Abstract
Increasing evidence points to a role for the protein quality control in the endoplasmic reticulum (ER) in maintaining intestinal homeostasis. However, the specific role for general ER chaperones in this process remains unknown. Herein, we report that a major ER heat shock protein grp94 interacts with MesD, a critical chaperone for the Wnt coreceptor low-density lipoprotein receptor-related protein 6 (LRP6). Without grp94, LRP6 fails to export from the ER to the cell surface, resulting in a profound loss of canonical Wnt signaling. The significance of this finding is demonstrated in vivo in that grp94 loss causes a rapid and profound compromise in intestinal homeostasis with gut-intrinsic defect in the proliferation of intestinal crypts, compromise of nuclear β-catenin translocation, loss of crypt-villus structure, and impaired barrier function. Taken together, our work has uncovered the role of grp94 in chaperoning LRP6-MesD in coordinating intestinal homeostasis, placing canonical Wnt-signaling pathway under the direct regulation of the general protein quality control machinery in the ER.
Heat shock protein (HSP) grp94 (1), also known as gp96 (2), encoded by HSP90b1 (3), is the endoplasmic reticulum (ER) paralog of cytosolic HSP90. Like other HSPs, grp94 is induced by the accumulation of misfolded proteins (4) and binds and hydrolizes ATP (5–7). As the most abundant protein in the ER lumen, it is phylogenically conserved (8) and ubiquitously expressed in all nucleated cells. An important function of the ER is the steady-state folding of nascent polypeptides into their mature tertiary/quaternary structures and the assembly of large multimeric protein complexes in the secretory pathway. Recent work demonstrates that grp94 is the critical chaperone for multiple Toll-like receptors (TLRs) and integrins (9–13) and that it participates in the unfolded protein response (UPR) (14). Without grp94, most integrins and TLRs are unable to fold properly and, thus, fail to exit the ER and traffic to their proper “post-ER” compartment. Misfolded proteins are actively retained in the ER as a part of the ER quality-control process, and their accumulation in the ER can result in ER stress and activation of the UPR. Up to 10% of cytosolic proteins are dependent on HSP90 for folding (15); it is, therefore, unlikely that grp94 clients are limited to TLRs and integrins.
The intestinal epithelium is continually replenished through the proliferation and differentiation of intestinal stem cells that reside within the intestinal crypts (16). Canonical Wnt signaling through the surface receptor, Frizzled, and its coreceptor low-density lipoprotein receptor-related protein 5 (LRP5) or LRP6, is instrumental for gut homeostasis (16). LRP5 and LRP6 are highly homologous to each other, and their function in the canonical Wnt pathway is redundant in response to some Wnt ligands but not so to others (17–21). Mesoderm development (MesD), an ER chaperone, was recently shown to be necessary for surface expression of LRP5/6 and Wnt signaling (22). Interestingly, like grp94 and MesD, LRP5 and -6 are also necessary for mesoderm formation and gastrulation in mice (20), suggesting a possible overlap among these proteins/pathways in early development. However, the functional connection between grp94 and Wnt pathway has not been reported. Furthermore, a direct role for MesD or LRP5/6 in controlling adult intestinal homeostasis has not been demonstrated because of lack of appropriate genetic models.
Here, we report that grp94 interacts with MesD, which is necessary for proper cell surface expression of LRP6 and canonical Wnt signaling. Consistent with a role for grp94 in Wnt signaling, proliferation in intestinal crypts and intestinal integrity in mice are critically dependent on grp94.
Results
grp94 Interacts with MesD and Chaperones LRP6.
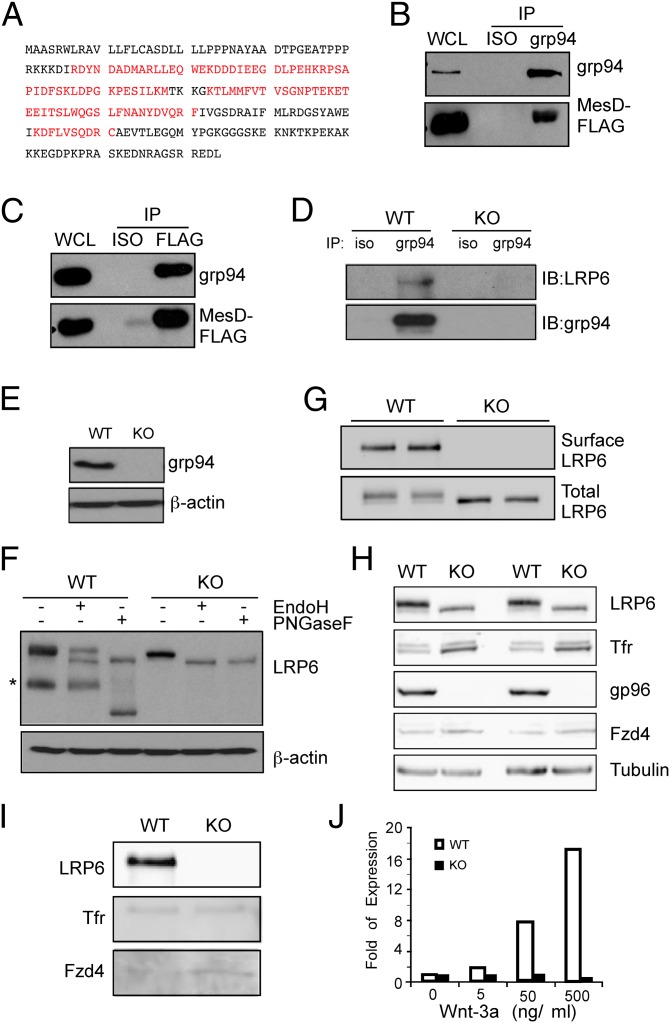
In an effort to expand the client network of grp94, we took an unbiased approach to immunoprecipitate grp94 clientale complex from a preleukemia B-cell line, 14.GFP (9). We then resolved the complex on SDS/PAGE and stained with Coomassie blue. We subjected the protein bands that were specifically associated with grp94 pull-down to trypsin digestion, followed by tandem mass spectrometry (MS/MS) to identify grp94-associated proteins. We unexpectedly discovered MesD as a grp94-interacting molecule (Fig. 1A). MesD, so named after its critical role in mesoderm formation during early embryogenesis, has been shown to be a critical chaperone for the surface expression of LRP5/6 (22). To confirm the interaction between grp94 and MesD, we FLAG-tagged MesD and expressed MesD-FLAG in HEK293 cells, followed by a coimmunoprecipitation study. Indeed, a strong interaction between the two was demonstrated (Fig. 1 B and C). Similarly, we found that grp94 interacts with LRP6 (Fig. 1D), which is a coreceptor for the cell surface Wnt receptor Frizzled and is required for canonical Wnt signaling (23). After maturation and surface expression, LRP6 undergoes γ-secretase–dependent regulated intramembrane proteolysis (RIP) to liberate its extracellular and intracellular domain (24). Indeed, in wild-type (WT) mouse embryonic fibroblasts (MEFs) (Fig. 1E), LRP6 undergoes RIP to release its extracellular domain (Fig. 1F). There are two forms of the full-length LRP6 in WT cells: Endoglycosidase H (Endo H)-resistant surface LRP6 and Endo H-sensitive LRP6 in the ER (Fig. 1F). However, we found that in grp94 knockout (KO) cells, LRP6 does not undergo RIP or transport to the cell surface, as indicated by the sensitivity of LRP6 to Endo H in grp94 KO cells (Fig. 1F). In further support of this conclusion, surface biotinylation followed by avidin pull-down and immunoblot failed to detect LRP6 on the surface of grp94 KO cells (Fig. 1G), although the same method detected similar amounts of transferrin receptor and Wnt receptor frizzled-4 (Fzd4) from WT and KO cells (Fig. 1 H and I). Finally, we tested WT and grp94 KO cells for their abilities to respond to a known Wnt ligand, Wnt-3a. Axin2 is a negative regulator of the Wnt-signaling pathway and is up-regulated in response to Wnt ligand. As expected, Wnt-3a treatment led to a dose-dependent up-regulation of Axin2 mRNA in WT cells but not in KO cells (Fig. 1J).
