Abstract
T cells expressing antigen-specific T-cell receptors (TCRs) can mediate effective tumor regression, but they often also are accompanied by autoimmune responses. To determine the TCR affinity threshold defining the optimal balance between effective antitumor activity and autoimmunity in vivo, we used a unique self-antigen system comprising seven human melanoma gp100(209–217)-specific TCRs spanning physiological affinities (1–100 μM). We found that in vitro and in vivo T-cell responses are determined by TCR affinity, except in one case that was compensated by substantial CD8 involvement. Strikingly, we found that T-cell antitumor activity and autoimmunity are closely coupled but plateau at a defined TCR affinity of 10 µM, likely due to diminished contribution of TCR affinity to avidity above the threshold. Together, these results suggest that a relatively low-affinity threshold is necessary for the immune system to avoid self-damage, given the close relationship between antitumor activity and autoimmunity. The low threshold, in turn, indicates that adoptive T-cell therapy treatment strategies using in vitro-generated high-affinity TCRs do not necessarily improve efficacy.
Keywords: adoptive cell transfer, kinetic threshold, tumor immunity, ocular autoimmunity
Adoptive cell therapy (ACT) has shown efficacy in tumor treatment (1–3), particularly in metastatic melanoma (4–6), achieving up to 72% objective response rates (7, 8) in patients with autologous tumor-infiltrating lymphocytes (TILs). Genetically modified lymphocytes expressing antigen-specific T-cell receptors (TCRs) (6, 9) are applicable to a wider range of patients when suitable TILs cannot be isolated (9), but they are less effective than TILs (6, 9). Therefore, it is necessary to enhance the effectiveness of gene-modified lymphocytes by using TCRs with optimal antitumor activity (10, 11). To this end, it is critical to determine what TCR properties correlate to antitumor activity. Substantial evidence indicates a correlation between T-cell functional activity and TCR affinity (12–18). Subsequently, efforts have been taken to generate modified high-affinity TCRs for clinical studies (19–21). However, this correlation remains controversial: high-affinity TCRs have been shown to lead to stronger (22), plateaued (12, 17), or even attenuated (23–25) T-cell responses. Underlying reasons for this controversy could be that antigens previously analyzed were derived from models that induce unusually strong T-cell responses that do not reflect immune responses against true self-antigens, which tend to be less robust. Other studies have used a panel of altered peptide ligands in which the lack of correlation between TCR affinity and T-cell activation observed for some peptides could be a result of reduced stability of the peptide–major histocompatibility complexes (pMHCs) over the course of the activation assay (18). Recent work by Rufer and colleagues (17, 24) studying T-cell responses and in vitro tumor reactivity to the cancer/testis antigen 1 (NY-ESO-1) showed a plateau of maximal T-cell function at a KD of ∼5 μM but did not determine how the plateau relates to tumor rejection in vivo. Therefore, quantitative analysis of TCR affinity and how it relates to in vivo antitumor activity in ACT is important because it remains unclear whether higher-affinity TCRs can render ACT more effective.
ACT is often associated with autoimmunity in mouse (26) and human (6, 27) melanoma due to the expression of tumor-associated antigens (TAAs) in normal tissues. One study of a diabetic mouse model using peptide vaccination indicated a correlation between ligand affinity and autoimmune response (28). However, it has not previously been determined whether T cells expressing higher-affinity TCRs lead to more severe autoimmunity in ACT or the kinetic threshold that sets this fine balance. Here we use a “true” self-antigen system, which allows us to systematically and directly investigate the interplay between TCR biophysical properties, antitumor activities, and autoimmune responses to solve this important basic immunological question.
The strength of the TCR/pMHC interaction on the cell surface is determined not only by TCR affinity, but also by other factors, such as TCR clustering and coreceptors (29). Coreceptors stabilize TCR/pMHC interactions and are required for functionality of low-affinity TCRs (16). pMHC tetramers (6, 14, 16, 30) have been used to measure the combined effects, which is referred to as avidity (29). Tetramer-based avidity measurements should not be confused with T-cell functional avidity (10, 11, 31), which refers to cellular responses in addition to binding. Here we use the term functional activity to describe T-cell functional outcomes, such as cytokine release, cytotoxicity, and antitumor response.
To understand the interplay between TCR affinity/avidity, antitumor activity, and autoimmune response, we studied gp100209–217 (gp209), a melanoma differentiation TAA presented by human leukocyte antigen (HLA)-A*0201 (HLA-A2) and abundantly expressed in metastatic melanomas (32). A modified epitope, gp209–2M (33), which enhances peptide stability ninefold while not altering pMHC structure (34), is significantly more immunogenic (35) and has been widely used in clinical studies (36, 37). Therefore, to closely mimic the clinical situation, we used this modified epitope in all subsequent in vitro and in vivo experiments. We established a unique system of seven gp209-specific TCRs isolated from melanoma patients immunized with gp209–2M, covering the physiological affinity range (1–100 µM). Our results demonstrate an affinity threshold (∼10 µM) for maximal antitumor activity and autoreactivity. We propose that such a threshold is necessary for the immune system to prevent self-destruction due to the close relationship between in vivo autoimmunity and antitumor response.
Results
Panel of gp209-Specific TCRs Shows Different Avidity and Functional Activities on Primary T Cells.
