Abstract
Design of a regulatable multistate protein is a challenge for protein engineering. Here we design a protein with a unique topology, called uniRapR, whose conformation is controlled by the binding of a small molecule. We confirm switching and control ability of uniRapR in silico, in vitro, and in vivo. As a proof of concept, uniRapR is used as an artificial regulatory domain to control activity of kinases. By activating Src kinase using uniRapR in single cells and whole organism, we observe two unique phenotypes consistent with its role in metastasis. Activation of Src kinase leads to rapid induction of protrusion with polarized spreading in HeLa cells, and morphological changes with loss of cell–cell contacts in the epidermal tissue of zebrafish. The rational creation of uniRapR exemplifies the strength of computational protein design, and offers a powerful means for targeted activation of many pathways to study signaling in living organisms.
Keywords: spatiotemporal control, cell motility, endothelial-mesenchymal transition
The past two decades have seen a revolution in computational protein design, with remarkable milestones including design of a helical protein from first principles (1), redesign of zinc finger proteins (2), and de novo design of an α/β protein (3). These studies highlighted, as a proof of principle, our ability to rationally control the structure of proteins by using basic physical principles and phenomenology. These approaches are based on finding an optimal sequence for a given single structure or ensemble of related states, and do not provide a strategy to construct a protein capable of large on-demand conformational transitions (4, 5). A number of multistate protein design algorithms (4, 6) have been proposed; however, designing an experimentally confirmed, regulatable multistate protein, or a conformational switch (5), still remains as a challenging task because of the necessity of engineering and controlling multiple protein states (4, 7, 8).
Such a conformational switch protein has great advantages in cell signaling, because it can be used as a universal regulatory domain (9) for precise, specific, and temporal control over rapidly activated signaling proteins (5, 10–15). Traditional genetically encoded methods for temporal protein control at the protein level have several drawbacks (5, 13). Recently developed protein switches, including derivatives of the light, oxygen, or voltage (LOV) domain (16, 17), can provide direct control at the protein level with light, but cannot be readily used in nontransparent animals. Our previous rapamycin regulated (RapR) kinase method (14) can potentially overcome this problem, but it requires expression and control of two proteins. The variable stoichiometry of these proteins renders the response more heterogeneous and essentially impractical in animals. Therefore, a single-chain, insertable, and transferable regulatory domain would be very valuable.
Here we design a ligand-controlled conformational switch, uniRapR, a potentially broadly applicable, single-chain regulatory domain. We first confirm its switching properties and control ability with molecular dynamics and in vitro enzymatic assays. Further, by temporally activating Src kinase with uniRapR in living single cells and zebrafish, we reveal two phenotypes related to the role of this kinase in metastasis.
Results
Design of uniRapR with Desired Stability and Conformational Dynamics.
Design of ligand binding proteins is still an unsolved problem in protein design (18); therefore, to design a ligand-controlled protein, we first use the binding pocket of one of the highest-affinity (19) protein–ligand complexes, 12-kDa FK506-binding protein (FKBP12) and FKBP12-rapamycin binding protein (FRB) in complex with rapamycin (20). We rationally rewired this complex to build a single-chain protein featuring a unique topology (21) (Fig. 1A and Fig. S1). To construct the subdomain-A of uniRapR, we used elements of insertable FKBP12 (iFKBP), a modified FKBP; we previously demonstrated that its insertion does not destroy the structure of a host protein (14). Subdomain-B of uniRapR contains the rapamycin-binding surface of FRB, and we stabilized this surface with two helices grafted from FRB. Thus, we expect the resulting design has all the desired properties of a universal regulatory domain featuring modularity, transferability, and robust switching ability.
