Abstract
Defects in the human protein TMEM165 are known to cause a subtype of Congenital Disorders of Glycosylation. Transmembrane protein 165 (TMEM165) belongs to an uncharacterized family of membrane proteins called Uncharacterized Protein Family 0016, which are well conserved throughout evolution and share characteristics reminiscent of the cation/Ca2+ exchanger superfamily. Gcr1 dependent translation factor 1 (Gdt1p), the budding yeast member of this family, contributes to Ca2+ homeostasis via an uncharacterized Ca2+ transport pathway localized in the Golgi apparatus. The gdt1Δ mutant was found to be sensitive to high concentrations of Ca2+, and interestingly, this sensitivity was suppressed by expression of TMEM165, the human ortholog of Gdt1p, indicating conservation of function among the members of this family. Patch-clamp analyses on human cells indicated that TMEM165 expression is linked to Ca2+ ion transport. Furthermore, defects in TMEM165 affected both Ca2+ and pH homeostasis. Based on these results, we propose that Gdt1p and TMEM165 could be members of a unique family of Golgi-localized Ca2+/H+ antiporters and that modification of the Golgi Ca2+ and pH balance could explain the glycosylation defects observed in TMEM165-deficient patients.
Keywords: glycosylation desorder, transporter, electrophysiology
Recently, Foulquier et al. (1) demonstrated that mutations of the gene coding for transmembrane protein 165 (TMEM165) are involved in a subtype of Congenital Disorders of Glycosylation (CDGs). CDGs are a family of inborn metabolic diseases affecting the glycosylation pathway. Most of these mutations are found in genes directly involved in glycosylation, however unique types of CDG have been found to be caused by deficiencies in vesicular Golgi trafficking (2–6) and Golgi pH homeostasis (7). TMEM165 belongs to a well-conserved, but uncharacterized, family of membrane proteins named UPF0016 (Uncharacterized Protein Family 0016; Pfam PF01169) and is localized in the Golgi apparatus (1). The members of this family are well conserved and are found in many organisms—for example, 919 different species of bacteria and 409 different eukaryotes.
Gcr1 dependent translation factor 1 (Gdt1p) the yeast ortholog of TMEM165, is a 280-residue membrane protein and is involved in tolerance to high concentrations of calcium (Ca2+) (8). In eukaryotic cells, Ca2+ is a ubiquitous intracellular messenger involved in many different biological processes (9). To allow the increases in cytosolic calcium concentration ([Ca2+]cyt) required for these signaling mechanisms, it is absolutely necessary that the resting Ca2+ levels are maintained below a certain threshold. Under normal conditions, the yeast [Ca2+]cyt is maintained between 50 and 200 nM (10). The maintenance of this basal level and the return to normal levels after stimulation are achieved by a series of Ca2+ pumps and exchangers located in different compartments of the cell: Pmr1p, the P-type Ca2+/Mn2+-ATPase localized in the medial-Golgi apparatus and responsible for the Ca2+ supply for the secretory pathway (11–13), and Pmc1p (14), a P-type Ca2+-ATPase, and Vcx1p (15, 16), a Ca2+/H+ exchanger (CAX), both responsible for the uptake of Ca2+ through the vacuolar membrane.
Yeast is a simple and convenient model for examining the basic mechanisms of Ca2+ signaling and homeostasis in eukaryotic cells, but is far less complex than animal cells. Different sets of Ca2+ sensors, effectors, and transporters are found in different animal cell types and are involved in Ca2+-signaling systems with different spatial and temporal properties (17). Basically, transient increases in the [Ca2+]cyt are produced either by uptake from the external medium, via a variety of plasma membrane channels, or by release from internal stores. Interestingly, calcium-proton exchange activity has been reported in different mammalian cell types such as synaptic vesicles of sheep brain cortex (18); however, although members of the CAX family are present in the genomes of some animals (e.g., fish and amphibians) (19), they are absent in mammalian genomes (20). This contradiction could be due to the existence of a still unidentified family of Ca2+/H+ antiporters.
Here, we show that, in yeast, Gdt1p is localized in the cis- and medial-Golgi apparatus, where it colocalizes with Pmr1p. Our findings indicate that Gdt1p and Pmr1p are involved in two different, but related, pathways of Ca2+ transport in the Golgi apparatus. Interestingly, the growth defect observed in the absence of Gdt1p in yeast could be partially restored by expression of a truncated version of human TMEM165, suggesting conservation of function between these two proteins. In human cells, we show that TMEM165 is involved in Ca2+ and pH homeostasis. Furthermore, patch clamp analyses showed that expression of TMEM165 resulted in ion transport, which was inhibited by EGTA, suggesting that the ion transported was Ca2+. Together, our results suggest that Gdt1p, TMEM165, and the other members of the UPF0016 family could be a group of Ca2+/H+ antiporters.
Results
Gdt1p Is Involved in Calcium Tolerance.
In a large-scale phenotype analysis in yeast (8), it was shown that gdt1Δ null mutants have a growth defect in the presence of high concentrations of calcium chloride (700 mM). We confirmed this phenotype and compared it with the growth of mutants pmr1Δ, pmc1Δ, and vcx1Δ (Fig. S1A and Table S1). On plates supplemented with 50 mM CaCl2, none of the strains showed sensitivity; interestingly, the slight growth defect observed for the pmr1Δ mutant under normal conditions was even partially overcome. However, in the presence of a high concentration of Ca2+ (750 mM), growth of the gdt1Δ and pmr1Δ mutants was reduced compared with that of the wild type, while the pmc1Δ null mutant was totally unable to grow (Fig. S1A). These differences have been confirmed when growth rates were compared in liquid medium. Interestingly, a slight defect is detected for gdt1Δ strain in YD medium (Fig. S1B). This result differs somewhat from that previously reported by Miseta et al. (21), who found that the growth of the pmr1Δ mutant was also totally abolished by high concentrations of Ca2+. This difference could be due to the different background of the strains used. In any case, these results show that Gdt1p is involved in Ca2+ homeostasis following exposure to extreme Ca2+ stress, but probably to a lesser extent than the Ca2+-ATPases, Pmr1p and Pmc1p.
