Abstract
Cellular reprogramming is a new and rapidly emerging field in which somatic cells can be turned into pluripotent stem cells or other somatic cell types simply by the expression of specific combinations of genes. By viral expression of neural fate determinants, it is possible to directly reprogram mouse and human fibroblasts into functional neurons, also known as induced neurons. The resulting cells are nonproliferating and present an alternative to induced pluripotent stem cells for obtaining patient- and disease-specific neurons to be used for disease modeling and for development of cell therapy. In addition, because the cells do not pass a stem cell intermediate, direct neural conversion has the potential to be performed in vivo. In this study, we show that transplanted human fibroblasts and human astrocytes, which are engineered to express inducible forms of neural reprogramming genes, convert into neurons when reprogramming genes are activated after transplantation. Using a transgenic mouse model to specifically direct expression of reprogramming genes to parenchymal astrocytes residing in the striatum, we also show that endogenous mouse astrocytes can be directly converted into neural nuclei (NeuN)-expressing neurons in situ. Taken together, our data provide proof of principle that direct neural conversion can take place in the adult rodent brain when using transplanted human cells or endogenous mouse cells as a starting cell for neural conversion.
The ability to reprogram somatic cells to pluripotent stem cells or other somatic cell types by expressing key combinations of genes has opened up new possibilities for disease modeling and cell therapy (1, 2). Using this technique, it is possible to directly reprogram mouse and human fibroblasts into functional neurons, also known as induced neurons (iNs), using viral delivery of the three neural conversion factors achaete-scute complex-like 1 (Ascl1), brain-2 (Brn2a), and myelin transcription factor-like 1 (Myt1l) (ABM) (3, 4). A growing number of studies now show that by altering the combination of genes used for reprogramming, different subtypes of neurons are obtained (3, 5, 6). Importantly, the resulting cells are nonproliferating, which makes them an interesting alternative to induced pluripotent stem cells as a source of patient-specific neurons for cell replacement therapy, once efficient grafting strategies for these cells are developed.
The adult brain has a very limited inherent capacity for repair, and new neurons are only formed in two discrete regions: the subventricular zone of the lateral ventricles, which generates neurons migrating to the olfactory bulb, and the hippocampus (7, 8). Experimental studies have shown that these endogenous progenitors can also be recruited to generate new neurons in other regions as well in response to injury (9–11). However, the number of new neurons is very low, their migration is hard to control, and the therapeutic implications are unclear. Several cell types residing outside the neurogenic niche, such as parenchymal astrocytes and pericytes, have been shown to form neurons in vitro (12–16). However, parenchymal astrocytes do not form neurons in vivo, which has been speculated to be at least partly because of the nonpermissive environment of the adult brain parenchyma.
Direct neural conversion presents a new possible route for generation of new neurons from parenchymal glia in the brain. Although direct in vivo conversion has already been successful in organs such as the pancreas and heart (17, 18), the method is yet to be explored in the brain. In this study, we show that transplanted human embryonic fibroblasts (hEFs), human fetal lung fibroblast (HFL1) cells, and human astrocytes expressing ABM can overcome these nonneurogenic cues and be converted into neurons while residing in the adult brain. The resulting neurons are stably reprogrammed, survive, and mature in the adult brain while not forming tumors or neural overgrowths. When adding dopamine (DA) fate determinants to the reprogramming procedure, tyrosine hydroxylase (TH)-expressing neurons can be obtained by in vivo conversion of transplanted cells. To establish that this conversion can also take place when resident glia cells are used as a substrate for neural conversion, we generated Cre-inducible lentiviral vectors (LVs) that, when injected to the striatum of transgenic mice expressing Cre from the GFAP promoter, express the reprogramming genes specifically in resident striatal astrocytes. Using this system, we show that iNs can also be generated from endogenous mouse astrocytes that are reprogrammed by viral delivery in situ.
Results
In the first part of this study, we used doxycycline-regulated LVs to deliver the neural conversion genes Ascl1, Brn2a, and Myt1l. These vectors efficiently convert human fibroblasts into functional neurons in the presence of doxycycline in vitro (3, 19) Thus, we can control initiation of conversion by regulating transgene expression via addition of doxycycline in the culture medium in vitro (Fig. S1 A and B). Similarly, it should be possible to initiate the conversion process via administration of doxycycline in the drinking water to activate expression of the neural conversion genes in vivo.