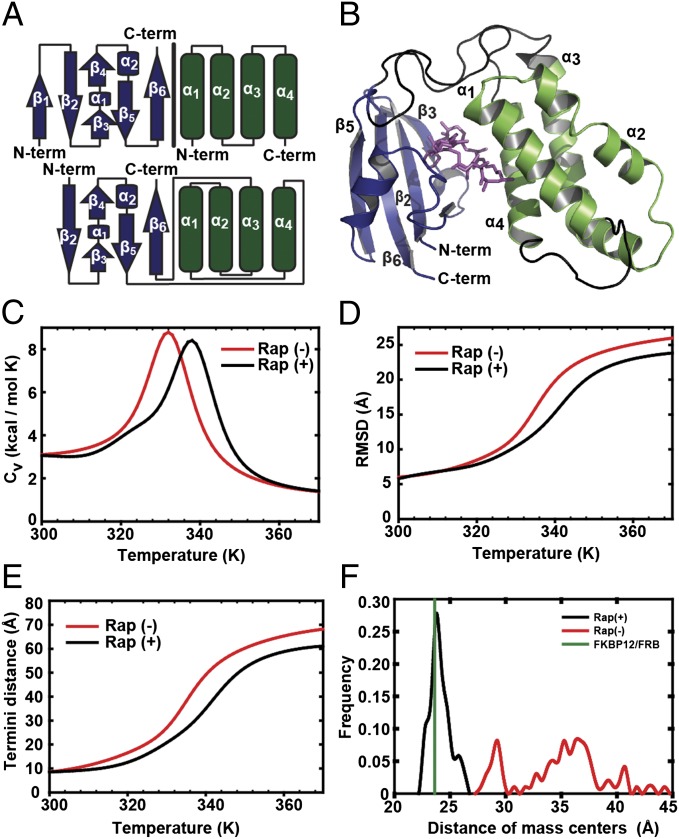
Fig. 1.
Design and thermodynamics of uniRapR domain. (A) The FKBP12 (blue)/FRB (green) complex was used to build the switch module (Fig. S1). While keeping the sequence from β2 to β5 of iFKBP, we linked β5 of subdomain-A to the carboxyl-terminal α-helix (α4) of subdomain-B by using an optimized GS linker to permit a hinge-like motion. Because the N terminus of α1 is relatively close to the C terminus of α4, we linked these two helices using a PPGPGSG linker. Sequences of helices α2 and α3 were kept as in WT FRB, and α3 was linked to the C-terminal β-strand (β6) of subdomain A, as the N terminus of β6 of FKBP is in the vicinity of the ternary complex interface. (B) A model of the holo-uniRapR (blue, subdomain-A; green, subdomain-B) protein was built based on the crystal structure of the FKBP12/FRB complex (Protein Data Bank ID code 1FAP) by using DMD. (C) Heat capacities of apo (red) and holo (black) forms of uniRapR were calculated by using WHAM (38). (D) rmsd values of apo (red) and holo (black) forms of subdomain-A were calculated for different temperatures by using WHAM. (E) Distance between Cα atoms of amino and carboxyl termini as a function of temperature for apo (red) and holo (black) forms of uniRapR. (F) Relative positions of uniRapR subdomains compared with the FKBP12/FRB proteins in complex. Distance between centers of masses of uniRapR subdomains A and B was calculated by using multiple molecular dynamics trajectories. In the presence of rapamycin (black), distance of centers of masses of uniRapR subdomains is ∼24 Å, close to that of FKBP12 and FRB complex (green). In the absence of rapamycin (red), uniRapR subdomains move randomly, and they are not in contact.
To test conformational switching features of uniRapR, we constructed a structural model of uniRapR based on complex structure of FKBP-FRB–rapamycin and performed replica exchange and equilibrium discrete molecular dynamics (DMD) simulations (22, 23) (Fig. 1B and Materials and Methods). Previous studies that used insertable FKBP showed that the transition between a folded and unfolded state could be used to control kinase activity when the FKBP was inserted at a conserved site in kinases. Therefore, desired switching properties of uniRapR were achieved by manipulating the subdomain motions and changing the relative stability between the folded and unfolded states. We estimated the stability of uniRapR states by characterizing its folding thermodynamics. We calculated its specific heat and the root-mean-square deviations (rmsds) from the native structure of iFKBP as a function of temperature. A peak in the specific heat curve corresponds to the folding transition, indicated by significant increase of rmsd (Fig. 1 C and D). We observed the stabilization of uniRapR upon rapamycin binding as a shift of the peak of the specific heat curve to a higher temperature. UniRapR can achieve regulatory function when inserted into a host kinase because its thermal stability and a change in its equilibrium amino- (N-) and carboxy- (C-) termini distance are dependent on rapamycin binding. Indeed, we observed reduced distance between Cα atoms of N- and C-termini upon binding of rapamycin (Fig. 1E). Equilibrium simulations confirmed that subdomain-A only interacts with subdomain-B in the presence of rapamycin (Fig. 1F and Movies S1 and S2). These observations suggest that the conformation of uniRapR depends on the presence of rapamycin, whereby binding of rapamycin to the pocket formed by the two subdomains stabilizes uniRapR.