Fig. 1.
grp94 interacts with MesD and is a critical molecular chaperone for LRP6. (A) Identification of MesD as a grp94-interacting protein. MS/MS analysis of grp94-interacting proteins identified MesD. The sequence in red reflects regions authenticated by MS. (B) Immunoblot of grp94 and MesD-FLAG following immunoprecipitation of MesD-FLAG from HEK293 cell lysates. (C) Immunoblot of grp94 and MesD-FLAG following immunoprecipitation of grp94 from HEK293 cell lysates. Immunoprecipitation with isotype control antibody (ISO) is also shown. Expression of grp94 and MesD in whole cell lysate (WCL) is indicated. Data are representative of two independent experiments. (D) Immunoblot of LRP6 and grp94 following immunoprecipitation of grp94 from lysates of WT or grp94 KO MEFs. (E) Immunoblot analysis of grp94 from WT and grp94 KO MEF lysates. (F) Expression of endogenous LRP6 and its sensitivity to N-glycase Endo H and peptide N glycosidase F (PNGaseF) in WT and grp94 KO MEFs. Asterisk indicates cleaved LRP6. β-Actin served as a loading control. (G) WT or grp94 KO MEFs (duplicates) were subjected to surface biotinylation and pull-down with avidin beads, followed by immunoblot for cell surface LRP6. Total LRP6 was also blotted as a control. (H) Immunoblot of various proteins from the total lysates of WT and grp94 MEFs. After incubation of WT and grp94 mutant cells with EZ-Link Sulfo-NHS-SS-Biotin, lysates from cells were examined by immunoblot analysis for LRP6, transferrin receptor (Tfr), grp94, Fzd4, and tubulin. (I) Cell surface biotinylation of WT and grp94 KO MEFs. Lysates from cells treating with EZ-Link Sulfo-NHS-SS-Biotin were immunoprecipitated with NeutrAvidin beads and harvested. The pull-down–biotinylated membrane surface proteins were analyzed by immunoblotting. (J) Expression of Wnt target gene, Axin2, in response to Wnt stimulation. WT and grp94 KO MEFs were stimulated with Wnt-3a for 24 h, and fold expression of the Axin2 relative to unstimulated cells was measured by quantitative PCR. Data are representative of more than two independent experiments.
To determine the roles of grp94 in LRP6-MesD interaction, we expressed the both LRP6 and MesD in WT or grp94 knockdown HEK293 cells, followed by examination of the complex formation between the two. We found that knocking down grp94 resulted in a significant reduction of LRP6-MesD complex (Fig. 2).
Fig. 2.
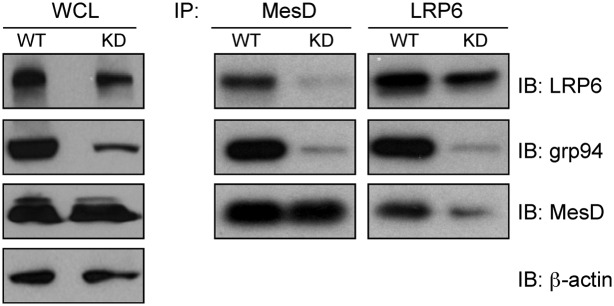
Knockdown of grp94 compromises LRP6-MesD interaction. LRP6-myc and MesD-FLAG were expressed in WT or grp94 knockdown (KD) HEK293 cells. Expression of indicated proteins in the whole-cell lysate of WT and KD cells was determined by immunoblot (IB). IP of LRP6-myc or MesD was then performed, followed by SDS/PAGE and IB for MesD-FLAG, grp94, and LRP6-myc. Two independent experiments were performed with similar findings.
Conditional Deletion of grp94 Results in Loss of Intestinal Homeostasis and Reduced β-Catenin Nuclear Translocation.
