Abstract
New neurons are continuously added to the dentate gyrus of the adult mammalian brain. During the critical period of a few weeks after birth when newborn neurons progressively mature, a restricted fraction is competitively selected to survive in an experience-dependent manner, a condition for their contribution to memory processes. The mechanisms that control critical stages of experience-dependent functional incorporation of adult newborn neurons remain largely unknown. Here, we identify a unique transcriptional regulator of the functional integration of newborn neurons, the inducible immediate early gene zif268/egr1. We show that newborn neurons in zif268-KO mice undergo accelerated death during the critical period of 2–3 wk around their birth and exhibit deficient neurochemical and morphological maturation, including reduced GluR1 expression, increased NKCC1/KCC2b chloride cotransporter ratio, altered dendritic development, and marked spine growth defect. Investigating responsiveness of newborn neurons to activity-dependent expression of zif268 in learning, we demonstrate that in the absence of zif268, training in a spatial learning task during this critical period fails to recruit newborn neurons and promote their survival, leading to impaired long-term memory. This study reveals a previously unknown mechanism for the control of the selection, functional maturation, and experience-dependent recruitment of dentate gyrus newborn neurons that depends on the inducible immediate early gene zif268, processes that are critical for their contribution to hippocampal-dependent long-term memory.
Keywords: memory consolidation, neurogenesis, plasticity, hippocampus, transcription factor
New neurons are continuously generated in adult life in discrete regions of the mammalian brain, including in the dentate gyrus (DG) of the hippocampus (1). After birth, the majority of newborn dentate granule cells (DGCs) follow a 2-mo race of development to become fully mature and integrate into existing circuits (2–4), a condition for their contribution to hippocampal functions, in particular to hippocampal-dependent learning and memory and spatial pattern discrimination (5). The cells’ involvement as part of neuronal networks supporting learning and memory is believed to be time-locked to a critical stage of maturation (6). From the second week after birth, axons of newborn DGCs elongate (3) and contact hilar and CA3 pyramidal cells (7), then dendrites extend in the molecular layer and spines start to appear (2, 3). Newborn DGCs are initially tonically activated by ambient depolarizing GABA and then hyperpolarized after the gradual conversion of Cl− cotransporter expression (8), along with expression of glutamate receptors and their progressive responsiveness to glutamatergic inputs (8, 9). At this stage, they are hyperexcitable, display properties of enhanced synaptic plasticity, and are prone to integrate the existing hippocampal neurocircuitry (10–12). As their functional maturation progresses, however, newborn DGCs compete to survive, leaving the majority eliminated by cell death (13). Several behavioral manipulations, in particular hippocampal-dependent learning (14), can regulate their rate of survival. To date, the mechanisms that govern maturation, selection, and experience-dependent integration of newborn DGCs remains largely unknown.
Immediate early genes (IEGs) encoding inducible transcription factors are interesting candidates because they are transiently and preferentially induced in newborn DGCs during their critical period of maturation in response to synaptic activity and behavioral experience (4, 15–22). Zif268 (egr1, early growth response 1) is one such IEG that plays a critical role in hippocampal functions and memory processes. The gene is rapidly induced in the hippocampus by learning and recall of several forms of hippocampal-dependent memories (23) and its inactivation severely compromises the formation of several types of memories (24–27), in particular when a high demand on hippocampal function is required (28). Importantly, several studies have shown that Zif268 is induced in young newborn DGCs upon the occurrence of synaptic plasticity (16) and during learning or recall of novel information (4, 15, 17, 21, 22), and can thus direct expression of selective gene programs in activated newborn neurons. These data raise the issue as to whether hippocampal-dependent memory deficits in zif268-KO mice may in part be because of altered neurogenesis. Therefore, we used the birthdating marker BrdU to follow the fate of newly generated cells in zif268-KO mice and asked whether the absence of zif268 has specific consequences for the neurogenic process and the recruitment of newborn DGCs in relation to learning and memory. We demonstrate that zif268 actively controls the survival of newborn DGCs during their critical period of maturation in the first 2–3 wk of their birth. We then demonstrate that in the absence of zif268 the occurrence of spatial learning during this critical period fails to recruit newborn DGCs and to promote their survival, and leads to impaired long-term memory, suggesting that zif268 is required for their functional incorporation into hippocampal memory networks. Mechanistically, we found that the absence of zif268 critically affects neurochemical and morphological maturation of newborn DGCs, a cellular phenotype likely related to their inability to be recruited during learning and participate in establishing long-term memory.
Results
Zif268 Controls the Selection of Newborn DGCs During a Critical Period of Maturation.
We examined the level of neurogenesis in the DG of adult WT and zif268-KO mice 2 h, 7, 14, 21, 28, 34, and 43 d postinjections (dpi) of BrdU and assessed the proportion of new DGCs at each age that express Zif268 in home-cage WT mice (Fig. 1A). In this basal condition, a small proportion of young WT newborn DGCs expressed Zif268, which increased significantly over time (F5,34 = 10.97, P < 0.001). Consistent with previous reports (4, 6), the proportion of Zif268+/BrdU+ cells before 21 dpi was lower than in mature preexisting neurons (Zif268+ in NeuN+ mature neurons: 1.9 ± 0.2%), but became significantly higher in 43-d-old newborn DGCs (Zif268+ in BrdU+ cells: 1.1 ± 0.3%; 0.8 ± 0.3%; 3.0 ± 0.9%; 6.9 ± 2.1%; 2.5 ± 0.6%; 25 ± 2.8%, at 7, 14, 21, 28, 34, and 43 dpi P = 0.007, respectively).
Fig. 1.