Change in ATP Binding Site Dynamics Confer Allosteric Control in Src Kinase.
We hypothesize that uniRapR renders a protein switchable when inserted into a site that is allosterically coupled to its active site. Allosteric sites can be identified by using information from experimental mutation studies in the literature or by using various computational methods (24–26). We inserted uniRapR into the Src kinase at a site known (14) to be allosterically coupled to the ATP binding site (Fig. 2). In equilibrium DMD simulations, we observed that uniRapR inserted into Src at residue 288 with optimized double-gly-pro-gly (GPG) linkers destabilized the ATP binding site through long-range interactions, suggesting inactivation of the kinase in the absence of rapamycin. Rapamycin binding to uniRapR reduced fluctuations in the G-loop (residues 276–279), which is part of the ATP binding site. The reduced fluctuations in the presence of rapamycin restored the G-loop dynamics of Src-uniRapR to a level identical to that of WT Src (Fig. 2B), implying activation of the kinase in the presence of rapamycin. Computational analysis thus suggests that Src kinase activity can be regulated by inserting uniRapR at a position that is allosterically coupled to the ATP binding site.
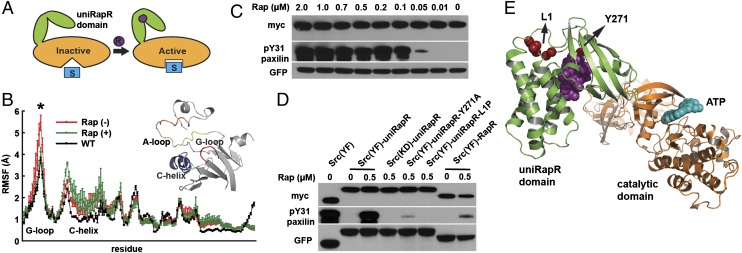
Fig. 2.
Control of Src kinase activity with uniRapR domain. (A) Schematic representation of activity control with the uniRapR domain. (B) Root mean square fluctuations of the ATP binding site (gray structure) based on multiple equilibrium DMD simulations for WT Src (black), apo (red), and holo (green) uniRapR-inserted Src (P < 0.01). (C) HEK293T cells expressing the Src-uniRapR-cerulean-myc construct were treated with different concentrations of rapamycin (0–2 μM), and lysates were assayed for expression of the construct with Western blotting by using anti-GFP. The construct was pulled down with anti-myc and mixed with the paxillin substrate in the presence of ATP for 10 min. Reaction suspensions were blotted and probed with anti-myc and anti–pY31-paxillin to confirm binding and phosphorylation of the substrate, respectively. (D) As controls, constitutively active Src (YF) without the uniRapR domain, kinase dead (YF/KD), Y271A and L1polyP Src mutants with the uniRapR domain, and our previous dimerization-based switch were tested. (E) Y271A and L1polyP substitutions shown on the Src-uniRapR model.
UniRapR Allows Specific and Robust Control over Various Kinases in Vitro.
We tested uniRapR functionality in vitro by using Src kinase expressed in HEK293T cells. By using paxillin and poly-Glu-Tyr (E4Y) as the Src substrates, we observed that constitutively active (YF) Src kinase (27) modified with uniRapR [uniRapR Src (YF)] demonstrated greatly enhanced activity in the presence of rapamycin, confirming that uniRapR functions as a specific on/off switch (Fig. 2C and Figs. S2–S7). A catalytically dead Src mutant (D388R) (14) with inserted uniRapR was inactive regardless of the presence of rapamycin, indicating that rapamycin-induced phosphorylation of paxillin or E4Y is caused by only Src-uniRapR catalytic activity (Fig. 2C and Fig. S7). Additionally, when we constrained the hinge motion of the uniRapR domain by substituting the optimized flexible linker (Fig. S2) between subdomains with a rigid poly-proline linker (uniRapR-L1P), catalytic activity in the presence of rapamycin was abolished (Fig. 2D). Based on our model and on the crystal structure of the FKBP12/FRB complex showing that Y271 [Y82 in the complex entered with Protein Data Bank ID code 1FAP] is in contact with rapamycin, we expected Y271A substitution to abolish rapamycin binding. We observed dramatically reduced activity of uniRapR Src (Y271A), further indicating that uniRapR Src switching activity is directly dependent on rapamycin binding to uniRapR Src (Fig. 2 D and E). Significantly, we observed that uniRapR Src has much higher switchable kinase activity than did our previous dimerization-based control of kinase activity (i.e., RapR), in which kinase activity was regulated by using coexpressed WT FRB and iFKBP (14) (Fig. 2D). These results indicate that insertion of the uniRapR switch enables us to robustly and specifically control Src kinase activity.
