fig. 5, day 3–6). Spontaneously switching to a longer division cycle (day 7) signals formation of a prebradyzoite, which is primed to develop into the end-stage bradyzoite (G1/G0 state) (6, 7). Studies in animals reinforce these basic outlines (29–31), which established the minimal timing of tissue cyst formation by using a sensitive cat bioassay (reviewed in ref. 32). In metazoans, semiconservative chromosome replication is postulated to provide the driving force behind development that results in altered epigenetic states and changes in gene expression (33, 34). As a framework, this model appears to fit our understanding of Toxoplasma development. It is well known that tissue cyst formation requires replication (25), enrichment for S and mitotic parasites characterizes the course of bradyzoite development (7), and peak expression of bradyzoite transcripts coincides with the S/M periods of the tachyzoite cell cycle (8). Thus, replicating forms in Toxoplasma can be envisioned as reprograming states required for changing one transmissible end stage (G0/G1 phase) into another to ensure survival in multiple hosts (e.g., sporozoite switch to bradyzoite). A precise relationship between chromosome replication and transcriptome remodeling is not established; however, a surprisingly short timeframe (1–4 d) produces infectious tissue cysts following bradyzoite inoculation of mice (32).
Fig. 5.
Model for control of bradyzoite development by the AP2IX-9 transcriptional repressor. Four major transitions are thought to occur in the intermediate life cycle: following infections with sporozoites (or bradyzoites) parasites rapidly differentiate into replicating tachyzoites (A), limited tachyzoite proliferation leads to a slower growing prebradyzoite state (B), and (C) the timing of commitment to the dormant bradyzoite in the mature tissue cyst is asynchronous (C and D*). AP2IX-9 balances the competing interests of parasite expansion and efficient transmission through direct inhibition of commitment to the mature bradyzoite. This model is based on studies here and published results with the VEG strain (6, 7) that has been maintained in the Jitender P. Dubey laboratory (US Department of Agriculture, Beltsville, MD) by exclusive mouse-cat serial passage to preserve the native Toxoplasma developmental pathway (32).
The prebradyzoite is an important, if also a poorly understood transition of the intermediate life cycle. Low passage strains readily form this developmental stage that is characterized by a spontaneous switch to slower growth, while retaining tachyzoite proteins on the parasite surface (7). Here, the activity of AP2IX-9 provides new insight into the molecular mechanisms regulating Toxoplasma development. We propose that the prebradyzoite stage produced by native parasite proliferation is held in the poised state by ApiAP2 factors like AP2IX-9 (see model in Fig. 5). As a transcriptional repressor, the role for AP2IX-9 could be to prevent premature commitment of the prebradyzoite in a less optimal host tissue or balance the parasite response to different immune system reactions. There is little evidence for quorum sensing mechanisms in apicomplexa infections, although if they occur, the AP2IX-9 mechanism could play a role here as well. Importantly, AP2IX-9 expression may be the first marker for this specific developmental transition. In providing a transient adaptive response, the AP2IX-9 is similar to the AP2 factor, abscisic acid-insensitive 4 (ABI4) of plants that is induced by stress (high sugar) where it reduces critical gene expression to protect these sensitive plants during early stages of development (35). In later stages of seedling development, ABI4 does not appear to be required and is no longer induced by stress (36) as much as AP2IX-9 is dispensable for the mature bradyzoite. Dividing tachyzoites appear to actively degrade AP2IX-9, which could reflect a protective response, or, alternatively, stabilization of AP2IX-9 may be tied to mechanisms active only during the prebradyzoite developmental transition. The latter explanation has some support because conditional overexpression of AP2IX-9 in the Shield-1 model was tolerated by tachyzoites (Fig. 3 and SI Appendix, Fig. S4A), and induction of this protein is tightly associated with strain developmental competency. In Prugniaud and CTG strains, AP2IX-9 is inducible by stress, whereas in the developmentally resistant RH strain, AP2IX-9 does not accumulate under any pH condition although the encoded mRNA is inducible. Based on transcriptome profile, RH strain is trapped upstream of the prebradyzoite (equivalent to D3-6; Fig. 5) (37), and supporting this assignment, the chromatin signature of bradyzoite promoters in RH indicates they are transcriptionally inactive (18). Therefore, we interpret the difference in AP2IX-9 expression in these strains to indicate that when the AP2IX-9 repressor complex is bound to chromatin it is stabilized and, therefore, the native transient pattern of AP2IX-9 expression is strongly influenced by histone modification at specific promoters. The changing epigenetic landscape during bradyzoite differentiation may also explain the decline of AP2IX-9 as development progresses to the mature tissue cyst. Here, other ApiAP2 factors likely replace AP2IX-9 to activate the bradyzoite transcriptome such as the newly described bradyzoite activator AP2XI-4 that binds GT/CA DNA repeats (14). We suspect the principle of ApiAP2 posttranscriptional control orchestrated by dynamic chromatin states will apply to many other family members including those in the large group of cycling ApiAP2 factors that act in the tachyzoite cell cycle (8). In conclusion, evidence presented here indicates AP2IX-9 is a major regulator of the intermediate life cycle of Toxoplasma where it may help control the balance between parasite expansion and transmission and, through the prevention of premature commitment to the bradyzoite end-stage, could help steer tissue cyst formation to a favorable host cell environment.