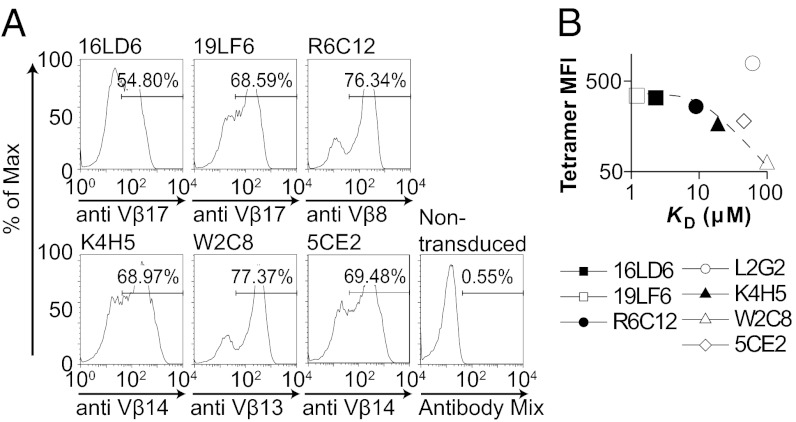
We selected seven gp209-specific TCRs isolated from melanoma patients previously vaccinated with gp209–2M (Table 1). The affinities of the TCRs, as measured the by surface plasmon resonance (SPR) (Table 1 and Fig. S1), span the entire affinity range of naturally selected TCRs (KD, ∼1–100 μM) (29) and thus provide a suitable system for studying the correlation between TCR affinity and functional activities. To investigate the antitumor activities of the TCRs, we characterized them in primary splenocytes isolated from A2–Kb transgenic mice (38), a model for studying HLA-A2–restricted TCRs (35, 39). The retrovirally transduced (40) T cells showed comparable TCR surface expression (Fig. 1A; Fig. S2G). Next, we determined the avidities of the TCRs using gp209–2M HLA-A2 tetramer staining. The avidity, which is estimated by the mean fluorescent intensity (MFI) of tetramer binding, although not a direct measurement of monomeric TCR/pMHC interaction, usually correlates with TCR affinity (14, 16, 30). In CD8− splenocytes, only cells transduced with the two highest-affinity TCRs, 16LD6 (KD, ∼2.6 μM) and 19LF6 (KD, ∼1.4 μM), bind tetramer (Fig. S2B), consistent with previous studies showing that tetramer binding independent of CD8 is governed by TCR affinity (16). We further analyzed the functional activities of the TCRs by measuring IL-2 secretion in response to gp209–2M antigen (Fig. S2C). Only 19LF6-transduced CD8− splenocytes secrete significant amounts of IFN-γ (Fig. S2C), suggesting an affinity threshold (1.4–2.6 μM) for TCR function independent of CD8. This threshold is comparable to the 2C TCR system (3 μM) (18), but quite different from the ILA1 TCR system (200 μM) (16).
Table 1.
Panel of gp100-specific TCRs isolated from melanoma patients
| TCR gene* |
Functional activity† on primary T cells |
SPR measurement of soluble TCRs at 25 °C‡ |
|||||||
| TCR | α | β | Antibody specific for Vβ chain | CD8− | CD8+ | ka, M-1s−1 × 104 | kd, s−1 | KD, μM | KD, steady state, μM |
| 19LF6§ | 19 | 19 | vβ17 | + | ++ | 0.14 | 0.002 | 1.4 | n.d.¶ |
| 16LD6§ | 3 | 19 | vβ17 | − | ++ | 4.2 (4.3) | 0.1 (0.1) | 2.6 (2.6) | 2.3 (1.8) |
| R6C12|| | 41 | 12-3 | vβ8 | − | ++ | 1.9 | 0.2 | 9.3 | 9.0 |
| K4H5|| | 17 | 27 | vβ14 | − | + | 8.9 | ≥1** | 12.8 | 18.9 |
| 5CE2|| | 12-1 | 27 | vβ14 | − | + | 0.43 | 0.2 | 47.4 | 45.7 |
| L2G2§ | 12-2 | 7-9 | n.a. | − | ++/+++ | 1.6 (1.0) | ≥1** (0.65) | 60.0 (62.9) | 61.2 (62.6) |
| W2C8|| | 2 | 6-2 | vβ13 | − | −/+ | Fast | Fast | n.d. | 99.0 |
n.a., not available; n.d., not determined due to low affinity.
TCR gene names are based on International ImMunoGeneTics Information System nomenclature (www.imgt.org).
Functional activities include cytokine release, calcium release, ERK phosphorylation, antitumor response, and autoimmune response.
Kinetic measurements are based on TCR immobilization (Fig. S1E); values in parentheses are data based on pMHC immobilization.
Peripheral blood lymphocytes of a patient previously vaccinated against gp209-2M, who had a gp100-downregularted melanoma reoccur following vaccination.
Steady-state fitting of 19LF6 was not performed because the binding at the tested concentration did not reach equilibrium.
Peripheral blood mononuclear cells (PBMCs) from different melanoma patients vaccinated with gp209-2M.
Too fast to be determined with confidence.
Fig. 1.
A panel of gp209-specific TCRs shows distinct avidities and affinities. (A) The gp209-specific TCRs were retrovirally transduced into splenocytes isolated from A2–Kb transgenic mice, and expression of TCRs was quantified by antibodies specific for the TCR Vβ chains. No commercial antibody is available for L2G2. However, splenocytes transduced with L2G2 showed strong gp209–2M–HLA-A2–Kb tetramer staining (Fig. S2D), which allowed us to use tetramer for quantification of the percentage of TCR expression. The expression level of L2G2 is comparable to other TCRs as demonstrated by intracellular staining of cMyc-tagged TCRs (Fig. S2 F and G and SI Methods). (B) The affinities of the soluble TCRs were measured by SPR (Fig. S1 and Table 1) and compared with the avidities estimated by the MFI of gp209–2M–HLA-A2–Kb tetramer staining (Fig. S2E) on CD8+ splenocytes. Data are representative of three or more experiments.
In the presence of CD8, the avidity of the TCRs is significantly enhanced (Fig. S2D). Interestingly, L2G2 becomes the highest-avidity TCR when expressed in CD8+ splenocytes (Fig. S2E), although the affinity of L2G2 is low (KD, ∼60 μM; Table 1). One possible explanation is that the contribution of CD8 in stabilizing TCR/pMHC is TCR-dependent: CD8 could more significantly enhance the L2G2/pMHC interaction. Indeed, L2G2 alone does not bind tetramer (Fig. S2B); however, in the presence of CD8, the tetramer-binding intensity is the highest of the panel of TCRs analyzed (Fig. S2E). The avidities of other TCRs in the panel are also enhanced by CD8, but to a lesser extent than L2G2. For comparison, 5CE2 (KD, ∼46 μM) has a comparable affinity to L2G2 but far lower avidity in the presence of CD8 (Fig. S2E). It is possible that the L2G2/pMHC binding is more dramatically enhanced by CD8 in the second stage than the rest of the TCRs. This hypothesis is based on the two-stage mode of CD8/TCR/pMHC interaction (41) observed in 2D affinity measurements (42). Together, these results highlight the contribution of CD8 to the TCR/pMHC interaction, which in some cases can be dependent on individual TCR structure. TCR avidity is affected by cellular environments and coreceptors in addition to TCR affinity (29).