To assess the transferability of the uniRapR switch, we inserted uniRapR into analogous sites of focal adhesion kinase (FAK) and mitogen activated kinase p38. Insertion of uniRapR into a constitutively active mutant (28) of FAK (YM) inhibited the activity of FAK (YM) for its substrate paxillin; addition of rapamycin rescued FAK activity (Fig. 3A). Addition of rapamycin did not rescue kinase-dead FAK (YM/KD) activity, suggesting that rapamycin-induced phosphorylation of paxillin is only due to FAK-uniRapR activity. A design with a different circularly permuted version of uniRapR (Fig. S8A) did not allow control of FAK activity (Fig. S8B). Insertion of uniRapR into p38 kinase with the same GPG linker abolished its activity, which could be restored by the addition of rapamycin. Activity of WT p38 was unchanged in the presence of rapamycin (Fig. 3B). Insertion of uniRapR into p38 with an optimized linker described in our previous study (14) resulted in a higher activity in the presence of rapamycin. Indeed, we observed activity similar to WT in the presence of rapamycin (Fig. 3B), indicating that optimal regulation of the host protein can be achieved by varying the connection linker. All these data suggest that uniRapR can be inserted as a transferable regulatory domain in a wide variety of kinases.
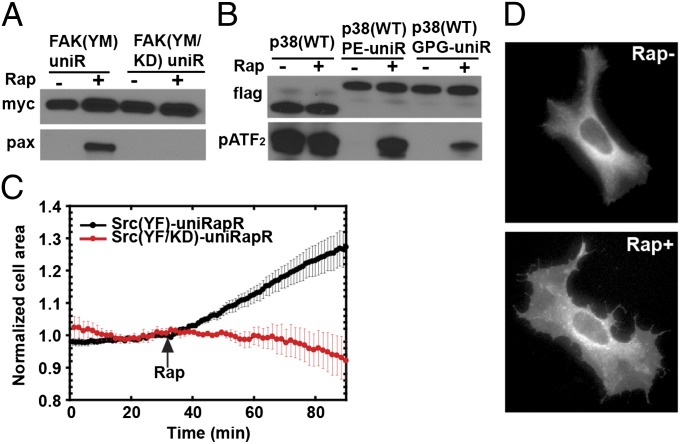
Fig. 3.
Testing uniRapR in different kinases and effects of Src activation in HeLa cells. Immunoprecipitation, in vitro FAK (A), and p38 (B) assays were performed similarly as for Src kinase. (C) Change in cell area of HeLa cells expressing Src (YF)-uniRapR (n = 8/8 cells) or Src (YF/KD)-uniRapR (n = 8/8 cells). (D) HeLa cells expressing Src-uniRapR (YF)-cerulean demonstrate spreading after the addition of rapamycin.
Specific Activation of Src Kinase Leads to Polarized Spreading in Single Cells.
Signaling cascades containing Src kinase play important roles in cell growth, proliferation, migration, and tumor invasiveness (27). However, the specific roles of Src catalytic activity, especially in cell migration, are unclear because of the limitations of existing chemical and genetic methods, including limited temporal control of activation or inactivation. For example, overexpression of constitutively active Src prevents the observation of events immediately following Src activation, as the cell compensates for Src expression, probably with other Src kinase family members, during gradual increase in expression level. Likewise, blocking Src expression with RNA interference is also a slow process. We overcome these limitations by using uniRapR Src (YF), which can reach maximal stimulation in less than 3 min.
To observe the effect of Src activation on cell motility, we expressed uniRapR Src (YF) in HeLa cells. In the absence of rapamycin, we observed only peripheral ruffles near the cell edge, a phenotype also seen in untransfected cells. After rapamycin addition, we observed a statistically significant increase in cell area for all cells examined, relative to those expressing catalytically dead Src (YF/KD)-uniRapR (area increase of 30 ± 5%, n = 8 cells; Fig. 3 C and D and Movies S3 and S4). Control cells showed no statistically significant change (area change of 8 ± 6%, n = 8 cells). In a control study, rapamycin alone did not have any effect on the phenotype of untransfected cells. Polarized spreading of HeLa cells following Src activation supports a role for Src in cell invasiveness.
