Abstract
Marine invertebrates commonly produce larvae that disperse in ocean waters before settling into adult shoreline habitat. Chemical and other seafloor-associated cues often facilitate this latter transition. However, the range of effectiveness of such cues is limited to small spatial scales, creating challenges for larvae in finding suitable sites at which to settle, especially given that they may be carried many kilometers by currents during their planktonic phase. One possible solution is for larvae to use additional, broader-scale environmental signposts to first narrow their search to the general vicinity of a candidate settlement location. Here we demonstrate strong effects of just such a habitat-scale cue, one with the potential to signal larvae that they have arrived in appropriate coastal areas. Larvae of the purple sea urchin (Strongylocentrotus purpuratus) exhibit dramatic enhancement in settlement following stimulation by turbulent shear typical of wave-swept shores where adults of this species live. This response manifests in an unprecedented fashion relative to previously identified cues. Turbulent shear does not boost settlement by itself. Instead, it drives a marked developmental acceleration that causes “precompetent” larvae refractory to chemical settlement inducers to immediately become “competent” and thereby reactive to such inducers. These findings reveal an unrecognized ability of larval invertebrates to shift the trajectory of a major life history event in response to fluid-dynamic attributes of a target environment. Such an ability may improve performance and survival in marine organisms by encouraging completion of their life cycle in advantageous locations.
Keywords: competence, energy dissipation rate, metamorphosis, surf zone, turbulence
Nearly 80% of marine invertebrate species that dwell along the shore possess a two-phase life cycle (1). Such life cycles typically are composed of an adult stage resident on the seafloor and a dispersing larval stage that develops in the plankton before returning to, and settling within, shoreline habitat. Because planktonic larval stages foster demographic connectivity among populations (2–4) and because the transition to the substratum often is irreversible and fraught with high mortality (5), considerable attention has focused on cues used by larvae to identify and settle in quality locations. In particular, research has centered on chemical compounds that induce settlement. A rich array of dissolved and surface-bound chemicals have been implicated, including amino acids and proteins, fatty acids, and carbohydrates (6–8). Such substances often are associated with food, prey, adults of the same species, or biofilms composed of microorganisms. Other habitat properties also may be assessed by larvae once they contact the seafloor. For example, larvae often prefer certain substratum textures and topographies, light intensities, or local water velocities (9–13).
Despite their clear importance as signals for larvae, chemical and other seafloor-associated cues provide useful information predominantly over scales of centimeters. This limitation raises the question of how larvae transported orders of magnitude farther in coastal currents (e.g., ref. 4) find their way to the often discrete patches of habitat that possess appropriate cues. If larvae rely exclusively on localized indicators, they retain little capacity to efficiently reject larger stretches of unviable shore, and large numbers of individuals therefore might waste precious time and resources conducting fruitless fine-scale searches. In contrast, if larvae use not only localized cues at the seafloor but also broader, habitat-scale signals characterizing suitable environments, settlement success might be improved considerably (see also ref. 14). Larvae might narrow in on potential destination sites, first identifying their approach to, and arrival into, appropriate habitat, then using behaviors or physical agents to convey them to the seafloor where local inducers become available.
Here we use direct, experimental approaches to explore the possibility that larvae might use information spanning a range of scales when settling. We focus our efforts on the purple sea urchin (Strongylocentrotus purpuratus), an established study organism for developmental biologists and dispersal ecologists alike. Although evidence suggests that chemical substances are involved in larval settlement by this species (15), its adult habitat also is characterized by specific physical traits. In particular, S. purpuratus lives in intertidal and shallow subtidal regions on rocky shores that experience breaking waves and exceptional turbulence (Fig. 1). Such turbulence often is indexed by the energy dissipation rate, a measure of how fast kinetic energy is exhausted by friction at the finest scales of fluid motion. Offshore, in the mixed layer of the ocean or under surface whitecaps, average energy dissipation rates range from 10−9 to 10−3 W/kg (16, 17). Energy dissipation rates within the surf zone often are larger, exhibiting average values as high as 10−1 W/kg on sandy beaches (18, 19) and up to 1 W/kg on exposed rocky coasts where waves break with extraordinary violence (20). These differences in magnitude raise the possibility that larvae might detect proximity to shoreline habitat purely through an evaluation of turbulence intensity, much as certain zooplankton use hydrodynamic-sensing capabilities for predator avoidance (21).
Fig. 1.
Key stages in the life cycle of the purple sea urchin, S. purpuratus, as individuals transit between open ocean and shoreline habitats. Persistent differences in the intensity of water motion are depicted qualitatively by the color gradient, with stronger turbulence indicated in white. (A) Adults release gametes that fertilize externally and develop into pluteus larvae. (B) Pluteus larva at the eight-arm stage with a juvenile rudiment on its left side, nearing the competence period for settlement. Four of its five primary podia are visible on the left. (C) Turbulence-exposed larva 1 h after induction with KCl-augmented sea water, showing the retraction of tissue from the tips of the skeletal arm rods, indicating the first stage of settlement. (D) Metamorphosed juvenile 24 h after turbulence exposure and settlement. The larval arms have been reabsorbed, the juvenile has everted, the calcitic spines are visible, and the individual moves using its tube feet. The juvenile stage continues for approximately a year or more before first reproduction. Diameter of the circular image equals 8 cm in A and 620 μm in B–D.
We use a three-step approach to examine how the habitat-scale attribute of surf-zone turbulence might influence larval settlement in S. purpuratus. First, we undertake an expanded analysis of previously collected turbulence data from rocky shores where purple sea urchins live, to better understand the most intense conditions experienced by settling larvae. Second, because interactions of microscopic larvae with flow are difficult to examine in the field, we reproduce key features of natural turbulence in the laboratory using a Taylor–Couette cell (22). This apparatus consists of two nested cylinders of different diameters that, when rotated fast enough relative to each other, generate turbulent flows within an intervening layer of fluid. Although this device does not recreate every aspect of fluid movement a larva would encounter in nature, it generates flow fields characterized by small-scale gradients in fluid velocity (i.e., turbulent shear) that rapidly change orientation and magnitude through time, a fundamental characteristic of how larvae experience turbulence at small scales (23). In the third component of the study, we expose purple sea urchin larvae to varying levels of turbulent shear in the Taylor–Couette cell, and assay their settlement responses.