By comparing TCR avidity and affinity, we found that TCR affinity correlates with avidity (except for L2G2; Fig. 1B). However, the contribution of affinity to avidity shows a diminishing return: The avidity does not improve significantly for TCRs with higher affinity than R6C12 (KD, ∼9 μM). Our observation parallels a previous study (16) using a panel of pMHC ligands that shows minimal avidity increase for high-affinity ligands. In summary, we have generated and characterized a panel of gp209-specific TCRs spanning the physiological affinity range. We showed that the avidity of the TCRs results from synergy between TCR, pMHC, and CD8, and that the contribution of CD8 is TCR-dependent.
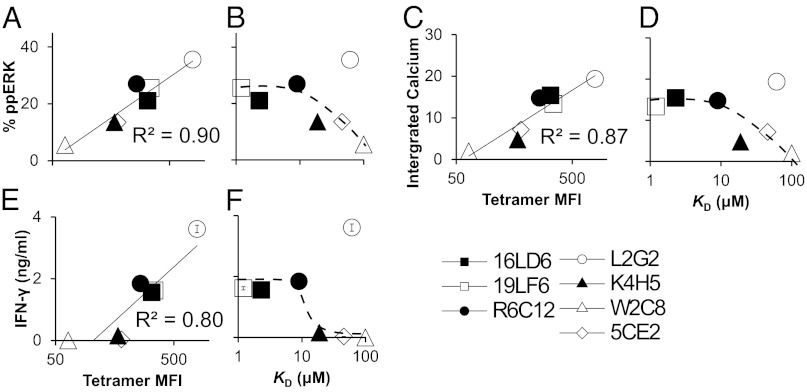
Strength of T-Cell Response Correlates with TCR Avidities and Affinities.
To define how the functional activities of the TCRs expressed on CD8+ splenocytes correlate with TCR avidity and affinity, we measured key proximal and distal T-cell activation events. The phosphorylation of extracellular signal-regulated kinase 1 (ERK-1; Figs. S3A and S4 A and B) and calcium elevation (Fig. S3B) are two proximal signaling events that occur seconds after T-cell/antigen-presenting cell (APC) engagement (43). There is a linear correlation between TCR avidity and the strength of proximal activation (Fig. 2 A and C). Because the contribution of TCR affinity to avidity is diminished above ∼10 μM (Fig. 1B), it is not surprising that the strength of T-cell response plateaus above this threshold (Fig. 2 B and D). Consistent with proximal T-cell responses, we observed that cytokine production (Fig. S3C), which occurs much later after T-cell/APC engagement, is linearly correlated with avidity (Fig. 2E) and plateaus above ∼10 μM affinity (Fig. 2F). Similar correlations between TCR avidity/affinity and the strength of T-cell responses were observed when the melanoma cell line B16/A2–Kb (44) was used as APCs (Fig. S4 C–K). Collectively, our data suggest a connection between TCR affinity, avidity, and functional activity. T-cell functional activity is determined by avidity, which is mainly determined by TCR affinity. A plateau of T-cell functional activity above a defined affinity threshold has been reported (12, 17). Here we show that one possible explanation for this phenomenon is the diminishing contribution of TCR affinity to avidity on the cell surface above a defined threshold. Interestingly, the avidity and functional activities of L2G2 are above the plateau, which we attributed to the strong stabilization of L2G2/pMHC complex by CD8.
Fig. 2.
The strength of the proximal and distal T-cell response is correlated with TCR avidity and affinity. (A and B) The percentage of ERK phosphorylation of CD8+ splenocytes mixed with T2/A2–Kb cells loaded with gp209–2M peptide (Fig. S3A) was correlated with TCR avidity (A) or affinity (B) at a representative gp209–2M concentration (10 μM). (C and D) The average calcium signal of transduced CD8+ splenocytes mixed with T2/A2–Kb cells loaded with 10 μM gp209–2M peptide (Fig. S3B) was correlated with TCR avidity (C) or affinity (D). (E and F) The maximal amounts of IFN-γ secretion of transduced CD8+ splenocytes mixed with T2/A2–Kb cells loaded with gp209–2M peptide (Fig. S3C) were correlated with TCR avidity (E) or affinity (F).
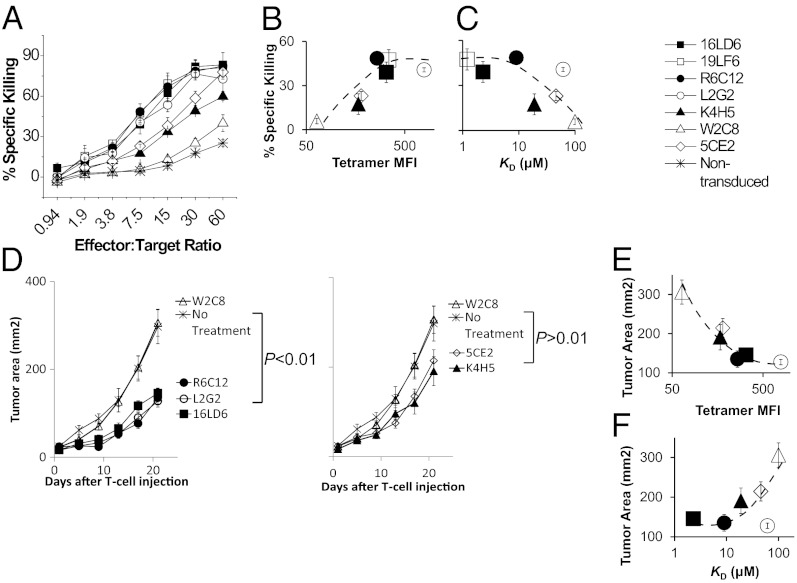
Strength of in Vitro and in Vivo Antitumor Activity Plateaus Above a Defined Avidity and Affinity Threshold.