Specific Activation of Src Kinase Causes Loss of Cell–Cell Contacts in Zebrafish Epidermis.
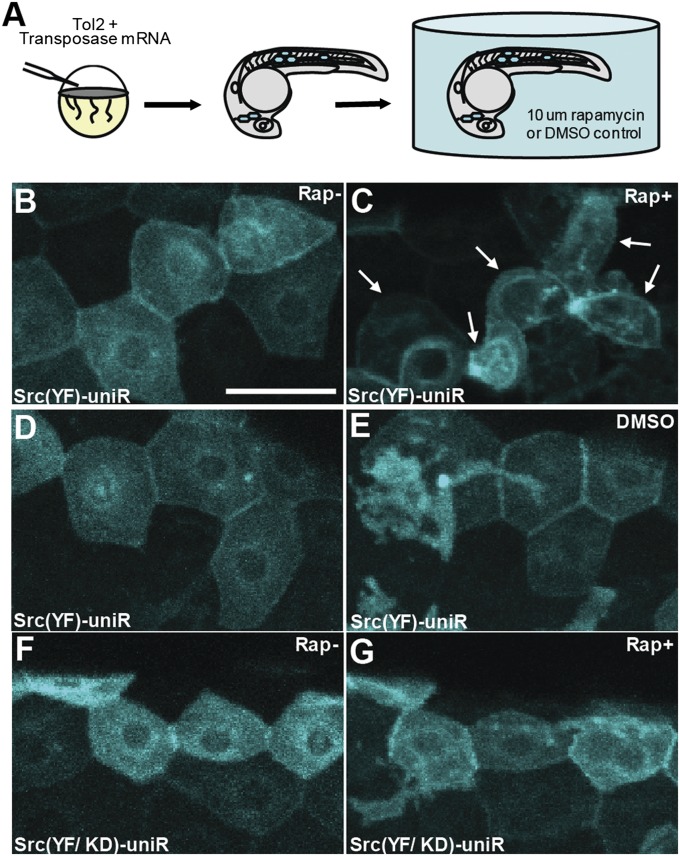
Although we observe a profound impact of Src activation in cultured cells, it is crucial to determine whether uniRapR Src (YF) enables Src activation to be studied in the context of a multicellular organism. To study the role of Src activation during development, we expressed uniRapR Src (YF) in zebrafish embryos (Fig. 4A). Epidermal cells expressing uniRapR Src (YF) demonstrated WT polygonal shape and formed tight connections with no gapping in the absence of rapamycin (Fig. 4B). When we activated uniRapR Src (YF) by adding rapamycin, the epidermal cells had extended protrusions and underwent significant morphological changes in 12 to 16 h. These morphological changes caused the loss of cell–cell contacts as cells became more rounded (Fig. 4C). In control experiments, we did not observe morphological changes when cells expressing uniRapR Src were treated only with vehicle (Fig. 4 D and E), or when we expressed the catalytically dead uniRapR Src (YF/KD) construct in zebrafish embryos in the presence of rapamycin (Fig. 4 F and G), demonstrating that the observed effects are caused specifically by uniRapR Src activation by rapamycin. This dramatic phenotype of altered cell morphology upon Src activation demonstrates the applicability of uniRapR in studying signaling pathways in whole organisms.
Fig. 4.
Activation of Src induces cell changes in zebrafish epidermal cells. (A) Synthetic transposase mRNA was coinjected with the Tol2 Krt4 Src (YF)-uniRapR-cerulean plasmid into one-cell zebrafish embryos, resulting in mosaic expression of Src-uniRapR in the epidermis. Epidermal cells with characteristic flat honeycomb morphology were selected and imaged before (B and F) and after (C and G) 16 h of rapamycin treatment. (C) Epidermal cells expressing Src (YF)-uniRapR-cerulean in zebrafish embryos exposed to 10 μM rapamycin become rounded and undergo dynamic cell shape changes. White arrows indicate an epidermal cell before and after each treatment. Control epidermal cells expressing Src (YF)-uniRapR-cerulean in vehicle (i.e., DMSO) (D and E) or expressing the kinase-dead construct Src (YF/KD)-uniRapR-cerulean in rapamycin (G) have a static morphology and do not undergo dynamic cell shape changes. (Scale bar: 30 μm.)