Results
Turbulence on Wave-Exposed Coasts.
Turbulence within the surf zones of steep rocky shores can be extremely intense. Energy dissipation rates within the uppermost 1% quantile of those we measured approach 10 W/kg (Fig. 2). Such values are one to two orders of magnitude greater than the average rates of energy dissipation we recorded simultaneously, and are substantially in excess of intensities reported for offshore regions (e.g., refs. 16 and 17). Consistent with expectations and theory (24), these exceptional dissipation rates also exhibited an increasing trend under larger waves, suggesting that even greater rates might arise during times of amplified sea state. Together, these findings suggest that levels of turbulence on exposed outer coasts might be distinguishable from those present in other nearshore or offshore areas, and therefore might serve larvae as useful environmental indicators of preferred habitat.
Fig. 2.

Measurements of surf-zone turbulence in rocky coast habitat of central California, indexed in terms of energy dissipation rates. Estimates of average rates (○) and uppermost 1% quantile rates (●) are plotted as a function of the heights of waves breaking on the shore. Color gradient is as in Fig. 1.
Re-Creation of Turbulence in the Laboratory.
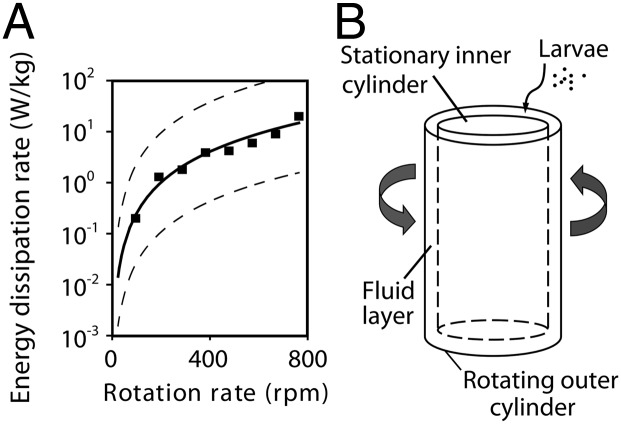
Intensities of turbulence produced within our Taylor–Couette cell corresponded to levels recorded in the field. A robust theory exists concerning turbulence in Taylor–Couette cells, which allows bounds to be placed on energy dissipation rates expected for a given apparatus geometry and rotation speed (25). Although these bounds are broad, Denny et al. (26) determined empirically the energy dissipation rates in a Taylor–Couette cell possessing a geometry and range of rotation speeds that serve well for testing invertebrate larvae. In our comparison of their data with theoretical values, we found that their estimates fell almost exactly on the midline between the expected low and high theoretical bounds (Fig. 3). This correspondence, and our use of a Taylor–Couette cell of identical geometry, allowed us to translate readily between rotation speed (rpm) and energy dissipation rate according to the midline regression of Fig. 3.
Fig. 3.
Energy dissipation rates produced within the Taylor–Couette cell used in the study. (A) Theoretical relationships (dashed lines) (25) establish upper and lower bounds as a function of apparatus rotation speed. Empirical measurements (▪) acquired in a Taylor–Couette cell of geometry identical to that used here (26) fall on the midline (solid line) between these predicted bounds. (B) Basic elements of the device.
Response of Larvae to Turbulence.
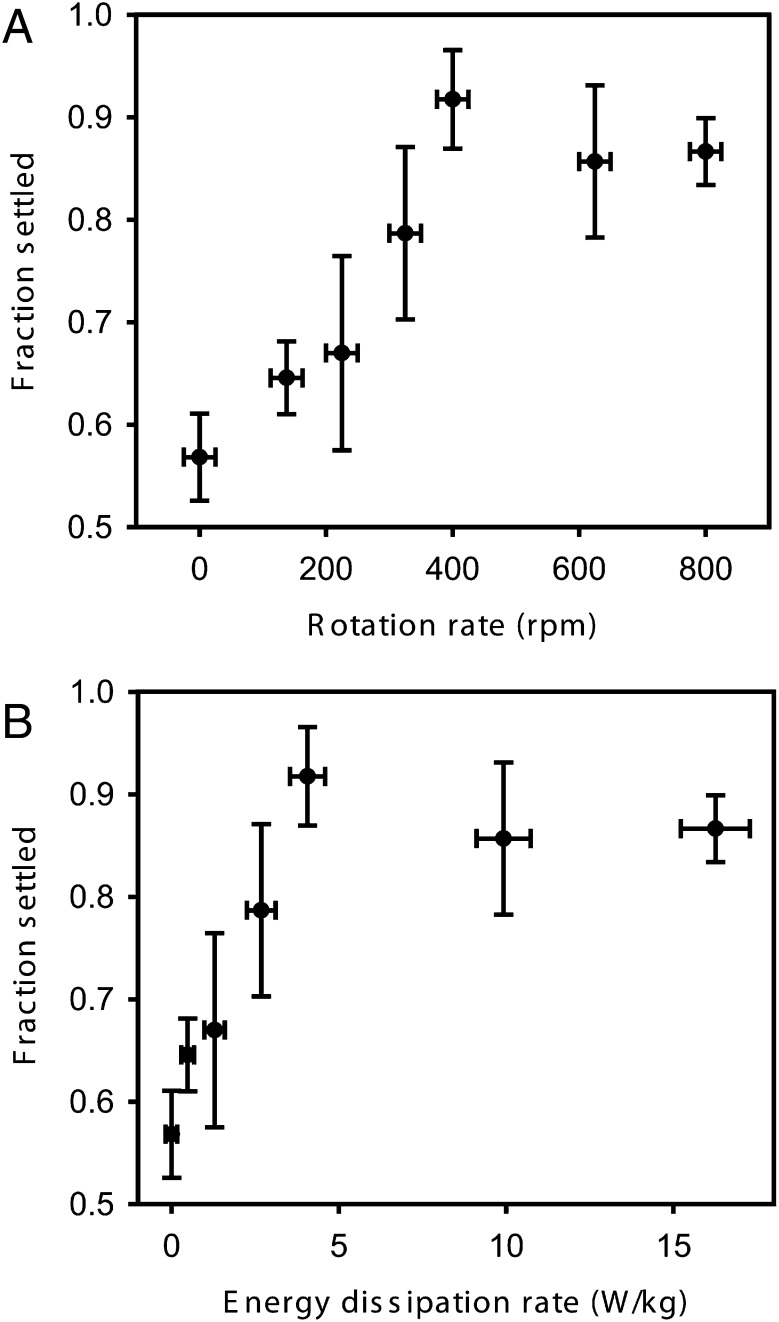
Purple sea urchin larvae exposed to elevated levels of turbulent shear in the Taylor–Couette cell settled at dramatically higher rates (Fig. 4). Larvae that settled 55% of the time in the absence of turbulence showed a nearly 90% propensity to settle and complete metamorphosis after they were subjected to 3 min of turbulent shear at rotation speeds above 400 rpm. Outcomes across rotation speed also exhibited a sigmoidal pattern reminiscent of classic dose–response curves, with an abrupt and significant increase in settlement occurring above a threshold rotation speed (n = 22 trials of 35–40 larvae each; likelihood ratio test, χ2 = 22.75, P < 0.0001). The treatment effect could not be explained by larval handling procedures during transfer into and out of the Taylor–Couette cell, as larvae inserted into the device at 0 rpm exhibited no elevation in settlement upon removal. Similarly, the effect could not be attributed to chemicals originating in the apparatus or to effects of turbulence on the filtered seawater in which the larvae were held, because larvae exposed to seawater spun in the Taylor–Couette cell also showed no increase in settlement. Indeed, there were no significant differences in settlement among our three controls (unmanipulated larvae, 0 rpm, spun seawater; n = 14 trials of 35–40 larvae each; ANOVA, F2,11 = 0.81, P > 0.47). The above responses to the treatment and control trials were determined through exposure of larvae to elevated potassium concentrations in seawater (i.e., through addition of KCl), a standard settlement test for echinoids (27, 28). In the treatment trials, these KCl assays were applied immediately after larvae were removed from the Taylor–Couette cell. We also conducted an additional set of trials in which turbulence-exposed larvae were challenged with a natural inducer composed of a combination of coralline algae and biofilm instead of KCl. These larvae also exhibited elevated settlement (59% compared with 41% in controls; t test, t4 = 4.504, P < 0.01), suggesting that effects of turbulence were not reliant on potassium-based induction.
Fig. 4.