To explore the feasibility of performing the neural conversion in vivo, GFP-labeled HFL1 cells (Fig. S1C) were transduced with neural conversion factors but never exposed to doxycycline in culture medium; that is, the conversion genes were never activated in vitro. The transduced cells were subsequently grafted as fibroblasts to the striatum and hippocampus of adult rats. Once fibroblasts were located in the brain parenchyma, the neural conversion process was initiated by administration of doxycycline in the drinking water. In one group, recipient animals were pretreated with doxycycline for 1 wk before grafting (n = 5), such that conversion was initiated once cells were placed in brain parenchyma, and in the second group, doxycycline was given 1 wk after grafting, such that initiation of conversion occurred 1–2 wk after grafting (n = 5). Two control groups were included: one group of animals was grafted with ABM-transduced cells but never treated with doxycycline (n = 6), and one group of animals was grafted with GFP-only labeled cells onto doxycycline-treated hosts (n = 6).
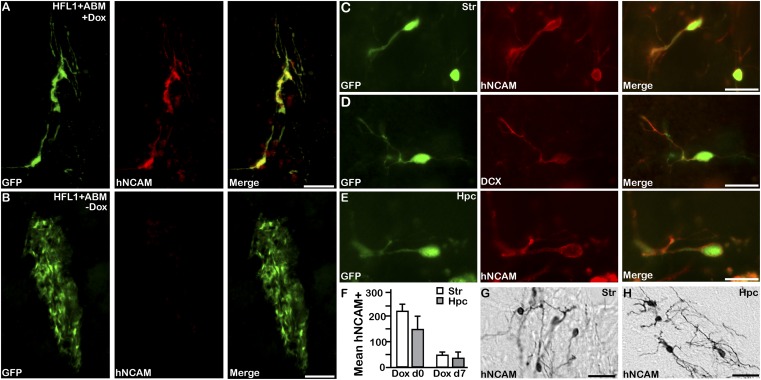
When analyzed after 4 wk in vivo, GFP-positive cells coexpressing human-specific neural cell adhesion molecule (hNCAM) could be detected in the animals exposed to doxycycline (Fig. 1 A, C, and E), which is indicative of successful fibroblast-to-neuron conversion. Such hNCAM-expressing cells were not present in the control (doxycycline-naive) animals transplanted with GFP-labeled, ABM-transduced fibroblasts but were found where the conversion genes were never activated in vivo (Fig. 1B), although the same cell preparation converted into iNs when parallel cultures were plated in doxycycline-containing culture medium in vitro (Fig. S1D). In addition, hNCAM-positive neurons were never detected in the control animals in which GFP-only transduced fibroblasts were grafted into doxycycline-treated hosts (Fig. S1E; n = 5).
Fig. 1.
Direct neural conversion from human fibroblasts takes place in vivo. (A) Induced neurons obtained from in vivo reprogramming of grafted GFP-labeled HFL1 cells express hNCAM (red). (Scale bars: 75 μm.) (B) Control grafts where reprogramming genes have not been activated are negative for hNCAM. (Scale bars: 75 μm.) (C–E) In vivo reprogrammed iN cells detected by GFP coexpress hNCAM (red) cells and DCX (red) in striatum and hippocampus. (Scale bars: 25 μm.) (F) Quantifications of hNCAM-positive cells where doxycycline administered in rats before transplant (Dox d0; n = 3) and 1 wk after transplantation (Dox d7; n = 3) into the striatum (G; white) and hippocampus (H; gray). (Scale bars: 50 μm.) Hpc, hippocampus; Str, striatum.
Animals in both experimental groups exposed to doxycycline (at the time of transplantation and 1 wk later) contained iNs in both striatum and hippocampus, as detected by immunolabeling for GFP combined with hNCAM and doublecortin (DCX) (Fig. 1 C–F). Quantifications based on hNCAM expression showed no major difference in cell number between cells converted in striatum or in hippocampus (Fig. 1F), although there were subtle differences in morphology of converted cells between the two regions (Fig. 1 G and H).
