Abstract
The migration and positioning of osteoclast precursor monocytes are controlled by the blood-enriched lipid mediator sphingosine-1-phosphate (S1P) and have recently been shown to be critical points of control in osteoclastogenesis and bone homeostasis. Here, we show that calcitriol, which is the hormonally active form of vitamin D, and its therapeutically used analog, eldecalcitol, inhibit bone resorption by modulating this mechanism. Vitamin D analogs have been used clinically for treating osteoporosis, although the mode of its pharmacologic action remains to be fully elucidated. In this study, we found that active vitamin D reduced the expression of S1PR2, a chemorepulsive receptor for blood S1P, on circulating osteoclast precursor monocytes both in vitro and in vivo. Calcitriol- or eldecalcitol-treated monocytoid RAW264.7 cells, which display osteoclast precursor-like properties, migrated readily to S1P. Concordantly, the mobility of circulating CX3CR1+ osteoclast precursor monocytes was significantly increased on systemic administration of active vitamin D. These results show a mechanism for active vitamin D in controlling the migratory behavior of circulating osteoclast precursors, and this action should be conducive to limiting osteoclastic bone resorption in vivo.
Keywords: chemotaxis, imaging, treatment, immunology, circulation
Bone is a highly dynamic organ, and it is continuously remodeled cooperatively by bone-resorbing osteoclasts and bone-replenishing osteoblasts (1). Osteoclasts, which have bone-resorbing capacity, are a unique cell type differentiated from monocyte/macrophage lineage hematopoietic precursor cells termed osteoclast precursors. Previous studies have identified key molecular signals, such as mediated by macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL), that regulate osteoclastic differentiation and function (2, 3). Unlike osteoblasts, which are of mesenchymal origin and essentially reside in bone tissues, osteoclasts and their precursor monocytes are highly dynamic. Their migratory mechanisms in systemic circulation and homing into bone spaces have recently emerged as critical points of control for osteoclastogenesis and thus, bone homeostasis. We have recently used intravital two-photon microscopy to visualize the bone tissues of live mice and found that sphingosine-1-phosphate (S1P), a lysophospholipid mediator enriched in blood, plays a vital role in regulating the migration and positioning of osteoclast precursors on the bone surface (4, 5).
Osteoclast precursor monocytes express S1PR1 (formerly designated as S1P1 or Edg-1), a cognate receptor for S1P, and can use this receptor to migrate from bone tissues to blood that contains S1P. The deletion of S1PR1 in monocytoid cells leads to an accumulation of osteoclast precursors and a resultant increase in bone resorption, which suggests that the S1P–S1PR1 interaction is essential for the recirculation of osteoclast precursors from bone to blood (4). The expression of S1PR1 was suppressed on stimulation with RANKL, representing a reasonable mechanism where monocytoid precursors, after initiating a commitment to osteoclast differentiation, can no longer recirculate into the blood.
Successive studies have shown that osteoclast precursors also express S1PR2 (S1P2 or Edg-5), another cognate receptor that negatively regulates S1P (4, 6). Although S1PR1 exerts positive chemotaxis to an S1P gradient, S1PR2 inhibits the positive chemotaxis induced by S1PR1 or induces migration in the inverse direction along the S1P gradient—so-called chemorepulsion (7). The deletion of S1PR2 led to moderate osteopetrosis attributable to a decrease in osteoclast recruitment onto the bone surface, indicating that S1PR2-mediated chemorepulsion against blood S1P contributes to the homing of osteoclast precursors into bone spaces (6). Most studies have shown that the migration of osteoclast precursors is reciprocally regulated by two counteracting receptors, circulation-attractive S1PR1 and bone-tropic S1PR2, and that their entrance into and exit from the bones are finely tuned by these accelerators and brakes. More importantly, it was shown that either activation of S1PR1 or blockade of S1PR2 could relieve bone density loss in a murine osteoporosis model by inhibiting bone homing of osteoclast precursor monocytes (4, 6). These results have garnered great attention regarding drug discovery. Many of the antibone-resorptive agents developed thus far, including bisphosphonate (8) and cathepsin K inhibitors (9, 10), target fully matured osteoclasts, whereas treatments targeting monocytoid early osteoclast precursors, such as S1P modulators, would provide a unique line of therapy for bone loss. Here, we reveal that an antibone-resorptive drug with this type of pharmacologic profile already exists—the vitamin D hormone.