Discussion
Design of a broadly applicable artificial regulatory domain has been challenging for the following reasons: (i) the protein should be able to adopt multiple states that can be ”switched” by exogenous stimulation, i.e., ligand binding; (ii) the designed switch should be transferable, i.e., its action must be predictable and reproducible in a variety of host proteins; and (iii) the protein must be readily insertable into the host protein without significantly destabilizing it. We have solved these problems by designing a single-chain protein that can be regulated by a small molecule. Control of activity via small-molecule binding can be achieved by harnessing protein–protein interactions that occur in the presence of ligands, for example type 2C protein phosphatase ABI1/abscisic acid/PYR1-like (PYL1) (29) or FKBP12/rapamycin/FRB (20). Here we use the FKBP12/rapamycin/FRB complex because a photoactivable version of rapamycin is available, potentially making uniRapR a tool for spatial as well as temporal control of protein activity (30). Also, nonimmunosuppressive rapamycin analogues (31), such as C-16-(S)-3-methylindolerapamycin (iRap), can be used for minimal perturbation of normal physiology in animal studies. The iRap was proven to be innocuous in living cells (32). Here we show that Src-uniRapR is also active in the presence of iRap (Fig. S7). uniRapR can potentially be applied to a wide range of rapidly activating, allosteric signaling proteins. The dynamic behavior of a particular protein in a signaling pathway can be investigated by using a uniRapR protein analogue to activate the proteins with a resolution of minutes. Moreover, by inserting uniRapR into putative allosteric loops in a protein of interest, allosteric sites that are coupled can be experimentally identified.
We selected Src kinase as a proof-of-concept system to be tested in HeLa cells and zebrafish tissue because the specific role of this Src family member in cell motility is unclear. The roles of individual Src family members have proven to be difficult to dissect by using traditional approaches because of the functional redundancy and structural similarity of the Src family kinases (SFKs). SFKs are involved in many cellular processes, including transcription, differentiation, proliferation, development, motility, and cell death, and the majority of them have been identified as cellular oncogenes (27, 33). By using a uniRapR version of SFK members including Yes, Fyn, Lyn, Lck, Hck, Blk, Fgr, and Yrk kinases, differential roles of these proteins in cell motility can ultimately be elucidated.
An important advantage of uniRapR is its practical use in animals. Light-dependent control can be useful at the single-cell level, but uniRapR can provide activity control with the small molecule rapamycin even within deep tissues, where light cannot penetrate. To investigate the effect of Src kinase activation on a higher-order process such as intercellular communication in animals, a significant process involved in tissue development, repair, immune response, and homeostasis (27, 33), we tested uniRapR Src in living zebrafish epidermal tissue. We demonstrated that activation of Src kinase leads to a decrease in communication between cells, consistent with its oncogenic role in metastasis. The dynamic behavior of proteins downstream of Src, such as connexin 43 or Cas, that are involved in intercellular communication (34), will be the subject of future study.
UniRapR is a unique example of an insertable, transferable, and ligand-controllable protein switch. This switch has widespread potential applications for understanding signaling pathways involving kinases, a glimpse of which is offered by the unique phenotypes we demonstrate in mammalian cells and zebrafish tissue upon Src kinase activation. The potentially wide applicability of uniRapR is underscored by the structural conservation of kinases and the allosteric properties of many signaling proteins.
Materials and Methods
Detailed experimental and computational procedures are presented in SI Materials and Methods. In short: (i) DNA construction of the uniRapR domain and kinase-uniRapR constructs with all mutants was performed by using QuikChange PCR (Table S1). (ii) Computational design, modeling, and computational analysis of uniRapR and Src-uniRapR structures were performed by using replica-exchange and equilibrium DMD simulations (35) and Medusa modeling kit (36, 37) (Fig. S1 and Table S2). To analyze replica-exchange DMD simulations, the weighted histogram analysis method (WHAM) (38) was used to estimate thermodynamic properties of the uniRapR domain. (iii) Immunoprecipitation and in vitro kinase assays were as performed previously described (14). HEK293T cells expressing constructs were lysed, and protein constructs were pulled down and tested with the paxillin and poly-Glu-Tyr (E4Y) as Src and FAK substrate, and ATF2 as p38 substrate. The binding of the proteins to antibody-conjugated beads and phosphorylation of substrates were confirmed with Western Blotting. The enzymatic activities of immunoprecipitated constructs were determined by measuring the transfer rate of γ-32P from radioactive ATP to substrate peptide E4Y. (iv) Live cell imaging was performed by using HeLa cells with an Olympus IX-81 microscope equipped with a ZDC focus drift compensator and a Photometrics CoolSnap ES2 CCD camera (Roper Photometrics). (v) Imaging of live zebrafish embryo epidermis tissue was performed with a confocal microscope (FluoView FV1000; Olympus).