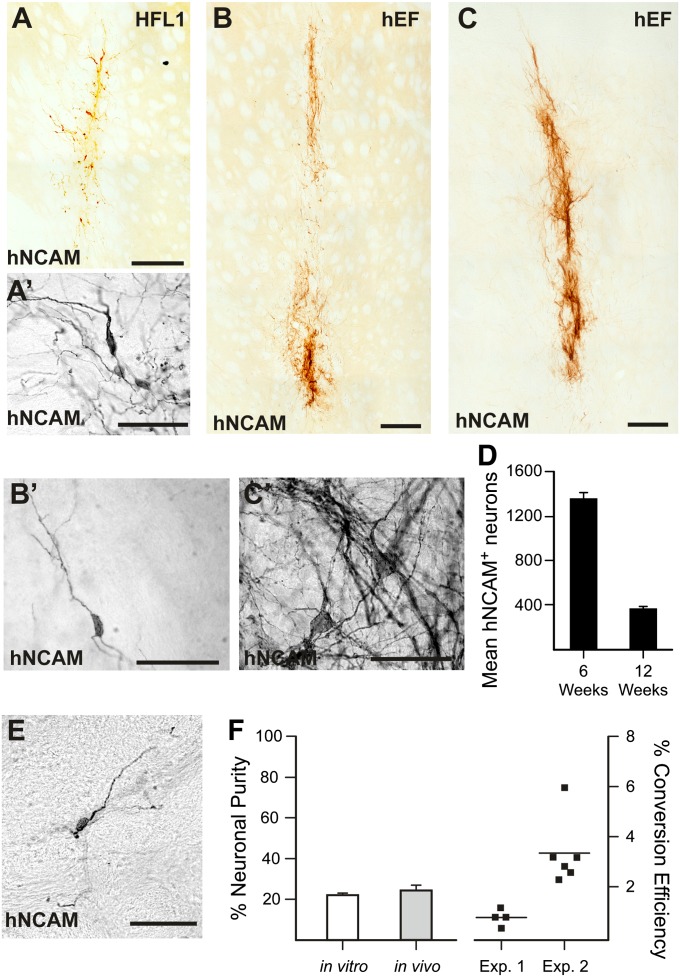
To test the robustness of in vivo neuronal reprogramming and to confirm cell survival and phenotypic stability, we transplanted doxycycline-pretreated animals with either hEFs or HFL1 cells that had been transduced with reprogramming genes (not activated) into the adult rat striatum. When analyzed 6 wk after initiation of conversion, both fibroblast types had efficiently converted into neurons, as detected by hNCAM immunolabeling (Fig. 2 A, A′, B, and B′). In animals analyzed 12 wk after initiation of conversion (the last 6 wk without doxycycline administration), converted iN cells could still be observed (Fig. 2 C and C′). At this time, the number of iNs was lower (Fig. 2D), but the neuronal morphology and extended processes were much more elaborate (compare Fig. 2 C′ with A′ and B′). A decrease in neurons between 6 and 12 wk could suggest that the iN phenotype is not stable once doxycycline is removed. However, in a parallel set of experiments, we grafted immunodeficient rats with transduced fibroblasts. In this experiment, doxycycline was administered for the first 6 wk and then removed for duration of experiment. When the animals were killed after 25 wk, iNs could still be detected (Fig. 2E), confirming the long-term stability of the neuronal phenotype in the absence of sustained exogenous gene expression.
Fig. 2.
Long-term survival and stability of iN cells generated from transplanted fibroblasts via conversion in vivo. hNCAM-positive cells in rats transplanted with (A and A′) hFL-1 and (B and B′) hEF 6 wk after in vivo conversion, and (C and C′) hEFs with more mature morphology 12 wk after initiation of conversion in vivo. (D) Quantifications of hNCAM-positive cells 6 wk (n = 6) and 12 wk (n = 5) after conversion. (E) hNCAM-positive cell 25 wk after transplantation where doxycycline was removed after 4 wk. (F) Quantification of the neuronal purity and conversion efficiency of direct neural conversion in vitro and in vivo. (Scale bar: 250 μm for A–C and 50 μm for A′–C′, and E.)
Quantifications of the in vivo reprogramming process show that the efficiency is similar to parallel cultures converted in vitro (Fig. 2F). When quantifying the neuronal purity (number of converted cells divided by total cell number), it was found to be similar in vivo and in vitro (Fig. 2F). The conversion efficiency (number of neurons related to number of fibroblasts grafted) ranged from 0.4% to 5.9% (n = 10; 2 independent experiments), which is in the range of previously published in vitro conversion efficiencies of human fibroblasts (3, 5, 20).
We and others have previously shown that dopaminergic iN cells (DA-iN) can be generated from human fibroblasts when neural conversion genes are combined with DA fate determinants such as FoxA2, Lmx1a, Lmx1b, Otx2, Ngn2, Pax2, Pax5, Nurr1, En1, and Gli1 or combinations thereof (3, 5). By performing a series of systematic screens, we found that none of these genes were essential for DA-iN conversion, as reducing a single factor at a time from the pool did not result in loss of TH expression (Fig. 3A). When adding a single factor at a time to our previously identified minimal combination of Lmx1a and FoxA2 (3), two additional genes, Otx2 and Lmx1b, were found to increase DA-iN conversion of hEFs (Fig. 3B). These genes, when combined with Lmx1a and FoxA2, yielded a higher conversion of dopaminergic human iN cells (hiN-DA) than previously reported combinations. (Fig. 3 B and C and Fig. S2A and refs. 3 and 5). Thus, we transduced both hEFs and HFL1 cells with neural reprogramming genes and the 4-factor DA determinant combination and transplanted the cells into rats prelesioned with 6-hydroxydopamine (6-OHDA) unilaterally to remove the host DA system. When analyzed after 6 wk, hNCAM-positive fibers innervated the host striatum, with processes extending a distance from the graft core comparable to the distance we observe for grafted human fetal cells and for human embryonic stem cells analyzed at the same time (Fig. 3 D–F). Despite a similar degree of morphological maturation at a time in which a substantial fraction of both grafted fetal cells and human embryonic stem cells express TH (21), few of the hiN cells obtained via conversion in vivo expressed TH at this stage (Fig. 3G). The TH-expressing cells observed appeared morphologically immature, with a small soma and short, stunted processes (Fig. 3G′). A subgroup of animals grafted with hEFs were kept for 12 wk (n = 6). When analyzing these animals, we did not detect an increase in the number of TH-expressing cells (Fig. 3H), but the TH-positive cells had a more mature morphology with longer, more elaborate processes (Fig. 3H′).