Vitamin D was first identified as an antirachitic factor and has been clinically shown to improve calcium balance in both young and elderly populations. Active vitamin D analogs have been used clinically in several countries for treating bone and mineral disorders associated with chronic kidney diseases or osteoporosis, although their direct pharmacologic mechanisms in bone are not fully understood (11, 12). Notably, active vitamin D metabolites, such as calcitriol [1α,25(OH)2D3 (1,25-D)], have been shown to increase the expression of RANKL in bone marrow stromal cells, thereby acting as osteoclastogenic, bone-resorbing factors (13, 14). These findings have raised an intractable paradox regarding the action on bone of vitamin D (15–17).
In the present study, we first show that the active form of vitamin D, 1,25-D, and its clinically used analog, eldecalcitol (ELD), significantly suppress the expression of bone-tropic S1PR2 in circulating osteoclast precursor monocytes and block osteoclastic bone resorption by mobilizing precursor monocytes from the bone to the blood. This study shows that the control of osteoclast precursor monocyte migration is a target of the antibone-resorptive action of vitamin D.
Results
Active Vitamin D, 1,25-D, and Its Therapeutically Used Analog, ELD, Suppress the Expression of S1PR2 in Circulating Osteoclast Precursor Monocytes.
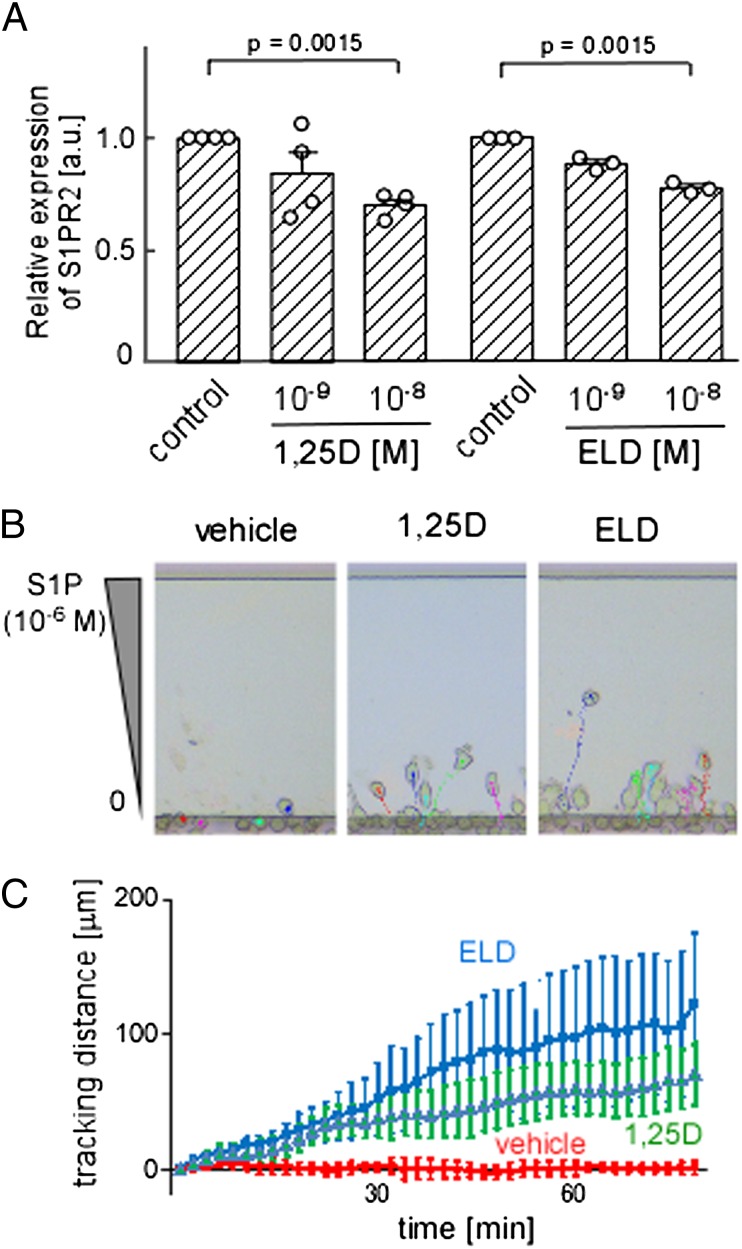
To investigate the potential role of vitamin D signaling in the control of osteoclast precursor monocyte mobilization, we examined the effects of the active form of vitamin D, 1,25-D, and its clinically used active vitamin D analog, ELD, on the expression of several chemokine receptors in a monocytoid cell line, RAW264.7, which because it can be differentiated into giant osteoclast-like cells on stimulation with RANKL, is considered to be a cell line with osteoclast precursor-like capacity (18). Both 1,25-D and ELD significantly suppressed the expression of S1PR2 in a dose-dependent manner (Fig. 1A), whereas the expression levels of other chemokine receptors, including S1PR1, CXCR4, and CX3CR1, were essentially unaltered (Fig. S1). This down-regulation of S1PR2 was observed only in RAW264.7 cells that were cultured under floating conditions; these cells tend to undergo transformation into macrophage-like cells when grown in adherent cultures (Fig. S2A), which leads to reduced expression of the vitamin D receptor (VDR) (Fig. S2B) and loss of responsiveness to VDR stimulation (Fig. S2C). These results suggest that this regulation of expression is critically dependent on VDR in these cell types.