Enhancement of settlement in sea urchin larvae exposed to turbulent shear in a Taylor–Couette cell, via precocious entry into the competent state. The fraction of larvae that settled after a 3-min exposure in the apparatus is shown as a function of (A) the apparatus rotation rate and (B) the intensity of turbulent shear produced within it (estimated after Fig. 3). Note that ∼45% of the larvae in these trials (i.e., the fraction that did not settle at 0 rpm) were formally precompetent. At high spin speeds, most of these precompetent larvae settled in response to a chemical inducer; these precompetent larvae thus became competent as a result of turbulence exposure. Data are means ± SEM.
Accelerated Entry into Competence.
The capacity of larvae to undertake settlement is generally thought to be controlled by a physiological shift from a “precompetent” state, in which settlement cues have no effect, to a “competent” state, operationally defined by the positive response of larvae to an inducer such as KCl (29). Based on this concept, we therefore expected larvae at very early stages of development to not undergo settlement even under high levels of turbulent shear, and indeed this is the pattern we found. When larvae we had identified individually as having no apparent calcification in their juvenile rudiments (a clear indication of early precompetence; 23 d after fertilization) were subjected to 0- or 600-rpm treatments for 3 min in the Taylor–Couette cell and then tested with KCl exposure, none settled.
Intriguingly, we also found that turbulent shear did not elicit settlement when imposed in isolation. In this regard, turbulence acted strikingly differently from a typical inducer. We determined this trend through a conservative examination of competent larvae (>70% settlement in a follow-up KCl test) that were 35 d old at the time of testing. Given results of Fig. 4, which focused on 28-d-old precompetent larvae, the default expectation was that the more advanced larvae would settle especially strongly in response to turbulence and if turbulence was acting as a typical cue, even in the absence of KCl. However, when the 35-d-old larvae were subjected to 0- or 450-rpm treatments in the Taylor–Couette cell (n = 25–30 larvae per trial) and afterward placed into clean glass dishes with Millipore filtered seawater (MFSW), none settled, even after 24 h. These results combined with the data of Fig. 4 demonstrate that turbulence exposure causes precompetent purple sea urchin larvae to settle in response to a strong chemical inducer but that turbulence itself does not result in settlement, even in fully competent larvae. Stated another way, turbulence does not directly induce settlement in already competent larvae, but rather accelerates the transition to competence, at which point larvae become precociously sensitive to local settlement inducers (see also the legend for Fig. 4).
Discussion
Our findings have two important implications. First, they experimentally document an expanded scope for hydrodynamic effects on larval settlement. It is well recognized that larvae use turbulence as a vertical transport agent to the substratum (e.g., refs. 30–32) and that they respond to characteristics of near-substratum flow as well as chemical factors when attaching (6–13). In contrast, limited attention has been focused on the possibility that attributes of water-column fluid movement might provide an assessment tool for larvae before their contact with the seafloor. Certain gastropods cease swimming and sink upon encountering turbulence, and theoretical modeling suggests that this behavior might enhance the delivery of larvae to suitable habitat (14). However, settlement ensuing from such behaviors has not been verified. Our study reveals clear effects of turbulence on settlement of sea urchins and demonstrates that these effects arise under ecologically relevant flow regimes. In particular, we show that large turbulence intensities >1 W/kg occur in the surf zones of rocky coasts, providing routine opportunity for larvae to experience such conditions as they approach suitable nearshore areas. An energy dissipation rate of 1 W/kg also corresponds to the threshold level at which turbulence effects on settlement arose in our experiments (Figs. 3 and 4). This matching implies a potentially beneficial environmental tuning of the larval response. We also note that although the most extreme levels of turbulence manifest most commonly as brief bursts lasting seconds or less, the accumulated exposure time from many bursts may be appreciable. For instance, in the event of a half-hour mixing time across the surf zone before settling, one can estimate that a larva would experience dissipation rates approaching 10 W/kg for a total duration of ∼3 min. This duration is equivalent to the exposure period used in our experimental protocol, with the caveat that differences in the temporal pattern of exposure (e.g., continuous vs. episodic) might well be relevant.