Supplementary Material
Acknowledgments
We thank Srinivas Ramachandran, Elizabeth Proctor, and Rachel Redler for critical comments on the manuscript. O.D. is a Howard Hughes Medical Institute International Student Research Fellow. This work was supported by National Institutes of Health Awards R01GM080742 (to N.V.D.), U01GM094663 (to K.M.H.), and R01GM102924 (to K.M.H.); National Institute of Environmental Health Sciences Award ES007015 (to C.M.F.); and National Cancer Institute Award CA157322 (to C.M.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1218319110/-/DCSupplemental.
References
- 1.Regan L, DeGrado WF. Characterization of a helical protein designed from first principles. Science. 1988;241(4868):976–978. doi: 10.1126/science.3043666. [DOI] [PubMed] [Google Scholar]
- 2.Dahiyat BI, Mayo SL. De novo protein design: Fully automated sequence selection. Science. 1997;278(5335):82–87. doi: 10.1126/science.278.5335.82. [DOI] [PubMed] [Google Scholar]
- 3.Kuhlman B, et al. Design of a novel globular protein fold with atomic-level accuracy. Science. 2003;302(5649):1364–1368. doi: 10.1126/science.1089427. [DOI] [PubMed] [Google Scholar]
- 4.Allen BD, Mayo SL. An efficient algorithm for multistate protein design based on FASTER. J Comput Chem. 2010;31(5):904–916. doi: 10.1002/jcc.21375. [DOI] [PubMed] [Google Scholar]
- 5.Ambroggio XI, Kuhlman B. Design of protein conformational switches. Curr Opin Struct Biol. 2006;16(4):525–530. doi: 10.1016/j.sbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Yanover C, Fromer M, Shifman JM. Dead-end elimination for multistate protein design. J Comput Chem. 2007;28(13):2122–2129. doi: 10.1002/jcc.20661. [DOI] [PubMed] [Google Scholar]
- 7.Havranek JJ, Harbury PB. Automated design of specificity in molecular recognition. Nat Struct Biol. 2003;10(1):45–52. doi: 10.1038/nsb877. [DOI] [PubMed] [Google Scholar]
- 8.Korendovych IV, et al. Design of a switchable eliminase. Proc Natl Acad Sci USA. 2011;108(17):6823–6827. doi: 10.1073/pnas.1018191108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostermeier M. Designing switchable enzymes. Curr Opin Struct Biol. 2009;19(4):442–448. doi: 10.1016/j.sbi.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kholodenko BN, Hancock JF, Kolch W. Signalling ballet in space and time. Nat Rev Mol Cell Biol. 2010;11(6):414–426. doi: 10.1038/nrm2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dueber JE, Yeh BJ, Chak K, Lim WA. Reprogramming control of an allosteric signaling switch through modular recombination. Science. 2003;301(5641):1904–1908. doi: 10.1126/science.1085945. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, et al. Surface sites for engineering allosteric control in proteins. Science. 2008;322(5900):438–442. doi: 10.1126/science.1159052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golynskiy MV, Koay MS, Vinkenborg JL, Merkx M. Engineering protein switches: Sensors, regulators, and spare parts for biology and biotechnology. ChemBioChem. 2011;12(3):353–361. doi: 10.1002/cbic.201000642. [DOI] [PubMed] [Google Scholar]
- 14.Karginov AV, Ding F, Kota P, Dokholyan NV, Hahn KM. Engineered allosteric activation of kinases in living cells. Nat Biotechnol. 2010;28(7):743–747. doi: 10.1038/nbt.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu YI, et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461(7260):104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huala E, et al. Arabidopsis NPH1: A protein kinase with a putative redox-sensing domain. Science. 1997;278(5346):2120–2123. doi: 10.1126/science.278.5346.2120. [DOI] [PubMed] [Google Scholar]
- 17.Harper SM, Neil LC, Gardner KH. Structural basis of a phototropin light switch. Science. 2003;301(5639):1541–1544. doi: 10.1126/science.1086810. [DOI] [PubMed] [Google Scholar]