To further characterize the antitumor activity of the panel of TCRs, we assessed their in vitro antigen-specific lysing of the B16/A2–Kb tumor cell line (Fig. 3A). The endogenous gp209 antigen expressed on this tumor line only elicits weak T-cell responses (Fig. S4L), and therefore B16/A2–Kb tumor cells were loaded with exogenous peptide ligand to more closely resemble in vivo tumor lysis conditions, because peptide vaccination is required for effective tumor regression in vivo both in mice (45) and human clinical studies (46). Consistent with T-cell responses (Fig. 2), the in vitro antitumor activity also plateaus above the ∼10-μM affinity threshold (Fig. 3C). Interestingly, T cells transduced with the highest-avidity TCR L2G2 exhibit similar level of cytotoxicity as lower-avidity TCRs, 16LD6, 19LF6, and R6C12 (Fig. 3 A and B), despite their higher strength of T-cell response (Fig. 2). Our data demonstrated a maximal antitumor activity for TCRs above a defined avidity (Fig. 3B) or affinity threshold (∼10μM) (Fig. 3C). The maximal activity is in contrast to stronger T-cell responses, such as calcium release and cytokine production, with higher-avidity TCRs (Fig. 2).
Fig. 3.
In vitro and In vivo antitumor activities plateau beyond a defined avidity and affinity threshold. (A) Cytotoxicity assay of transduced CD8+ splenocytes mixed with B16/A2–Kb melanoma cell line loaded with 10 μM gp209–2M peptide at various effector/target ratios. The percentage of specific killing of B16/A2–Kb melanoma cell line was measured by 51Cr release assay. Error bars represent the SD of triplicate measurements. (B and C) The percentage of specific killing at a representative effector/target ratio (7.5) was correlated with TCR avidity (B) or affinity (C). (D) ACT of A2–Kb transgenic mice bearing B16/A2–Kb melanoma. Error bars represent SEM of six mice per group. (E and F) The average size of tumors at day 21 was correlated with TCR avidity (E) or affinity (F). Data are representative of two independent experiments.
To assess whether a threshold for maximal antitumor activity also exists in vivo in the ACT setting (35, 39, 47, 48), we treated A2–Kb transgenic mice bearing B16/A2–Kb melanoma by a combination of vaccination with gp209–2M fowlpox virus (49) and with CD8+ T cells transduced with selected TCRs (16LD6, R6C12, 5CE2, K4H5, L2G2, and W2C8) representing the entire avidity/affinity range. Treatment with CD8+ splenocytes expressing the low-affinity/-avidity TCR W2C8 did not result in differences in tumor growth compared with the untreated group (Fig. 3D), indicating that T cells, vaccine, and IL-2 alone have no effect on tumor growth in A2–Kb mice, consistent with previous studies (39). In contrast, treatment with three high-avidity TCRs, 16LD6, R6C12, and L2G2, resulted in significant delay in tumor growth (P < 0.01; Fig. 3D Left). In addition, tumor growth after L2G2 treatment showed no differences compared with 16LD6 or R6C12, suggesting a maximal in vivo antitumor activity, consistent with the in vitro cytotoxicity data (Fig. 3A). Treatment with intermediate avidity/affinity TCRs, K4H5, and 5CE2, mediated moderate delay in tumor growth (Fig. 3D Right); however, it is not statistically significant (P > 0.5) compared with the untreated group. The antitumor activity of the TCRs plateaus above an avidity (Fig. 3E) and affinity threshold (KD, ∼10 μM; Fig. 3F), similar to the in vitro cytotoxicity (Fig. 3A). In summary, both in vitro and the in vivo antitumor activities of gp209-specific TCRs reach a maximum at a defined avidity and affinity threshold (KD, ∼10μM). Previous studies of the relationship between TCR affinity and T-cell function have led to controversial conclusions (12, 17, 22–25). Here, we demonstrated a plateaued correlation in a self-antigen system in both in vivo and in vitro settings, which has far broader implications for clinical applications.
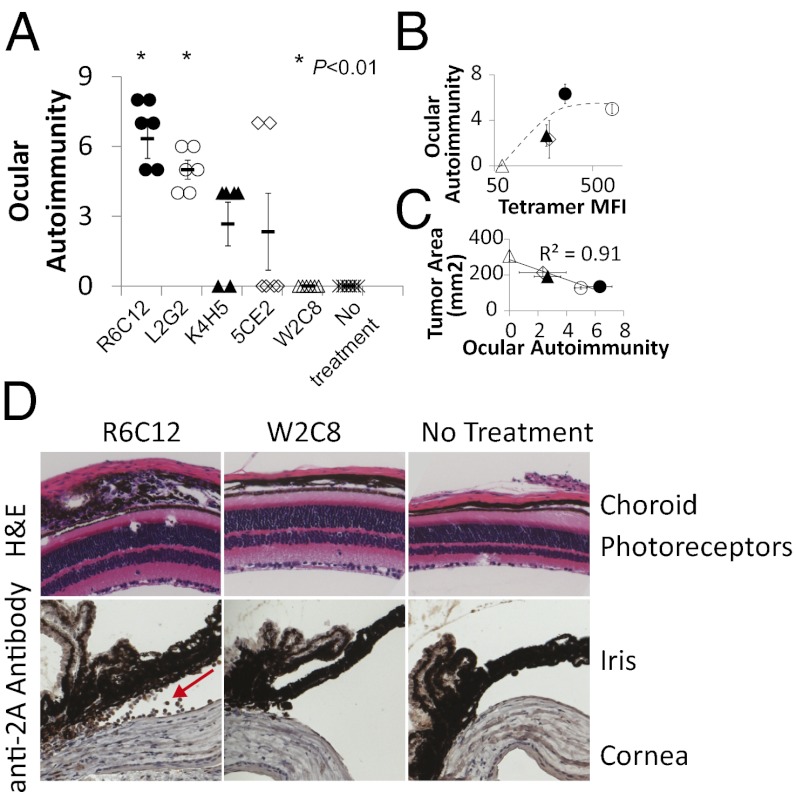
Autoimmunity Is Correlated with TCR Antitumor Activities.