Fig. 3.
Dopaminergic fate determinants, innervation, and in vivo conversion of human astrocytes. (A) Subtractive screen for DA determinants in which a single factor was removed at a time and (B) the additive screen, where DA fate determinants were added to the previously identified minimal combination Lmx1a and FoxA2 one at a time or in combinations of two. (C) Quantifications of DA-iNs obtained using the combination ABM+Lmx1a/b, Foxa2, and Otx2 (white) (n = 3) compared with ABM+Lmx1a and Foxa2 (gray) (n = 9) and A+Lmx1a and Nurr1 (black) (n = 3). Y-axis shows the mean number of neurons per random field (A–C). ***P < 0.0003; one-way ANOVA, Bonferroni post hoc test. Innervation of the striatum comparing (D) human fetal cells, (E) human embryonic stem cell–derived neurons, and (F) human DA-iN cells 6 wk after transplantation/initiation of conversion. Distance between dotted lines, 200 μm. (Scale bar: 250 μm.) Dotted lines mark zones in host striatum at 200 μm from graft TH-positive cells 6 wk (G and G′) and 12 wk (H and H′) after transplantation. (I) Grafted human astrocytes expressing a human-specific GFAP antibody and (J) hNCAM-positive cells converted from transplanted human astrocytes in vivo by administration of doxycycline in the drinking water. (Scale bar: 100 μm for G, H, and I; 50 μm for G′ and H′; and 25 μm for J.)
The ability to convert nonneuronal cells into subtype-specific neurons directly in the diseased brain opens up novel strategies for brain repair using resident brain cells as starting material for conversion. One cell type that could be a potential target for in vivo conversion of endogenous cells in the brain is astrocytes. To test the feasibility of astrocyte-to-neuron conversion taking place in vivo, we obtained human cortical astrocytes (Inoprot Inc.). At the time of grafting, the astrocytes express GFAP and brain lipid binding protein (BLBP) (Fig. S2 B and C), but not hNCAM, βIII-tubulin, or sex determining region Y box 2 (Sox2), markers of neurons or neural progenitors, respectively (Fig. S2 D–F). Following the same protocol as for fibroblast conversion, the astrocytes were transduced with reprogramming genes in vitro but were never exposed to doxycycline while in culture. Once transplanted, the reprogramming genes were activated via doxycycline delivered in the drinking water. When analyzed 6 wk after activation of reprogramming genes in vivo, the grafted human astrocytes could be detected by immunostaining, using a human-specific GFAP antibody (Fig. 3I). Some cells that had converted into iNs expressing hNCAM in vivo could be detected (Fig. 3J), providing additional proof of principle that in vivo neural conversion of human somatic cells is possible.