Fig. 1.
Suppression of S1PR2 expression and gain of chemotactic activity to S1P in RAW264.7 osteoclast precursors after treatment with 1,25-D or ELD. (A) Quantitative real-time PCR analysis of S1PR2 mRNA expressed by RAW264.7 cells in floating cultures. Both 1,25-D and ELD suppress the expression of S1PR2 in a dose-dependent manner in floating cultures. Error bars represent ± SEM (n = 3 for each). (B) In vitro S1P-directed chemotaxis of RAW264.7 cells pretreated with vehicle (Left) (Movie S1), 1,25-D (Center) (Movie S2), or ELD (Right) (Movie S3) and dynamically visualized using EZ-Taxiscan. The cells are loaded into the lower chamber. The upper chamber is filled with medium that contains 10−6 M S1P. Cells migrate into the terrace between the loading chambers. The height from the bottom to the top of the terrace is 8 μm. (Scale bar: 100 μm.) (C) Migration distances derived from microscopy analyses of RAW264.7 cells pretreated with vehicle (red), 1,25-D (green), or ELD (blue). The experiments were independently performed three times, and the data are largely consistent. Each dot represents the mean value of 10 independent cells, and error bars represent ± SEM.
S1P-mediated chemotaxis of osteoclast precursor monocytes was previously shown to be reciprocally regulated by two counteracting cognate receptors, S1PR1 and S1PR2 (4, 6). S1PR1 is a major high-affinity receptor that induces positive migration along an S1P concentration gradient, whereas S1PR2 has a lower affinity and inhibits S1PR1-mediated chemotaxis and/or induces migration in an inverse direction (chemorepulsion). At lower doses of S1P (10−8 M), osteoclast precursor monocytes readily moved to the S1P gradient. Their movement was abolished at higher doses of S1P (10−6 M), because the negative receptor S1PR2 was activated. Concordant with previously obtained results, the RAW264.7 cells hardly moved in 10−6 M S1P under the control condition (Fig. 1 B, Left and C). Conversely, the cells that were preincubated with 1,25-D (Fig. 1 B, Center and C) or ELD (Fig. 1 B, Right and C) migrated to the higher concentration (10−6 M) of S1P; a similar finding was reported for S1PR2-knockdown RAW264.7 cells (6). These results clearly show that 1,25-D– or ELD-induced down-regulation of S1PR2 leads to enhanced migration to a high concentration of S1P, such as found in the blood (19).
Although the definition of osteoclast precursors remains controversial, monocytoid cells that express colony stimulating factor 1 receptor (CSF1R)/c-Fms are thought to contain precursor cells that can differentiate into osteoclasts (20). In addition, CX3CR1, which is a cognate receptor for CX3CL1/fractalkine that is preferentially expressed in monocytoid cell types, is considered a good marker of osteoclast precursors (6, 21, 22). We sorted CSF1R- or CX3CR1-positive cells from CSF1R-EGFP transgenic (20) or CX3CR1-EGFP knockin (21) mice, respectively, and treated the sorted cells with ELD (10−9 to 10−8 M) in floating cultures. The expression levels of S1PR2 in both cell types were significantly and dose-dependently suppressed by VDR stimulation (Fig. S3). The decrease in S1PR2 expression was more prominent in these cases compared with the decrease in expression observed in floating RAW264.7 cells.
1,25-D and ELD Ameliorate Ovariectomy-Induced Bone Density Loss and Suppress the Expression of S1PR2 in Circulating Monocytoid Osteoclast Precursors in Vivo.