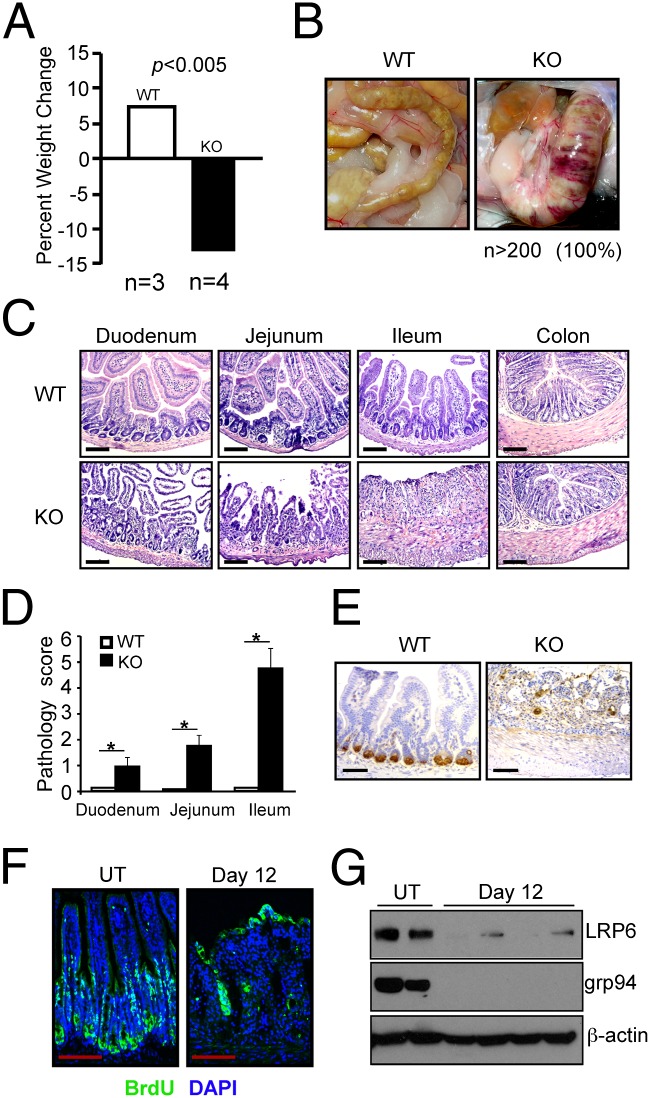
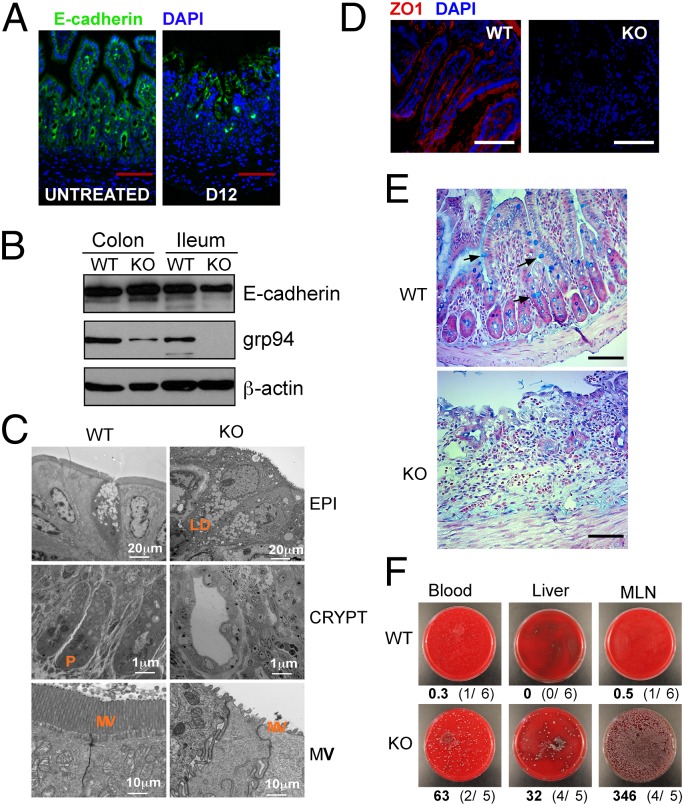
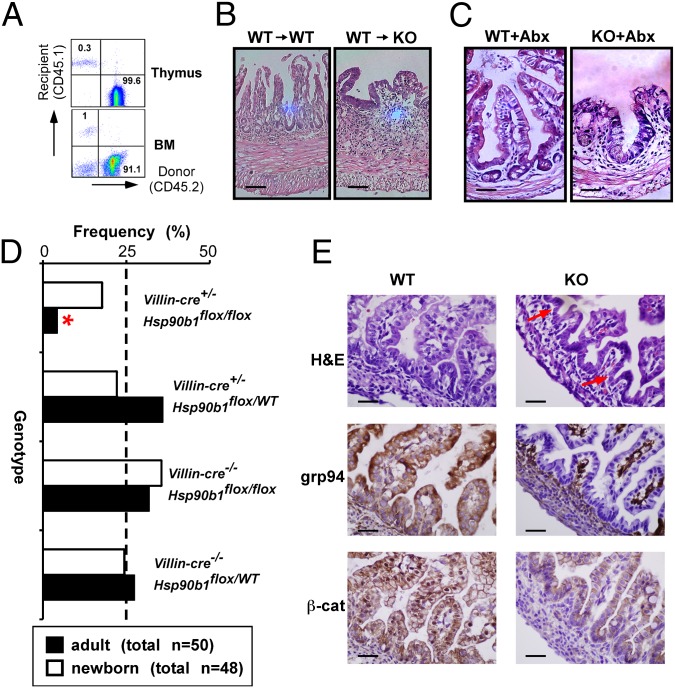
Intestinal homeostasis is critically dependent on the Wnt/β-catenin signaling pathway (25). For example, genetic deletion of β-catenin (26), LRP5/6 (27), and transcription factor 4 (Tcf4) (28) in mice results in profound loss of intestinal villus structure and death. If grp94 participates in Wnt coreceptor biogenesis, we would expect to see a similar intestinal phenotype in grp94 KO mice. We addressed this hypothesis using tamoxifen-inducible knockout strategy by crossing Hsp90b1flox/flox mice with a Rosa26ERT-cre mouse (29). Comparing with WT mice, we found that the KO mice experienced rapid weight loss, diarrhea, and hematochezia and ultimately death 12–14 d post-tamoxifen injection (PTI) in 100% of more than 200 mice studied (Fig. 3A). Gross pathological examination revealed significant intestinal dilatation, edema and hemorrhage, fecal obstruction, and thickening of the intestinal wall (Fig. 3B). Likely because of differences in the turnover rate of epithelium and the villus length (30), disease was often more pronounced in the terminal ileum, although the duodenum and jejunum were also affected to varying degrees without significant pathology in the colon. Like other Wnt-pathway KO mice, the pathology of the large bowel came later and was not easily observed because of death of the mice. The histopathological analysis revealed marked necrosis, neutrophil infiltration, and frank loss of intestinal villi and crypts in the ileum of grp94 KO mice (Fig. 3C). The pathology was apparent as further demonstrated by the pathology score to combine the degree of architectural loss and neutrophil infiltration (Fig. 3D). Importantly, loss of the gut epithelium is not limited to enterocytes. We observed near-total loss of Paneth cells by lysozyme immunohistochemistry (Fig. 3E). By BrdU pulsing, we found that there was significant reduction of BrdU uptake in the KO crypts (Fig. 3F), consistent with growth arrest and loss of β-catenin signaling (26). Remarkably, grp94 KO ileum had an almost complete loss of full-length LRP6 protein expression 12 d PTI (Fig. 3G), likely reflecting the increased sensitivity of unfolded LRP6 to protease-rich environment in the gut. The expression of E-cadherin, an important structural protein in the intestine (31), was not affected (Fig. 4 A and B). As expected, ultrastructural analysis of the grp94-null ileum revealed the complete loss of microvilli and Paneth cells (Fig. 4C). The severe compromise of the gut integrity was also evident from the observation of loss of gap junction protein ZO1 (Fig. 4D) and absence of Goblet cells by Alcian blue stain (Fig. 4E). Consequently, systemic dissemination of bacteria to the blood, liver, and mesenteric lymph node was evident with KO but not WT mice (Fig. 4F).