A second major insight from our work pertains to the manner in which turbulence affects larval settlement. No clues from prior research existed to suggest that water movement might alter the developmental trajectory underlying a larva’s transition to shoreline life. Our findings reveal, however, that brief, acute exposure to intense fluid motion sparks an abruptly accelerated entry into competence that promotes the response of larvae to standard chemical inducers. This altered trajectory then culminates in elevated rates of settlement and subsequent completion of metamorphosis to the juvenile stage. As a consequence, turbulence acts not only as a potential indicator of suitable habitat, as discussed above, but also signals to a larva that it might be time to transition to the seafloor, even if the larva has not yet reached competence. Such precocious entry into the benthos might represent a tradeoff against the alternative possibility that larvae might fail to get an additional opportunity to settle if they postpone the process.
Our interpretation of the results of our study relies on the assumption that increased potassium concentrations in seawater provide an accurate means of assessing larval competence. In certain groups of marine invertebrates, ionic chemical inducers such as KCl sometimes yield settlement responses that differ from those associated with presumed natural cues (33) (but see ref. 34). In echinoids, however, multiple studies have addressed and rejected the potential for biased outcomes due to elevated potassium. In all cases examined, rates of settlement and survival following KCl exposure closely resemble those arising in the presence of a potent natural inducer (28, 35–38). The successful, turbulence-caused metamorphic transition we observe therefore stands in marked contrast to consequences of transient exposure to other physical or chemical stimulants (e.g., H2S, petrochemicals, hypoxia). Such agents superficially appear to induce settlement but do not result in normal completion of metamorphosis (39).
The detailed physiological basis for and taxonomic breadth of the larval response to turbulent shear remain unclear and are important areas for future investigation. Valuable insights might emerge from experimental manipulation of mechanoreceptors [e.g., blocking of stretch-activated ion channels (40)], efforts that would be aided by the already sequenced genome of S. purpuratus. Comparative studies of species found in habitats subjected to predictably distinct flow regimes also would address the hypothesis that the turbulence response of larvae is calibrated to their preferred settlement environment. Such a hydrodynamically tuned system for distinguishing nearshore waters from those further offshore might provide substantial selective advantage by allowing larvae to complete the transition to adult life in locations likely to maximize later performance, survival, and reproduction. Benefits of such an ability might be amplified further if, as our data suggest, exposure to strong turbulence triggers rapid entry into the competence period, allowing previously refractory precompetent larvae to respond to local inducers immediately upon arrival at a suitable area. These possibilities beg reevaluation of the scope of cues facilitating settlement and metamorphosis in marine larvae. In particular, they suggest a need for increased attention to fluid-dynamic factors.
Materials and Methods
Larval Culture.
Purple sea urchin (S. purpuratus) larvae used in the study originated from adults collected in the intertidal zone at Clallam Bay, WA, and maintained in subtidal cages suspended off the dock at Friday Harbor Laboratories (Friday Harbor, WA). These adults were fed kelp (mainly blades of Nereocystis luetkeana) ad libitum approximately every 2 wk, year round. During each of three larval rearing periods, we spawned adults by shaking them, then fertilized extruded eggs with dilute sperm suspension (January 2012, two males × two females set up in four pairwise crosses; April 2012, two crosses separated by 1 wk of three males × one female) by standard methods (27). We washed embryos once to remove sperm, then cultured embryos and ensuing larvae at 16 °C in 0.45 µm MFSW for 24 h. At this point, we combined all hatched embryos into a single culture flask at an initial density of approximately one larva per milliliter and continued to rear them with gentle stirring at 16 °C. On the third day after fertilization, we began feeding larvae a mixture of Dunaliella tertiolecta and Rhodomonas sp. at 3:2.5 cells per microliter. Every 2 d, we changed >95% of the water in the culture vessel by reverse filtration and fed the larvae as above. On the eighth day after fertilization (six-arm pluteus stage), we reduced the larval density to one larva per 2 mL, and on day 14 (eight-arm stage), we decreased the density again to one larva per 5 mL. Larvae were maintained at this density until they were transported to Bodega Marine Laboratory (BML; Bodega Bay, CA) between 14 and 26 d post fertilization. The January 2012 and the later set of April 2012 larvae were moved directly to BML; the earlier set of April 2012 larvae were moved in two stages separated by 4 d: first to Hopkins Marine Station (HMS; Pacific Grove, CA) and then to BML. During each of these transport steps, none of which lasted longer than 24 h, larvae were maintained at one larva per milliliter at 12–14 °C. Immediately following arrival at HMS or BML, larval densities again were reduced to one larva per 5 mL, the culture water was changed, and the larvae were fed as before. Larvae always were stirred gently for at least 7 d at BML in seawater held at 14–16 °C before settlement trials began, to allow them to recover from any transport shock. All animals were handled according to approved HMS and BML protocols.
Analysis of Field Turbulence.