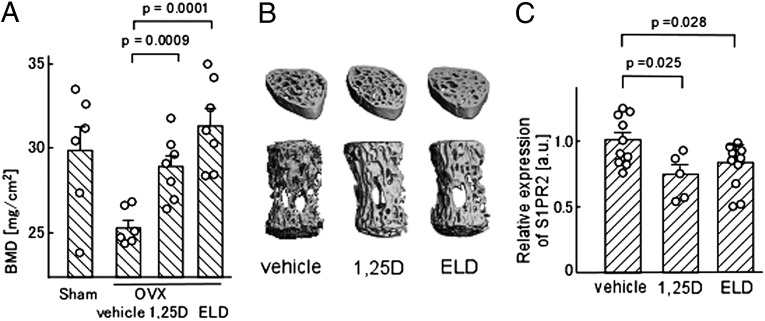
Next, we examined the effects on mice of in vivo treatment with 1,25-D and ELD. According to the previously reported protocol, 1,25-D or ELD (dosage of 50 ng/kg body weight for each) dissolved in a vehicle [medium chain triglyceride (MCT)] was orally administered to the mice for 4 wk. Concordant with the previous data (23, 24), oral treatment with 1,25-D or ELD prevented ovariectomy-induced bone loss and significantly recovered the bone mineral densities compared with vehicle control (Fig. 2 A and B). Under this condition, we examined the expression of S1PR2 in CD11b+-circulating monocytes, including osteoclast precursors. The CD11b+ cells in mice that were treated orally with 1,25-D or ELD expressed lower levels of S1PR2 than cells that were subjected to the control conditions (Fig. 2C). These results suggest that oral administration of active vitamin D, or analogs thereof, suppresses S1PR2 expression in osteoclast precursor monocytes in vivo.
Fig. 2.
In vivo impacts of 1,25-D and ELD on bone remodeling. (A) Preventive effects of 1,25-D and ELD on ovariectomy-induced osteoporosis. Lumbar vertebrae were collected from mice that were sham-operated; ovariectomized and vehicle-treated; ovariectomized and 1,25-D–treated; and ovariectomized and ELD-treated. Bone mineral density was measured by dual-energy X-ray absorptiometry. Error bars represent ± SEM (n = 6 or 7 for each). (B) Microcomputed tomography images of lumbar vertebrae from ovariectomized mice treated with vehicle (Left), 1,25-D (Center), and ELD (Right). (C) Quantitative real-time PCR analysis of S1PR2 mRNA expressed by CD11b+ cells sorted from WT mice that were treated orally with vehicle only, 1,25-D, or ELD daily for 5 d. Error bars represent ± SEM (n = 10 for vehicle-treated; n = 5 for 1,25-D–treated; n =10 for ELD-treated).
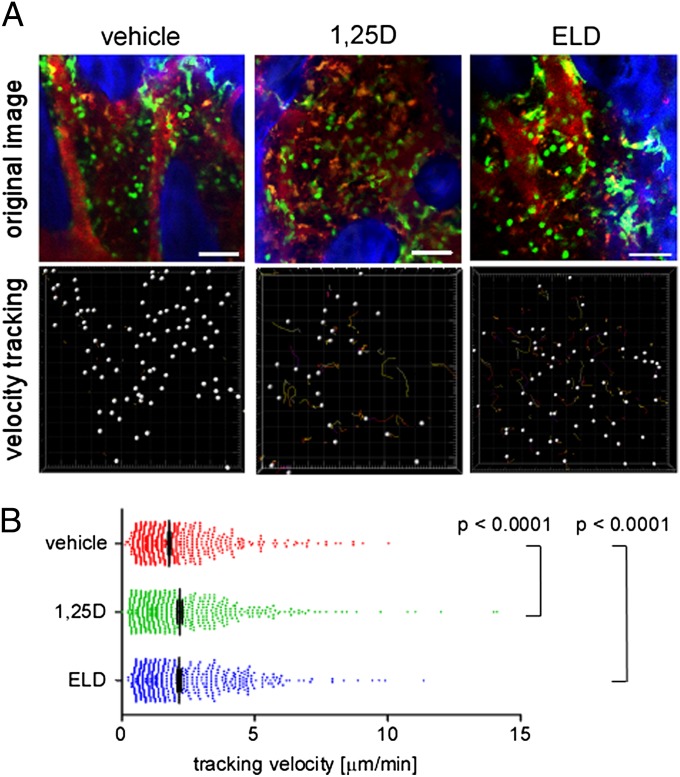
Finally, we examined the in vivo mobility of CX3CR1-EGFP+ osteoclast precursor monocytes in living bone tissues using intravital multiphoton bone microscopy according to methods that we originally established (4, 6). CX3CR1-EGFP knockin mice were treated orally with 1,25-D or ELD (50 ng/kg body weight) or vehicle (MCT) for 5 d, and the bone tissues of the mice were visualized to assess the mobilities of the EGFP+ cells (Fig. 3). The mobility of the CX3CR1-EGFP+ monocytoid cells was significantly increased in mice that were treated with ELD, which is similar to what was observed after treatment with an S1PR2 antagonist (6). These results clearly show that in vivo oral treatment with 1,25-D or ELD suppresses S1PR2 expression and the mobilization of osteoclast precursor monocytes from the bone to the blood circulation, thereby contributing to limiting osteoclastic bone destruction, which is the main therapeutic effect of active vitamin D drugs.