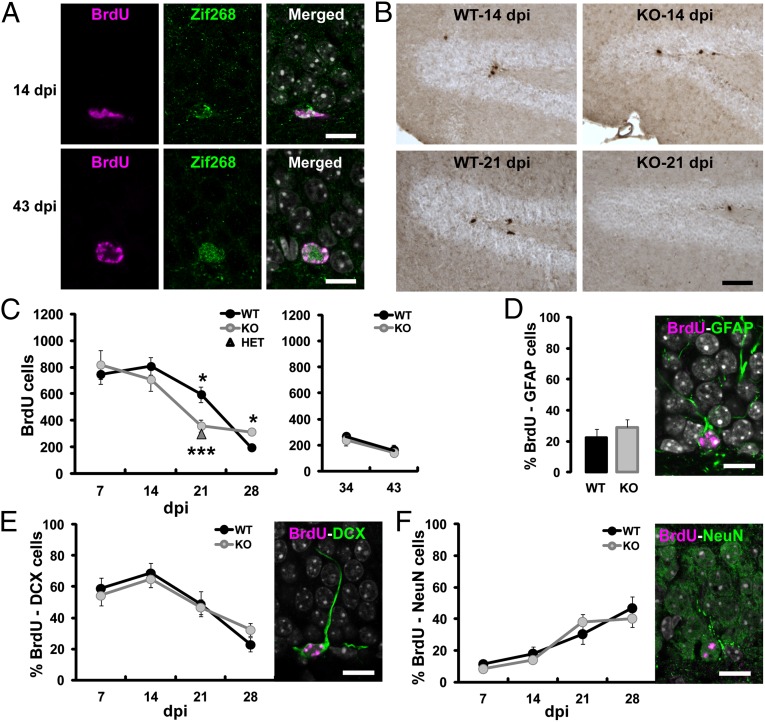
The selection of newborn DGCs during a critical period of maturation is impaired in zif268-KO mice. (A) Confocal micrographs of 14-d-old and 43-d-old newborn cells (BrdU) expressing Zif268 in WT mice. (B) Photomicrographs showing BrdU staining in the DG of WT and zif268-KO mice at 14 and 21 dpi. (C) Total number of BrdU+ cells at different dpi. Zif268-KO and zif268-HET mice showed significantly less BrdU+ cells than WT mice at 21 dpi. *P < 0.05, ***P < 0.005. (D) The proportion of BrdU+/GFAP+ cells at 21 dpi was similar between genotypes. (E and F) The proportion of BrdU+ cells expressing the immature neuronal marker DCX (E) or the mature neuronal marker NeuN (F) over time was identical in zif268-KO and WT mice. (Scale bars: 10 µm in A and D–F; 50 µm in B.) Number of mice: 7 dpi 7 WT, 8 KO; 14 dpi 8 WT, 8 KO; 21 dpi 6 WT, 7 KO; 28 dpi 7 WT, 7 KO; 34 and 43 dpi 6 WT, 6 KO; (HET): 21 dpi n = 5. Data are means ± SEM in this and subsequent figures. *P < 0.05, ***P < 0.005.
Following progenitor cells proliferation, which was not affected in zif268-KO mice (Fig. S1A), a high rate of newborn cells was eliminated both in WT and zif268-KO mice within the first few weeks of their birth (F1,3 = 23, P < 0.001) (Fig. 1 B and C). Whereas WT mice showed a progressive loss of BrdU+ cells between 14 and 43 d (14 vs. 21 dpi, P = 0.05; 21 vs. 28 dpi, P = 0.0002; 34 vs. 43 dpi, P = 0.037) with a peak between 14 and 28 d, as previously reported in mice as opposed to the more progressive elimination in rats (4), the selection was drastically accelerated in zif268-KO mice, with a salient loss between 14 and 21 d (14 vs. 21 dpi, P = 0.009; 21 vs. 28 dpi, P = 0.39; 34 vs. 43 dpi, P = 0.065; genotype effect at 21 dpi, P = 0.011). Remarkably, however, despite a transient homeostatic effect in zif268-KO mice observed at 28 dpi (P = 0.013), likely because of the preceding accelerated apoptosis, the number of cells that did survive at longer delays was in the end similar for the two genotypes (Fig. 1C) (43 dpi P = 0.66). The alteration of neurogenesis in zif268-KO mice is unlikely to be a result of developmental anatomic modifications of the hippocampus, because neuronal architecture and basic DG synaptic functions (25)—as well as DG volume—were not affected (Fig. S1B). The implication of zif268 in adult neurogenesis during a critical window around 21 d was further reinforced by the finding of a similar accelerated loss in the DG of zif268 heterozygous mice (Fig. 1C) (WT vs. heterozygous at 21 dpi P = 0.001), indicating there is no gene-dosage effect, and by a similar reduction of olfactory bulb neurogenesis, the other neurogenic brain area, in zif268-KO mice (Fig. S1 C and D). Double labeling with the glial marker GFAP and the early and late neuronal markers, doublecortin (DCX) and NeuN, revealed no difference between genotypes, indicating normal differentiation rate and neuronal maturation over time among the surviving neurons (4) (Fig. 1 D–F) (GFAP: genotype P = 0.40; DCX: F1,3 = 0.63, time P < 0.0001, genotype P = 0.94; NeuN: F1,3 = 0.87, time P < 0.0001, genotype P = 0.64).
Overall, these results indicate that zif268 has a key role in the survival of adult newborn DGCs during the critical time window within which they are selected to die or survive for long-term functional integration. In the absence of zif268, however, the accelerated death does not affect the whole population of newborn neurons as the number of surviving DGCs several weeks later appears equivalent to that of WT mice.
Zif268 Is Required for Long-Term Recruitment of Newborn DGCs into Spatial Memory Networks.