Gdt1p Is Localized in the Early Golgi Apparatus.
To determine the subcellular localization of Gdt1p, wild-type yeast cells were grown in 1 L cultures, lysed gently, and the different organelles fractionated on a 10-step sucrose gradient. Gdt1p was found in the same fractions as markers of the Golgi (Emp47p) and endosomes (Pep12p), but not in fractions containing markers for the ER (Sec22p), plasma membrane (Pma1p), or vacuoles (carboxypeptidase Y) (Fig. S2A). To distinguish between a Golgi or an endosomal localization, we assessed the colocalization of Gdt1p and Pep12p by immunofluorescence microscopy in the wild-type strain using antibodies specific for each protein. Gdt1p did not colocalize with the endosomal marker, suggesting that it is probably located in the Golgi apparatus (Fig. S2B). We then immunostained Gdt1p in strains expressing GFP-fused markers of the different cisternae of the Golgi, namely Sed5p (cis-Golgi), Gos1p (medial-Golgi), and Sec7p (trans-Golgi) (22). Partial superimposition of the signals was visible only with Sed5p and Gos1p (Fig. S2C), showing that Gdt1p is present in the early compartments of the Golgi apparatus, but not in the trans-Golgi.
GDT1 Genetically Interacts with PMR1.
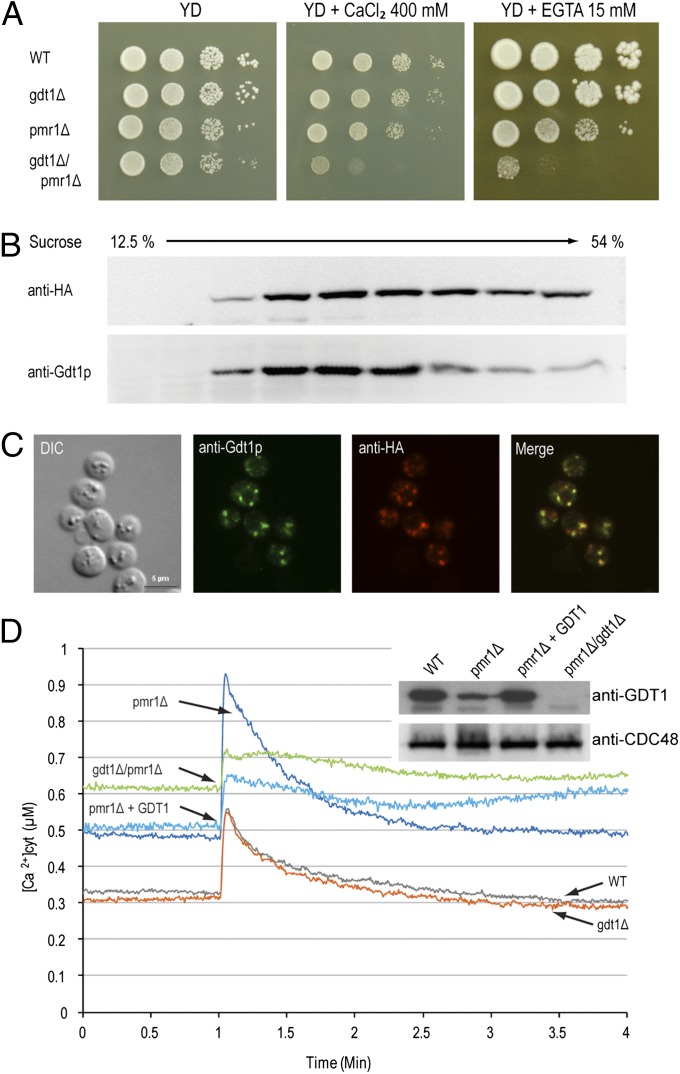
Because of their common Golgi localization and involvement in Ca2+ tolerance, we wanted to investigate possible interactions between GDT1 and PMR1. A high-throughput analysis previously revealed a synergistic genetic interaction between those two genes (23). The growth of the gdt1Δ/pmr1Δ double deletant was slightly decreased compared with that of the single mutants under normal conditions, and this synergistic effect was greatly enhanced in the presence of 400 mM Ca2+ (Fig. 1A). No effect was observed when Mg2+ or Mn2+ was added on solid medium (Fig. S3). This result suggests that both Gdt1p and Pmr1p are involved in high Ca2+ stress tolerance but via two distinct pathways and that the absence of one can be compensated for by the other pathway, but the simultaneous suppression of both is highly deleterious for cell growth. Interestingly, this synergistic effect was also visible on medium containing a very low concentration of Ca2+ (about 3 µM after addition of 15 mM EGTA at pH 6.0). It was already known that the pmr1Δ mutant is hypersensitive to EGTA (24), showing that Pmr1p is necessary for the adequate supply of Ca2+ to the Golgi apparatus (12). The fact that the single gdt1Δ mutant did not show any growth difference compared with the wild type under those conditions probably means that the role of Gdt1p in maintaining an adequate Ca2+ supply is only marginal in the presence of Pmr1p, but becomes essential when Pmr1p is absent.
Fig. 1.