Fig. 3.
In vivo S1PR2-mediated control of migration of osteoclast precursor monocytes visualized using intravital multiphoton imaging. (A) Intravital multiphoton imaging of the skull bone tissues of heterozygous CX3CR1-EGFP knockin mice that were treated orally with vehicle only (Left) (Movie S4), 1,25-D (Center) (Movie S5), or ELD (Right) (Movie S6) daily for 5 d. CX3CR1-EGFP–positive cells appear green. The microvasculature was visualized by i.v. injection of Texas Red-conjugated 70 kDa dextran (red). The blue color indicates the bone surface (Upper). The movements of the CX3CR1-EGFP–positive cells were tracked for 20 min. Colored lines show the associated trajectories of the cells (Lower). (Scale bars: 50 μm.) (B) Summary of the mean tracking velocities of CX3CR1-EGFP–positive cells treated with vehicle (red circles), 1,25-D (green circles), and ELD (blue circles). Data points (n = 708 for vehicle-treated; n = 507 for 1,25-D–treated; n = 520 for ELD-treated) represent the values for individual cells compiled from six independent experiments, and error bars represent ± SEM.
Discussion
Active vitamin D analogs have been used for treating bone and mineral disorders, mainly osteoporosis. The precise pharmacologic action has remained elusive, although previous reports have indicated that c-fos, an osteoclastogenic transcription factor (23), and RANKL expression in osteoblasts (24) may be the targets of vitamin D action. In the present study, we reveal a point of control by active vitamin D: the control of osteoclast precursor monocyte migration. Because this therapeutic point is independent of the points exploited by conventional antibone-resorptive drugs, such as bisphosphonate, it may provide a unique avenue of treatment for bone diseases. Currently, combination therapy with active vitamin D and bisphosphonate is the most effective treatment (25–27), which suggests synergistic therapeutic effects of bisphosphonate and vitamin D acting at respectively different therapeutic points.
ELD has been screened for its high therapeutic potency in treating bone diseases (28–30). However, because its pharmacologic profile, which includes binding affinity for VDR, is similar to the profiles of the active form of vitamin D, 1,25-D, and other active vitamin D analogs, such as alfacalcidol, the mechanism underlying its superior therapeutic effect is currently unknown (29, 31). One characteristic of ELD is its high binding affinity for vitamin D binding protein in serum (32), suggesting that ELD is more readily maintained in the blood. This point of action of vitamin D would be particularly advantageous in relation to its actions on circulating osteoclast precursor monocytes.
Recently, we reported that a high concentration of S1P in blood is critically maintained by an S1P transporter, spns2, which is expressed in vascular endothelial cells (33). Genetic ablation of spns2 in mice led to an almost 50% decrease in blood S1P, and the migration rates of T and B lymphocytes, which express S1P receptors, were greatly impaired (33). Moreover, in these mice, the bone mineral density was largely changed, suggesting the significance of S1P for physiologic bone metabolism. More importantly, a recent clinical investigation has shown that the serum concentration of S1P is critically correlated with bone mineral density and the incidence of bone fractures in humans (34). These lines of in vivo evidence in mice and humans strongly support our notion that ELD, which is stably present in circulating blood, could be beneficial for suppressing S1PR2 in circulating monocytes and blocking osteoclastogenesis and bone resorption.
Vitamin D is a multifunctional hormone that is essential for humans. Vitamin D has been reported to play crucial roles not only in the endocrine system and metabolism but also, in immunity (35), cancer biology (36), and neuroscience (37), although the pharmacologic actions have not been fully clarified in most cases. S1P and its cognate receptors, such as S1PR2, are also multifunctional and have been shown to be involved in immune regulation by controlling the chemotactic activities of various immune cells (38, 39). Now that active vitamin D has been shown to control the S1P system in bone metabolism, the actions of vitamin D related to S1P signaling should be examined in various other biological systems. These analyses may contribute to additional development of active vitamin D analogs for the treatment of several human diseases in addition to bone diseases.