The accelerated death of newborn DGCs during a critical period of maturation in the absence of zif268 does not indicate whether zif268 also controls activity-dependent functional integration of the remaining pool of newborn DGCs when Zif268 is robustly and transiently expressed in these cells upon learning or recall. To address this issue, we examined whether the absence of Zif268 alters the recruitment of newborn DGCs by spatial learning and their subsequent long-term survival and functional incorporation. We used the spatial Morris water-maze task with a massed protocol that results in long-lasting spatial memory and increased incorporation of new neurons in the adult DG (22). Guided by evidence indicating that new neurons within 1–3 wk after birth have a unique role in hippocampal-dependent memory formation (6), and by the differential requirement for zif268 observed above in populations of newborn DGCs of different ages (Fig. 1C), we submitted WT and zif268-KO mice to spatial learning 9 or 18 d after BrdU injections and analyzed memory performance and survival rate 25 d after acquisition (Fig. 2A).
Fig. 2.
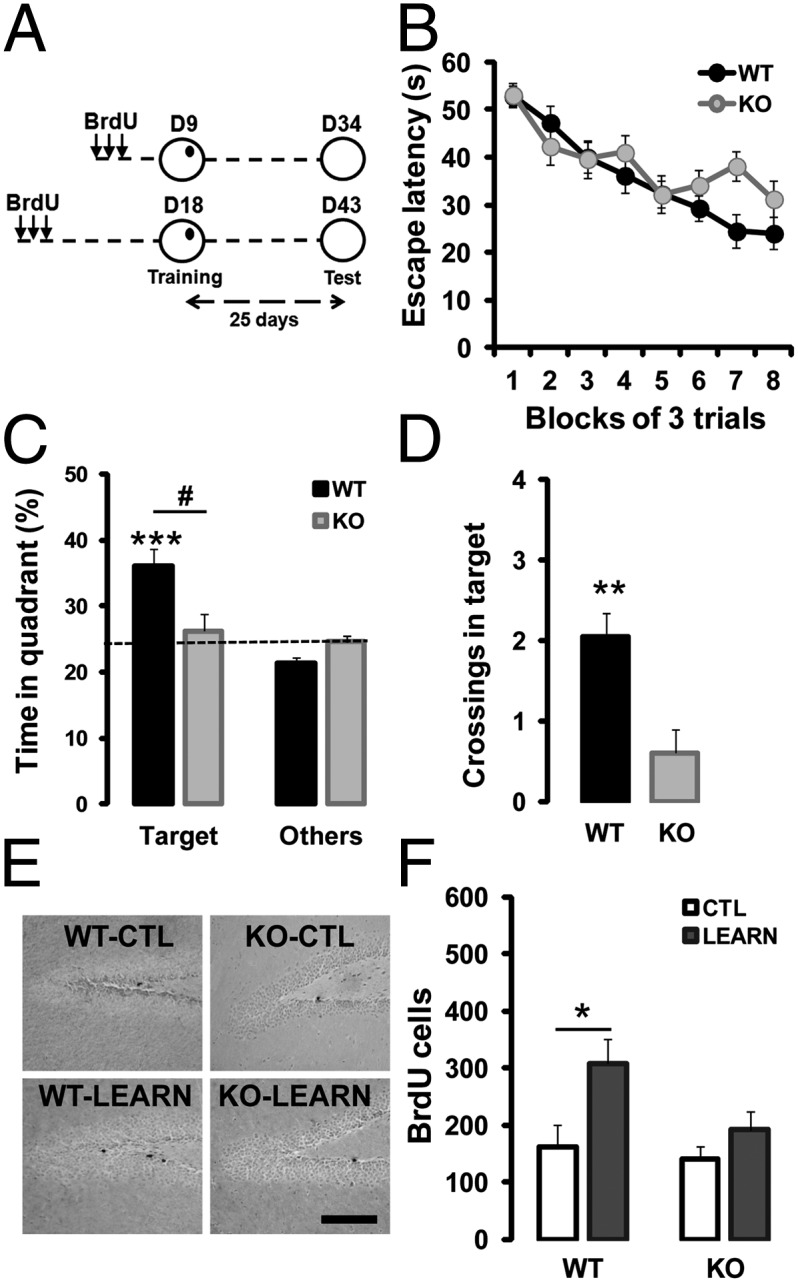
Zif268 is required for spatial learning-induced survival of 18-d-old adult-born DGCs. (A) Mice received three injections of BrdU on day 1, were submitted to spatial learning in the water maze on day 9 (D9) or 18 (D18) and to retention test 25-d later. (B) During training, latency to find the hidden platform declined equally in WT (n = 16) and zif268-KO mice (n = 14). (C) Twenty-five days after acquisition, WT mice spent significantly more time searching in the target quadrant compared with other quadrants, unlike zif268-KO mice that did not. (D) Significantly higher number of crossings over the target location in WT than in zif268-KO mice. (E) Photomicrographs showing BrdU staining in WT and zif268-KO mice at 43 dpi in control (CTL) and 25 d after spatial learning (LEARN). (Scale bar, 100 µm.) (F) Training promoted long-term survival of BrdU+ newborn cells aged 18 d at the time of acquisition in WT mice, but not in zif268-KO. CTL: WT n = 6; KO n = 6; LEARN: WT n= 10; KO n = 8. *,#P < 0.05, **P < 0.01, ***P < 0.005.