Fig. 3.
Deletion of grp94 in mouse results in compromise of gut homeostasis and loss of Wnt coreceptor LRP6. (A) Percent weight changes in WT and grp94 KO mice 12 d PTI. (B) Gross pathology showing bowel dilatation (∼three times of the diameter of the WT mice), obstruction, edema, and hemorrhage in the small intestine of grp94 KO mice (one representative mouse over more than 120 is shown). (C) H&E staining of gut sections of grp94 WT and grp94 KO mice 12 d PTI. The KO mice have fewer and shorter villi, especially in the ileum, which shows almost complete loss of villi with marked reduction of crypt. Multiple experiments (more than 30) were done with similar findings. (Scale bar: 100 μm.) (D) Quantification of gut pathology of KO (n = 5) and WT (n = 6) mice 12 d PTI. *P < 0.05. (E) Lysozyme stain of Paneth cells in the crypts of the ileum section. There was loss of crypt-villus structure and absence of Paneth cells in the KO ileum. (F) Immunofluorescence for BrdU and DAPI in the ileum of untreated (UT) and 12-d tamoxifen-treated mice. (Scale bar: 100 μm.) Comparing to the ileum of untreated mice, the tamoxifen-treated mice show significant loss of villi and crypts with markedly decreased BrdU uptake in the crypt. (G) Immunoblot for endogenous LRP6 at day 0 (WT mice) and day 12 (grp94 KO mice). grp94 and β-actin (loading control) expression is shown.
Fig. 4.
Loss of intestinal barrier function and bacterial translocation in grp94 KO mice. (A) Normal expression of E-cadherin in ileum of grp94 KO mice. Immunofluorescence microscopy for E-cadherin (green) on day 0 (untreated) (WT) and day 12 (D12) grp94 KO mice. DAPI staining is shown (blue). (Scale bar: 100 μm.) (B) Western blot analysis of E-cadherin in colon and ileum of WT and KO mice. grp94 and β-actin (loading control) expression is shown. Data are representative of multiple mice per group and experiments. (C) Ultrastructural analysis of small intestine of grp94 KO mice. Hsp90b1flox/wtR26RERT-cre (WT) and Hsp90b1flox/foxR26RERT-cre (KO) mice were treated for 12 d with tamoxifen. Distal ileum was dissected, carefully rinsed with PBS, and immediately fixed in 4% paraformaldehyde in PBS at 4 °C before processing for transmission electron microscope. LD, lipid droplets; MV, microvilli; P, Paneth cells. Loss of MV and Paneth cells can be readily seen. Data are representative of four mice per group. (D) Loss of gap junction protein Z01 in the KO ileum by immunofluorescence. (Scale bar: 100 μm.) (E) grp94 deletion leads to significant reduction of Goblet cells in all mice examined. (F) Presence of bacteria in cultured tissue lysate from mesenteric lymph nodes (MLN), liver (LIV), and blood (BLD) of grp94 KO mice. Representative images from each condition are shown. Numbers underneath these images represent average number of colonies after overnight culture from six WT and five KO mice, followed by the frequency of mice with positive bacteria culture in parenthesis.
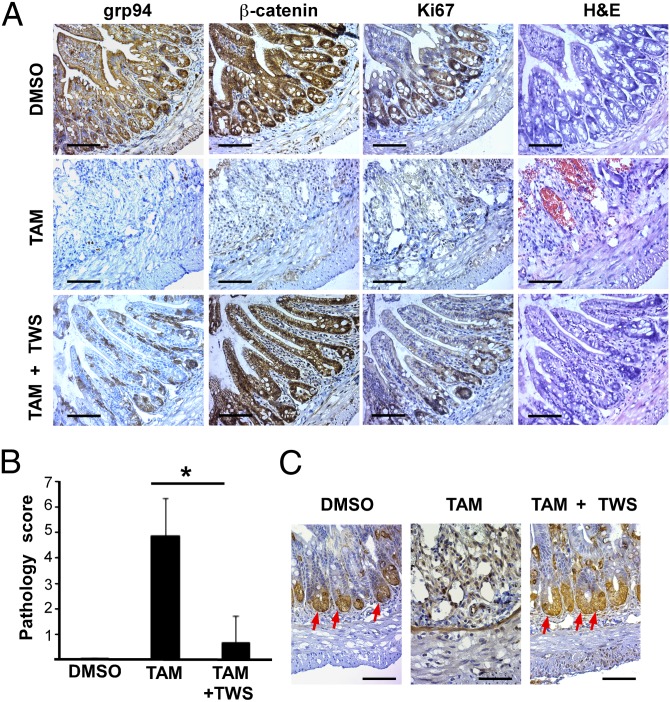
To determine whether loss of canonical Wnt signaling is responsible for the observed gut phenotype, we subjected grp94 KO mice to treatment with glycogen synthase kinase 3 beta (GSK3β) inhibitor TWS119 (32). Inhibition of GSK3β is expected to liberate β-catenin from its destruction complex and activate downstream Wnt target genes in a Wnt receptor/LRP6-independent manner. We found indeed that TWS119 treatment rescued the gut pathology in KO mice, which correlated with increased crypt cell proliferation (indexed by Ki67) and restored β-catenin nuclear translocation in the intestinal epithelial cells (Fig. 5A). TWS119 treatment significantly improved the pathology score (Fig. 5B) and resulted in restoration of the Paneth cells in the crypt (Fig. 5C).
Fig. 5.