Materials and Methods
Mice.
C57BL/6 mice were obtained from CREA Japan. CSF1R (M-CSF receptor) -EGFP transgenic mice (20) and CX3CR1-EGFP knockin mice (21) were obtained from Jackson Laboratory. All mice were bred and maintained under specific pathogen-free conditions at the animal facilities of Osaka University (Osaka, Japan), and all animal experiments were performed according to institutional animal experimental guidelines under approved protocols from the Animal Experimental Committee of Osaka University.
Cell Culture.
RAW264.7, which is a mouse macrophage/monocyte lineage cell line, was cultured with or without 1,25-D or ELD (Chugai Pharmaceuticals Co., Ltd.) for 2 d using a low-cell binding dish (Nalge Nunc), the surface of which was coated with 2-methacryloxyethyl phosphorylcholine. After incubation, total RNA was extracted from the cells, and quantitative real-time PCR was performed. Bone marrow cells were isolated from CSF1R-EGFP transgenic or CX3CR1-EGFP knockin mice, and the EGFP-positive cells were sorted using the FACS Aria flow cytometer (BD Biosciences). The cells were cultured in a low-cell binding dish with 25 ng/mL M-CSF (PeproTech) for 5 d; 1,25-D or ELD was added to the medium, and the cells were incubated for an additional 2 d. After incubation, total RNA was extracted from the cells, and quantitative real-time PCR was performed.
Quantitative real-time PCR.
Quantitative teal-time PCR was performed using the Thermal Cycler Dice Real-Time System TP800 (Takara) and the following specific primer pairs (forward and reverse, respectively): S1PR2 (5′-CCAAGGAGACGCTGGACATG-3′ and 5′-TGCCGTAGAGCTTGACCTTGTCGAA-3′); VDR (5′-AACGCTATGACCTGTGAAGGC-3′ and 5′-CCTGTACTTACGTCTGCACGA-3′); and GAPDH (5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′).
EZ-Taxiscan Chemotaxis Assay.
Chemotaxis experiments were conducted in an EZ-Taxiscan chamber according to the manufacturer’s protocol (GE Healthcare). The EZ-Taxiscan is a visually accessible chemotactic chamber, in which one compartment that contains the ligand (S1P) and another compartment that contains the cells are connected by a microchannel. A stable concentration gradient of chemoattractant can be reproducibly formed and maintained through the channel without medium flow. Phase-contrast images of migrating cells were acquired at 1-min intervals. Sequential image data were processed using the ImageJ program (National Institutes of Health) with an add-on program, MT Track J.
Ovariectomy and Bone Histomorphometry.
Nine-week-old female C57BL/6J mice, which were ovariectomized or sham-operated, were injected orally with 1,25-D (50 ng/kg body weight) dissolved in vehicle (MCT; Nisshin Oillio), ELD (50 ng/kg body weight) in vehicle, or vehicle only daily for 4 wk. At the end of the treatment period, the mice were euthanized, and the lumbar vertebrae were excised and fixed in 70% (vol/vol) ethanol. The uteri of all of the animals were excised and weighed to evaluate the effect of ovariectomy. Bone mineral density was measured by dual-energy X-ray absorptiometry (DCS-600EX; Aloka) at the second to fifth vertebrae. 3D trabecular analysis of the lumbar vertebral body was performed by microcomputed tomography (μCT 40; Scanco Medical).
1,25-D and ELD Treatment.
C57BL6/J mice were treated orally with 1,25-D (50 ng/kg body weight) in vehicle, ELD (50 ng/kg body weight) in vehicle, or vehicle only daily for 5 d. In some experiments, the mice were then killed, and CD11b+ cells were separated using autoMACS (Miltenyi Biotec) from the spleen and bone marrow, which contain a high number of circulating monocytoid cells. Total RNA was extracted from the cells, and quantitative real-time PCR was performed.
Multiphoton Intravital Bone Tissue Imaging.