GDT1 genetically interacts with PMR1. (A) The different mutants were precultured in YD medium to an OD600 of 0.3, then serial 10-fold dilutions were dropped onto solid YD medium alone or supplemented with 400 mM CaCl2 or 15 mM EGTA and the plates incubated at 28 °C for 4–6 d. Colocalization of Gdt1p and HA-Pmr1p as shown by subcellular fractionation (B) or immunostaining (C). (B) The fractions collected from the top of a discontinuous 10-step sucrose gradient were analyzed by Western blotting using antibodies against HA-Pmr1p (anti-HA) or Gdt1p. (C) Gdt1p and HA-Pmr1p in yeast were labeled using rabbit or rat primary antibodies, respectively, and fluorescein-conjugated anti-rabbit IgG and Alexa Fluor 546–conjugated anti-rat IgG antibodies. Differential interference contrast (DIC) and merged (merge) pictures are also presented. (Scale bar, 5 µm.) (D) The different strains expressing apo-aequorin from a plasmid were incubated overnight in minimal medium containing coelenterazine to reconstitute the holoenzyme. When the OD600 reached 3.0, 200 µl of each culture was transferred to a luminometric tube and the baseline luminescence monitored for 60 s, then CaCl2 was injected to a final concentration of 133 mM and the signal monitored for 3 min. The [Ca2+]cyt values were derived from luminometric units using the equation from Allen et al. (1977) (37). All displayed results are representative of those seen with at least three replicates. (Inset) Gdt1p and Cdc48p (loading control) levels in the different mutants measured by Western blotting of total extracts using anti-Gdt1p or anti-Cdc48p antibody. “pmr1Δ + GDT1” corresponds to the pmr1Δ mutant overexpressing GDT1.
To determine whether Gdt1p and Pmr1p exert their function in the same compartments, we performed colocalization experiments using a functional and correctly localized HA-tagged version of Pmr1p (25). Subcellular fractionation revealed that Gdt1p and HA–Pmr1p were found in the same fractions of the sucrose gradient (Fig. 1B) and immunofluorescence microscopy showed extensive colocalization between these proteins (Fig. 1C). Taken together, these results suggest the presence in the Golgi apparatus of two distinct machineries involved in Ca2+ supply to the secretory pathway and the detoxification of the cytosol upon high Ca2+ stress, a Pmr1p-dependent pathway, which is probably the more active under normal conditions, and a secondary, Gdt1p-dependent, pathway, which becomes essential in the absence of Pmr1p.
Gdt1p Regulates Cytosolic Ca2+ Levels in the pmr1Δ Mutant.
Using an aequorin-based assay, we monitored changes in the [Ca2+]cyt after exposure of the cells to high Ca2+ stress (133 mM). The absence of Gdt1p alone did not alter the response compared with that in the wild type (Fig. 1D). Both strains had a basal [Ca2+]cyt of about 0.3 µM, showed a sharp increase in the Ca2+ concentration after application of stress, then rapidly recovered, reaching their basal level after 3 min. In contrast, and in line with previous findings (26), the absence of Pmr1p markedly increased the initial [Ca2+]cyt to about 0.5 µM, indicating that active Ca2+ transport into the Golgi apparatus by Pmr1p is essential in maintaining the resting [Ca2+]cyt under a certain threshold. The intensity of the response was also markedly increased, suggesting a decreased rate of Ca2+ uptake from the cytosol, consistent with the absence of the major Ca2+ pump, or an increased rate of Ca2+ entry across the plasma membrane. However, we found that Gdt1p levels were markedly reduced in the pmr1Δ mutant compared with the wild type (Fig. 1D, Inset), and this decrease might be partially responsible for the large changes observed in this mutant. To verify this hypothesis, we overexpressed GDT1 in the pmr1Δ mutant to produce Gdt1p levels comparable to those in the wild type and found that, although the basal [Ca2+]cyt did not change, the intensity of the peak was markedly decreased, suggesting that the rate of Ca2+ uptake from the cytosol is higher in the pmr1Δ mutant when Gdt1p levels are increased. Finally, in the gdt1Δ/pmr1Δ double deletant, the resting [Ca2+]cyt was even higher (0.6 µM) than in the other strains, probably reflecting severe deregulation of Ca2+ homeostasis when both Ca2+ uptake pathways in the Golgi are disrupted, consistent with our phenotype results. After the stress, the [Ca2+]cyt decreased slowly and reached a new higher steady-state, indicating less efficient adaptability of this double deletant to environmental Ca2+ changes.
These results emphasize the role of Gdt1p in Ca2+ homeostasis. Although its absence did not seem to affect the cells in the presence of Pmr1p, which is known to be the major Ca2+ pump under normal conditions (27), the Gdt1p-dependent Ca2+ uptake pathway markedly affected the response to high environmental Ca2+ shock when Pmr1p was absent.
Gdt1p and Its Human Ortholog, TMEM165, Are Functionally Related.
The members of the UPF0016 family are highly conserved (Fig. S4A). Most of them possess two copies of a hydrophobic region that contains a highly conserved internal ExGD(KR)(TS) motif. These two homologous regions are probably the result of an ancient gene-duplication event. They contain three predicted transmembrane spans and surround a central hydrophilic loop usually rich in acidic residues (Fig. S4B). Due to the number of transmembrane domains, the two repeats are oriented in an antiparallel topology, a feature commonly observed in secondary transporters (28).
We wanted to examine conservation of function and tried to suppress the Ca2+ sensitivity of the gdt1Δ yeast mutant by expression of its human ortholog, TMEM165. As shown in Fig. S4C, unlike the full-length version of TMEM165, which was not able to complement the absence of GDT1, a truncated version of the protein lacking the first 55 residues, corresponding to an N-terminal extension that is not found in the yeast protein, partially restored growth in the presence of a high concentration of Ca2+, suggesting that these proteins exert the same function in their respective hosts. The N-terminal extension might alter the localization and/or activity of the protein when expressed in the yeast.
TMEM165 Is Involved in Ca2+ Movement Across Membranes.