β-Catenin activation rescues the intestinal pathology in grp94 KO mice. (A) After 10 d of treatment with DMSO or tamoxifen (TAM) with or without TWS119 (TWS), ileum tissues were stained for grp94, β-catenin, and Ki67 by immunohistochemistry. Shown also is an H&E-stained section. (Scale bar: 100 μm.) The marked ileum injury (mucosal ulceration, loss of villi, and decreased crypts) in TAM mice is reversed on H&E stain, and the immunohistochemical staining pattern of indicated proteins are restored to that of DMSO-treated control mice with TWS119 treatment (TAM+TWS). (B) Quantification of gut pathology of KO mice treated with indicated conditions (n = 5 per group). *P < 0.05. (C) Restoration of crypt-villus structure and appearance of Paneth cells (arrows) in the crypts of the ileum section of TAM-treated KO mice with TWS119.
Intestine-Specific Knockout of grp94 Recapitulates the Gut Pathology.
grp94 deletion in the hematopoietic system can cause granulocytosis and defective lymphopoiesis (12). To rule out the possibility that KO immune system contribute indirectly to the development of the gut pathology, we reconstituted grp94 KO recipients with WT bone marrow (12). Over 90% chimerism of the gut-associated lymphoid tissues was confirmed by congenic marker (Fig. 6A). We found that WT hematopoietic system was unable to rescue the KO gut phenotype (Fig. 6B). Moreover, administration of broad-spectrum antibiotics, which effectively eliminated over 90% of the gut flora (33), failed to alter the severity or kinetics of the intestinal disease (Fig. 6C), suggesting that grp94 plays a critical and gut-intrinsic role in gut homeostasis via regulation of the canonical Wnt-signaling pathway. To further address this hypothesis, we crossed Hsp90b1flox/flox mice with a Villincre transgenic mouse (34) to allow for gut-specific deletion of grp94. Canonical Wnt pathway in the gut is dependent on the transcriptional activation complex between nuclear β-catenin and Tcf4, to transactivate downstream genes. Tcf4 plays indispensable roles in maintaining the crypt stem cells of the small intestine (28). Similar to Tcf4 KO mice, we found that gut-specific deletion of grp94 does not affect embryogenesis. The KO mice were born at expected Mendelian ratios (Fig. 6D). However, the intestinal epithelium-specific deletion of grp94 was associated with postnatal death of mice (Fig. 6D). The only 2 survived mice out of 12 expected KO mice did so because of only partial knockdown of grp94. No mice with complete KO of grp94 survived. Examination of the newborn mice euthanized before death demonstrated the concurrent failure of β-catenin nuclear translocation (Fig. 6E). Histological analysis demonstrated decreased number of villi, significant reduction of cells in the crypt regions between the villi, decreased mitosis figures, and frequent “lifting” of the epithelial layer, which is reminiscent of Tcf4 KO mice (28). The complete phenocopy of gut-specific grp94 KO mice with Tcf4 KO mice led us to further conclude that the gut pathology associated with grp94 deletion is attributable to gut-intrinsic loss of Wnt signaling.
Fig. 6.
Gut-intrinsic roles of grp94 in gut homeostasis. (A) Flow-cytometric analysis of donor cells based on congenic marker CD45.2. (B) H&E staining of ileum sections of WT→WT and WT→KO bone marrow chimeric mice 12 d PTI. (Scale bar: 10 μm.) (C) H&E staining of ileum sections of grp94 WT and KO mice treated with antibiotics before deletion of grp94 15 d PTD. Data are representative of two independent experiments with multiple mice (n > 5) per group. (Scale bar: 25 μm.) (D) Loss of intestine-specific grp94 KO mice during the postnatal period. Villin-cre+/−Hsp90b1flox/WT mice were crossed with Villin-cre−/−Hsp90b1flox/flox mice. The genotypes of newborn mice, as well as that of the postweaning adult mice, were determined. The observed frequency of Villin-cre+/−Hsp90b1flox/flox adult mice was significantly reduced from the expected value of 25%. *P < 0.05. (E) H&E and immunohistochemistry staining of grp94 and β-catenin of ileum sections from the villincreHsp90b1flox/flox and the control villincreHsp90b1flox/WT mice. (Scale bar: 25 μm.) H&E stain of the ileum section of the KO newborn mice shows shorter villi with decreased cell number in intervillus crypt region (arrows) and mitosis, indicating less proliferation activity. Immunohistochemistry stain reveals complete loss of grp94 expression in KO ileum with concurrent loss of β-catenin nuclear translocation compared with WT mice. A representative image from four mice is shown.
Discussion
Herein, we report the surprising and indispensable role for a major ER-resident molecular chaperone, grp94, in chaperoning LRP6 and in intestinal homeostasis. grp94 binds to MesD and LRP6, and it plays a critical role for the maturation and surface expression of LRP6. The essential roles of grp94 in LRP6 expression and function in canonical Wnt pathway are established both in vitro and in vivo.
We demonstrated that grp94 interact with both LRP6 and the ER resident “chaperone” MesD (22). MesD does not appear to play any other role in ER function besides its unique function in promoting LRP5/6 surface expression. Interestingly, grp94 also appears to play an important role in mesoderm formation during development (35). Thus, our data strongly indicate that full LRP6 maturation and cell surface expression is dependent on the coordinated action of both grp94 and MesD. The conditional grp94 KO mouse now provides a unique experimental system to study the roles of LRPs in the biology of intestinal homeostasis in both physiological and pathological conditions.
The proliferation and differentiation of the intestinal epithelium are tightly controlled by the Wnt/β-catenin pathway. The loss of enterocytes/villi and crypts in grp94 KO intestine closely resembled that of mouse models of gut-specific deletion of β-catenin (26) and LRP5/6 (27). Both β-catenin and LRP5/6 conditional KO mice developed severe intestinal pathology ∼4 d after deletion. grp94 KO mice developed pathology around 10 d PTI, reflecting the requirement for 1 additional week to delete grp94. Our focus on LRP5/6 also came from two other observations: that grp94 was found to complex with MesD and that LRP6 has some structural similarities to integrins, a known family of grp94-dependent client proteins (11). We found, indeed, that LRP6 expression in the intestine was significantly compromised in the absence of grp94. More importantly, LRP6 in grp94 KO cells is unable to acquire Endo H resistance and fails to export to cell surface, indicating that LRP6 is trapped in the ER in the absence of grp94. We also demonstrated that grp94 is required for optimal interaction between LRP6 and MesD. Further study is necessary to understand the precise molecular mechanism of grp94-MesD-LRP interaction and its regulation during steady-state and pathological conditions.