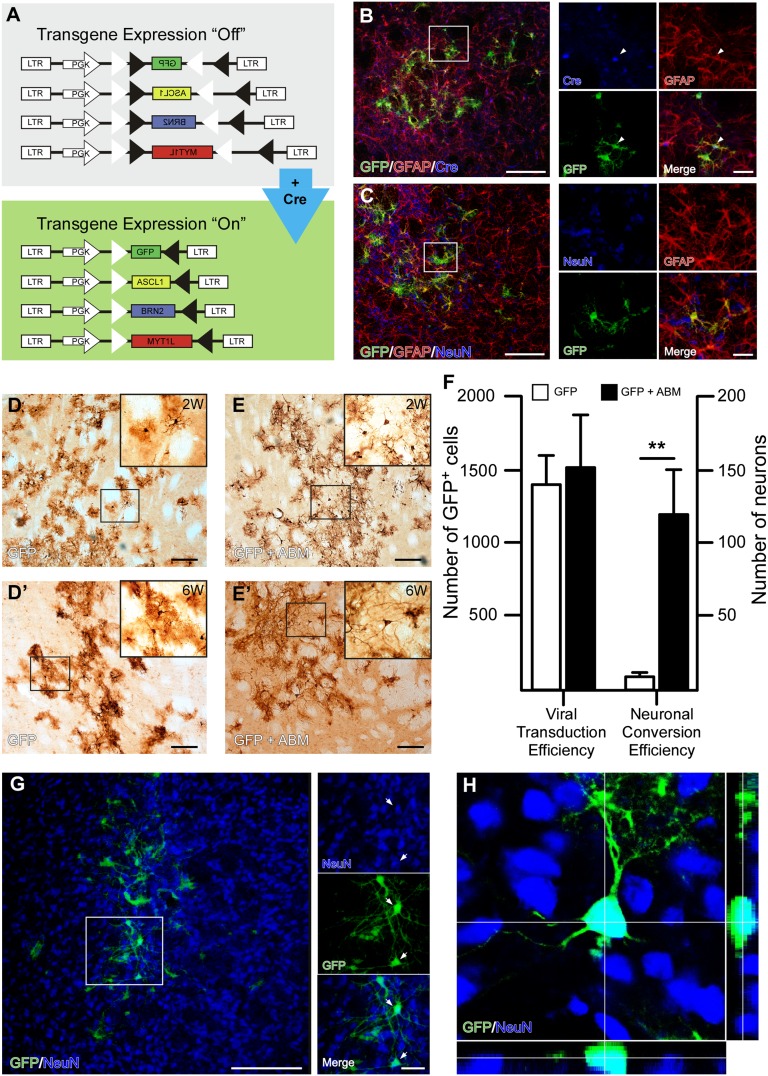
To establish the feasibility of in vivo conversion from resident glia cells, we developed a set of Cre-inducible LVs that contain a reporter (GFP) and reprogramming genes (ABM) (22). With these vectors (Fig. 4A), transgene expression is activated only in cells in which Cre recombinase is present (Fig. 1A and Fig. S3A). Stereotaxic injections of the Cre-inducible GFP-LVs into the striatum of GFAP-Cre heterozygous mice resulted in expression of GFP (Fig. S3B) in the injection area, whereas no GFP was expressed when vectors were injected into wild-type littermates (Fig. S3B), establishing a tight Cre-dependent control of gene expression in vivo. In the adult striatal parenchyma, GFAP is expressed by resident astrocytes; in accordance with this, GFP expression was found to be localized to cells with glial morphology and coexpressing Cre and GFAP (Fig. 4 B and C and representative diaminobenzidine (DAB) images in Fig. 4D, Left). Therefore, our experimental set-up allows us to specifically target parenchymal glia in the adult mouse striatum. We did, however, observe very few cells (<5/animal) with neuronal morphology in 2 mice throughout the time course of the experiments (Fig. 4F), which is most likely explained by Cre expression in nonglial cells at extremely low frequency in these mice.
Fig. 4.
Neural conversion of striatal astrocytes in situ. (A) Schematic of Cre-inducible lentiviral vectors used for conversion of endogenous astrocytes. The cDNA for GFP, Ascl1, Brn2, and Myt1l was cloned in the reverse orientation between two pairs of heterotypic, antiparallel loxP-sequences. This vector design results in “flipping” and activation of the transgene exclusively in Cre-expressing cells. (B and C) Immunostaining for GFAP/CRE/GFP and GFAP/GFP/NeuN after injection into GFAP-Cre mice confirms vector targeting astrocytes specifically. (D and E) GFP-expressing cells with neuronal morphology could be detected in animals injected with GFP and ABM 2 wk and 6 wk (D′ and E′) after injection, but not in control animals injected with GFP only. (F) Quantification of in vivo viral transduction efficiency and in vivo neural conversion. White bars are the control group transduced with GFP (n = 5), and black bars are the experimental group transduced with GFP and ABM (n = 5). ** P < 0.01, GFP+ABM group significantly different compared with GFP only. (G and H) GFP and NeuN stainings confirming neuronal identity of iNs generated via in vivo conversion.
In the next set of experiments, we injected the GFAP-Cre mice with two different combinations of genes, GFP and ABM (n = 12), known to convert mouse fibroblasts and hepatocytes into neurons (4, 23), and with GFP only (n = 12) as control. Assessment of the viral transduction efficiency 6 wk after injection showed no difference in transduction volume or number of transduced cells per animal between the 2 groups (Fig. 4F) but revealed the appearance of GFP-expressing cells with neuronal morphology in animals injected with ABM (Fig. 4 D and E). Quantification of the number of neurons formed after injection of GFP or GFP plus ABM showed that a significant number of reprogrammed neurons could be detected in the ABM-transduced but not the GFP-transduced animals: 117.4 ± 30.6 for ABM compared with 6.6 ± 3.3 for control (P < 0.01; n = 5 for each group). To confirm neuronal identity of the GFP-expressing cells with neuron-like morphology, we colabeled cells with GFP and the neuronal marker NeuN. When analyzing these stainings, we detected an overlap of GFP and NeuN in animals killed 6 wk after injection (Fig. 4G), which was confirmed by confocal microscopy (Fig. 4H).