Materials and Methods
Information about parental and transgenic strains; culture conditions; plasmid construction; and more detailed descriptions of the assays used for IFA, Western blot analysis, and EMSAs, and luciferase activity, can be found in SI Appendix. T. gondii genomic sequences were accessed via http://ToxoDB.org.
Development and Conditional Expression Models.
To induce bradyzoite differentiation in vitro, infected cultures were subjected to alkaline media (pH 8.2) for indicated time periods according to ref. 22 with media replaced every 24 h to maintain pH. Conditional expression of the AP2IX-9 protein fused to the DD domain (DD = FKBP 106 amino acid peptide plus 3×HA epitope tag is 16.2 kDa) used the small molecule Shield-1 at concentrations in media <500 nM (24), which had no adverse effect on parasite invasion or growth rate. A dose of 100–500 nM Shield-1 gave equivalent levels of the DDAP2IX-9 protein in pH 8.2 media supplemented with Shield-1.
Protein and RNA Microarray Analysis.
The AP2IX-9 DNA binding domain (amino acids 1142–1204) was cloned in pGEX4T3, expressed as a GST-fusion in BL21DE3 cells (Novagen), and purified by glutathione affinity column. Protein binding microarrays were performed as described (12). All RNA samples were collected as independent biological replicates. RNA quality was determined by using the Agilent Bioanalyzer 2100. Total parasite RNA was collected and prepared for hybridization on the ToxoGeneChip as described in ref. 8. Data were analyzed in Genespring GX software (version 11.5, Agilent) and in Bioconductor (38). Microarray data are available in the Gene Expression Omnibus repository under accession no. GSE16037.
Chromatin Immunoprecipitation and qPCR.
Parasites were inoculated at 3:1 multiplicity of infection in T175cm2 flasks, allowed to invade for 3 h, rinsed three times with HBSS to remove free floating parasites, and media was replaced with pH 8.2 media supplemented with 200 nM Shield-1 (Clontech). ChIP-qPCR was performed by published methods (39) with the modifications fully described in SI Appendix.
Supplementary Material
Acknowledgments
We thank Kami Kim for reviewing the manuscript and Sarah Abney for help with GST-IX-9 protein production. This work was supported by National Institutes of Health (NIH) Grants R01 AI077662 and R01 AI089885 (to M.W.W.), R01-AI076276 (to M.L.), R01 AI077502 (to W.J.S.), and R01 AI095094 (to L.M.W.). This work was also supported by multiinvestigator NIH Grant RC4 AI092801 (Kami Kim, Principal Investigator).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The microarray data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE36300).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300059110/-/DCSupplemental.