grp94 is an endoplasmic reticulum resident HSP and a master chaperone for TLRs and integrins (8–11, 13, 36). We also considered the possibility that loss of grp94-dependent client proteins TLRs and/or integrins could partially contribute to the development of the intestinal pathology in grp94 KO mice. However, mice that lack the TLR-signaling molecules myeloid differentiation primary response 88 (MyD88) or TIR-domain-containing adapter-inducing interferon beta (TRIF), although more sensitive to experimental colitis, do not develop spontaneous enteritis (37). Although loss of β1 integrin in the intestine results in the development of colitis (38), β1 integrin expression is not dependent on grp94 (12). Moreover, many of the integrin α-chains that pair with β1 are not dependent on grp94 either, with the exception of α2 (11). α2 knockout mice do not develop gut disease, and although α2 is expressed in the mouse intestine, the specific function of α2 in the gut is unknown. Neither TLRs nor grp94-dependent integrins have been directly linked to the gut homeostasis intrinsically. Most importantly, we demonstrated the complete phenocopy of gut-specific grp94 KO mice with Tcf4 KO mice and that the gut phenotype associated with grp94 loss can be rescued by activation of canonical Wnt-signaling pathway via GSK3β inhibitor. Thus, the pathogenesis of gut diseases in grp94 KO mice is primarily attributable to the loss of gut-intrinsic canonical Wnt pathway, rather than the lack of other functional clients of grp94.
In summary, we have uncovered a function of a major ER luminal HSP grp94 in chaperoning LRP6 and Wnt signaling. Given the importance for Wnt in tumorigenesis and other aspects of development (25, 39), a previously unappreciated and critical role for grp94 in the Wnt signaling may have fundamental implications in linking the general ER protein homeostasis with Wnt signaling. Elucidation of the precise mechanism of grp94 in chaperoning LRP6 will also facilitate grp94-targeted therapeutics for cancer.
Methods
Mice and Genotyping.
Conditional Hsp90b1-deficient mice were described previously (10, 11, 40). villincre mice were purchased from The Jackson Laboratory. Animal use was approved by the Medical University of South Carolina Animal Care Committee.
Plasmids.
grp94 constructs in MigR were described previously (10). Human LRP6-myc was a generous gift from Cristof Neihrs (Deutsches Krebsforshungszentrum, Heidelberg) and Yonghe Li (Southern Research Institute, Birmingham, AL). Mouse MesD-FLAG and mouse LRP6-myc plasmids were a generous gift from Janet Lighthouse and Bernadette Hoeldner (Stony Brook University, Stony Brook, NY). Site-directed mutagenesis was performed on parental grp94/MigR using a commercially available kit (Stratagene) and verified by sequencing. Transient transfection of HEK293 cells was performed using 5–10 μg of plasmid DNA and Lipofectamine 2000. Cells were analyzed 48 h after transfection.
Cell Lines.
HEK293 cells were originally obtained from the ATCC. WT grp94 and tamoxifen-inducible KO MEFs were generated and immortalized using transduction of large-T antigen retrovirus. After cloning, MEF cells were either treated with ethanol (vehicle) or 1 mM hydroxy-tamoxifen (Sigma-Aldrich) to generate grp94-null MEFs. MEF cells were maintained as WT or grp94 KO.
Antibodies.
Most antibodies were purchased from Sigma-Aldrich except the antibodies against the following antigens: Lysozyme (Abcam), β-cat (Cell Signaling), grp94 (Enzo Life Sciences), Ki67 (Nova Biologicals), ZO1 (Abcam), BrdU (Invitrogen), transferrin (Invitrogen), and LRP6 (Cell Signaling).
Tamoxifen-Inducible Deletion of grp94 and Model of Acute Intestinal Disease and Bone Marrow Chimeras.
grp94 KO mice and bone marrow chimeras were described previously (12). Tamoxifen citrate (Sigma-Aldrich) was dissolved at 10 mg/mL in peanut or corn oil and further diluted to 1 mg/mL for injections. Low-dose tamoxifen (5 μg/g body weight) was injected intraperitoneally to Hsp90b1flox/wtR26RERT-cre (WT), Hsp90b1flox/flox, or Hsp90b1flox/foxR26RERT-cre (KO) mice for 0–12 consecutive days. For rescue experiment, GSK3β inhibitor TWS119 (Cayman Chemical) was administered intraperitoneally after day 3 at 15 mg/kg body weight daily for 7 d (32).
Antibiotic Treatment and Commensal Depletion.
Mice were treated with 1 g/L ampicillin (Sigma), 1 g/L neomycin (Sigma), 1 g/L metronidazole (Sigma), and 0.5 g/L vancomycin (RPI Corp) in the drinking water for 1 mo. Commensal depletion was verified by fecal plating on LB agar without antibiotics (33). Water was changed every third day, and antibiotic treatment was continued throughout tamoxifen treatment. Tamoxifen was administered to mice at 5 μg/g body weight as above. Mice were killed at day 12, or their survival was tracked indefinitely. Bacterial load from mice was quantified by inoculating 300 μL of diluted blood (1:10 in PBS), homogenate of liver (50 mg/mL), or mesenteric lymph node (25 mg/mL) to modified trypticase soy agar (TSA II) plates with 5% (vol/vol) sheep blood (BD Biosciences), followed by overnight culture at 37 °C and counting of bacterial colonies.
Reverse Transcription and Quantitative PCR.
Approximately 1-cm segments of intestinal tissue were snap frozen and stored at −80 °C. RNA was extracted by TRIzol (Gibco) method. cDNA was made by reverse-transcription PCR using SuperScript polymerase II (Invitrogen). Quantitative PCR was performed using Sybr Green (Applied Biosystems) method using the primer sets. Expression level was calculated using the formula 2−ΔCT and multiplied by a factor of 10. β-Actin was used as an internal control.
Histopathology, Immunofluorescence, and Immunohistochemistry.