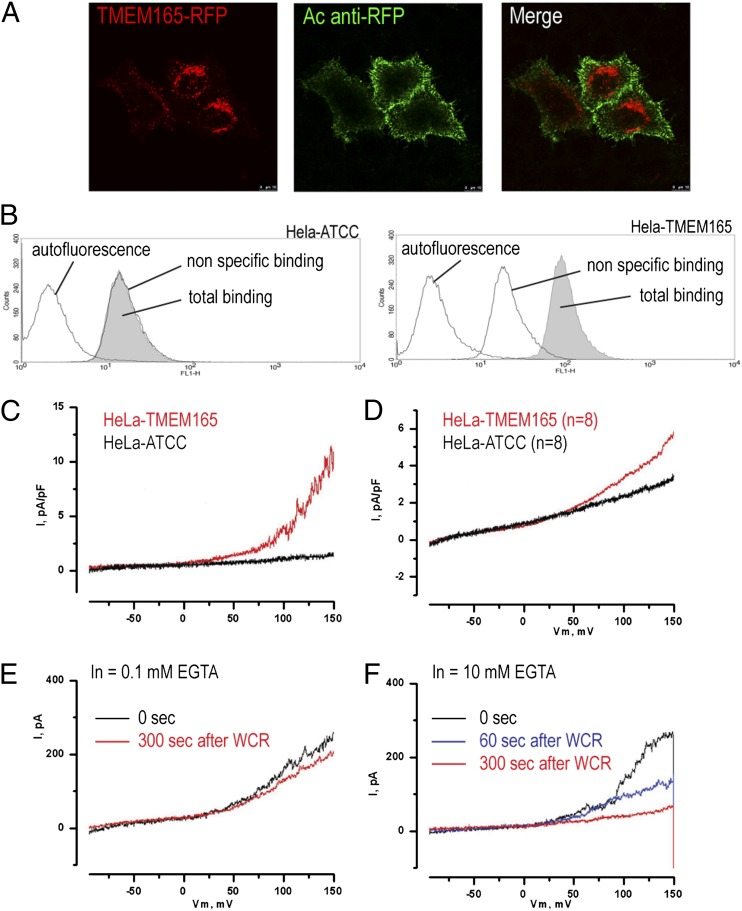
Based on the predicted topology of TMEM165 and Gdt1p, it is likely that they act as transporters. We performed electrophysiological measurements on mammalian cells (HeLa cells) stably expressing TMEM165 C-terminally tagged with red fluorescent protein (RFP) (TMEM165-RFP). To perform patch-clamp analysis, we first checked that wild-type TMEM165-RFP could reach the plasma membrane. For this, nonpermeabilized TMEM165-RFP–expressing cells were labeled with polyclonal antibodies against RFP (anti-RFP), then, after fixation, were labeled with Alexa Fluor 488–conjugated secondary antibody to visualize TMEM165-RFP at the plasma membrane. Alexa Fluor 488 labeling (green) of the plasma membrane was clearly observed in TMEM165-RFP–expressing cells (Fig. 2A). To confirm this result, the plasma membrane localization of TMEM165-RFP was assessed by flow cytometry. In contrast to the specific binding for HeLa cells overexpressing TMEM165-RFP, no binding to the parental HeLa cells was observed (Fig. 2B). While in normal conditions TMEM165 is localized in the Golgi apparatus (1), this result confirmed the plasma membrane localization likely resulting from the overexpression of the fusion protein.
Fig. 2.
Ionic movements are monitored across the plasma membrane when TMEM165 is overexpressed. (A) Living cells stably expressing TMEM165-RFP were incubated for 10 min at 18 °C with rabbit anti-RFP antibody and then were fixed and immunostained with Alexa Fluor 488–conjugated anti-rabbit IgG antibodies. (B) Presence of TMEM165 at the plasma membrane of HeLa cells stably expressing TMEM165-RFP investigated by flow cytometry. As described in Materials and Methods, fixed cells were incubated with rabbit anti-RFP antibodies and stained with Alexa Fluor 488–conjugated anti-rabbit IgG antibody. The plots show the green fluorescence (FL1-H) of wild-type HeLa cells (Left) and TMEM165-RFP–expressing HeLa cells (Right). Each plot shows the fluorescence of cells incubated without antibodies (autofluorescence; black line on left), only with Alexa Fluor 488–conjugated anti-rabbit IgG antibody (nonspecific binding; black line on right), or with both anti-RFP rabbit antibodies and Alexa Fluor 488–conjugated anti-rabbit antibody (total binding; gray shaded area). (C) Whole-cell membrane currents were measured in nontransfected HeLa cells (HeLa–ATCC) and HeLa cells overexpressing TMEM165. (D) Averaged membrane currents for n = 8. (E and F) Membrane currents in HeLa cells overexpressing TMEM165 in the presence of 0.1 mM EGTA (E) or 10 mM EGTA (F) (n = 4). WCR, whole-cell recording.
Given the nonnegligible fraction of TMEM165-RFP found at the plasma membrane, the cells were then subjected to patch clamp analyses. Whole-cell ionic currents across the plasma membrane were measured in HeLa cells overexpressing wild-type TMEM165-RFP and in nontransfected HeLa cells. Potassium currents were inhibited using 10 mM tetra-ethyl ammonium (TEA) (a nonspecific inhibitor of potassium channels) in the external medium. Membrane currents were measured for a voltage ramp ranging from –100–150 mV. No significant current was observed in nontransfected HeLa cells (HeLa–American Type Culture Collection), while in HeLa cells overexpressing TMEM165-RFP (HeLa-TMEM165), outward rectifying currents were observed (Fig. 2C). Thus, expression of TMEM165 was associated with ion transport. At 150 mV, membrane currents were increased by almost twofold in TMEM165-HeLa cells (n = 8) compared with nontransfected cells (5.8 ± 1.2 pA/pF vs. 3.2 ± 0.8 pA/pF) (Fig. 2D). To assess whether the membrane currents observed in HeLa cells were dependent on Ca2+, we used two different concentrations of EGTA (0.1 and 10 mM, respectively) in the patch-pipette, which should reduce the cytosolic-free Ca2+ concentration to 1–10 nM. Using an EGTA concentration of 10 mM, the outward rectifying currents progressively decreased after breaking into whole-cell configuration as EGTA diffused into the cytosol (Fig. 2F), whereas using an EGTA concentration of 0.1 mM, membrane currents did not decrease over the duration of the experiment (Fig. 2E).