References
- 1.Dubey JP, Miller NL, Frenkel JK. Toxoplasma gondii life cycle in cats. J Am Vet Med Assoc. 1970;157(11):1767–1770. [PubMed] [Google Scholar]
- 2.Kruszon-Moran D, McQuillan GM. Seroprevalence of six infectious diseases among adults in the United States by race/ethnicity: Data from the third national health and nutrition examination survey, 1988--94. Adv Data. 2005;(352):1–9. [PubMed] [Google Scholar]
- 3.Jones JL, Kruszon-Moran D, Sanders-Lewis K, Wilson M. Toxoplasma gondii infection in the United States, 1999 2004, decline from the prior decade. Am J Trop Med Hyg. 2007;77(3):405–410. [PubMed] [Google Scholar]
- 4.Bahia-Oliveira LM, et al. Highly endemic, waterborne toxoplasmosis in north Rio de Janeiro state, Brazil. Emerg Infect Dis. 2003;9(1):55–62. doi: 10.3201/eid0901.020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adomako-Ankomah Y, Wier GM, Boyle JP. Beyond the genome: Recent advances in Toxoplasma gondii functional genomics. Parasite Immunol. 2012;34(2-3):80–89. doi: 10.1111/j.1365-3024.2011.01312.x. [DOI] [PubMed] [Google Scholar]
- 6.Jerome ME, Radke JR, Bohne W, Roos DS, White MW. Toxoplasma gondii bradyzoites form spontaneously during sporozoite-initiated development. Infect Immun. 1998;66(10):4838–4844. doi: 10.1128/iai.66.10.4838-4844.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radke JR, Guerini MN, Jerome M, White MW. A change in the premitotic period of the cell cycle is associated with bradyzoite differentiation in Toxoplasma gondii. Mol Biochem Parasitol. 2003;131(2):119–127. doi: 10.1016/s0166-6851(03)00198-1. [DOI] [PubMed] [Google Scholar]
- 8.Behnke MS, et al. Coordinated progression through two subtranscriptomes underlies the tachyzoite cycle of Toxoplasma gondii. PLoS ONE. 2010;5(8):e12354. doi: 10.1371/journal.pone.0012354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozdech Z, et al. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 2003;1(1):E5. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balaji S, Babu MM, Iyer LM, Aravind L. Discovery of the principal specific transcription factors of Apicomplexa and their implication for the evolution of the AP2-integrase DNA binding domains. Nucleic Acids Res. 2005;33(13):3994–4006. doi: 10.1093/nar/gki709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altschul SF, Wootton JC, Zaslavsky E, Yu YK. The construction and use of log-odds substitution scores for multiple sequence alignment. PLOS Comput Biol. 2010;6(7):e1000852. doi: 10.1371/journal.pcbi.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell TL, De Silva EK, Olszewski KL, Elemento O, Llinás M. Identification and genome-wide prediction of DNA binding specificities for the ApiAP2 family of regulators from the malaria parasite. PLoS Pathog. 2010;6(10):e1001165. doi: 10.1371/journal.ppat.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwanaga S, Kaneko I, Kato T, Yuda M. Identification of an AP2-family protein that is critical for malaria liver stage development. PLoS ONE. 2012;7(11):e47557. doi: 10.1371/journal.pone.0047557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker R, et al. The Toxoplasma nuclear factor TgAP2XI-4 controls bradyzoite gene expression and cyst formation. Mol Microbiol. 2013;87(3):641–655. doi: 10.1111/mmi.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuda M, Iwanaga S, Shigenobu S, Kato T, Kaneko I. Transcription factor AP2-Sp and its target genes in malarial sporozoites. Mol Microbiol. 2010;75(4):854–863. doi: 10.1111/j.1365-2958.2009.07005.x. [DOI] [PubMed] [Google Scholar]
- 16.Yuda M, et al. Identification of a transcription factor in the mosquito-invasive stage of malaria parasites. Mol Microbiol. 2009;71(6):1402–1414. doi: 10.1111/j.1365-2958.2009.06609.x. [DOI] [PubMed] [Google Scholar]
- 17.Flueck C, et al. A major role for the Plasmodium falciparum ApiAP2 protein PfSIP2 in chromosome end biology. PLoS Pathog. 2010;6(2):e1000784. doi: 10.1371/journal.ppat.1000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behnke MS, Radke JB, Smith AT, Sullivan WJ, Jr, White MW. The transcription of bradyzoite genes in Toxoplasma gondii is controlled by autonomous promoter elements. Mol Microbiol. 2008;68(6):1502–1518. doi: 10.1111/j.1365-2958.2008.06249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bohne W, Parmley SF, Yang S, Gross U. Bradyzoite-specific genes. Curr Top Microbiol Immunol. 1996;219:81–91. doi: 10.1007/978-3-642-51014-4_9. [DOI] [PubMed] [Google Scholar]