- 18.Schreier B, Stumpp C, Wiesner S, Höcker B. Computational design of ligand binding is not a solved problem. Proc Natl Acad Sci USA. 2009;106(44):18491–18496. doi: 10.1073/pnas.0907950106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banaszynski LA, Liu CW, Wandless TJ. Characterization of the FKBP⋅rapamycin⋅FRB ternary complex. J Am Chem Soc. 2005;127(13):4715–4721. doi: 10.1021/ja043277y. and erratum (2006) 128(49):15928. [DOI] [PubMed] [Google Scholar]
- 20.Choi J, Chen J, Schreiber SL, Clardy J. Structure of the FKBP12-rapamycin complex interacting with the binding domain of human FRAP. Science. 1996;273(5272):239–242. doi: 10.1126/science.273.5272.239. [DOI] [PubMed] [Google Scholar]
- 21.Grishin NV. Fold change in evolution of protein structures. J Struct Biol. 2001;134(2-3):167–185. doi: 10.1006/jsbi.2001.4335. [DOI] [PubMed] [Google Scholar]
- 22.Ding F, Tsao D, Nie H, Dokholyan NV. Ab initio folding of proteins with all-atom discrete molecular dynamics. Structure. 2008;16(7):1010–1018. doi: 10.1016/j.str.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dagliyan O, Proctor EA, D’Auria KM, Ding F, Dokholyan NV. Structural and dynamic determinants of protein-peptide recognition. Structure. 2011;19(12):1837–1845. doi: 10.1016/j.str.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suel GM, Lockless SW, Wall MA, Ranganathan R. Evolutionarily conserved networks of residues mediate allosteric communication in proteins. Nat Struct Biol. 2003;10(1):59–69. doi: 10.1038/nsb881. [DOI] [PubMed] [Google Scholar]
- 25.Sharma S, Ding F, Dokholyan NV. Multiscale modeling of nucleosome dynamics. Biophys J. 2007;92(5):1457–1470. doi: 10.1529/biophysj.106.094805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kidd BA, Baker D, Thomas WE. Computation of conformational coupling in allosteric proteins. PLOS Comput Biol. 2009;5(8):e1000484. doi: 10.1371/journal.pcbi.1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23(48):7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 28.Lietha D, et al. Structural basis for the autoinhibition of focal adhesion kinase. Cell. 2007;129(6):1177–1187. doi: 10.1016/j.cell.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melcher K, et al. Identification and mechanism of ABA receptor antagonism. Nat Struct Mol Biol. 2010;17(9):1102–1108. doi: 10.1038/nsmb.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karginov AV, et al. Light regulation of protein dimerization and kinase activity in living cells using photocaged rapamycin and engineered FKBP. J Am Chem Soc. 2011;133(3):420–423. doi: 10.1021/ja109630v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickman DA, et al. Antifungal rapamycin analogues with reduced immunosuppressive activity. Bioorg Med Chem Lett. 2000;10(13):1405–1408. doi: 10.1016/s0960-894x(00)00184-0. [DOI] [PubMed] [Google Scholar]
- 32.Inoue T, Heo WD, Grimley JS, Wandless TJ, Meyer T. An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nat Methods. 2005;2(6):415–418. doi: 10.1038/nmeth763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 34.Shen YQ, et al. SRC utilizes Cas to block gap junctional communication mediated by connexin43. J Biol Chem. 2007;282(26):18914–18921. doi: 10.1074/jbc.M608980200. [DOI] [PubMed] [Google Scholar]
- 35.Dokholyan NV, Buldyrev SV, Stanley HE, Shakhnovich EI. Discrete molecular dynamics studies of the folding of a protein-like model. Fold Des. 1998;3(6):577–587. doi: 10.1016/S1359-0278(98)00072-8. [DOI] [PubMed] [Google Scholar]
- 36.Yin SY, Ding F, Dokholyan NV. Eris: An automated estimator of protein stability. Nat Methods. 2007;4(6):466–467. doi: 10.1038/nmeth0607-466. [DOI] [PubMed] [Google Scholar]
- 37.Ding F, Yin SY, Dokholyan NV. Rapid flexible docking using a stochastic rotamer library of ligands. J Chem Inf Model. 2010;50(9):1623–1632. doi: 10.1021/ci100218t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar S, Bouzida D, Swendsen RH, Kollman PA, Rosenberg JM. The weighted histogram analysis method for free-energy calculations on biomolecules. 1. The method. J Comput Chem. 1992;13(8):1011–1021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.