The context for testing effects of turbulence on larval settlement and metamorphosis was established through an expanded analysis of previously collected surf-zone velocity recordings (20, 41, 42), which represent to our knowledge the only available data from steep rocky shores such as those where the purple sea urchin lives. The approach may be summarized as follows. Horizontal water velocities were measured at three locations along a 500-m stretch of shore at HMS, sampling at 1,000 Hz using drag-sphere flow probes (41–43). These devices have limitations in spatial resolution (i.e., order 10−2 m vs. 10−4 m for techniques such as laser Doppler imaging), but at present are the only turbulence-sensing instruments that have a proven capability to survive field deployment on exposed wave-swept coasts. Velocities were recorded at an elevation of 2 cm above the substratum. Energy dissipation rates, ε, were determined from the velocity data using methods outlined by George et al. (18), who examined similar features of surf-zone turbulence on a sandy beach (related analyses also appear in more recent publications; e.g., refs. 19, 44, and 45). Across an intermediate range of scales of fluid motion [i.e., across the inertial subrange (46)], flow structures interact with little viscous loss and velocity fluctuations aligned with the mean flow follow the relationship
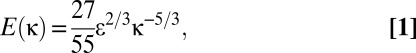 |
where E(κ) is the power spectrum of velocity in the so-called wavenumber domain, which quantifies the energy contained in flow structures of a given spatial dimension, l (represented by a wavenumber, κ = 2π/l). In the presence of a strong mean velocity, U, the wavenumber spectrum can be determined from a standard frequency spectrum by invoking a relationship called Taylor’s hypothesis (46), which assumes that a stationary sensor encounters a flow structure of spatial extent l for the duration τ = l/U. Under these conditions,
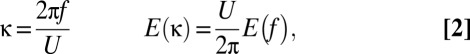 |
where f (=1/τ) is frequency and E(f) is the frequency power spectrum of the velocity fluctuations. Based on Eq. 1, the energy dissipation rate can then be calculated from the y-intercept of a regression line fit to the plot of the logarithm of E(κ) vs. the logarithm of κ. Multiple dissipation rate estimates, quantified over brief periods during which conditions are relatively constant, follow an approximately lognormal cumulative probability distribution when considered in ensemble. This property is well known and tied to the strong “intermittency” of field turbulence (47). Average dissipation rates, <ε>, therefore may be estimated as
 |
where σlnε2 is the variance of the natural logarithm of the ε values, and μlnε is the mean of the normally distributed ln(ε) values.
The above relationships provide the framework by which the velocity recordings were analyzed. Multiple hours-long time series of velocity were divided into successive 0.128-s segments, and each of the resultant 138,000 segments was evaluated for adherence to Taylor’s hypothesis (see ref. 20 for details). For segments meeting this criterion, a frequency power spectrum was computed and converted to a wavenumber spectrum using Eq. 2. A line of −5/3 slope was fit to the log-transform of the first 32 spectral estimates, which corresponded to the low-wavenumber end of the inertial subrange and covered frequencies of ∼8–250 Hz. Higher frequencies were eliminated from the regression because the upper end of the spectrum had already been discarded because of earlier low-pass filtering, and because the 0.01-m spatial resolution of the flow probe yielded a maximum detectable turbulent frequency of ∼300 Hz (f ∼ U/l ∼ 3/0.01 for typical mean flows in the field). Frequencies below 8 Hz were excluded to remove from the regression any low-frequency components of flow associated with residual features of the overall wave motion [f ∼ U/l, with U ∼ 2H and l ∼ 0.25H, where H is local wave height (48)]. Individual estimates of energy dissipation rate were computed for each 0.128-s segment from the intercept, b, of the −5/3 fitted line, as ε = [(55/27)(10b)]3/2 using Eq. 1. In the ensuing summary analyses, all segments associated with flows produced by a given 0.05-m range in wave height were then assembled, and the resulting distribution of the logarithm of these values was plotted on a standard probability scale. The slope (= 1/σlnε) and intercept (= −μlnε/σlnε) of the resulting line provided estimates of the parameters in Eq. 3 that define <ε>, the average energy dissipation rate for a given wave height. Energy dissipation rates in the uppermost 1% quantile then were computed by assuming a lognormal distribution for the individual ε values and invoking the definitional quantile expression, exp(μlnε + pσlnε), where p is the standard normal deviate for 0.99 (numerically = 2.33).
Taylor–Couette Apparatus.
The Taylor–Couette cell used in the present study was composed of two vertically oriented coaxial cylinders separated by a narrow gap containing seawater (Fig. 3). The stationary inner cylinder was held at constant temperature (14–16 °C) by means of a circulating water stream from a temperature-controlled water bath passing through the cylinder’s interior while the outer cylinder was rotated at a prescribed speed. The relative motion between cylinders thereby sheared the seawater between them. At rotation speeds used for testing sea urchin larvae, the sheared flow was turbulent. The geometry of the apparatus (inner cylinder, 50.5-mm outside radius; outer cylinder, 54-mm inside radius) was constructed to match that of Denny and colleagues (26, 49), allowing us to use their empirical determinations of energy dissipation rate as a function of cylinder rotation speed.
Larval Exposure to Turbulent Shear.
Exposure trials were conducted over 2–3-d blocks during February 2012 and May 2012, and consisted of several steps: selecting precompetent larvae into experimental batches, exposing larvae to treatment or control conditions, and testing for settlement responses. Larval cultures were concentrated by reverse filtration and visually inspected to determine the developmental stage of the larvae. Most larvae were synchronous in their development and appeared morphologically precompetent at the time of selection, with only short or incipient spines. Individuals that exhibited delayed or advanced developmental stages were excluded from the main experiment. Selected precompetent larvae were subdivided into individual beakers containing 35–40 individuals at a density of one larva per 5 mL. Each batch of larvae then was randomly assigned to a single treatment or control condition.
For each treatment run, a single batch of 35–40 larvae was concentrated twofold by reverse filtration and gently introduced with a glass Pasteur pipette into 150 mL of MFSW maintained at 14–16 °C within the Taylor–Couette cell. The entire water volume within the apparatus then was subjected to a specified level of turbulent shear for 3 min. Immediately following each treatment run, the larvae within the Taylor–Couette cell were decanted gently into a glass beaker and the Taylor–Couette cell was rinsed twice with 15 °C MFSW to capture any remaining larvae. All recovered larvae were used in ensuing settlement assays. The Taylor–Couette cell then was rinsed thoroughly with distilled water to ensure that no larvae were transferred to subsequent trials, and the next trial was initiated.
