Abstract
Transgene-based genetic sexing methods are being developed for insects of agricultural and public health importance. Male-only rearing has long been sought in sericulture because males show superior economic characteristics, such as better fitness, lower food consumption, and higher silk yield. Here we report the establishment of a transgene-based genetic sexing system for the silkworm, Bombyx mori. We developed a construct in which a positive feedback loop regulated by sex-specific alternative splicing leads to high-level expression of the tetracycline-repressible transactivator in females only. Transgenic animals show female-specific lethality during embryonic and early larval stages, leading to male-only cocoons. This transgene-based female-specific lethal system not only has wide application in sericulture, but also has great potential in lepidopteran pest control.
Keywords: Lepidoptera, doublesex
The mulberry silkworm, Bombyx mori, is a completely domesticated insect and is the foundation of sericulture, an endeavor of great economic importance. It is believed that sericulture originated in China and has been conducted there for more than 5,000 y (1). Male-only rearing techniques for B. mori are desirable because males show higher resistance to disease, lower food consumption, and better silk quality (2). To this end, several B. mori strains for male-only rearing have been developed by classical genetics. In the last century, Strunnikov (3) established a sex-linked, balanced-lethal system using radiation-induced chromosome translocations. However, conventional approaches involving selective breeding or irradiation for developing male-only silkworm strains are time and labor consuming. Thus, novel approaches are desired to improve modern silkworm breeding. In recent years, advances in B. mori genetic manipulation, notably genetic transformation, have been successfully established and applied extensively in gene function analysis and the production of bioreactors (1, 4–7). These technologies provide a potential basis for improvements in sericulture including the development of a male-only rearing system.
Transgene-based genetic sexing systems have been developed in Drosophila melanogaster (8, 9) and several medically and agriculturally important insect species (10–13) as part of a series of genetics-based improvements and alternatives to the sterile insect technique (14). Systems based on sex-specific lethality for improving silk production are preferable to those using differential expression of a marker gene, for example fluorescence, which then would require manual or automated examination of each individual as part of the sorting process. Molecular designs developed for Diptera should be able to provide genetic sexing in B. mori, as the ability to transfer systems from one species to another is a key advantage of transgenic approaches over classical genetic methods (14–16). Furthermore, despite early reports to the contrary, more recent work has established clearly that transgenic strains can be developed with good fitness properties, even where the transgene induces conditional lethality as in sexing strains based on the use of female-specific conditional lethal transgenes (17–23).
Here we describe the development of a transgene-based, female-specific conditional lethal system in B. mori. The tetracycline-repressible transactivator (tTAV) protein is expressed only in females through the use of a sex-specific alternative splicing module (11, 24). Accumulation of tTAV in females, designed to occur through a positive feedback loop with the tetracycline response element (tetO) (13, 17, 25, 26), induces female-specific lethality during embryonic and early larval stages in the absence of dietary tetracycline, whereas males survive the whole life cycle without lethal tTAV expression. Therefore, a long-term goal of male-only rearing in sericulture has been achieved through this approach.
Results
We cloned and sequenced the alternatively spliced region of doublesex from the pink bollworm (Pgdsx), Pectinophora gossypiella, a lepidopteran pest of cotton. We used this sequence (KC588523) to build a Pgdsx minigene and incorporated it in the construct, OX4319, which comprised a conditional female-specific lethal gene and a fluorescent marker gene (Fig. 1) (13). A total of 768 preblastoderm Nistari eggs were injected with a mixture of OX4319 and helper plasmids (27). Strong transient expression of DsRed2 driven by the ie1 promoter could be detected by fluorescence microscopy at 48 h after microinjection (Fig. 2 A and E). Of the injected embryos, 492 (64%) hatched, and 242 larvae survived to adulthood. These moths were mated to WT moths, and 95 egg batches were collected and examined for fluorescence. Screening at the late embryonic (Fig. 2 B and F) and larval stages (Fig. 2 C and G) detected one or more individuals showing red fluorescence in 12 of these batches. Transformation efficiency, defined as the proportion of fertile injection survivors giving one or more transgenic offspring, was therefore 12.6%.