- 20.Fox BA, et al. type II Toxoplasma gondii KU80 knockout strains enable functional analysis of genes required for cyst development and latent infection. Eukaryot Cell. 2011;10(9):1193–1206. doi: 10.1128/EC.00297-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchholz KR, et al. Identification of tissue cyst wall components by transcriptome analysis of in vivo and in vitro Toxoplasma gondii bradyzoites. Eukaryot Cell. 2011;10(12):1637–1647. doi: 10.1128/EC.05182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohne W, Roos DS. Stage-specific expression of a selectable marker in Toxoplasma gondii permits selective inhibition of either tachyzoites or bradyzoites. Mol Biochem Parasitol. 1997;88(1-2):115–126. doi: 10.1016/s0166-6851(97)00087-x. [DOI] [PubMed] [Google Scholar]
- 23.Bohne W, et al. Targeted disruption of the bradyzoite-specific gene BAG1 does not prevent tissue cyst formation in Toxoplasma gondii. Mol Biochem Parasitol. 1998;92(2):291–301. doi: 10.1016/s0166-6851(97)00236-3. [DOI] [PubMed] [Google Scholar]
- 24.Herm-Götz A, et al. Rapid control of protein level in the apicomplexan Toxoplasma gondii. Nat Methods. 2007;4(12):1003–1005. doi: 10.1038/nmeth1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohne W, Heesemann J, Gross U. Reduced replication of Toxoplasma gondii is necessary for induction of bradyzoite-specific antigens: A possible role for nitric oxide in triggering stage conversion. Infect Immun. 1994;62(5):1761–1767. doi: 10.1128/iai.62.5.1761-1767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahl A, et al. A novel multifunctional oligonucleotide microarray for Toxoplasma gondii. BMC Genomics. 2010;11:603. doi: 10.1186/1471-2164-11-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yant L, Mathieu J, Schmid M. Just say no: Floral repressors help Arabidopsis bide the time. Curr Opin Plant Biol. 2009;12(5):580–586. doi: 10.1016/j.pbi.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Song CP, et al. Role of an Arabidopsis AP2/EREBP-type transcriptional repressor in abscisic acid and drought stress responses. Plant Cell. 2005;17(8):2384–2396. doi: 10.1105/tpc.105.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubey JP. Reshedding of Toxoplasma oocysts by chronically infected cats. Nature. 1976;262(5565):213–214. doi: 10.1038/262213a0. [DOI] [PubMed] [Google Scholar]
- 30.Dubey JP. Bradyzoite-induced murine toxoplasmosis: Stage conversion, pathogenesis, and tissue cyst formation in mice fed bradyzoites of different strains of Toxoplasma gondii. J Eukaryot Microbiol. 1997;44(6):592–602. doi: 10.1111/j.1550-7408.1997.tb05965.x. [DOI] [PubMed] [Google Scholar]
- 31.Dubey JP. Oocyst shedding by cats fed isolated bradyzoites and comparison of infectivity of bradyzoites of the VEG strain Toxoplasma gondii to cats and mice. J Parasitol. 2001;87(1):215–219. doi: 10.1645/0022-3395(2001)087[0215:OSBCFI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Dubey JP. Unexpected oocyst shedding by cats fed Toxoplasma gondii tachyzoites: in Vivo stage conversion and strain variation. Vet Parasitol. 2005;133(4):289–298. doi: 10.1016/j.vetpar.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Göndör A, Ohlsson R. Replication timing and epigenetic reprogramming of gene expression: A two-way relationship? Nat Rev Genet. 2009;10(4):269–276. doi: 10.1038/nrg2555. [DOI] [PubMed] [Google Scholar]
- 34.Henery CC, Miranda M, Wiekowski M, Wilmut I, DePamphilis ML. Repression of gene expression at the beginning of mouse development. Dev Biol. 1995;169(2):448–460. doi: 10.1006/dbio.1995.1160. [DOI] [PubMed] [Google Scholar]
- 35.Rook F, Hadingham SA, Li Y, Bevan MW. Sugar and ABA response pathways and the control of gene expression. Plant Cell Environ. 2006;29(3):426–434. doi: 10.1111/j.1365-3040.2005.01477.x. [DOI] [PubMed] [Google Scholar]
- 36.Arroyo A, Bossi F, Finkelstein RR, León P. Three genes that affect sugar sensing (abscisic acid insensitive 4, abscisic acid insensitive 5, and constitutive triple response 1) are differentially regulated by glucose in Arabidopsis. Plant Physiol. 2003;133(1):231–242. doi: 10.1104/pp.103.021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radke JR, et al. The transcriptome of Toxoplasma gondii. BMC Biol. 2005;3:26. doi: 10.1186/1741-7007-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gentleman RC, et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004;5(10):R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gissot M, Kelly KA, Ajioka JW, Greally JM, Kim K. Epigenomic modifications predict active promoters and gene structure in Toxoplasma gondii. PLoS Pathog. 2007;3(6):e77. doi: 10.1371/journal.ppat.0030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.