In concert with the treatment exposures, randomly selected batches of larvae were exposed to one of three control conditions. Larvae assigned to “unmanipulated controls” were concentrated by reverse filtration and immediately subjected to settlement assays. Larvae assigned to “spun-seawater controls” were concentrated twofold by reverse filtration and placed for 3 min in a beaker of water that had been subjected to 3 min of the highest level of experimental turbulent shear in the Taylor–Couette cell. Thus, these larvae never experienced turbulent shear themselves but were exposed to sheared water as a test for possible effects of turbulent shear on water chemistry (e.g., as a result of hypothetical liberation of a settlement inducer from the apparatus or of the effects of shear on any microorganisms not removed by 0.45 μm Millipore seawater filtration). Larvae assigned to “handling controls” (0-rpm treatments) were treated the same as larvae assigned to spin treatments, except the Taylor–Couette cell was not rotated during the 3 min larvae were within it, thereby controlling for manipulations associated with transfer of larvae into and out of the apparatus.
Definitions of Settlement, Metamorphosis, and Competence.
Settlement in most invertebrate taxa is connected intimately with metamorphosis, a dramatic remodeling of body form during which many structures present during the larval phase are lost and the overall juvenile/adult body form is assumed (50). In fact, settlement and metamorphosis are sufficiently intertwined that some researchers merge them conceptually (6). In S. purpuratus, metamorphosis by our definition (51, 52) (but also see ref. 29) begins more than a week before settlement, with the formation of the pentameral juvenile rudiment, which grows and develops calcified juvenile skeletal structures (including test and spines) within the larval body (Fig. 1B; the state of development of these structures is among the morphological features we used to identify precompetent and competent larvae). Settlement, the most dramatic phase of metamorphosis, starts when a larva attaches permanently to the substratum. The larval skin then is withdrawn from the larval skeletal arm rods within minutes (Fig. 1C) and the juvenile everts, exposing the spines. Over the next days, the mouth opens and a fully functioning juvenile is revealed (Fig. 1D). However, settlement and the subsequent final metamorphic stages are initiated only if a larva is sufficiently developmentally mature (i.e., if it has reached competence). In echinoids, the competence of larvae (53) and thus their capacity to settle and complete metamorphosis, is traditionally and operationally assayed through exposure to dilute KCl in seawater (<0.1 M excess). In competent individuals, KCl induces rapid settlement and the completion of metamorphosis, whereas it induces no such change in precompetent individuals (27, 28). When we selected larvae, we used the morphological features indicated above but always confirmed this assessment with KCl tests.
Quantification of Settlement.
Following turbulence treatments, settlement was assayed by exposing larvae to 70 mM excess KCl in MFSW for 1 h at 14 °C, followed by recovery in MFSW, as in standard protocols (27, 36–38). A larva was scored as settled after this 1-h exposure if it had begun to withdraw skin from the tips of the larval skeletal rods (Fig. 1C). Continued withdrawal of skin was verified over the next several hours, and each larva was checked for eversion and eventual adoption of the definitive juvenile morphology, with active emergent tube feet (primary podia) and juvenile and adult-type spines (Fig. 1D) (54).
In one set of trials examining whether turbulence effects were associated exclusively with KCl induction or would manifest more broadly (i.e., with a natural inducer), groups of 25 larvae were transferred into the Taylor–Couette cell and spun at either 0 or 600 rpm for 3 min. These larvae then were exposed for 2 h to 8 mL MFSW in well plates coated with a 7-d-old biofilm grown in the presence of S. purpuratus adults, and to which 100 mg of coralline algae had been added (Calliarthron tuberculosum, collected in the intertidal zone at HMS). Although it is not known what the true natural settlement cue for S. purpuratus larvae might be, there is substantial precedent for the use of biofilm and coralline algae in laboratory studies (e.g., refs. 15, 37, 38, and 52). Larvae subsequently were scored for settlement as above at the conclusion of the 2-h exposure period.
In a final experiment, we examined whether turbulence exposure itself could result in settlement. For these trials, we selected groups of 25–30 morphologically competent larvae and randomly assigned them to either 0- or 450-rpm treatments. Larvae were recovered following turbulence exposure as before, but instead of being transferred to KCl, they were placed directly into 10 mL MFSW in glass dishes. After 24 h, none of the larvae subjected to either treatment had settled. To confirm our initial scoring of these larvae as competent, we subsequently exposed all larvae to 70 mM excess KCl for 1 h as described previously.
Note that even the earliest precompetent larvae that settled (in either KCl or a natural inducer) following turbulence exposure had functional tube feet and at least incipient spines. These structures continued to develop in the resulting juveniles regardless of the treatment, settlement inducer, or stage at which they settled. Furthermore, although the settlement process with KCl differs in some respects from the process associated with a natural inducer (main difference: limited muscular and ciliary movement in KCl), both follow a similar trajectory of arm retraction and eversion of the juvenile rudiment.
Quantification of the Completion of Metamorphosis.
To confirm our scoring of settlement in the core experimental trials and to ensure that the settled larvae would complete metamorphosis normally, all settled larvae were reexamined following an additional 24 h of recovery in MFSW at 14–16 °C. This evaluation verified that >99% of all larvae had been scored correctly the previous day. These larvae also continued to metamorphose into juveniles, and no postsettlement mortality was detected. Larvae that were scored as not settled also recovered normally and were swimming after 24 h, with no apparent ill effects of the treatment.
Statistical Analyses.
The effect of turbulent shear on larval settlement rate (Fig. 4) was evaluated using logistic regression implemented with a likelihood ratio test. The category of response (enhanced or unaltered settlement) was determined for each experimental run by assessing whether the proportion of larvae settling exceeded or fell below the global median. Potential differences among control runs were tested using ANOVA applied to the arcsin-transformed proportional settlement data. The effect of turbulence on settlement, as assayed with a natural inducer in place of KCl, was examined with a t test. All statistical analyses were conducted using JMP (version 10.0.0) software.