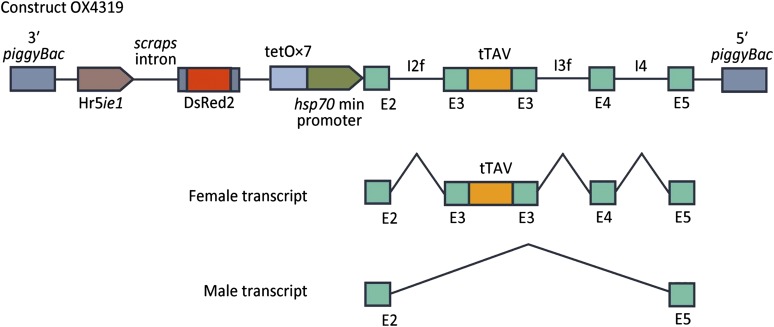
Fig. 1.
Female-specific lethal construct OX4319. OX4319 contains a conditional female-specific lethal gene and a fluorescent marker gene within a piggyBac-based nonautonomous transposon vector. The nonautonomous piggyBac vector allows germ-line transformation of insects via microinjection with separately supplied piggyBac transposase. The fluorescent marker is DsRed2 under the control of a baculovirus-derived promoter, Hr5ie1; this provides strong red fluorescence in a range of insect species (25, 29, 33, 34). The conditional female-specific gene is designed to derive its lethal effect via hyperaccumulation of tTAV from a positive feedback loop, leading to transcriptional suppression (25, 26). Lethality is repressed by dietary tetracycline, which inhibits the binding of tTA to tetO. Female specificity is provided by an alternative splicing element derived from the pink bollworm homolog of doublesex (dsx). tTAV is cloned into a female-specific exon (E3) so that only females produce mRNA encoding tTAV. Two relevant splice variants are indicated. Additional splice variants are known for both Bmdsx and Pgdsx (13, 35, 36). E, exons; I, introns.
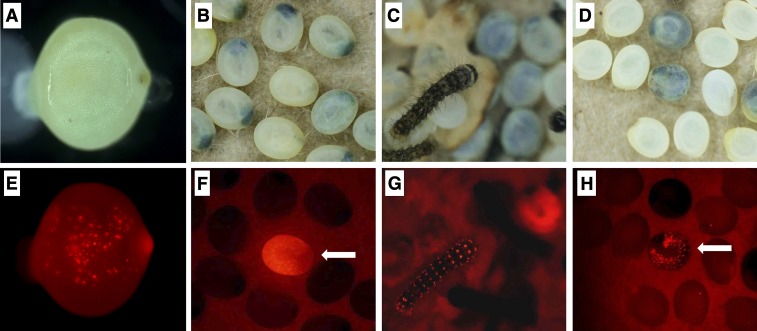
Fig. 2.
DsRed2 fluorescence phenotypes of transformed silkworms. Insects in A–D and E–H photographed with normal light and UV fluorescence, respectively. (A and E) Transient expression of DsRed2 in a G0 embryo. (B and F) G1 late embryo positive for DsRed2 (indicated by arrow). (C and G) G1 first-instar larva positive for DsRed2. (D and H) Embryonic lethality in G1 transgenic animals; some transgenic embryos developed normally at first but died at late embryonic stage (indicated by arrow).
Three distinct transgenic lines, OX4319-A, OX4319-B, and OX4319-C, were used in the subsequent experiments. The insertion of the transgenes into the genome was confirmed by inverse-PCR using DNA isolated from whole-body homogenates of DsRed2-positive G1 moths. To confirm precise integration at the TTAA site of piggybac-based OX4319, genomic junction sequences flanking the 5′- and 3′-ends of piggyBac inverted terminal repeat sequences were determined in the three lines. Searches of the silkworm genome database (http://kaikoblast.dna.affrc.go.jp) localized transgenes to distinct chromosomes (Fig. S1).
RT-PCR results using sex-specific primer sets showed sex-specific splice variants in males and females, supporting the interpretation that an alternative splicing event occurs in the different sexes (Fig. S2). Furthermore, immunoblot analysis also demonstrated that tTAV could be detected only in female transgenic animals, supporting the conclusion that female-specific lethality was induced by mass accumulation of tTAV protein (Fig. S2).