Together, these experiments show that TMEM165 overexpression is associated with outward rectifying currents, which might be due to cation efflux from the cell or anion influx into the cytosol. Since these currents were still observed in the absence of potassium and the presence of TEA, it is unlikely that these currents are carried by potassium fluxes.
To determine whether Ca2+ homeostasis was perturbed in HeLa cells overexpressing TMEM165, the [Ca2+]cyt was measured by loading the cells with the Ca2+ probe Fura–2. In the absence of extracellular Ca2+, the [Ca2+]cyt was lower in TMEM165 overexpressing HeLa cells than in control cells (Fig. S5). Exposure of Fura-2–loaded cells to 1 μM thapsigargin produced in HeLa–ATCC cells a steep increase in the cytosolic Ca2+ levels as a result of the depletion of intracellular stores. Interestingly, thapsigargin-induced Ca2+ release was greatly reduced in HeLa-TMEM165 cells. This confirms that TMEM165 overexpression not only disturbs the [Ca2+]cyt homeostasis but also the luminal Ca2+ stores.
TMEM165 Deficiency Impairs Lysosomal pH Homeostasis.
So far, four different mutations in the TMEM165 gene have been identified in five patients (1). The first is an intronic mutation causing the activation of a cryptic splice site (c.792+182G > A), the second a missense mutation resulting in an Arg to His substitution (c.377G > A; p.R126H), the third a different missense mutation in the same codon (c.376C > T; p.R126C), and the fourth another missense mutation resulting in a Gly to Arg substitution (c.910G > A; p.G304R).
We investigated the effect of the natural mutated versions of the protein on pH homeostasis using fibroblasts from the different patients. After loading with the acidotropic dye LysoTracker Red, differences in fluorescence intensities were noted between control and three patients’ cells (Fig. S6 A and B). The lysosomal accumulation of LysoTracker was confirmed by colocalization with LAMP2 (lysosomal-associated membrane protein 2) (Fig. S6C).
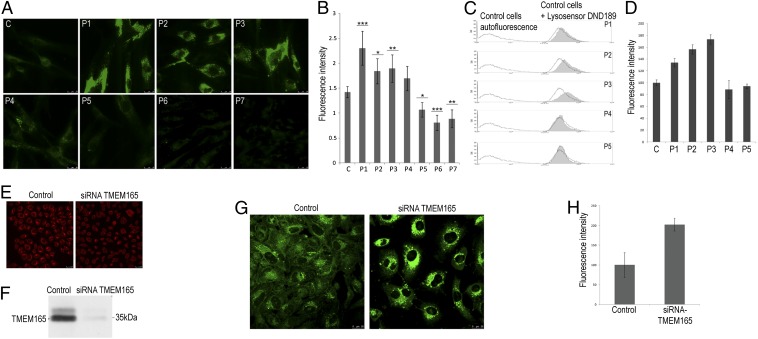
To investigate the pH properties of these compartments in living cells, patients’ cells were stained with a pH-sensitive fluorescent dye LysoSensor DND189, a membrane-permeating weak base with an acid- dependent fluorescence (pKa 5.1), and analyzed by fluorescence microscopy and flow cytometry. Compared with control cells, intracellular fluorescence was significantly increased in the cells from three patients (Fig. 3 A and B), consistent with a decrease in the lysosomal pH in the TMEM165-deficient cells and the LysoTracker results. As a negative control, cells from two V0-ATPase–deficient patients (patients P6 and P7) (7) were included in the assay and both showed decreased fluorescence compared with control cells (Fig. 3 A and B). These results for the TMEM165-deficient cells were confirmed by FACS analysis (Fig. 3 C and D). The differences in the quantification for patients P4 and P5 suggest that the missense mutations have a weaker effect on TMEM165 activity than the splicing mutants (P1, P2, and P3).
Fig. 3.
Lysosomal pH homeostasis is disturbed in TMEM165-deficient patients’ cells. (A) Live cell fluorescent images of lysosomal pH using LysoSensor green DND189 in fibroblasts from a control (C), five TMEM165-deficient patients (P1 to P5), and two V0-ATPase–deficient patients (P6 and P7). (B) Quantification of the fluorescence intensities observed in A. Error bars indicate mean ± 95% confidence interval (n = 3, around 10 cells per experiments). Data were analyzed by one-way ANOVA followed by Student post hoc test to identify mean differences between treatment and positive control. P values less than 0.05, 0.01, or 0.001 were indicated by *, **, or ***, respectively. Statistical analyses were performed using the software JMP 10 (SAS Institute). (C) Control and patients’ fibroblasts were incubated with LysoSensor green DND189, harvested, and analyzed by flow cytometry. Each histogram shows the autofluorescence (black line on the left) and the fluorescence (FL1-H) for the control cells (black line on the right) and one of the TMEM165-deficient patients’ cells (filled gray area). (D) Quantification of fluorescence intensities. The results were expressed as the percentage of the specific staining of the control cells—that is, the difference between the total and nonspecific binding peaks (autofluorescence). (E and F) HeLa cells were transfected with nontargeting siRNA (control) or siRNA targeted against TMEM165 (siRNA TMEM165); then, 7 d later, indirect immunofluorescence (E) and Western blotting analysis (F) were performed using anti-TMEM165 antibodies. (G) Live cell fluorescent images of the lysosomal pH using LysoSensor Green DND189 in control HeLa cells and TMEM165 siRNA knock-down HeLa cells. The results shown are representative of multiple cells assessed in independent experiments. (H) Quantification of the green fluorescence intensity (error bars indicate mean ± SEM, n = 3, >15 cells per experiments).