As expected (25), both WT and zif268-KO mice from the two groups learned to locate the hidden platform (Fig. 2B) (F1,28 = 1.22, time P < 0.0001; genotype P = 0.28; interaction P = 0.061). In the 18-d group, performance during probe tests in the absence of the platform 25 d after training showed that WT mice formed a long-term spatial memory, whereas zif268-KO mice showed no evidence of long-term memory [(Fig. 2C) target quadrant: WT P = 0.0024, KO P = 0.66; genotype P = 0.035 (Fig. 2D) crossings: genotype P = 0.007] as previously reported (25). Counting BrdU+ cells after retention, we found that survival of new cells aged 18 d at the time of learning was largely increased (by ∼twofold) in WT mice compared with WT home cage controls (Fig. 2 E and F) (F1,14 = 4.78, P = 0.046). In contrast, training did not promote survival of newborn cells of the same age in zif268-KO mice (Fig. 2 E and F) (F1,12 = 1.35, P = 0.27). In the 9-d group, performance at test replicated the above results showing long-term spatial memory impairment in zif268-KO mice (Fig. S2A). Although survival of 9-d-old neurons can be enhanced after more prolonged training regimes, such as in distributed spatial learning (29), neither in WT nor in zif268-KO mice did massed training have any enhancing effect on survival of 9-d-old cells (Fig. S2 B and C). These results indicate that zif268 plays an essential role for spatial training-induced survival of 18-d-old, but not 9-d-old newborn neurons.
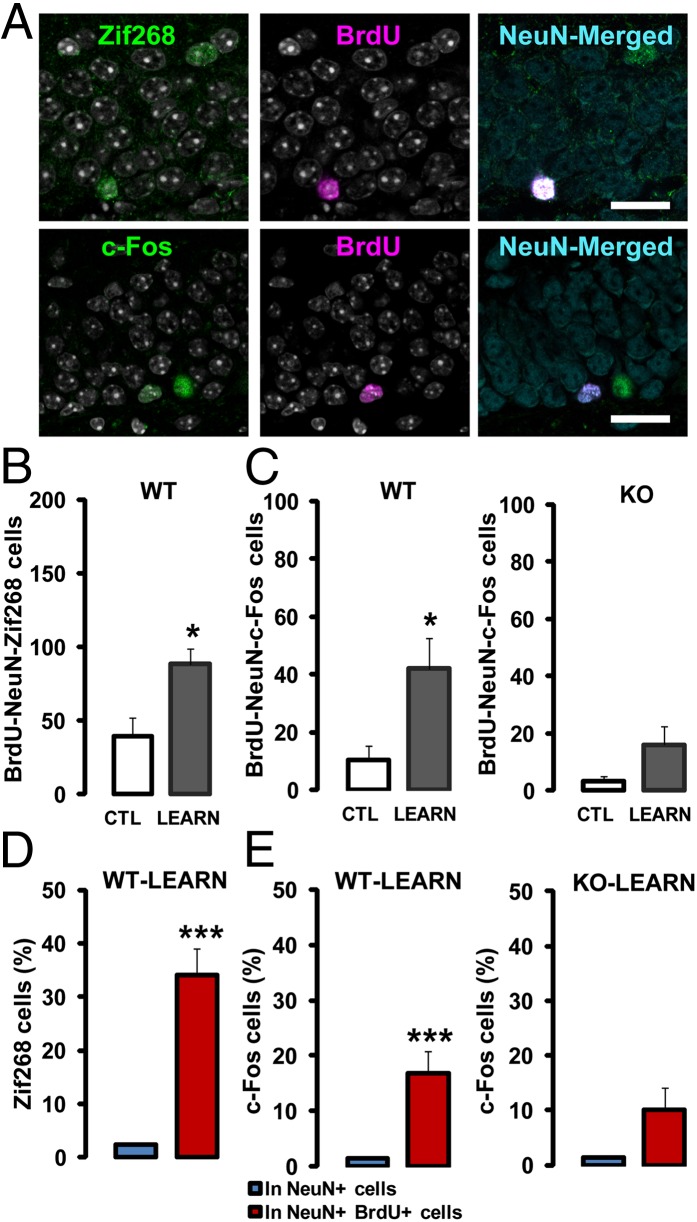
We next asked whether zif268 is required for the functional recruitment of newborn neurons by mapping the expression of Zif268 and c-Fos in BrdU+/NeuN+ DGCs, 90 min after memory recall (19, 22). In WT mice, a much higher number of 18-d-old BrdU+/NeuN+ DGCs expressed Zif268 and c-Fos upon retrieval compared with WT controls (Fig. 3 A–C) (Zif268: P = 0.011, c-Fos: P = 0.034). Interestingly, the percentage of newborn DGCs expressing Zif268 or c-Fos postrecall was for both genes much higher than the percentage of preexisting DGCs expressing Zif268 or c-Fos [(Fig. 3D) Zif268: P < 0.001; (Fig. 3E) c-Fos: P = 0.002], suggesting preferential activation of newborn neurons during recall, as reported in other tasks (19, 22). In contrast, the proportion of activated, BrdU+/NeuN+/c-Fos+ cells in zif268-KO mice was low and not significantly different from that observed in home-cage zif268-KO controls and there was no evidence of preferential activation of newborn DGCs [(Fig. 3C) P = 0.13; (Fig. 3E) P = 0.053], indicating that the long-term memory deficit in these mice is associated with the absence of long-lasting newborn DGCs recruitment. Younger newborn neurons, aged 9 d at the time of learning—besides not being more incorporated during acquisition (Fig. S2 B and C)—were neither more recruited (Fig. S3 A–C) nor preferentially activated (Fig. S3 D and E) upon recall in either WT mice, as previously described (22), or zif268-KO mice. In all, these findings indicate that zif268 controls the long-lasting recruitment of newborn DGCs during the critical period when they are prone to being incorporated for spatial memory formation and long-term consolidation.
Fig. 3.
Zif268 is required for the recruitment of 18-d-old neurons by spatial learning. (A) Confocal micrographs of 43-d-old BrdU+/NeuN+ neurons expressing Zif268 or c-Fos in a WT mouse. (Scale bars, 30 µm.) (B) Upon spatial memory recall, the number of BrdU+/NeuN+ DGCs expressing Zif268 was substantially increased in WT mice. (C) The number of BrdU+/NeuN+ DGCs expressing c-Fos upon recall was also increased in WT mice, but not in zif268-KO mice. (D) Upon recall, a higher proportion of newborn DGCs in WT mice expressed Zif268, compared with preexisting neurons. (E) Similarly, the proportion of c-Fos–expressing neurons was higher for newborn than for preexisting DGCs in WT mice, in contrast to zif268-KO mice for which the number of c-Fos+ newborn DGCs was only slightly, but not significantly higher than that of preexisting DGCs. CTL: WT n = 6; KO n = 6; LEARN: WT n = 9; KO n = 8. *P < 0.05, ***P < 0.005.