Approximately 1-cm segments of tissue were embedded in OCT freezing medium (Thermo Scientific) and immediately frozen on dry ice or fixed in 4% formaldehyde/PBS, rehydrated in 30% sucrose/PBS, before embedding in OCT medium. Samples were stored at −80 °C until sectioning. Five to 7-μm sections were cut on a cryostat onto poly-L lysine-coated slides (Sigma). For histological examination, slides were immediately stained with hematoxylin/eosin (H&E). Gut pathology score is defined by summation of two parameter scores to achieve a range of 0–6: inflammation (0, no inflammation; 1, neutrophil rarely discernible; 2, presence of neutrophils in every high power field; 3, collection of >10 neutrophils in any given area); tissue destruction (0, normal structure; 1, villus length shortened by 50%; 2, flattened mucosa with loss of villi; 3, evidence of submucosa ulceration). For staining Goblet cells, fixed tissue was stained with Alcian blue solution for 20 min at room temperature and then rinsed thoroughly in tap water, followed by counterstain with Kernechtrot’s nuclear fast red solution for 5 min. After rinsing in distilled water, the tissue was dehydrated and mounted. For immunofluorescence, slides were fixed in ice-cold acetone. Alternatively, for grp94 intracellular staining, slides were fixed in 4% formalin/PBS for 20 min and permeabilized with ice-cold methanol for 20 min. Slides were washed in PBS; Fc receptor was blocked and/or slides were blocked in 10% goat serum/PBS for 30 min. Primary antibodies were diluted in 2% BSA/PBS, and slides were stained for 45 min. After washing in PBS, slides were stained with secondary antibody (or directly conjugated antibodies) diluted in 2% BSA/PBS for 30 min. Slides were washed again and counterstained with nuclear stain, DAPI. For BrdU staining, fixed-tissue samples were cut into 10-μm sections and fixed a second time in 10% formalin/PBS for 30 min. After washing, slides were treated in 2 M HCl/H2O for 20 min at 37 °C. Slides were rinsed in PBS and neutralized in two washes in 0.1 M sodium borate buffer (pH 8.5). After thorough washing, slides were stained with anti-BrdU antibody (MoBU-1 or ZBU-30; 1:25; Invitrogen).
Electron Microscopy.
Tissues were dissected and fixed in 4% buffered paraformaldehyde, osmicated, stained in block with uranyl acetate, dehydrated, and embedded in Poly/Bed resin. Thick 1-μm sections were cut on a Leica EM UC7 ultramicrotome and examined in the light microscope. Thin 70- to 80-nm sections were cut and collected on 200 Cu/Rh mesh grids, stained with uranyl acetate and lead citrate, and observed in a Hitachi H-7650 transmission electron microscope.
BrdU Pulse.
Mice were injected intraperitoneally with BrdU (1 mg) in PBS 24 h before euthanasia.
Protein Extraction, Immunoprecipitation, and Western Blot.
Protein extraction, immunoprecipitation, and Western blot were carried out as described previously (10). Briefly, HEK293 or MEF cells were harvested by trypsin-EDTA or 5 μM EDTA, subjected to dithiobis (succinimidyl propionate) (Thermo Scientific) for 30 min at room temperature, washed in PBS, and lysed on ice in radioimmunoprecipitation assay (RIPA) lysis buffer plus protease inhibitor mixture (Sigma-Aldrich). Nuclear-free protein lysate was quantified by Bradford assay, and an equal amount of lysate was incubated with antibodies or isotype control as indicated. grp94 was immunoprecipitated using 9G10 antibody (Stressgen) and Protein-G beads (Pierce), and MesD-FLAG was immunoprecipitated using anti-FLAG antibody (BioM2; Sigma) and Neutravidin beads (Thermo Scientific).
In-Gel Digestion and Nano–LC-MS/MS Analysis of grp94-Associated Proteins.
grp94 immunoprecipitates were resolved on SDS/PAGE. In-gel digestion of unique bands was performed using trypsin, followed by microcapillary HPLC and LC-MS/MS analysis on the linear trap quadrupole. The obtained MS/MS data were subjected to database searches of mouse proteome using SEQUEST software, as described (41).
Cell Surface Biotinylation.
After washing cells three times with 1× PBS CM [10 mM potassium phosphate (pH 7.5), 140 mM NaCl, 0.1 mM CaCl2, and 1 mM MgCl2], cells were incubated for 30 min with the 1× PBS CM containing 0.5 mg/mL of EZ-Link Sulfo-NHS-SS-Biotin (Thermo Fisher Scientific; catalog no. 21331) on ice with rocking. The biotinylation reactions were then incubated with 1× PBS CM containing 50 mM NH4Cl on ice for 5 min to quench free biotin. Then, the cells were washed three times with 1× PBS CM and lysed in lysis buffer containing 1.25% Triton X-100, 0.25% SDS, 50 mM Tris⋅HCl (pH 8.0), 150 mM NaCl, 5 mM EDTA, 5 mg/mL iodoacetamide, 10 μg/mL APMSF, and protease inhibitor mixture (Roche). Whole-cell lysates were centrifuged at 15,000 × g at 4 °C for 15 min, and the supernatant was collected and incubated with NeutrAvidin beads (Thermo Fisher Scientific; catalog no. 29200) at 4 °C overnight while rotating. The NeutrAvidin beads were centrifuged at 1,000 × g at 4 °C for 3 min and washed with wash buffer [0.5% Triton X-100, 0.1% SDS, 50 mM Tris⋅HCl (pH 8.0), 150 mM NaCl, 5 mM EDTA). The proteins in NeutrAvidin beads were eluted with SDS sample buffer and subjected to Western blot analysis.
Wnt Signaling.
WT and KO MEF cells were seeded at 1 × 105 cells per well in a 12-well plate the night before. The next day, cells were either stimulated with Wnt 3a (Peprotech) at the indicated dose or left unstimulated. Quantitative PCR for Axin2 was performed using the following primers: 5′-TGACTCTCCTTCCAGATCCCA-3′ (forward) and 5′-TGCCCACACTAGGCTGACA-3′ (reverse). Relative fold expression was calculated by the ΔΔCT method relative to β-actin control using the following formula: 2−ΔΔCT.
ELISA.
Serum was collected at the time of euthanasia. TNFα, IL-6, and IL-12p40 ELISAs (BD Bioscience) were performed according to the manufacturer’s protocol.
Statistics.
Error bar represents standard deviation. The Student’s t test or χ2 test was used to determine whether the difference between two groups was statistically significant (P < 0.05).