Acknowledgments
We thank C. Bishop, B. S. Cheng, M. W. Denny, R. B. Emlet, M. G. Hadfield, P. M. Schulte, and three anonymous reviewers for comments. J. R. Koseff and S. G. Monismith assisted with turbulence analysis; W. Newman helped construct the Taylor–Couette cell; M. B. Hille and C. J. Lowe provided facilities access; K. Uhlinger helped prepare natural inducers and shared algae; K. Laughlin assisted with larval culturing; and E. Sanford, E. S. Chang, S. Nimitkul, G. N. Cherr, C. A. Vines, and N. A. McGinn loaned equipment. This work was funded by National Science Foundation Grant OCE-0927255 and by an award under the Federal Coastal Zone Management Act, administered by the National Oceanic and Atmospheric Administration’s Office of Ocean and Coastal Resource Management (awarded to San Francisco State University).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Mileikovsky SA. Types of larval development in marine bottom invertebrates, their distribution and ecological significance: A re-evaluation. Mar Biol. 1971;10(3):193–213. [Google Scholar]
- 2.Jackson GA, Strathmann RR. Larval mortality from offshore mixing as a link between precompetent and competent periods of development. Am Nat. 1981;118(1):16–26. [Google Scholar]
- 3.Gaylord B, Gaines SD. Temperature or transport? Range limits in marine species mediated solely by flow. Am Nat. 2000;155(6):769–789. doi: 10.1086/303357. [DOI] [PubMed] [Google Scholar]
- 4.Cowen RK, Sponaugle S. Larval dispersal and marine population connectivity. Annu Rev Mar Sci. 2009;1:443–466. doi: 10.1146/annurev.marine.010908.163757. [DOI] [PubMed] [Google Scholar]
- 5.Gosselin LA, Qian P-Y. Juvenile mortality in benthic marine invertebrates. Mar Ecol Prog Ser. 1997;146(1-3):265–282. [Google Scholar]
- 6.Pawlik JR. Chemical ecology of the settlement of benthic marine invertebrates. Oceanogr Mar Biol Annu Rev. 1992;30:273–335. [Google Scholar]
- 7.Hadfield MG, Paul VJ. In: Marine Chemical Ecology. McClintock JB, Baker BJ, editors. Boca Raton, FL: CRC; 2001. pp. 431–462. [Google Scholar]
- 8.Thiyagarajan V. A review on the role of chemical cues in habitat selection by barnacles: New insights from larval proteomics. J Exp Mar Biol Ecol. 2010;392(1-2):22–36. [Google Scholar]
- 9.Crisp DJ. In: Chemoreception in Marine Organisms. Grant PT, Mackie AM, editors. New York: Academic; 1974. pp. 177–265. [Google Scholar]
- 10.Mullineaux LS, Garland ED. Larval recruitment in response to manipulated field flows. Mar Biol. 1993;116(4):667–683. [Google Scholar]
- 11.Walters LJ, Wethey DS. Settlement and early post-settlement survival of sessile marine invertebrates on topographically complex surfaces: The importance of refuge dimensions and adult morphology. Mar Ecol Prog Ser. 1996;137(1-3):161–171. [Google Scholar]
- 12.Reidenbach MA, Koseff JR, Koehl MAR. Hydrodynamic forces on larvae affect their settlement on coral reefs in turbulent, wave-driven flow. Limnol Oceanogr. 2009;54(1):318–330. [Google Scholar]
- 13.Koehl MAR, Hadfield MG. Hydrodynamics of larval settlement from a larva’s point of view. Integr Comp Biol. 2010;50(4):539–551. doi: 10.1093/icb/icq101. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs HL, Solow AR, Mullineaux LS. Larval responses to turbulence and temperature in a tidal inlet: Habitat selection by dispersing gastropods? J Mar Res. 2010;68(1):153–188. [Google Scholar]
- 15.Rowley RJ. Settlement and recruitment of sea urchins (Strongylocentrotus spp.) in a sea-urchin barren ground and a kelp bed—are populations regulated by settlement or post-settlement processes. Mar Biol. 1989;100(4):485–494. [Google Scholar]
- 16.Oakey NS, Elliott JA. Dissipation within the surface mixed layer. J Phys Oceanogr. 1982;12(2):171–185. [Google Scholar]
- 17.Terray EA, et al. Estimates of kinetic energy dissipation under breaking waves. J Phys Oceanogr. 1996;26(5):792–807. [Google Scholar]
- 18.George R, Flick RE, Guza RT. Observations of turbulence in the surf zone. J Geophys Res. 1994;99:801–810. [Google Scholar]
- 19.Raubenheimer B, Elgar S, Guza RT. Observations of swash zone velocities: A note on friction coefficients. J Geophys Res. 2004;109:C01027. doi: 10.1029/2003JC001877. [DOI] [Google Scholar]
- 20.Gaylord B. Hydrodynamic context for considering turbulence impacts on external fertilization. Biol Bull. 2008;214(3):315–318. doi: 10.2307/25470672. [DOI] [PubMed] [Google Scholar]
- 21.Fields DM, Yen J. The escape behavior of marine copepods in response to a quantifiable fluid mechanical disturbance. J Plankton Res. 1997;19(9):1289–1304. [Google Scholar]
- 22.Taylor GI. Stability of a viscous liquid contained between two rotating cylinders. Phil Trans R Soc A. 1923;223:289–343. [Google Scholar]
- 23.Jumars PA, Trowbridge JH, Boss E, Karp-Boss L. Turbulence-plankton interactions: A new cartoon. Mar Ecol (Berl) 2009;30(2):133–150. [Google Scholar]
- 24.Thornton EB, Guza RT. Transformation of wave height distribution. J Geophys Res. 1983;88:5925–5938. [Google Scholar]
- 25.Doering CR, Constantin P. Energy dissipation in shear driven turbulence. Phys Rev Lett. 1992;69(11):1648–1651. doi: 10.1103/PhysRevLett.69.1648. [DOI] [PubMed] [Google Scholar]
- 26.Denny MW, Nelson EK, Mead KS. Revised estimates of the effects of turbulence on fertilization in the purple sea urchin, Strongylocentrotus purpuratus. Biol Bull. 2002;203(3):275–277. doi: 10.2307/1543570. [DOI] [PubMed] [Google Scholar]