To further substantiate these findings, TMEM165 expression in HeLa cells was knocked down using siRNA, then the cells were stained with LysoSensor DND189. HeLa cells treated with siRNA showed a marked (> 90%) decrease in TMEM165 levels, as shown by immunofluorescence (Fig. 3E) and immunoblot analysis (Fig. 3F). Intense LysoSensor DND189 staining, corresponding to a general decrease in the pH in acidic compartments, was observed in these siRNA-targeted HeLa cells, which was about twice as intense as that in control cells (Fig. 3 G and H). Together these data show that late endosomal/lysosomal pH homeostasis is disturbed in cells from TMEM165-deficient patients.
Discussion
In this study, we characterized two members of an uncharacterized and highly conserved family of membrane proteins, the UPF0016 family. These proteins are involved in Ca2+ and pH homeostasis, suggesting that they could be members of Golgi-localized Ca2+/H+ antiporters. First, we showed that Gdt1p, the yeast member of the family, was localized in the cis- and medial-Golgi apparatus and was involved in tolerance to high environmental Ca2+ concentrations. Since these features are shared with Pmr1p, the Golgi Ca2+/Mn2+-ATPase, we tested the consequences of the lack of both proteins and found that sensitivity to Ca2+ was markedly increased in the gdt1Δ/pmr1Δ double mutant. This synergistic effect implies the presence of two distinct, but related, Ca2+ transport pathways, showing that Gdt1p is involved in a previously undescribed Ca2+ transport system in the Golgi apparatus. Moreover, the fact that tolerance to Ca2+ starvation was also reduced in the gdt1Δ/pmr1Δ mutant indicates that these two pathways are important for an adequate Ca2+ supply to the Golgi apparatus.
To confirm that the function of members of the UPF0016 family was well conserved, we tried to compensate for the absence of GDT1 by expressing the human ortholog of Gdt1p, TMEM165, and we showed that a truncated version of the protein, Δ55TMEM165, could at least partially overcome the Ca2+ sensitivity of the gdt1Δ mutant. The presence of a nonconserved 55-residue extension at the N terminus of TMEM165 prevented it from working properly when expressed in yeast. It is possible that this region affects the expression, targeting, and/or stability of the protein in yeast. Another explanation could be that an N-terminal autoinhibitory domain has appeared during evolution, requiring the action of other proteins to activate TMEM165. These domains are commonly found in Ca2+ transporters: for example, the plant and animal orthologs of Pmc1p have a calmodulin-binding autoinhibitory domain, which is absent in the yeast protein (29). Similarly, the Arabidopsis thaliana ortholog of Vcx1p, CAX1, has an N-terminal autoinhibitory tail that interacts with activator proteins (29).
In human cells, it was previously shown that endogenous TMEM165 is localized in the Golgi apparatus (1). In our study, deficiency or absence of TMEM165 was associated with an acidification of the lysosomal and endosomal compartments. In eukaryotic cells, the secretory pathway is progressively acidified and the pH of the different organelles results from a balance between proton pumping via the V-type H+ ATPase and proton leakage—for example, via transporters using the proton motive force. If TMEM165 is involved in the exit of H+ from the Golgi apparatus, its absence could affect this equilibrium and lead to acidification of the Golgi apparatus and, gradually, of all of the downstream acidic compartments. Alternatively, a defect in calcium homeostasis could indirectly affect organelle pH homeostasis probably via signaling perturbation.
We found that, when overexpressed in HeLa cells, a fraction of the total TMEM165 was relocalized to the plasma membrane. In this situation, whole-cell patch-clamp analysis was feasible and demonstrated that the presence of TMEM165 was linked to ion transport. The outward rectifying currents observed correspond to either a net export of positive charges or import of negative charges, showing that the transport is electrogenic. Furthermore, transport was inhibited by addition of EGTA, which suggests the involvement of Ca2+ ions.
Several features in the sequences of Gdt1p and TMEM165 strengthen the hypothesis that they are CAXs. Their predicted topology includes six transmembrane spans distributed in two hydrophobic clusters surrounding a large cytosolic loop that is rich in acidic residues. Except for the number of transmembrane spans, this topology corresponds exactly to the definition of the cation/Ca2+ exchanger superfamily (30). In this superfamily, the two hydrophobic clusters are homologous as a probable result of an ancient gene-duplication event. Each of these domains displays a highly conserved internal motif, called α-1 and α-2, and containing one conserved acidic residue (D/E). These acidic residues are believed to neutralize the two positive charges of a Ca2+ ion, and may thus help to overcome the energy barrier encountered by ions crossing a hydrophobic membrane. In the vicinity of this acidic residue, there are numerous serine and threonine residues, the side chain oxygen of which could help create a hydrophilic microenvironment around the Ca2+ transport pathway. These motifs also contain glycine and alanine residues that may provide the flexibility needed for potential conformational changes (30, 31). All of these features, without exception, are found in Gdt1p/TMEM165, and by analogy, we can call their two typical ExGD(KR)(TS) motifs α-1 and α-2, respectively.
An acidic residue-rich domain is found in several Ca2+-binding proteins, such as calsequestrin and calreticulin (32), or Ca2+ transporters, such as the yeast vacuolar CAX Vcx1p. This acidic motif seems to be essential for the activity and Ca2+ specificity of these proteins. For example, Cagnac et al. (33) recently showed that yeast Vnx1p, a membrane protein highly similar to Vcx1p, does not display any Ca2+/H+ exchange activity but does display Na+/H+ and K+/H+ exchange activity. According to the authors, this difference in substrate specificity could partially be explained by the absence of an acidic motif in Vnx1p. Although this is not direct evidence, these structural data, together with our experimental results, strongly justify the hypothesis that Gdt1p and TMEM165 are Ca2+/H+ antiporters. Further studies are needed to confirm this hypothesis by direct transport assays.