Discussion
The neural conversion genes ABM have previously been shown to convert mouse fibroblasts and hepatocytes, as well as human fibroblasts, into functional neurons in vitro (3, 5, 19, 23). In this study, we show that mouse and human somatic cells can be converted into neurons in vivo, using the same combination of genes. The human fibroblasts and human astrocytes used in this study were transduced in vitro, but the reprogramming genes were not activated until after the cells were residing in the adult brain parenchyma. We know from this and previous studies that the transduction with the reprogramming genes does not result in iN conversion in the absence of doxycycline (3). Thus, the complete fibroblast-to-neuron and astrocyte-to-neuron conversion process takes place in the brain parenchyma, which provides important proof of principle that direct neural conversion in vivo is possible. We next investigated the ability to reprogram endogenous brain cells by directing expression of the neural conversion genes to parenchymal astrocytes in the striatum, using stereotaxic injections of Cre-regulated LVs into GFAP-Cre mice. We could show that combinatorial expression of ABM also is sufficient to convert resident GFAP-expressing glia into NeuN-expressing neurons in situ.
Parkinson disease is one of the degenerative brain diseases in which cell replacement via transplantation has been shown to be effective in animal studies as well as in some patients (24, 25). Therefore, the midbrain DA neurons (i.e., the type of neuron that degenerates in Parkinson disease) are of particular interest from a therapeutic perspective, as well as when envisioning future replacement strategies based on in vivo conversion. We have previously identified two genes (Lmx1a and FoxA2) that, when expressed in combination with the neural conversion genes in human fibroblasts, result in formation of DA-iN cells (3). Another group identified the combination of Ascl1, Lmx1a, and Nurr1, which also results in the conversion of human fibroblasts into DA neurons (5). In this study, we performed a series of systematic screens and defined an optimized combination of factors (Lmx1a, FoxA2, Lmx1b, and Otx2) that significantly increase the DA conversion of human fibroblasts in vitro compared with previously published gene combinations (3, 5). By adding this optimized combination of DA fate determinants to the fibroblasts in vitro, TH-positive, putative DA neurons could be generated when converting the transplanted fibroblasts in vivo. Although the frequency of TH-expressing neurons was low in these experiments, which is to be expected given that each of the seven genes is delivered using separate viral vectors, the finding provides important proof of principle that subtype-specific neurons can be generated when transplanted fibroblasts are converted in vivo.
Taken together, our data provide the first evidence that neural conversion can take place in vivo, similar to what has already been shown for other organ systems (17, 18). The ability to convert resident mouse astrocytes into neurons in vivo points toward the feasibility of using direct conversion of endogenous cells in the brain for future brain repair strategies. In further support of this approach, transplanted human cells were also shown to convert into neurons in vivo when the neural conversion genes were activated once the cells were residing in the brain parenchyma. To reach clinical application, however, it is important to design glia-targeted in vivo gene delivery systems that are nonintegrating and in which the expression of reprogramming genes can be turned off. It is also necessary to determine optimal gene combinations that result in a high number of subtype-specific neurons that integrate into neuronal circuitry and restore lost function.
Experimental Procedures
Tissue Sources and Cell Preparations.
hEFs were obtained as previously described (3), HFL1 (ATCC-CCL-153) cells were obtained from the American Type Culture Collection, and human astrocytes (P10251) were obtained from Innoprot. For GFP labeling, HFL1 cells were plated at a density of 50,000 cells/cm2 in 10-cm2 culture Petri dishes (Nunc), transduced with a GFP lentivirus, and expanded until confluent. Cells were then split and replated in T75 bottles at a density of 5,000 cells/cm2, incubated at 37°C in 5% (vol/vol) CO2 until confluent, and then frozen. Cells were then thawed, plated in DMEM (Gibco) with 10% (vol/vol) fetal bovine serum and 1% Penstrep (vol/vol) (all from Sigma) and FACS analyzed for GFP expression, using an Accuri c6 flow cytometer (BD Biosciences).
Before transplantations, human fibroblasts were plated in MEF medium at a density of 50,000 cells/cm2 in 10-cm2 tissue culture dishes coated with 0.1% gelatin and human astrocytes in astrocyte medium at a density of 50,000 cells/cm2 in 10-cm 2 tissue culture dishes coated with 1% poly-L-lysine (Sigma).