Next, we investigated the possible biological significance of the observed plateaued correlation between TCR affinity and antitumor activity. This issue has not been addressed in previous studies, and we hypothesized that it could be attributed to autoimmunity. Autoimmune destruction of melanocytes in the eye (ocular autoimmunity) has been observed both in human (6, 27) and mouse (26) melanoma ACT. To determine whether autoimmunity correlates with TCR antitumor activities, we selected five TCRs showing various antitumor activities (high, L2G2 and R6C12; intermediate, K4H5 and 5CE2; and low, W2C8; Fig. 3D) and evaluated ocular autoimmunity 5 d after treatment (26). No signs of autoimmunity were observed for the low-avidity/-affinity TCR W2C8; in contrast, the two TCRs that mediate the most effective antitumor responses (R6C12 and L2G2; Fig. 3D) also showed the highest average ocular autoimmunity scores (26) (P < 0.01 vs. control; Fig. 4A). The average score of the highest-avidity TCR (L2G2) was similar to that of the lower-avidity TCR (R6C12; Fig. 4B), indicating a maximum avidity threshold for ocular autoimmunity. Consistent with previous observations in the pmel mouse model (26), we observed that tumor size is correlated with the severity of ocular autoimmunity (Fig. 4C) in our A2–Kb model. Histological examination of eye samples from mice receiving ACT revealed destruction of the choroid and iris and infiltration of T cells in mice treated with high-affinity or -avidity TCRs (R6C12 and L2G2), but not with low-affinity/-avidity TCR (W2C8; Fig. 4D). Conversely, T cells expressing both high- and low-avidity TCRs were able to traffic to the tumor and spleen (Fig. S5). Our data suggest that high-affinity/-avidity TCRs are required to compromise the immune privilege of the eyes. Together, our data indicate that, although ACT with higher-affinity/-avidity TCRs can lead to more efficient tumor regression, it is also accompanied by more severe autoimmunity. Therefore, we suggest that the plateaued correlation between affinity/avidity and tumor rejection observed for high-affinity/-avidity TCRs could be due to an internal protection mechanism in the immune system to prevent overt self-damage.
Fig. 4.
Ocular autoimmunity correlates with TCR affinity/avidity and is plateaued above an avidity threshold. (A) Eyes from A2–Kb mice receiving ACT were H&E stained on day 5 after ACT, and the ocular autoimmunity scores were evaluated as described (26). *P < 0.01 vs. control group that did not receive treatment. Error bars represent SEM (n = 6). (B and C) The ocular autoimmunity score was correlated with TCR avidity (B) or the average tumor size at day 21 (C). (D) Eyes from A were stained with H&E or anti-2A antibody specific for the 2A epitope tag (Fig. S2A). The samples were examined for inflammation in the choroid, photoreceptors, iris, or cornea and infiltration of T cells (highlighted by red arrow). Data are representative of two independent experiments.
Discussion
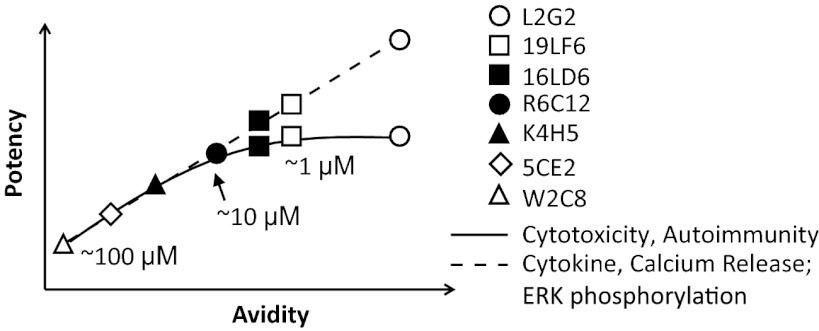
Using seven gp209-specific TCRs spanning the physiological affinity range, we have delineated the relationship between TCR affinity, avidity, and functional potency (Fig. 5). TCR potency is determined by TCR avidity, which is contributed both by TCR affinity and CD8, rather than affinity alone. Although in vitro T-cell responses, such as cytokine and calcium release, linearly correlate with TCR avidity, the killing of target cells, including in vitro/in vivo lysing of tumor cells and autoimmunity, plateau at an affinity threshold of ∼10 μM. Our results suggest that there can be a discrepancy between in vitro and in vivo TCR potency. Therefore, previous in vitro studies (17, 24) may not necessarily translate to clinical settings. In contrast, our studies have direct implication to human T-cell immunotherapy: Recent ACT clinical trials (6, 9) demonstrated a trend of improved efficacy for higher-affinity TCR DMF5 (KD = 5.6 μM) (50) compared with the lower-affinity TCR DMF4 (KD = 29 μM; ref. 50) (Table S1). However, it is unclear whether this trend is significant because of low patient sample size. Further studies are warranted to draw conclusions about the correlation of affinity and clinical efficacy.
Fig. 5.
TCR functional potency is defined by TCR avidity and affinity. Schematic plot of the correlations between T-cell functional activities (in vitro T-cell response, antitumor activity, and autoimmunity), TCR avidities, and affinities.
Our data clearly demonstrate that functional activities are not determined by TCR affinity alone, but by avidity determined by the combined contribution of TCR and CD8. The combined contribution is supported by the observation that the low-affinity TCR L2G2 showed the highest avidity and functional activity in our system. Previous studies (16, 41) indicate that the contribution of CD8 to TCR/pMHC interaction is dependent on ligand affinity. However, it is unknown whether the properties of TCRs can determine the magnitude of CD8 contribution (29). It has been demonstrated that CD8 engages in the TCR/pMHC interaction in the second stage after the TCR/pMHC complex is formed (41). Previous studies were not able to look into this phenomena; however, here, by using seven unique TCRs, we show that the CD8 contribution to TCR/pMHC interaction might be dependent on individual TCRs, because we have clearly demonstrated that CD8 stabilizes L2G2/pMHC interaction more profoundly than any of the other TCRs. However, further investigation using 2D measurements (42) is needed to dissect the underlying mechanism of TCR-dependent CD8 contribution.