Adequate Ca2+ uptake into acidic Ca2+ stores is known to be essential for both the function of the organelles and the maintenance of cytosolic Ca2+ homeostasis in human cells. For example, mutation of secretory pathway Ca2+-ATPase isoform 1 (SPCA1), the human ortholog of yeast PMR1, causes a rare autosomal-dominant skin disorder, called Hailey–Hailey disease, which is accompanied by an increase of the [Ca2+]cyt and a reduction in the Golgi luminal Ca2+ concentration (19, 34, 35). Mutations in the a2 subunit of the V-type H+ ATPase, encoded by ATP2V0A2, have also been shown to result in abnormal glycosylation (CDG) and skin diseases (7, 36). In these patients, defects in the pH regulation of acidic organelles or vesicular trafficking within the cell are thought to be the molecular cause of the pathology. These two examples emphasize the importance of regulation of Ca2+ and pH homeostasis both in the cytosol and in the lumen of acidic organelles that is essential for the activity of several enzymes, and which may, as in the case of the V-type H+ ATPase and TMEM165 deficiencies, affect glycosylation.
In conclusion, we believe that Gdt1p, TMEM165, and the other members of the UPF0016 family could be a unique group of Ca2+/H+ antiporters regulating Ca2+ and pH homeostasis in acidic Ca2+ stores. This study provides information on previously uncharacterized Ca2+ transporters in yeast, which remains a convenient model for understanding the Ca2+ homeostasis mechanisms in all eukaryotic cells. Moreover, this study provides insights into the molecular causes of defects of glycosylation observed in TMEM165-deficient patients.
Materials and Methods
Different haploid yeast strains were routinely grown at 28 °C in the proper culture medium (as they carry a plasmid or not) and submitted to growth assays, subcellular fractionation, immunolocalization, or aequorin-based luminometric assays as extensively described in SI Materials and Methods. Electrophysiological recordings were performed on HeLa cells stably overexpressing a RFP-tagged version of TMEM165, and lysosomal pH analysis was performed on fibroblasts from different TMEM165-deficient patients or siRNA-treated HeLa cells. All these experiments are fully described in SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors thank Professors S. Loukin, A. Nakano, and M. Ghislain for providing plasmids and all the members of their groups for helpful discussions. The work was supported by grants from the Fonds National de la Recherche Scientifique (FNRS), the Communauté française de Belgique–Actions de Recherches Concertées (to P. Morsomme), Grants G.0553.08 and G.0505.12 from the Fonds Wetenschappelijk Onderzoek (to G.M.), an Agence Nationale Recherche Jeune Chercheur grant (to F.F.), and Grant ERARE (European Network Research Programs on Rare Diseases) 11-135 from the Equipe de Recherche Associée–Net for Research Programmes (European Network on Congenital Disorders of Glycosylation) (to F.F., E.V.S., and G.M.). L.G. was a postdoctoral research fellow at the FNRS, and D.D. and A.-S.C. are research fellows at the “Fonds pour la Formation à la Recherche dans l’Industrie et dans l’Agriculture.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219871110/-/DCSupplemental.
References
- 1.Foulquier F, et al. TMEM165 deficiency causes a congenital disorder of glycosylation. Am J Hum Genet. 2012;91(1):15–26. doi: 10.1016/j.ajhg.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu X, et al. Mutation of the COG complex subunit gene COG7 causes a lethal congenital disorder. Nat Med. 2004;10(5):518–523. doi: 10.1038/nm1041. [DOI] [PubMed] [Google Scholar]
- 3.Reynders E, et al. Golgi function and dysfunction in the first COG4-deficient CDG type II patient. Hum Mol Genet. 2009;18(17):3244–3256. doi: 10.1093/hmg/ddp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foulquier F, et al. A new inborn error of glycosylation due to a Cog8 deficiency reveals a critical role for the Cog1-Cog8 interaction in COG complex formation. Hum Mol Genet. 2007;16(7):717–730. doi: 10.1093/hmg/ddl476. [DOI] [PubMed] [Google Scholar]
- 5.Reynders E, Foulquier F, Annaert W, Matthijs G. How Golgi glycosylation meets and needs trafficking: The case of the COG complex. Glycobiology. 2011;21(7):853–863. doi: 10.1093/glycob/cwq179. [DOI] [PubMed] [Google Scholar]
- 6.Foulquier F, et al. Conserved oligomeric Golgi complex subunit 1 deficiency reveals a previously uncharacterized congenital disorder of glycosylation type II. Proc Natl Acad Sci USA. 2006;103(10):3764–3769. doi: 10.1073/pnas.0507685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kornak U, et al. ARCL Debré-Type Study Group Impaired glycosylation and cutis laxa caused by mutations in the vesicular H+-ATPase subunit ATP6V0A2. Nat Genet. 2008;40(1):32–34. doi: 10.1038/ng.2007.45. [DOI] [PubMed] [Google Scholar]
- 8.Dudley AM, Janse DM, Tanay A, Shamir R, Church GM. A global view of pleiotropy and phenotypically derived gene function in yeast. Mol Syst Biol. 2005;1:2005.0001. doi: 10.1038/msb4100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 10.Cui J, Kaandorp JA, Sloot PMA, Lloyd CM, Filatov MV. Calcium homeostasis and signaling in yeast cells and cardiac myocytes. FEMS Yeast Res. 2009;9(8):1137–1147. doi: 10.1111/j.1567-1364.2009.00552.x. [DOI] [PubMed] [Google Scholar]