Viral Vectors and Neural Conversion.
Doxycycline-regulated LVs expressing mouse cDNAs for ABM have been described elsewhere (4). The doxycycline-regulated system (TET-ON) includes a separate lentiviral vector expressing a TET-ON transactivator (FUW.rtTA-SM2, Addgene) that was always cotransduced in the conversion experiments. LVs expressing mouse ORFs for DA determinants (En1, Foxa2, Gli1, Lmx1a, Lmx1b, Msx1, Nurr1, Otx2, Pax2, Pax5, and Ngn2) were generated as previously described. Cre-inducible lentiviral vectors were generated by inserting the cDNA for GFP, Ascl1, Brn2, and Myt1l in the reverse orientation between two pairs of heterotypic, antiparallel loxP-sequences (22). This vector design results in “flipping” and activation of the transgene exclusively in Cre-expressing cells. Third-generation LVs were produced as previously described and titrated by quantitative PCR analysis (3). The titers of the vectors used in this study were in the range of 1 × 108 to 2 × 109 transduction units (TU)/mL A multiplicity of infection (MOI) of 5 was used for LVs ABM, a MOI of 10 for the transactivator on hEF and HFL1 cells, and a MOI of 5 for LVs of DA determinants for in vivo studies.
Neural conversion was initiated by transduction of ABM and Fuw, as previously described (3), but the reprogramming process were activated with doxycycline in vivo at times described earlier.
Animal Surgery and Lesions.
Adult female Sprague-Dawley rats (225–250 g, Charles River) and athymic nude rats (10 wk old, Charles River) were housed in a 12-h light/dark cycle with ad libitum access to food and water. Rats were administered doxycycline (1 mg/mL, Saveen & Werner) through the drinking water. All experimental procedures were conducted following the guidelines put in place by the Ethical Committee for the Use of Laboratory Animals at Lund University. Surgical procedures were conducted under general anesthesia, using a 20:1 fentanyl and medetomidine solution injected intraperitoneally (Apoteksbolaget).
Transplantation.
Cells were transplanted into the striatum or hippocampus, using a microtransplantation approach as described in ref. 26. For HFL1 cells and hEFs 2 × 1–2 deposits of 100,000–150,000 cells/µL (total, 200,000–400,000 cells) were transplanted, and for astrocytes, 50,000 cells/µL (total, 100,000 cells). Immunosuppressive treatment was given to the Sprague-Dawley rats for the duration of the experiment in the form of daily i.p. injections of ciclosporin (10 mg/kg) starting 1 d before transplantation.
Lesions.
In some rats, a 6-OHDA lesion of the ascending medial forebrain bundle was performed using the same anesthetic procedures detailed earlier. A total of 14 μg freebase 6-OHDA was injected into the right hemisphere at the following coordinates in relation to bregma: −4.4 anterior/posterior (A/P), −1.2 medial/lateral (M/L), −7.8 dorsal/ventral (D/V), tooth bar, −2.4.
Vector injections.
GFAP-Cre mice (B6.Cg-Tg(Gfap-cre)77.6Mvs/J from Jackson mice) were used for the injection of Cre-inducible LVs. One microliter of LV was injected into the striatum at the following coordinates: A/P = +0.5, M/L = −2.1, D/V = −2.7, tooth bar = flat. For the GFP+ABM group, the 4 viruses were mixed at a similar ratio based on their functional titer. For GFP-only control injections, the vector was diluted in PBS to match the concentration of GFP vector in the GABM mix.
Immunostaining and Imaging.
Animals and cells were processed for histology as previously described (3, 27). For a complete list of antibodies and dilutions used in this study, see Table S1. All fluorescent images were captured using a Leica inverted microscope (Leica DFC360 FX + DMI 6000B) or a Leica DMRE confocal microscope equipped with green helium/neon, standard helium/neon, and argon lasers.
Quantifications and Statistical Analysis.
For in vitro experiments, the total number of DAPI, βIII, and microtubuli associated protein-2 (MAP2)-positive cells was counted in 50 randomly selected 10× fields (spiral fashion, from center to outside), using the Cellomics array scan (Array Scan VTI, Thermo Fischer; target activation program). For the screen of DA determinants, hEFs (Passage 3-5) were converted as described previously (3), while simultaneously overexpressing combinations of LVs expressing mouse ORFs for DA determinants (reductive screen: MOI, 2–3; additive screen: MOI, 5), and analyzed based on their phenotype 15d after transgene activation. For comparing different pools of DA determinants, a one-way ANOVA was performed. The neuronal purity is reported as total number of cells expressing βIII-tubulin and MAP2 out of DAPI-positive nuclei. The total number of TH neurons was obtained by manually counting all TH and MAP2 double-positive cells with a neuronal morphology in a Leica DM6000 inverted fluorescent microscope. All pooled data are presented as mean ± SEM.