Acknowledgments
We thank the past and present members of our laboratories for their input throughout the course of this work. B.L. is a National Institutes of Health (NIH) KL2 scholar and is supported by the South Carolina Clinical and Translational Research Institute at the Medical University of South Carolina (NIH Grants KL2RR029880 and UL1RR029882). The work was also supported, in part, by the Flow Cytometry and Cell Sorting Shared Resource, Hollings Cancer Center, Medical University of South Carolina (NIH Grant P30 CA138313). Z.L., C.E.C., S.T., and D.W. are supported by NIH grants. Z.L. is the Abney Chair Remembering Sally Abney Rose in Stem Cell Biology and Therapy.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Lee AS, Delegeane A, Scharff D. Highly conserved glucose-regulated protein in hamster and chicken cells: Preliminary characterization of its cDNA clone. Proc Natl Acad Sci USA. 1981;78(8):4922–4925. doi: 10.1073/pnas.78.8.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srivastava PK, DeLeo AB, Old LJ. Tumor rejection antigens of chemically induced sarcomas of inbred mice. Proc Natl Acad Sci USA. 1986;83(10):3407–3411. doi: 10.1073/pnas.83.10.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen B, Piel WH, Gui L, Bruford E, Monteiro A. The HSP90 family of genes in the human genome: Insights into their divergence and evolution. Genomics. 2005;86(6):627–637. doi: 10.1016/j.ygeno.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332(6163):462–464. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Srivastava PK. Tumor rejection antigen gp96/grp94 is an ATPase: Implications for protein folding and antigen presentation. EMBO J. 1993;12(8):3143–3151. doi: 10.1002/j.1460-2075.1993.tb05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frey S, Leskovar A, Reinstein J, Buchner J. The ATPase cycle of the endoplasmic chaperone Grp94. J Biol Chem. 2007;282(49):35612–35620. doi: 10.1074/jbc.M704647200. [DOI] [PubMed] [Google Scholar]
- 7.Dollins DE, Warren JJ, Immormino RM, Gewirth DT. Structures of GRP94-nucleotide complexes reveal mechanistic differences between the hsp90 chaperones. Mol Cell. 2007;28(1):41–56. doi: 10.1016/j.molcel.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morales C, Wu S, Yang Y, Hao B, Li Z. Drosophila glycoprotein 93 Is an ortholog of mammalian heat shock protein gp96 (grp94, HSP90b1, HSPC4) and retains disulfide bond-independent chaperone function for TLRs and integrins. J Immunol. 2009;183(8):5121–5128. doi: 10.4049/jimmunol.0900811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Randow F, Seed B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol. 2001;3(10):891–896. doi: 10.1038/ncb1001-891. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, et al. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26(2):215–226. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu B, Li Z. Endoplasmic reticulum HSP90b1 (gp96, grp94) optimizes B-cell function via chaperoning integrin and TLR but not immunoglobulin. Blood. 2008;112(4):1223–1230. doi: 10.1182/blood-2008-03-143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staron M, et al. gp96, an endoplasmic reticulum master chaperone for integrins and Toll-like receptors, selectively regulates early T and B lymphopoiesis. Blood. 2010;115(12):2380–2390. doi: 10.1182/blood-2009-07-233031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu B, et al. Folding of Toll-like receptors by the HSP90 paralogue gp96 requires a substrate-specific cochaperone. Nat Commun. 2010;1:79. doi: 10.1038/ncomms1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christianson JC, Shaler TA, Tyler RE, Kopito RR. OS-9 and GRP94 deliver mutant alpha1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nat Cell Biol. 2008;10(3):272–282. doi: 10.1038/ncb1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao R, et al. Navigating the chaperone network: An integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell. 2005;120(5):715–727. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 16.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 17.Tamai K, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407(6803):530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 18.He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/beta-catenin signaling: Arrows point the way. Development. 2004;131(8):1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- 19.Goel S, et al. Both LRP5 and LRP6 receptors are required to respond to physiological Wnt ligands in mammary epithelial cells and fibroblasts. J Biol Chem. 2012;287(20):16454–16466. doi: 10.1074/jbc.M112.362137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly OG, Pinson KI, Skarnes WC. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131(12):2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- 21.van Amerongen R, Berns A. Knockout mouse models to study Wnt signal transduction. Trends Genet. 2006;22(12):678–689. doi: 10.1016/j.tig.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh JC, et al. Mesd encodes an LRP5/6 chaperone essential for specification of mouse embryonic polarity. Cell. 2003;112(3):355–367. doi: 10.1016/s0092-8674(03)00045-x. [DOI] [PubMed] [Google Scholar]
- 23.Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 2009;174(3):715–721. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mi K, Johnson GV. Regulated proteolytic processing of LRP6 results in release of its intracellular domain. J Neurochem. 2007;101(2):517–529. doi: 10.1111/j.1471-4159.2007.04447.x. [DOI] [PubMed] [Google Scholar]
- 25.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27(21):7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong Z, Baker JJ, Zylstra-Diegel CR, Williams BO. Lrp5 and Lrp6 play compensatory roles in mouse intestinal development. J Cell Biochem. 2012;113(1):31–38. doi: 10.1002/jcb.23324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Korinek V, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19(4):379–383. doi: 10.1038/1270. [DOI] [PubMed] [Google Scholar]
- 29.Ventura A, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445(7128):661–665. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 30.Creamer B, Shorter RG, Bamforth J. The turnover and shedding of epithelial cells. I. The turnover in the gastro-intestinal tract. Gut. 1961;2:110–118. doi: 10.1136/gut.2.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303(5663):1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gattinoni L, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15(7):808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu B, et al. TLR4 up-regulation at protein or gene level is pathogenic for lupus-like autoimmune disease. J Immunol. 2006;177(10):6880–6888. doi: 10.4049/jimmunol.177.10.6880. [DOI] [PubMed] [Google Scholar]
- 34.Madison BB, et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277(36):33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 35.Wanderling S, et al. GRP94 is essential for mesoderm induction and muscle development because it regulates insulin-like growth factor secretion. Mol Biol Cell. 2007;18(10):3764–3775. doi: 10.1091/mbc.E07-03-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu S, et al. The molecular chaperone gp96/GRP94 interacts with Toll-like receptors and integrins via its C-terminal hydrophobic domain. J Biol Chem. 2012;287(9):6735–6742. doi: 10.1074/jbc.M111.309526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Jones RG, et al. Conditional deletion of beta1 integrins in the intestinal epithelium causes a loss of Hedgehog expression, intestinal hyperplasia, and early postnatal lethality. J Cell Biol. 2006;175(3):505–514. doi: 10.1083/jcb.200602160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8(5):387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 40.Staron M, et al. Heat-shock protein gp96/grp94 is an essential chaperone for the platelet glycoprotein Ib-IX-V complex. Blood. 2011;117(26):7136–7144. doi: 10.1182/blood-2011-01-330464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu L, et al. Global survey of human T leukemic cells by integrating proteomics and transcriptomics profiling. Mol Cell Proteomics. 2007;6(8):1343–1353. doi: 10.1074/mcp.M700017-MCP200. [DOI] [PubMed] [Google Scholar]