- 11.Schröder S, Schimmöller F, Singer-Krüger B, Riezman H. The Golgi-localization of yeast Emp47p depends on its di-lysine motif but is not affected by the ret1-1 mutation in alpha-COP. J Cell Biol. 1995;131(4):895–912. doi: 10.1083/jcb.131.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dürr G, et al. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol Biol Cell. 1998;9(5):1149–1162. doi: 10.1091/mbc.9.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strayle J, Pozzan T, Rudolph HK. Steady-state free Ca(2+) in the yeast endoplasmic reticulum reaches only 10 microM and is mainly controlled by the secretory pathway pump pmr1. EMBO J. 1999;18(17):4733–4743. doi: 10.1093/emboj/18.17.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunningham KW, Fink GR. Calcineurin-dependent growth control in Saccharomyces cerevisiae mutants lacking PMC1, a homolog of plasma membrane Ca2+ ATPases. J Cell Biol. 1994;124(3):351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham KW, Fink GR. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16(5):2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pozos TC, Sekler I, Cyert MS. The product of HUM1, a novel yeast gene, is required for vacuolar Ca2+/H+ exchange and is related to mammalian Na+/Ca2+ exchangers. Mol Cell Biol. 1996;16(7):3730–3741. doi: 10.1128/mcb.16.7.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 18.Gonçalves PP, Meireles SM, Neves P, Vale MG. Distinction between Ca(2+) pump and Ca(2+)/H(+) antiport activities in synaptic vesicles of sheep brain cortex. Neurochem Int. 2000;37(4):387–396. doi: 10.1016/s0197-0186(00)00009-7. [DOI] [PubMed] [Google Scholar]
- 19.Manohar M, et al. Zebrafish (Danio rerio) endomembrane antiporter similar to a yeast cation/H(+) transporter is required for neural crest development. Biochemistry. 2010;49(31):6557–6566. doi: 10.1021/bi100362k. [DOI] [PubMed] [Google Scholar]
- 20.Patel S, Docampo R. Acidic calcium stores open for business: Expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010;20(5):277–286. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miseta A, Fu L, Kellermayer R, Buckley J, Bedwell DM. The Golgi apparatus plays a significant role in the maintenance of Ca2+ homeostasis in the vps33Delta vacuolar biogenesis mutant of Saccharomyces cerevisiae. J Biol Chem. 1999;274(9):5939–5947. doi: 10.1074/jbc.274.9.5939. [DOI] [PubMed] [Google Scholar]
- 22.Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A. Live imaging of yeast Golgi cisternal maturation. Nature. 2006;441(7096):1007–1010. doi: 10.1038/nature04737. [DOI] [PubMed] [Google Scholar]
- 23.Costanzo M, et al. The genetic landscape of a cell. Science. 2010;327(5964):425–431. doi: 10.1126/science.1180823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudolph HK, et al. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 1989;58(1):133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- 25.Antebi A, Fink GR. The yeast Ca(2+)-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol Biol Cell. 1992;3(6):633–654. doi: 10.1091/mbc.3.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peiter E, et al. The Medicago truncatula DMI1 protein modulates cytosolic calcium signaling. Plant Physiol. 2007;145(1):192–203. doi: 10.1104/pp.107.097261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marchi V, Sorin A, Wei Y, Rao R. Induction of vacuolar Ca2+-ATPase and H+/Ca2+ exchange activity in yeast mutants lacking Pmr1, the Golgi Ca2+-ATPase. FEBS Lett. 1999;454(3):181–186. doi: 10.1016/s0014-5793(99)00803-0. [DOI] [PubMed] [Google Scholar]
- 28.Vinothkumar KR, Henderson R. Structures of membrane proteins. Q Rev Biophys. 2010;43(1):65–158. doi: 10.1017/S0033583510000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pittman JK. Vacuolar Ca(2+) uptake. Cell Calcium. 2011;50(2):139–146. doi: 10.1016/j.ceca.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Cai X, Lytton J. The cation/Ca(2+) exchanger superfamily: Phylogenetic analysis and structural implications. Mol Biol Evol. 2004;21(9):1692–1703. doi: 10.1093/molbev/msh177. [DOI] [PubMed] [Google Scholar]
- 31.Liao J, et al. Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science. 2012;335(6069):686–690. doi: 10.1126/science.1215759. [DOI] [PubMed] [Google Scholar]
- 32.Ivey DM, et al. Cloning and characterization of a putative Ca2+/H+ antiporter gene from Escherichia coli upon functional complementation of Na+/H+ antiporter-deficient strains by the overexpressed gene. J Biol Chem. 1993;268(15):11296–11303. [PubMed] [Google Scholar]
- 33.Cagnac O, Leterrier M, Yeager M, Blumwald E. Identification and characterization of Vnx1p, a novel type of vacuolar monovalent cation/H+ antiporter of Saccharomyces cerevisiae. J Biol Chem. 2007;282(33):24284–24293. doi: 10.1074/jbc.M703116200. [DOI] [PubMed] [Google Scholar]
- 34.Hu Z, et al. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet. 2000;24(1):61–65. doi: 10.1038/71701. [DOI] [PubMed] [Google Scholar]
- 35.Behne MJ, et al. Human keratinocyte ATP2C1 localizes to the Golgi and controls Golgi Ca2+ stores. J Invest Dermatol. 2003;121(4):688–694. doi: 10.1046/j.1523-1747.2003.12528.x. [DOI] [PubMed] [Google Scholar]
- 36.Guillard M, et al. Vacuolar H+-ATPase meets glycosylation in patients with cutis laxa. Biochim Biophys Acta. 2009;1792(9):903–914. doi: 10.1016/j.bbadis.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Allen DG, Blinks JR, Prendergast FG. Aequorin luminescence: Relation of light emission to calcium concentration—A calcium-independent component. Science. 1977;195(4282):996–998. doi: 10.1126/science.841325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.