Intravital microscopy of mouse calvaria bone tissues was performed using a protocol modified from a previous study (4); 10- to 14-wk-old mice were anesthetized using isoflurane [Escain; 2.0% (vol/vol) vaporized in 100% (vol/vol) oxygen], the frontoparietal region of the skull bone was exposed, and the internal surfaces of bones adjacent to the bone marrow cavity were observed using multiphoton excitation microscopy. The imaging system was composed of a multiphoton microscope (A1-MP; Nikon) driven by a laser (Chameleon Vision II Ti: Sapphire; Coherent) tuned to 880 nm together with an upright microscope equipped with a 25× water immersion objective (APO, N.A. 1.1; Nikon). Fluorescent cells were detected through bandpass emission filters at 500/50 nm (for EGFP). Vessels were visualized by intravenously injecting Texas Red-conjugated 70 kDa dextran (detected using a 601/56-nm filter) immediately before imaging. Image stacks were collected at a 5-μm vertical step size at a depth of 100–150 μm below the skull bone surface. Raw imaging data were processed using Imaris (Bitplane) with a Gaussian filter for noise reduction. Automatic 3D object tracking with Imaris Spots was aided by manual corrections to retrieve cell spatial coordinates over time.
Statistical Analysis.
The Mann–Whitney rank-sum test was used to calculate the P values for highly skewed distributions. For Gaussian-like distributions, two-tailed t tests were used.
Supplementary Material
Acknowledgments
This study was supported by Grant-in-Aid 22689030 for Encouragement of Young Scientists (A; to M.I.), Grant-in-Aid 22113007 for Scientific Research on Innovative Areas (to M.I.), a Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST Program) from the Ministry of Education, Science, Sports and Culture of Japan (to M.I.), and Grants CDA-00059/2009 and RGY0077/2011 from the International Human Frontier Science Program (to M.I.).
Footnotes
Conflict of interest statement: S.S. and H.S. are full-time employees of Chugai Pharmaceutical Co., Ltd. All other authors have no conflicts of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/1218799110/-/DCSupplemental.
References
- 1.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 2.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4(8):638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 3.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2(4):389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 4.Ishii M, et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature. 2009;458(7237):524–528. doi: 10.1038/nature07713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klauschen F, et al. Quantifying cellular interaction dynamics in 3D fluorescence microscopy data. Nat Protoc. 2009;4(9):1305–1311. doi: 10.1038/nprot.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ishii M, Kikuta J, Shimazu Y, Meier-Schellersheim M, Germain RN. Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med. 2010;207(13):2793–2798. doi: 10.1084/jem.20101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamoto H, et al. Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol Cell Biol. 2000;20(24):9247–9261. doi: 10.1128/mcb.20.24.9247-9261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell RG, et al. Bisphosphonates: An update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci. 2007;1117:209–257. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]
- 9.Bone HG, et al. Odanacatib, a cathepsin-K inhibitor for osteoporosis: A two-year study in postmenopausal women with low bone density. J Bone Miner Res. 2010;25(5):937–947. doi: 10.1359/jbmr.091035. [DOI] [PubMed] [Google Scholar]
- 10.Eisman JA, et al. Odanacatib in the treatment of postmenopausal women with low bone mineral density: Three-year continued therapy and resolution of effect. J Bone Miner Res. 2011;26(2):242–251. doi: 10.1002/jbmr.212. [DOI] [PubMed] [Google Scholar]
- 11.Bouillon R, Okamura WH, Norman AW. Structure-function relationships in the vitamin D endocrine system. Endocr Rev. 1995;16(2):200–257. doi: 10.1210/edrv-16-2-200. [DOI] [PubMed] [Google Scholar]
- 12.Plum LA, DeLuca HF. Vitamin D, disease and therapeutic opportunities. Nat Rev Drug Discov. 2010;9(12):941–955. doi: 10.1038/nrd3318. [DOI] [PubMed] [Google Scholar]
- 13.Roodman GD, Ibbotson KJ, MacDonald BR, Kuehl TJ, Mundy GR. 1,25-Dihydroxyvitamin D3 causes formation of multinucleated cells with several osteoclast characteristics in cultures of primate marrow. Proc Natl Acad Sci USA. 1985;82(23):8213–8217. doi: 10.1073/pnas.82.23.8213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasuda H, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95(7):3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suda T, et al. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20(3):345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 16.Suda T, Ueno Y, Fujii K, Shinki T. Vitamin D and bone. J Cell Biochem. 2003;88(2):259–266. doi: 10.1002/jcb.10331. [DOI] [PubMed] [Google Scholar]