It is unclear how high-avidity TCRs (such as L2G2) can lead to stronger T-cell response (Fig. 2) but not stronger cytotoxicity (Figs. 3 and 4). One possible explanation is that T-cell response and cytotoxicity are mediated by different mechanisms. The affinity-based model (12–14, 16–18, 29) suggests that the strength of T-cell signaling is determined by the number of TCR/pMHC complexes formed at the synapse. In addition, distal events, such as cytokine production, require maintenance of T-cell/APC synapse for maximum effect (51). High-avidity TCRs can potentially engage more pMHC ligands and maintain such engagement longer, resulting in stronger responses. Conversely, T-cell killing of target cells only requires three pMHC complexes (52), and the delivery of lytic granules to target cells is rapid and insensitive to antigen density (53). Therefore, above a defined threshold, higher-avidity TCRs may not be at an advantage over lower-avidity TCRs in the exertion of cytotoxic function.
It is intriguing that TCRs with affinity higher than the 10-µM threshold do not lead to more potent antitumor activities. There are a number of reasons that could explain this observation. Our binding data demonstrate that TCR affinity contributes to avidity up to the 10-μM threshold (Fig. 1B), after which further increase does not lead to higher avidity and consequent stronger T-cell functions. We propose that the observed plateaued avidity is due to the fact that TCR clustering is required both for multivalent tetramer binding and T-cell activation (29, 54). At the 10-µM threshold, all clustered TCRs may be occupied; further increase in affinity only leads to monovalent TCR/pMHC interaction, which does not contribute to tetramer binding or T-cell functions (29, 55). Furthermore, the high sensitivity of T-cell killing discussed above could be responsible for the observed plateau in vivo. Finally, we and others have proposed a negative regulation mechanism for T cells in response to high-avidity antigen stimulation in vivo, where the potency of high-avidity TCRs could be further curtailed due to negative feedback mechanisms (25, 56, 57). Consequently, it is likely that several mechanisms are involved that influence the T-cell response to high-avidity ligands in vivo.
One requirement for T-cell immunotherapy to be effective and safe for patients is the identification of an optimal TCR affinity range that leads to efficient tumor regression while inducing minimal autoimmune response. Tumor regression without induction of autoimmunity has been reported (9, 58, 59) using T cells with relatively low reactivity against tumor antigens; high-avidity T cells are often associated with autoimmunity (6, 27, 45, 59). Our results showed that antitumor activity and autoimmunity are coupled and have a similar kinetic threshold; reducing autoimmunity cannot be accomplished without sacrificing efficacy of tumor killing. The coupled relationship is supported by recent ACT clinical trials showing that the treatment with the higher-affinity TCR DMF5, but not with the lower-affinity TCR DMF4, leads to autoimmunity in patients (Table S1) (6). However, the severity of autoimmune response is not likely to increase further because it is also plateaued similar to what was observed for antitumor activity. In light of the close relationship we observed between tumor reactivity and autoimmunity, we propose that the relevance of the kinetic threshold as defined by our studies is a mechanism that could control for T-cell sensitivity in vivo to balance beneficial tumor immunity with overt reactivity.
Methods
DNA Constructs.
TCR genes were isolated from PBMCs as described (31) by using 5′ RACE–PCR (SMART RACE cDNA Amplification Kit; Clontech). Human/mouse chimeric TCR constructs were generated by PCRs as described (60) and subcloned into retroviral vector pMSGV [murine stem cell virus (MSCV)-based splice-gag vector] (61).
Transduction.
Retroviral transduction of splenocytes were performed as described (40, 62) (SI Methods).
Tetramer Staining.
Cells were stained with gp209–HLA-A2–Kb tetramer for 2 h at room temperature in PBS with 5% (vol/vol) FBS and 0.2% fresh sodium azide as described (14). The tetramer was titrated to obtain a selected concentration at which the highest-avidity TCR did not reach saturation.
Cytotoxicity Assays and ERK Phosphorylation Assays.
Cytotoxicity assays were performed as described (31) with modification (SI Methods), and ERK phosphorylation assays were performed as described (63) with modifications (SI Methods).
Fluorescent Imaging.
Imaging acquisition and data analysis were performed as described (55) (SI Methods).
Cytokine ELISA.
For cytokine ELISA, 1 × 105 T cells and 1 × 105 APCs (T2/A2–Kb or B16/A2–Kb) were mixed with various concentrations of gp209–2M and incubated overnight at 37 °C and 5% CO2. IL-2 or IFN-γ production was measured by standard sandwich ELISA. All antibodies and cytokine standards were from eBioscience. Streptavidin–HRP was from BD Biosciences and tetramethylbenzidine ELISA substrate was from Sigma.
ACT.
Immunohistochemistry.
A2–Kb transgenic mice bearing B16/A2–Kb tumor were killed 5 d after receiving ACT, and tissues (eyes, spleen, and tumor) were harvested, fixed in 4% paraformaldehyde, washed twice in PBS, embedded in paraffin, sectioned, and mounted on slides at the New York University (NYU) Immunohistochemistry Core. For ocular autoimmunity, the eyes were H&E stained and evaluated in a masked fashion as described (26). For immunohistochemistry, tissues slides were stained with anti-2A antibody (Millipore, 1:5,000 dilution) and developed with the VECTASTAIN Elite ABC system (Rabbit IgG; Vector Laboratories) according to the manufacturer’s instructions. Images were obtained on a Zeiss Axiophot microscope at 20× magnification and analyzed in the AxioVision software (Zeiss).
Statistics.
Tumor growth curves between different treatment groups were compared by using the repeated-measure ANOVA test. Correction for multiple testing was made by using Tukey’s method. Statistical analyses were conducted by using SAS (Version 9.20. Student t test was used to compare two samples. A P value of 0.05 or lower is considered statistically significant.