In Vivo Quantifications.
The total number of surviving human cells [human nuclei (HuNu)+] or human neurons (hNCAM+) were estimated in a series of every eighth coronal section of each animal. Cells were counted under a 20× objective, using a bright-field microscope. Neuronal purity was estimated from the number of hNCAM+ cells as a percentage of the HuNu+ population. Conversion efficiency was calculated as the percentage of hNCAM+ neurons out of the predicted survival of transplanted fibroblasts, corrected using a survival factor of 5% of total grafted cells surviving posttransplantation. Viral transduction efficiency was quantified by counting the number of GFP-labeled cells in striatum, and neural conversion was quantified by counting the number of GFP-labeled cells with neuronal morphology. All quantifications were performed in blind.
Supplementary Material
Acknowledgments
We thank Ingar Nilsson, Christina Isaksson Ulla Jarl, Anneli Josefsson, AnnaKarin Olden, Sandra Smiljanic, and Michael Sparrenius for technical assistance and Anna Speidel for experimental help. This study was supported by the European Community’s Seventh Framework Programme through NeuroStemcell (Grant 222943), the Swedish Research Council (Grants K2011-62X-20390-05-6 and 70862601), and the Swedish Parkinson Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303829110/-/DCSupplemental.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Chambers SM, Studer L. Cell fate plug and play: Direct reprogramming and induced pluripotency. Cell. 2011;145(6):827–830. doi: 10.1016/j.cell.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 3.Pfisterer U, et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci USA. 2011;108(25):10343–10348. doi: 10.1073/pnas.1105135108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vierbuchen T, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463(7284):1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caiazzo M, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476(7359):224–227. doi: 10.1038/nature10284. [DOI] [PubMed] [Google Scholar]
- 6.Son EY, et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9(3):205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Ihrie RA, Alvarez-Buylla A. Lake-front property: A unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70(4):674–686. doi: 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ming GL, Song H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weinandy F, Ninkovic J, Götz M. Restrictions in time and space—new insights into generation of specific neuronal subtypes in the adult mammalian brain. Eur J Neurosci. 2011;33(6):1045–1054. doi: 10.1111/j.1460-9568.2011.07602.x. [DOI] [PubMed] [Google Scholar]
- 11.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8(9):963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 12.Karow M, et al. Reprogramming of pericyte-derived cells of the adult human brain into induced neuronal cells. Cell Stem Cell. 2012;11(4):471–476. doi: 10.1016/j.stem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Berninger B, et al. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci. 2007;27(32):8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinrich C, et al. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 2010;8(5):e1000373. doi: 10.1371/journal.pbio.1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heins N, et al. Glial cells generate neurons: The role of the transcription factor Pax6. Nat Neurosci. 2002;5(4):308–315. doi: 10.1038/nn828. [DOI] [PubMed] [Google Scholar]
- 16.Heinrich C, et al. Generation of subtype-specific neurons from postnatal astroglia of the mouse cerebral cortex. Nat Protoc. 2011;6(2):214–228. doi: 10.1038/nprot.2010.188. [DOI] [PubMed] [Google Scholar]
- 17.Qian L, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485(7400):593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455(7213):627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfisterer U, et al. Efficient induction of functional neurons from adult human fibroblasts. Cell Cycle. 2011;10(19):3311–3316. doi: 10.4161/cc.10.19.17584. [DOI] [PubMed] [Google Scholar]
- 20.Pang ZP, et al. Induction of human neuronal cells by defined transcription factors. Nature. 2011;476(7359):220–223. doi: 10.1038/nature10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirkeby A, et al. 2012. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep 1(6):703–714.
- 22.Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28(28):7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marro S, et al. Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell. 2011;9(4):374–382. doi: 10.1016/j.stem.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindvall O, Björklund A. Cell therapy in Parkinson’s disease. NeuroRx. 2004;1(4):382–393. doi: 10.1602/neurorx.1.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindvall O, Björklund A. Cell therapeutics in Parkinson’s disease. Neurotherapeutics. 2011;8(4):539–548. doi: 10.1007/s13311-011-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nikkhah G, Winkler C, Rödter A, Samii M (1999) Neural transplantation methods: Microtransplantation of nigral dopamine neurons: A step-by-step recipe. Neuromethods 36:207–231.
- 27.Grealish S, et al. The A9 dopamine neuron component in grafts of ventral mesencephalon is an important determinant for recovery of motor function in a rat model of Parkinson’s disease. Brain. 2010;133(Pt 2):482–495. doi: 10.1093/brain/awp328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.