- 17.Suda T, Takahashi F, Takahashi N. Bone effects of vitamin D - Discrepancies between in vivo and in vitro studies. Arch Biochem Biophys. 2012;523(1):22–29. doi: 10.1016/j.abb.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Ishii M, et al. RANKL-induced expression of tetraspanin CD9 in lipid raft membrane microdomain is essential for cell fusion during osteoclastogenesis. J Bone Miner Res. 2006;21(6):965–976. doi: 10.1359/jbmr.060308. [DOI] [PubMed] [Google Scholar]
- 19.Maeda Y, Seki N, Sato N, Sugahara K, Chiba K. Sphingosine 1-phosphate receptor type 1 regulates egress of mature T cells from mouse bone marrow. Int Immunol. 2010;22(6):515–525. doi: 10.1093/intimm/dxq036. [DOI] [PubMed] [Google Scholar]
- 20.Burnett SH, et al. Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol. 2004;75(4):612–623. doi: 10.1189/jlb.0903442. [DOI] [PubMed] [Google Scholar]
- 21.Jung S, et al. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20(11):4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koizumi K, et al. Role of CX3CL1/fractalkine in osteoclast differentiation and bone resorption. J Immunol. 2009;183(12):7825–7831. doi: 10.4049/jimmunol.0803627. [DOI] [PubMed] [Google Scholar]
- 23.Takasu H, et al. c-Fos protein as a target of anti-osteoclastogenic action of vitamin D, and synthesis of new analogs. J Clin Invest. 2006;116(2):528–535. doi: 10.1172/JCI24742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harada S, et al. Daily administration of eldecalcitol (ED-71), an active vitamin D analog, increases bone mineral density by suppressing RANKL expression in mouse trabecular bone. J Bone Miner Res. 2012;27(2):461–473. doi: 10.1002/jbmr.555. [DOI] [PubMed] [Google Scholar]
- 25.Sakai S, Endo K, Takeda S, Mihara M, Shiraishi A. Combination therapy with eldecalcitol and alendronate has therapeutic advantages over monotherapy by improving bone strength. Bone. 2012;50(5):1054–1063. doi: 10.1016/j.bone.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Sugimoto M, Futaki N, Harada M, Kaku S. Effects of combined treatment with eldecalcitol and alendronate on bone mass, mechanical properties, and bone histomorphometry in ovariectomized rats: A comparison with alfacalcidol and alendronate. Bone. 2013;52(1):181–188. doi: 10.1016/j.bone.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 27.Ringe JD, Farahmand P, Schacht E, Rozehnal A. Superiority of a combined treatment of Alendronate and Alfacalcidol compared to the combination of Alendronate and plain vitamin D or Alfacalcidol alone in established postmenopausal or male osteoporosis (AAC-Trial) Rheumatol Int. 2007;27(5):425–434. doi: 10.1007/s00296-006-0288-z. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto T, et al. A new active vitamin D, ED-71, increases bone mass in osteoporotic patients under vitamin D supplementation: A randomized, double-blind, placebo-controlled clinical trial. J Clin Endocrinol Metab. 2005;90(9):5031–5036. doi: 10.1210/jc.2004-2552. [DOI] [PubMed] [Google Scholar]
- 29.Sanford M, McCormack PL. Eldecalcitol: A review of its use in the treatment of osteoporosis. Drugs. 2011;71(13):1755–1770. doi: 10.2165/11206790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto T, et al. A new active vitamin D3 analog, eldecalcitol, prevents the risk of osteoporotic fractures—a randomized, active comparator, double-blind study. Bone. 2011;49(4):605–612. doi: 10.1016/j.bone.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Ito M, Nakamura T, Fukunaga M, Shiraki M, Matsumoto T. Effect of eldecalcitol, an active vitamin D analog, on hip structure and biomechanical properties: 3D assessment by clinical CT. Bone. 2011;49(3):328–334. doi: 10.1016/j.bone.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Okano T, et al. A novel synthetic vitamin D3 analogue, 2-beta-(3-hydroxypropoxy)-calcitriol (ED-71): Its biological activities and pharmacological effects on calcium metabolism. Contrib Nephrol. 1991;91:116–122. doi: 10.1159/000420166. [DOI] [PubMed] [Google Scholar]
- 33.Fukuhara S, et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J Clin Invest. 2012;122(4):1416–1426. doi: 10.1172/JCI60746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SH, et al. Higher circulating sphingosine 1-phosphate levels are associated with lower bone mineral density and higher bone resorption marker in humans. J Clin Endocrinol Metab. 2012;97(8):E1421–E1428. doi: 10.1210/jc.2012-1044. [DOI] [PubMed] [Google Scholar]
- 35.Bouillon R, et al. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vuolo L, Di Somma C, Faggiano A, Colao A. Vitamin D and cancer. Front Endocrinol (Lausanne) 2012;3:58. doi: 10.3389/fendo.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 38.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: An autocrine and paracrine network. Nat Rev Immunol. 2005;5(7):560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 39.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.