- 27.Strathmann MF. Reproduction and Development of Marine Invertebrates of the Northern Pacific Coast. Seattle: Univ Washington Press; 1987. [Google Scholar]
- 28.Pearce CM, Scheibling RE. Induction of metamorphosis of larval echinoids (Strongylocentrotus droebachiensis and Echinarachnius parma) by potassium chloride (KCl) Invertebr Reprod Dev. 1994;26(3):213–220. [Google Scholar]
- 29.Hadfield MG, Carpizo-Ituarte EJ, del Carmen K, Nedved BT. Metamorphic competence, a major adaptive convergence in marine invertebrate larvae. Am Zool. 2001;41(5):1123–1131. [Google Scholar]
- 30.Eckman JE. Hydrodynamic processes affecting benthic recruitment. Limnol Oceanogr. 1983;28(2):241–257. [Google Scholar]
- 31.Butman CA. Larval settlement of soft-bottom invertebrates—the spatial scales of pattern explained by active habitat selection and the emerging role of hydrodynamical processes. Oceanogr Mar Biol. 1987;25:113–165. [Google Scholar]
- 32.Koehl MR. Mini review: Hydrodynamics of larval settlement into fouling communities. Biofouling. 2007;23(5-6):357–368. doi: 10.1080/08927010701492250. [DOI] [PubMed] [Google Scholar]
- 33.Pechenik JA, Gee CC. Onset of metamorphic competence in larvae of the gastropod Crepidula fornicata (L.), judged by a natural and an artificial cue. J Exp Mar Biol Ecol. 1993;167(1):59–72. [Google Scholar]
- 34.Eyster LS, Pechenik JA. Comparison of growth, respiration, and feeding of juvenile Crepidula fornicata (L) following natural or KCl-triggered metamorphosis. J Exp Mar Biol Ecol. 1988;118(3):269–279. [Google Scholar]
- 35.Cameron RA, Tosteson TR, Hensley V. The control of sea urchin metamorphosis: Ionic effects. Dev Growth Differ. 1989;31(6):589–594. doi: 10.1111/j.1440-169X.1989.00589.x. [DOI] [PubMed] [Google Scholar]
- 36.Carpizo-Ituarte E, Salas-Garza A, Pares-Sierra G. Induction of metamorphosis with KCl in three species of sea urchins and its implications in the production of juveniles. Cienc Mar. 2002;28(2):157–166. [Google Scholar]
- 37.Amador-Cano G, Carpizo-Ituarte E, Cristino-Jorge D. Role of protein kinase C, G-protein coupled receptors, and calcium flux during metamorphosis of the sea urchin Strongylocentrotus purpuratus. Biol Bull. 2006;210(2):121–131. doi: 10.2307/4134601. [DOI] [PubMed] [Google Scholar]
- 38.Sutherby J, et al. Histamine is a modulator of metamorphic competence in Strongylocentrotus purpuratus (Echinodermata: Echinoidea) BMC Dev Biol. 2012;12:14. doi: 10.1186/1471-213X-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubilier N. H2S—A settlement cue or toxic substance for Capitella sp. I larvae? Biol Bull. 1988;174(1):30–38. doi: 10.2307/1541756. [DOI] [PubMed] [Google Scholar]
- 40.Christensen AP, Corey DP. TRP channels in mechanosensation: Direct or indirect activation? Nat Rev Neurosci. 2007;8(7):510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- 41.Gaylord B. Detailing agents of physical disturbance: Wave-induced velocities and accelerations on a rocky shore. J Exp Mar Biol Ecol. 1999;239(1):85–124. [Google Scholar]
- 42.Gaylord B. Biological implications of surf-zone flow complexity. Limnol Oceanogr. 2000;45(1):174–188. [Google Scholar]
- 43.Donelan MA, Motycka J. Miniature drag sphere velocity probe. Rev Sci Instrum. 1978;49(3):298–304. doi: 10.1063/1.1135395. [DOI] [PubMed] [Google Scholar]
- 44.Trowbridge J, Elgar S. Turbulence measurements in the surf zone. J Phys Oceanogr. 2001;31(8):2403–2417. [Google Scholar]
- 45.Feddersen F. Observations of the surf-zone turbulent dissipation rate. J Phys Oceanogr. 2012;42(3):386–399. [Google Scholar]
- 46.Tennekes H, Lumley JL. A First Course in Turbulence. Cambridge, MA: MIT Press; 1972. [Google Scholar]
- 47.Baker MA, Gibson CH. Sampling turbulence in the stratified ocean: Statistical consequences of strong intermittency. J Phys Oceanogr. 1987;17(10):1817–1836. [Google Scholar]
- 48.Svendsen IA. Analysis of surf zone turbulence. J Geophys Res. 1987;92:5115–5124. [Google Scholar]
- 49.Mead KS, Denny MW. The effects of hydrodynamic shear stress on fertilization and early development of the purple sea urchin Strongylocentrotus purpuratus. Biol Bull. 1995;188(1):46–56. doi: 10.2307/1542066. [DOI] [PubMed] [Google Scholar]
- 50.Bishop CD, et al. What is metamorphosis? Integr Comp Biol. 2006;46(6):655–661. doi: 10.1093/icb/icl004. [DOI] [PubMed] [Google Scholar]
- 51.Chia F-S. In: Settlement and Metamorphosis of Marine Invertebrate Larvae. Chia F-S, Rice ME, editors. New York: Elsevier; 1978. pp. 283–285. [Google Scholar]
- 52.Hodin J. Expanding networks: Signaling components in and a hypothesis for the evolution of metamorphosis. Integr Comp Biol. 2006;46(6):719–742. doi: 10.1093/icb/icl038. [DOI] [PubMed] [Google Scholar]
- 53.Bishop CD, Huggett MJ, Heyland A, Hodin J, Brandhorst BP. Interspecific variation in metamorphic competence in marine invertebrates: The significance for comparative investigations into the timing of metamorphosis. Integr Comp Biol. 2006;46(6):662–682. doi: 10.1093/icb/icl043. [DOI] [PubMed] [Google Scholar]
- 54.Miller BA, Emlet RB. Development of newly metamorphosed juvenile sea urchins (Strongylocentrotus franciscanus and S. purpuratus): Morphology, the effects of temperature and larval food ration, and a method for determining age. J Exp Mar Biol Ecol. 1999;235(1):67–90. [Google Scholar]