Zif268 Controls the Functional and Morphological Maturation of Newborn DGCs.
To understand how lack of zif268 has such profound consequences on survival and recruitment of newborn DGCs, we evaluated their functional maturation at this delay (21 dpi) and thereafter (43 dpi). Around 3 wk of age, new DGCs undergo extensive morphological and synaptic changes that are essential for their recruitment and survival (6). We hypothesized that in the absence of zif268, the functional maturation of differentiated newborn DGCs at the time of learning may be incomplete, resulting in their recruitment failure during learning and, hence, deficient survival. To test this hypothesis, we first analyzed GluR1 expression in 21-dpi DGCs, when they start to receive glutamatergic inputs (7, 9), and at the later age of 43 dpi. We found that the proportion of 21-d-old newborn DGCs expressing GluR1 was reduced in zif268-KO mice (Fig. 4A) (P = 0.036), indicating immature glutamatergic function, although attaining WT level of expression at 43 dpi (P = 0.60). Then, we evaluated the expression pattern of the Cl− ionic cotransporters NKCC1 and KCC2 implicated in the conversion from GABA-induced depolarization to hyperpolarization, a mechanism crucial for synaptic integration of 3-wk-old DGCs (8). We found an imbalance in the expression of the two Cl− cotransporters in 21-dpi DGCs of zif268-KO mice, with a larger expression of NKCC1 associated with reduced expression of KCC2 (Fig. 4 B and C) (NKCC1: P = 0.027; KCC2: P = 0.025), again attaining WT expression levels at 43 dpi (P = 0.93 and P = 0.54, respectively). Thus, the absence of Zif268 results in a delayed maturation of GABAergic and glutamatergic functions of newborn DGCs.
Fig. 4.
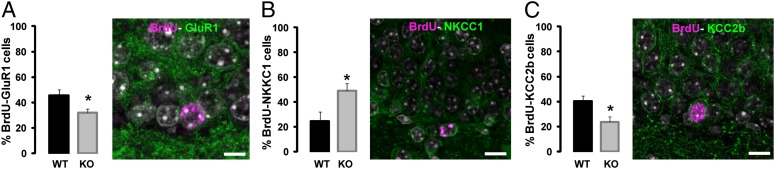
Zif268 regulates the functional maturation of newborn DGCs. (A) Expression of GluR1 was impaired in 21-d-old newborn BrdU+ DGCs in zif268-KO mice. (Scale bar, 8 µm.) (B and C) Expression of NKCC1 is higher in 21-d-old newborn BrdU+ DGCs in zif268-KO mice (B), whereas expression of KCC2b is impaired (C). (Scale bars, 10 µm.) WT n = 6; KO n = 7. *P < 0.05.
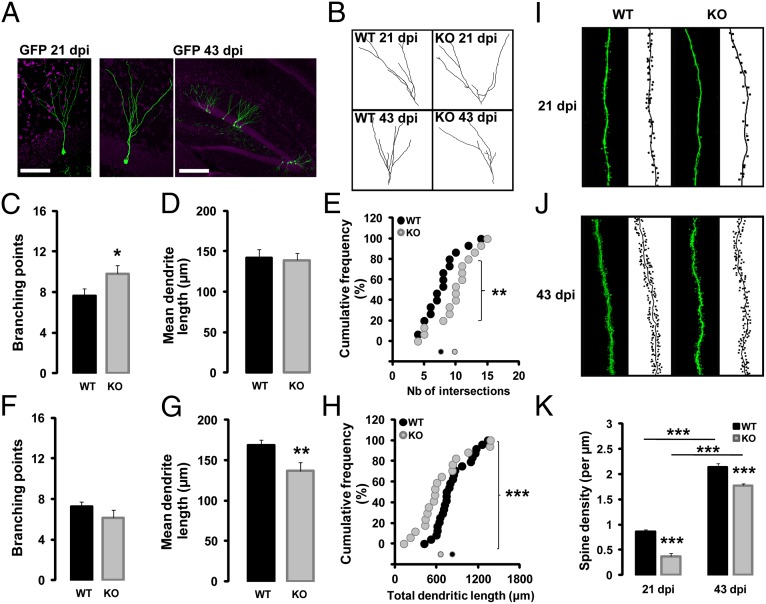
Next, using a retroviral vector strategy to express GFP specifically in proliferating cells and their progeny, we assessed morphological maturation of newborn DGCs in WT and zif268-KO mice at 21 and 43 dpi because dendritic development and spine growth are maximal during this time interval (3, 7). At 21 and 43 dpi, GFP-labeled DGCs were located in the innermost part of the granule cell layer and dendritic processes arborized extensively in the molecular layer (Fig. 5A). There was no apparent alteration in zif268-KO mice in axonal projections of new DGCs in area CA3 at these delays (Fig. S4A), indicating normal efferent connectivity. There were, however, clear differences in neuronal morphology between WT and zif268-KO mice (Fig. 5B). The majority of 21-dpi DGCs from zif268-KO mice exhibited increased branching points (Fig. 5C) (P = 0.046); (Fig. 5E), (P = 0.007), without modification of dendritic length (Fig. 5D) (P = 0.84) (Fig. S4 B–D). The increased number of nodes appeared for a large part because of the first intersection after the primary dendrite, which in zif268-KO mice was closer to the soma (Fig. S4E). Dendritic growth continued for 3 wk in WT mice but not in zif268-KO mice, which ended up by having 43-dpi DGCs with shorter dendritic arborization [(Fig. 5 D vs. G) 21 vs. 43 dpi, WT: P = 0.025; KO: P = 0.86; (Fig. 5 F–H) genotype: (Fig. 5F) P = 0.21; (Fig. 5G) P = 0.008; (Fig. 5H) P = 0.003) (Fig. S4 F–I). Defective dendritic development in zif268-KO mice was not the consequence of impaired formation of primary cilia occurring around 21 d after birth of newborn DGCs and which controls dendrites formation (30) (Fig. S5).