Different dosages of dietary tetracycline were tested to assess the repressible effect of tetracycline on female-specific lethality. It is important that the tetracycline concentration used to repress the lethal system during normal rearing is not itself deleterious to the developing larvae. In preliminary experiments, 200 μg/mL tetracycline in the artificial diet was found to have no deleterious effect during normal silkworm rearing. This concentration is higher than the 30–100 µg/mL successfully used to repress tetracycline-dependent lethality in Drosophila and several pest insects (8, 9, 13, 17, 25, 26, 28, 29), so we considered it likely that it would be sufficient to suppress a similar transgene in B. mori.
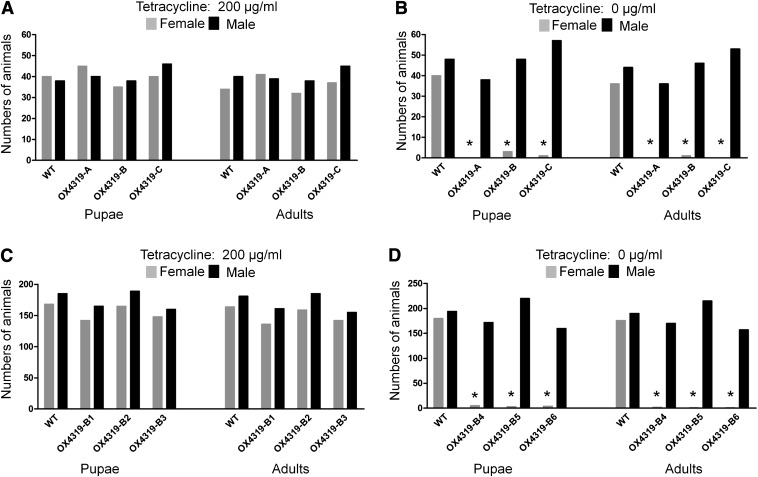
Embryonic lethality was observed in ∼10% of the G2 heterozygotes silkworms (Fig. 2 D and H). Gene amplification from the dead embryos failed to detect the male-specific splice variant supporting the conclusion that they were female. These results also support the hypothesis that mass accumulation of tTAV during embryogenesis could induce lethality, in contrast to similar positive-feedback constructs in various Diptera and other Lepidoptera, which appear to confer postembryonic lethality (13, 17, 25, 26). Larvae carrying the transgene were selected for testing by their red fluorescence. Most of the transgenic silkworms in all three independent transgenic lines reared on diet supplemented with tetracycline survived to adulthood, and the sex ratios in each of these lines did not differ significantly from that of WT Nistari (Fig. 3A). However, when reared on diet without tetracycline, approximately half of the transgenic individuals died during early larval stages (Fig. 4). Scoring sex ratios revealed that almost all females died during larval stages; only a few females survived to pupal and adult stages (Fig. 3B). A similar experiment conducted on a larger scale in G4 homozygous lines confirmed the conditional female-specific lethal phenotype (Fig. 3 C and D).
Fig. 3.
Repressible female-specific lethality in transgenic silkworms. Newly hatched first-instar transgenic (fluorescent) and WT larvae were reared on a diet with (A and C) or without (B and D) 200 μg/mL tetracycline. These larvae were either heterozygous G2 larvae from each of three independent lines OX4319-A, OX4319-B, and OX4319-C (A and B) or G4 homozygotes (C and D) derived from OX4319-B (six batches OX4319B1-6). Survival of these larvae to pupae and adults was analyzed. Neither heterozygous nor homozygous transgenic lines showed large deviation in sex ratio from WT when reared on diet with tetracycline (A and C), but very few transgenic females survived when reared on diet without tetracycline (B and D). Significant female-specific lethality is marked with asterisk (χ2 test, P < 0.01).
Fig. 4.
Female-specific lethality in OX4319 transgenic lines. In the absence of dietary tetracycline, males survived the whole life cycle, whereas females died at embryonic or early larval stages. (Left) Male larvae that have developed normally to the third instar. (Right) Larvae from the same brood, presumably females that could not molt at the end of first larval instar. (Scale bar: 1 cm.)