Supplementary Material
Acknowledgments
We thank Zahid Dewan and Mark J. Alu (NYU Research Histology Core) for preparing mice tissue samples; NYU Flow Cytometry Core and NYU animal facility for technical assistance; Evan Newell and Mark M. Davis for protocols and advice on soluble TCR expression and purification; the Jeff Ravetch Laboratory for the use of the Biacore T100; and Nina Bhardwaj, William L. Carroll, Arup Chakraborty, Herman Eisen, Joel Ernst, Duane Moogk, Patrick Ott, and Cenk Sumen for helpful discussions and reading of the manuscript. M.K. was a Pew Scholar in the Biomedical Sciences supported by the Pew Trust. This work was supported by National Institutes of Health (NIH) Grants from National Cancer Institute 1U01CA137070 and National Institute of General Medical Sciences 5R01GM085586 (to M.K.); American Cancer Society Research Scholar Grant RSG-09-070-01-LIB (to M.K); a Cancer Research Investigator grant (to M.K.); an American Heart Association Fellowship (to K.M.); an NIH Molecular Biophysics T32 Training Grant (to K.M.); the NYU Cancer Institute; NYU Cancer Institute Cancer Center Support Grant 5P30CA016087-27 (to I.O.), and the Marc Jacobs campaign to support the Interdisciplinary Melanoma Cooperative Group.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221609110/-/DCSupplemental.
References
- 1.June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: Tools, trials and tribulations. Nat Rev Immunol. 2009;9(10):704–716. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: A clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber J, et al. Immunotherapy Task Force of the NCI Investigational Drug Steering Committee White paper on adoptive cell therapy for cancer with tumor-infiltrating lymphocytes: A report of the CTEP subcommittee on adoptive cell therapy. Clin Cancer Res. 2011;17(7):1664–1673. doi: 10.1158/1078-0432.CCR-10-2272. [DOI] [PubMed] [Google Scholar]
- 4.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson LA, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3):535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morgan RA, Dudley ME, Rosenberg SA. Adoptive cell therapy: Genetic modification to redirect effector cell specificity. Cancer J. 2010;16(4):336–341. doi: 10.1097/PPO.0b013e3181eb3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21(2):233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexander-Miller MA, Leggatt GR, Berzofsky JA. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc Natl Acad Sci USA. 1996;93(9):4102–4107. doi: 10.1073/pnas.93.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeh HJ, 3rd, Perry-Lalley D, Dudley ME, Rosenberg SA, Yang JC. High avidity CTLs for two self-antigens demonstrate superior in vitro and in vivo antitumor efficacy. J Immunol. 1999;162(2):989–994. [PubMed] [Google Scholar]
- 12.Tian S, Maile R, Collins EJ, Frelinger JA. CD8+ T cell activation is governed by TCR-peptide/MHC affinity, not dissociation rate. J Immunol. 2007;179(5):2952–2960. doi: 10.4049/jimmunol.179.5.2952. [DOI] [PubMed] [Google Scholar]
- 13.Corse E, Gottschalk RA, Allison JP. Strength of TCR-peptide/MHC interactions and in vivo T cell responses. J Immunol. 2011;186(9):5039–5045. doi: 10.4049/jimmunol.1003650. [DOI] [PubMed] [Google Scholar]
- 14.Krogsgaard M, et al. Evidence that structural rearrangements and/or flexibility during TCR binding can contribute to T cell activation. Mol Cell. 2003;12(6):1367–1378. doi: 10.1016/s1097-2765(03)00474-x. [DOI] [PubMed] [Google Scholar]
- 15.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458(7235):211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laugel B, et al. Different T cell receptor affinity thresholds and CD8 coreceptor dependence govern cytotoxic T lymphocyte activation and tetramer binding properties. J Biol Chem. 2007;282(33):23799–23810. doi: 10.1074/jbc.M700976200. [DOI] [PubMed] [Google Scholar]
- 17.Schmid DA, et al. Evidence for a TCR affinity threshold delimiting maximal CD8 T cell function. J Immunol. 2010;184(9):4936–4946. doi: 10.4049/jimmunol.1000173. [DOI] [PubMed] [Google Scholar]
- 18.Holler PD, Kranz DM. Quantitative analysis of the contribution of TCR/pepMHC affinity and CD8 to T cell activation. Immunity. 2003;18(2):255–264. doi: 10.1016/s1074-7613(03)00019-0. [DOI] [PubMed] [Google Scholar]
- 19.Robbins PF, et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J Immunol. 2008;180(9):6116–6131. doi: 10.4049/jimmunol.180.9.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chinnasamy N, et al. A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J Immunol. 2011;186(2):685–696. doi: 10.4049/jimmunol.1001775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robbins PF, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29(7):917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varela-Rohena A, et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med. 2008;14(12):1390–1395. doi: 10.1038/nm.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMahan RH, et al. Relating TCR-peptide-MHC affinity to immunogenicity for the design of tumor vaccines. J Clin Invest. 2006;116(9):2543–2551. doi: 10.1172/JCI26936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irving M, et al. Interplay between T cell receptor binding kinetics and the level of cognate peptide presented by major histocompatibility complexes governs CD8+ T cell responsiveness. J Biol Chem. 2012;287(27):23068–23078. doi: 10.1074/jbc.M112.357673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corse E, Gottschalk RA, Krogsgaard M, Allison JP. Attenuated T cell responses to a high-potency ligand in vivo. PLoS Biol. 2010;8(9):e1000481. doi: 10.1371/journal.pbio.1000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer DC, et al. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc Natl Acad Sci USA. 2008;105(23):8061–8066. doi: 10.1073/pnas.0710929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh S, et al. Ocular and systemic autoimmunity after successful tumor-infiltrating lymphocyte immunotherapy for recurrent, metastatic melanoma. Ophthalmology. 2009;116(5):981–989. doi: 10.1016/j.ophtha.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gronski MA, et al. TCR affinity and negative regulation limit autoimmunity. Nat Med. 2004;10(11):1234–1239. doi: 10.1038/nm1114. [DOI] [PubMed] [Google Scholar]
- 29.Stone JD, Chervin AS, Kranz DM. T-cell receptor binding affinities and kinetics: Impact on T-cell activity and specificity. Immunology. 2009;126(2):165–176. doi: 10.1111/j.1365-2567.2008.03015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savage PA, Boniface JJ, Davis MM. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity. 1999;10(4):485–492. doi: 10.1016/s1074-7613(00)80048-5. [DOI] [PubMed] [Google Scholar]