Fig. 5.
Zif268 regulates the morphological maturation and spine formation of newborn DGCs. (A) Confocal 3D reconstruction of dendrites of newborn GFP-labeled DGCs, 21 and 43 d after retroviral vector injection in the DG of WT mice. (Scale bars: 21 dpi 50 µm; 43 dpi 150 µm.) (B) Skeletons of newborn neurons in WT and zif268-KO mice at 21 and 43 dpi. (C) Number of branching points at 21 dpi was increased in zif268-KO mice. (D) Mean dendrite length at 21 dpi was similar between genotypes. (E) Quantification of branch number in 21-d-old newborn DGCs. Each symbol represents data from a single newborn DGC. (F) The number of branching points at 43 dpi was similar between genotypes. (G) Mean dendrite length at 43 dpi was decreased in zif268-KO mice. (H) Quantification of total dendritic length in 43-d-old newborn DGCs. (I) Confocal 3D images of dendrites with spines and their skeletons of 21 and (J) 43-d-old newborn DGCs in WT and zif268-KO mice. (K) Spine density was impaired in zif268-KO mice, both at 21 and 43 dpi. WT n = 16; KO n = 16 in C–E; WT n = 25; KO n = 18 in F–H. *P < 0.05, **P < 0.01, ***P < 0.005.
To explore terminal stages of maturation and assess whether newborn DGCs reach a fully mature state in mutant mice, we compared spine density and expansion during the exponential phase of spine growth (3, 7). Spine quantification on apical dendrites of 21-dpi newborn DGCs revealed a deficit in spine density in zif268-KO mice compared with WT mice (Fig. 5 I–K). There was an increase in spine density between 21 and 43 d in both groups, but even at 43 dpi spine density in zif268-KO mice remained significantly lower than in WT mice (Fig. 5 I–K) (F1,68 = 10.51, time P < 0.001, genotype P < 0.001, interaction P = 0.0018). This alteration in spine density was observed across the whole population of 21- and 43-dpi DGCs examined (Fig. S6 A and B), indicating a robust effect, not because of a particular sensitivity of a subpopulation of newborn DGCs to zif268 deficiency.
Discussion
The number of surviving newborn neurons in the adult brain is thought to depend largely on their maturation and functional integration into the preexisting networks, an experience-dependent process that contributes to learning and memory (5). The mechanisms that govern critical stages of experience-dependent functional incorporation of adult newborn DGCs remain largely unknown. The present study is unique in showing that one IEG encoding an inducible transcription factor, zif268, controls major processes in the time-frame of maturation of adult newborn DGCs during the critical period within which they are selected to survive for long-term functional integration into preexisting hippocampal networks (6). The zif268-dependent selection process takes place between 2 and 3 wk of their birth, when they start to receive synaptic glutamatergic input (7, 9), undergo conversion of GABA-mediated depolarization to hyperpolarization (8), and maximally develop dendritic arbors and spines (3, 7); selective maturation processes that are all impaired in the absence of zif268. This stalled and incomplete maturation of surviving newborn DGCs within this critical period is likely to be a salient functional cause of their unsuccessful recruitment and functional incorporation into memory networks, as evidenced by the failure of training in a spatial learning task to promote their survival and lack of activation upon memory recall. Although we cannot formally rule out a developmental effect of zif268 mutation, present data showing normal DG volume, neurogenic niche, plasticity marker expression, and previous results indicating normal neural functions, such as basic place cell properties, synaptic transmission and cell-signaling up to the zif268 promoter, neuronal excitability, presynaptic functions, recurrent inhibition, short-term plasticity, as well as normal learning and short-term memory in various tasks despite impaired long-term memory, argue against developmental defects in these mice (24, 25, 31).
Several extrinsic and intrinsic mechanisms have been identified as important regulators of the survival, maturation, and functional integration of adult-born DGCs (32, 33). These mechanisms include NMDA receptors implicated in glutamate-dependent survival and synaptic plasticity of newborn DGCs, GABA-induced depolarization required for dendritic development and synapse formation, the neurotrophic factors BDNF/TrkB, FGF-2, and NT3 [the absence of which decreases neurogenesis, impairs long-term potentiation (LTP), increases anxiety-like behavior, and impairs spatial memory], as well as the transcription regulators CREB, Cdk5, disc1, Klf-9, and the epigenetic factors Gadd45, miR132, miR124, and repressor element 1 (RE-1) protein-silencing transcription factor (REST)/neuron-restrictive silencing factor (NRSF) that differentially modulate survival, dendritic growth, synaptic plasticity and integration of newborn neurons in the adult hippocampus. Interestingly, the majority of these factors could be downstream transcriptional targets of zif268. BDNF/NT3/FGF have been identified as direct (34) and indirect targets of Zif268 via a Gadd45-dependent DNA demethylation pathway (35). The role of Cdk5-p35 complex in neurogenesis requires ERK activation and induction of zif268 (36). The qualitative change in GABA-mediated responses from depolarizing to hyperpolarizing in adult-born DGCs is related to increased KCC2b expression, which can be directly controlled by stimulating EGR consensus DNA binding sites on KCC2b promoter (37). Finally, the negative regulator REST/NRSF that controls the rate of adult neurogenesis by partly orchestrating the expression of miR124 and miR132 (38), might regulate transcriptional responses induced by Zif268 expression (39). These findings provide potential molecular routes by which Zif268 could control various stages of the neurogenic process in a cell-autonomous manner, a hypothesis consistent with the reported normal cortico-hippocampal architecture, synaptic transmission, and neuronal excitability in zif268-KO mice (25), as well as with the parallel reported here between the normal early and impaired late stages of DGCs maturation in relation to Zif268 expression in adult-born DGCs. However, at this point we cannot discard a noncell-autonomous function of Zif268. Cell type-specific manipulation of zif268 using genetic approaches will be one step further for dissociating cell-autonomous and noncell-autonomous contribution of zif268.