Discussion
We describe the successful development of a repressible and female-specific lethal transgenic system in B. mori. In this autoregulatory tTAV system, tTAV serves as both the transactivator and the effector, and high-level expression of tTAV from a proposed positive feedback configuration provides a lethal effect in B. mori, as was seen previously for the dipterans, Ceratitis capitata, Bactrocera oleae, and Aedes aegypti (17, 25, 26), and the lepidopterans, pink bollworm, and diamondback moth Plutella xylostella (29). The incorporation of a sex-specific, alternative-splicing module from the pink bollworm ortholog of doublesex makes the effect sex-specific (13). As a result, female lethality is induced during embryonic and early larval stages caused by mass accumulation of tTAV in the absence of dietary tetracycline. Interestingly, embryonic lethality was not observed in transgenic strains of other pest insects carrying molecular constructs of similar design (13, 17, 25, 26, 29); the difference may result from the relatively slow development of silkworm allowing more time for accumulation of the toxic protein. Female lethality can be repressed when animals are provided with dietary tetracycline, and females and males survive to adult in approximately equal proportions. Male viability was not affected irrespective of the presence of dietary tetracycline. The interspecies alternate splicing of dsx between pink bollworm and the silkworm shows promise for the similar function of this construct in other Lepidoptera, particularly in significant pest species.
Early-acting lethality is useful for mass male-only silkworm rearing in sericulture to reduce the considerable costs of rearing females. Once a female-specific lethality system is established in one B. mori strain, it can be transferred rapidly by standard breeding methods to production strains for commercial sericulture. Moreover, genetic transformation of commercial strains with OX4319 is underway to establish stable transgenic commercial lines by the more direct means. Compared with conventional silkworm breeding and selection for sex-linked lethality systems, this transgene-based approach is easily managed and is time and labor saving. It also is significant that the lethal effect is dominant. Silkworms for commercial silk production are usually F1 hybrids between two pure-breeding parental strains. A dominant female-killing gene could be established in either one of these strains, the single copy of the transgene present in the F1 then being sufficient to eliminate females when these are reared on normal mulberry leaves in the absence of tetracycline.
The results of this study provide a unique strategy of genetic sexing, extending previous work to an economically important nonpest insect where sex separation is a potentially valuable trait. Some of the concerns and hypothetical risks that have been articulated in relation to the use of similar technology in pest insects (30, 31) might not apply to B. mori, a domesticated insect with no wild form. Furthermore, demonstration of this system in a model lepidopteran insect will facilitate the future development of genetic approaches for lepidopteran pest management.
Materials and Methods
Plasmid Construction.
OX4319 was constructed by inserting tTAV coding sequence into the female-specific exon 2 [named “exon 3,” according to B. mori dsx nomenclature (32)] of a Pgdsx minigene construct containing four ligated fragments derived from four exons and flanking introns (Fig. 1). The background gene structure was derived from a plasmid with tetO21-VP16 and, also adjacent to tetO21 but in inverse orientation, Pgdsx-tTAV. tetO14-VP16 was deleted, leaving tetO7-Pgdsx-tTAV. The tTAV sequence begins with a translation start signal comprising the trinucleotide ATG; all endogenous ATG sequences of Pgdsx between this engineered ATG and the transcription start of Pgdsx-tTAV were removed to inhibit premature initiation of translation.
Silkworm Rearing and Genetic Transformation.
A multivoltine, nondiapausing silkworm strain, Nistari, was used for germ-line transformation using the method of Tan et al. (27). DNA was microinjected into preblastoderm G0 embryos that then were incubated at 25 °C in a humidified chamber for 10–12 d until larval hatching. Larvae were reared on fresh mulberry leaves or an artificial diet (Nihonnosanko) under standard conditions (27). Putative transgenic adult G0 were mated to WT moths, and G1 progeny were scored for the presence of the marker gene using a fluorescence microscopy (Nikon AZ100).
Inverse PCR and Junction Sequences.