- 31.Johnson LA, et al. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177(9):6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 33.Parkhurst MR, et al. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157(6):2539–2548. [PubMed] [Google Scholar]
- 34.Borbulevych OY, Baxter TK, Yu Z, Restifo NP, Baker BM. Increased immunogenicity of an anchor-modified tumor-associated antigen is due to the enhanced stability of the peptide/MHC complex: Implications for vaccine design. J Immunol. 2005;174(8):4812–4820. doi: 10.4049/jimmunol.174.8.4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu Z, et al. Poor immunogenicity of a self/tumor antigen derives from peptide-MHC-I instability and is independent of tolerance. J Clin Invest. 2004;114(4):551–559. doi: 10.1172/JCI21695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg SA, et al. Impact of cytokine administration on the generation of antitumor reactivity in patients with metastatic melanoma receiving a peptide vaccine. J Immunol. 1999;163(3):1690–1695. [PMC free article] [PubMed] [Google Scholar]
- 37.Walker EB, et al. gp100(209-2M) peptide immunization of human lymphocyte antigen-A2+ stage I-III melanoma patients induces significant increase in antigen-specific effector and long-term memory CD8+ T cells. Clin Cancer Res. 2004;10(2):668–680. doi: 10.1158/1078-0432.ccr-0095-03. [DOI] [PubMed] [Google Scholar]
- 38.Vitiello A, Marchesini D, Furze J, Sherman LA, Chesnut RW. Analysis of the HLA-restricted influenza-specific cytotoxic T lymphocyte response in transgenic mice carrying a chimeric human-mouse class I major histocompatibility complex. J Exp Med. 1991;173(4):1007–1015. doi: 10.1084/jem.173.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frankel TL, et al. Both CD4 and CD8 T cells mediate equally effective in vivo tumor treatment when engineered with a highly avid TCR targeting tyrosinase. J Immunol. 2010;184(11):5988–5998. doi: 10.4049/jimmunol.1000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhong S, Malecek K, Perez-Garcia A, Krogsgaard M. Retroviral transduction of T-cell receptors in mouse T-cells. J Vis Exp. 2010;(44):2307. doi: 10.3791/2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang N, et al. Two-stage cooperative T cell receptor-peptide major histocompatibility complex-CD8 trimolecular interactions amplify antigen discrimination. Immunity. 2011;34(1):13–23. doi: 10.1016/j.immuni.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang J, et al. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 2010;464(7290):932–936. doi: 10.1038/nature08944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Irvine KR, et al. Recombinant virus vaccination against “self” antigens using anchor-fixed immunogens. Cancer Res. 1999;59(11):2536–2540. [PMC free article] [PubMed] [Google Scholar]
- 45.Overwijk WW, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198(4):569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith FO, Klapper JA, Wunderlich JR, Rosenberg SA, Dudley ME. Impact of a recombinant fowlpox vaccine on the efficacy of adoptive cell therapy with tumor infiltrating lymphocytes in a patient with metastatic melanoma. J Immunother. 2009;32(8):870–874. doi: 10.1097/CJI.0b013e3181b36b69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hinrichs CS, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci USA. 2009;106(41):17469–17474. doi: 10.1073/pnas.0907448106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gattinoni L, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15(7):808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberg SA, et al. Recombinant fowlpox viruses encoding the anchor-modified gp100 melanoma antigen can generate antitumor immune responses in patients with metastatic melanoma. Clin Cancer Res. 2003;9(8):2973–2980. [PMC free article] [PubMed] [Google Scholar]
- 50.Borbulevych OY, Santhanagopolan SM, Hossain M, Baker BM. TCRs used in cancer gene therapy cross-react with MART-1/Melan-A tumor antigens via distinct mechanisms. J Immunol. 2011;187(5):2453–2463. doi: 10.4049/jimmunol.1101268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4(8):749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 52.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5(5):524–530. doi: 10.1038/ni1058. [DOI] [PubMed] [Google Scholar]
- 53.Wiedemann A, Depoil D, Faroudi M, Valitutti S. Cytotoxic T lymphocytes kill multiple targets simultaneously via spatiotemporal uncoupling of lytic and stimulatory synapses. Proc Natl Acad Sci USA. 2006;103(29):10985–10990. doi: 10.1073/pnas.0600651103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stone JD, Cochran JR, Stern LJ. T-cell activation by soluble MHC oligomers can be described by a two-parameter binding model. Biophys J. 2001;81(5):2547–2557. doi: 10.1016/S0006-3495(01)75899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krogsgaard M, et al. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434(7030):238–243. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 56.Slansky JE, Jordan KR. The Goldilocks model for TCR-too much attraction might not be best for vaccine design. PLoS Biol. 2010;8(9):e1000482. doi: 10.1371/journal.pbio.1000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hebeisen M, et al. SHP-1 phosphatase activity counteracts increased T cell receptor affinity. J Clin Invest. 2013;123(3):1044–56. doi: 10.1172/JCI65325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eck SC, Turka LA. Adoptive transfer enables tumor rejection targeted against a self-antigen without the induction of autoimmunity. Cancer Res. 2001;61(7):3077–3083. [PubMed] [Google Scholar]
- 59.Morgan DJ, et al. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J Immunol. 1998;160(2):643–651. [PubMed] [Google Scholar]
- 60.Bowne WB, et al. Coupling and uncoupling of tumor immunity and autoimmunity. J Exp Med. 1999;190(11):1717–1722. doi: 10.1084/jem.190.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen CJ, Zhao Y, Zheng Z, Rosenberg SA, Morgan RA. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 2006;66(17):8878–8886. doi: 10.1158/0008-5472.CAN-06-1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Y, et al. Primary human lymphocytes transduced with NY-ESO-1 antigen-specific TCR genes recognize and kill diverse human tumor cell lines. J Immunol. 2005;174(7):4415–4423. doi: 10.4049/jimmunol.174.7.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kerkar SP, et al. Genetic engineering of murine CD8+ and CD4+ T cells for preclinical adoptive immunotherapy studies. J Immunother. 2011;34(4):343–352. doi: 10.1097/CJI.0b013e3182187600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feinerman O, Veiga J, Dorfman JR, Germain RN, Altan-Bonnet G. Variability and robustness in T cell activation from regulated heterogeneity in protein levels. Science. 2008;321(5892):1081–1084. doi: 10.1126/science.1158013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.