Interestingly, zif268 in the DG is expressed in an exclusively activity-dependent manner. As such, it is to our knowledge unique in being a gene with this property of having a strong and specific role in survival and maturation of adult-born DGCs during the critical period within which they establish appropriate connectivity and have unique functional properties relevant to hippocampal information processing (6, 40, 41). At any given time at the basal level, very few Zif268+ DGCs can be found, reflecting the activity-dependent transient expression and rapid degradation of the protein. In contrast, Zif268 is conspicuously expressed in a sparse, although reliable population of DGCs, including newborn DGCs, time-locked with the occurrence of synaptic plasticity (16) or of learning or recall (4, 15, 17, 21, 22), as also observed here. This feature appears critical for newborn DGC function in memory formation. From the present findings, we propose that in the behaving animal under low solicitation transient expression of Zif268 occurring in 2–3-wk-old DGCs is critical to their selection and progression of their functional and morphological maturation (Fig. S7A). When a specific learning experience occurs, a number of 3-wk-old newborn DGCs—because of their high intrinsic excitability, reduced GABAergic inhibition, and high capacity for undergoing synaptic potentiation (40, 42, 43)—would be preferentially activated by activity patterns entering the DG and express Zif268 (and other IEGs) (4, 15–22), promoting their recruitment, maturation, survival, and functional integration into memory networks, and hence their subsequent recruitment upon memory recall (Fig. S7C). In the absence of zif268, 3-wk-old newborn DGCs harbor less mature functional and morphological features (Fig. S7B). Their reduced potential for being activated by glutamatergic inputs would impede their recruitment by training (Fig. S7D), preventing their contribution to long-term spatial memory (44). Our previous studies in zif268-KO mice have shown that zif268 is required for the expression of late-phase hippocampal LTP (25), long-term stability of hippocampal place cell representations (31), and consolidation of several forms of long-term memory (25, 28). Memory deficits in the absence of zif268 are unlikely to be caused exclusively by the altered neurogenic function in the DG. In this area, information processing will hence be subjected to at least two types of plasticity disorders: that affecting neurogenesis and that affecting synaptic plasticity. There is evidence to suggest that adult-generated DGCs play a significant role in DG synaptic plasticity (11, 12) and, conversely, the induction of synaptic plasticity promotes neurogenesis (16), suggesting these two forms of plasticity are tightly coupled. The cause and consequence nature in the neurogenesis–LTP relationship, whether there is a prime and secondary cause underlying the memory deficits in the absence of zif268 or whether both directions of causation operate simultaneously, remains a challenge for future research.
Methods
Animals and BrdU Injections.
Zif268-KO mice, zif268-heterozygous, and WT male mice (2- to 3-mo-old) were used. Mice received one intraperitoneal BrdU injection (50 mg/kg) for cell proliferation, five every 2 h (50 mg/kg) for cell survival, and three every 4 h (100 mg/kg) for cell survival after learning experiments.
Morris Water-Maze Training.
Mice were trained to locate a hidden platform at a fixed location using a massed-training procedure and retention was tested 25 d later, as detailed in SI Methods.
Retrovirus-Mediated Labeling of New Neurons.
Moloney murine retroviruses expressing GFP were injected in the DG. Labeled neurons were analyzed using NeuronStudio software, as described in SI Methods.
Immunohistochemistry.
Tissue preparation, immunochemistry, and cell counting were carried out as described in SI Methods.
Statistical Analyses.
Data were expressed as mean ± SEM. Statistical comparisons were conducted by two-way ANOVA followed by t test.
Supplementary Material
Acknowledgments
We thank N. Spassky for gift of adenylate cyclase III antibody, S. Scotto-Lomassesse for her help in morphological analysis, Lydie Collet for art work, and P. Veyrac and N. Devignes for animal care and genotyping. Zif268-KO mice were bred in Orsay from breeders generously provided by P. Charnay, P. Topliko, and S. Garel. This research was funded by Centre National de la Recherche Scientifique, Université Paris-Sud, and supported by Agence Nationale Recherche Grant ANR-2010-BLAN-1413-01 (to S.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1220558110/-/DCSupplemental.
References
- 1.Ming GL, Song H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snyder JS, et al. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29(46):14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng W, Aimone JB, Gage FH. New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11(5):339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aasebø IE, Blankvoort S, Tashiro A. Critical maturational period of new neurons in adult dentate gyrus for their involvement in memory formation. Eur J Neurosci. 2011;33(6):1094–1100. doi: 10.1111/j.1460-9568.2011.07608.x. [DOI] [PubMed] [Google Scholar]
- 7.Toni N, Sultan S. Synapse formation on adult-born hippocampal neurons. Eur J Neurosci. 2011;33(6):1062–1068. doi: 10.1111/j.1460-9568.2011.07604.x. [DOI] [PubMed] [Google Scholar]
- 8.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439(7076):589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagihara H, Ohira K, Toyama K, Miyakawa T. Expression of the AMPA receptor subunits GluR1 and GluR2 is associated with granule cell maturation in the dentate gyrus. Front Neurosci. 2011;5:100. doi: 10.3389/fnins.2011.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mongiat LA, Schinder AF. Adult neurogenesis and the plasticity of the dentate gyrus network. Eur J Neurosci. 2011;33(6):1055–1061. doi: 10.1111/j.1460-9568.2011.07603.x. [DOI] [PubMed] [Google Scholar]
- 11.Saxe MD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103(46):17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85(6):2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 13.Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291(1):17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- 14.Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2(3):260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 15.Tashiro A, Makino H, Gage FH. Experience-specific functional modification of the dentate gyrus through adult neurogenesis: A critical period during an immature stage. J Neurosci. 2007;27(12):3252–3259. doi: 10.1523/JNEUROSCI.4941-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruel-Jungerman E, Davis S, Rampon C, Laroche S. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci. 2006;26(22):5888–5893. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jessberger S, Kempermann G. Adult-born hippocampal neurons mature into activity-dependent responsiveness. Eur J Neurosci. 2003;18(10):2707–2712. doi: 10.1111/j.1460-9568.2003.02986.x. [DOI] [PubMed] [Google Scholar]
- 18.Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26(47):12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kee N, Teixeira CM, Wang AH, Frankland PW. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10(3):355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 20.Snyder JS, Radik R, Wojtowicz JM, Cameron HA. Anatomical gradients of adult neurogenesis and activity: young neurons in the ventral dentate gyrus are activated by water maze training. Hippocampus. 2009;19(4):360–370. doi: 10.1002/hipo.20525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snyder JS, Clifford MA, Jeurling SI, Cameron HA. Complementary activation of hippocampal-cortical subregions and immature neurons following chronic training in single and multiple context versions of the water maze. Behav Brain Res. 2012;227(2):330–339. doi: 10.1016/j.bbr.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trouche S, Bontempi B, Roullet P, Rampon C. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proc Natl Acad Sci USA. 2009;106(14):5919–5924. doi: 10.1073/pnas.0811054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis S, Bozon B, Laroche S. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav Brain Res. 2003;142(1–2):17–30. doi: 10.1016/s0166-4328(02)00421-7. [DOI] [PubMed] [Google Scholar]
- 24.Bozon B, Davis S, Laroche S. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron. 2003;40(4):695–701. doi: 10.1016/s0896-6273(03)00674-3. [DOI] [PubMed] [Google Scholar]
- 25.Jones MW, et al. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4(3):289–296. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- 26.Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304(5672):839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- 27.Malkani S, Wallace KJ, Donley MP, Rosen JB. An egr-1 (zif268) antisense oligodeoxynucleotide infused into the amygdala disrupts fear conditioning. Learn Mem. 2004;11(5):617–624. doi: 10.1101/lm.73104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bozon B, Davis S, Laroche S. Regulated transcription of the immediate-early gene Zif268: Mechanisms and gene dosage-dependent function in synaptic plasticity and memory formation. Hippocampus. 2002;12(5):570–577. doi: 10.1002/hipo.10100. [DOI] [PubMed] [Google Scholar]
- 29.Sisti HM, Glass AL, Shors TJ. Neurogenesis and the spacing effect: Learning over time enhances memory and the survival of new neurons. Learn Mem. 2007;14(5):368–375. doi: 10.1101/lm.488707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumamoto N, et al. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat Neurosci. 2012;15(3):399–405. doi: 10.1038/nn.3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Renaudineau S, Poucet B, Laroche S, Davis S, Save E. Impaired long-term stability of CA1 place cell representation in mice lacking the transcription factor zif268/egr1. Proc Natl Acad Sci USA. 2009;106(28):11771–11775. doi: 10.1073/pnas.0900484106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun J, Sun J, Ming GL, Song H. Epigenetic regulation of neurogenesis in the adult mammalian brain. Eur J Neurosci. 2011;33(6):1087–1093. doi: 10.1111/j.1460-9568.2011.07607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mu Y, Lee SW, Gage FH. Signaling in adult neurogenesis. Curr Opin Neurobiol. 2010;20(4):416–423. doi: 10.1016/j.conb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumgärtel K, et al. Changes in the proteome after neuronal zif268 overexpression. J Proteome Res. 2009;8(7):3298–3316. doi: 10.1021/pr801000r. [DOI] [PubMed] [Google Scholar]
- 35.Ma DK, et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323(5917):1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harada T, Morooka T, Ogawa S, Nishida E. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat Cell Biol. 2001;3(5):453–459. doi: 10.1038/35074516. [DOI] [PubMed] [Google Scholar]
- 37.Ludwig A, et al. Early growth response 4 mediates BDNF induction of potassium chloride cotransporter 2 transcription. J Neurosci. 2011;31(2):644–649. doi: 10.1523/JNEUROSCI.2006-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao Z, Ding P, Hsieh J. Profiling of REST-dependent microRNAs reveals dynamic modes of expression. Front Neurosci. 2012;6:67. doi: 10.3389/fnins.2012.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Loo KM, et al. Transcriptional regulation of T-type calcium channel CaV3.2: Bi-directionality by early growth response 1 (Egr1) and repressor element 1 (RE-1) protein-silencing transcription factor (REST) J Biol Chem. 2012;287(19):15489–15501. doi: 10.1074/jbc.M111.310763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marín-Burgin A, Mongiat LA, Pardi MB, Schinder AF. Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science. 2012;335(6073):1238–1242. doi: 10.1126/science.1214956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aimone JB, Deng W, Gage FH. Resolving new memories: A critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70(4):589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429(6988):184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 43.Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54(4):559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arruda-Carvalho M, Sakaguchi M, Akers KG, Josselyn SA, Frankland PW. Posttraining ablation of adult-generated neurons degrades previously acquired memories. J Neurosci. 2011;31(42):15113–15127. doi: 10.1523/JNEUROSCI.3432-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.