Genomic DNA was extracted from G1 transgenic moths by standard SDS lysis-phenol treatment after incubation with proteinase K, followed by RNase treatment and purification. DNA was digested with Sau3AI and circularized by ligation for 30 min at 16 °C. The 3ʹ- and 5′-end genome:transgene junction sequences were amplified using the following primers designed to anneal to the 3′ or the 5′ arm of the piggyBac vector—3′F1: 5′-CGCTTACGCATAAACGATGA-3′ and 3′R1: 5′- GCCAAATGAAGTGCCTGGTA-3′ for the first PCR and 3′F2: 5′-TGCGGTTTACCGGTACTTTC-3′ and 3′R2: 5′-CCGATAAAACACATGCGTCA-3′ for the second PCR; 5′F1: 5′-CACGCGGTCGTTATAGTTCA-3′ and 5′R1: 5′-TCCTCTCTGCTCTTCTGC AA-3′ for the first PCR and 5′F2: 5′-ACGGATTCGCGCTATTTAGA-3′ and 5′R2: 5′-GATGACGAGCTTGTTGGTGA-3′ for the second PCR. The PCR conditions were as follows: 94 °C for 2 min, 35 cycles of 94 °C for 30 s, 55 °C for 30 s, and 65 °C for 2 min, followed by a final extension period of 65 °C for 10 min. Amplified fragments were gel-purified and sequenced directly.
RT-PCR.
RT-PCR was performed to analyze transcripts in transgenic animals. Total RNA was extracted from three whole bodies of larvae using TRIzol Reagent (Invitrogen) followed by DNase treatment to remove genomic DNA contamination. cDNA was prepared using the RevertAid First-Strand cDNA synthesis kit (Fermentas). The following primer pair was used to investigate sex-specific alternative splicing in transgenic animals—OX4319F: 5′-GCTGAACAAGCTAAACAATCTGCCCTAG-3′ and OX4319R: 5′-GCGGGGATGATCTCTGCGTGGTAA-3′. Sex-specific splicing of the endogenous Bmdsx was detected in both WT and OX4319-B animals with the following primers: BmdsxF: 5′-CTTGGATGAGGCTTCAAGGAAAATCT-3′ and BmdsxR 5′-GTGGCGAGTGTCGCCCCGTCGTCA-3′. Another primer pair amplifies a 136-bp fragment from the B. mori ribosomal protein 49 (Bmrp49) as an internal control—Bmrp49F: 5′-TCAATCGGATCGCTATGACA-3′ and Bmrp49R: 5′-ATGACGGGTCTTCTTGTTGG-3′. The PCR conditions were the same as inverse-PCR, and amplified fragments were gel-purified and sequenced directly.
Immunoblot Analysis.
Three G1 larval whole-body homogenates were resolved by 10% SDS/PAGE and transferred to a PVDF membrane. The tTAV protein on the membrane was detected with rabbit anti-TetR antibody (1:1,000 dilution; Abcam) as the primary antibody and alkaline phosphatase conjugated goat anti-rabbit IgG (1:5,000 dilution; ABmart) as the secondary antibody. Signal detection was performed using the ECL Plus Western Blotting Detection Kit (GE Healthcare).
Supplementary Material
Acknowledgments
We thank Lang You, Dan Liu, and Jianhao Jiang for technical assistance. This work was supported by grants from the National Science Foundation of China (31030060, 30825007, and 31272037) and the National Basic Research Program of China (2012CB114101 and 2012CB721103). L.J. was supported by a Biotechnology and Biological Sciences Research Council Collaborative Awards in Science and Engineering doctoral studentship.
Footnotes
Conflict of interest statement: G.F., N.I.M., and L.A. have employment and equity interest in Oxitec Ltd. Oxitec and Oxford University hold intellectual property related to the subject matter of this paper. All other authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1221700110/-/DCSupplemental.
References
- 1.Goldsmith MR, Shimada T, Abe H. The genetics and genomics of the silkworm, Bombyx mori. Annu Rev Entomol. 2005;50:71–100. doi: 10.1146/annurev.ento.50.071803.130456. [DOI] [PubMed] [Google Scholar]
- 2.Traut W, Sahara K, Marec F. Sex chromosomes and sex determination in Lepidoptera. Sex Dev. 2007;1(6):332–346. doi: 10.1159/000111765. [DOI] [PubMed] [Google Scholar]
- 3.Strunnikov V. Sex control in silkworms. Nature. 1975;255(5504):111–113. doi: 10.1038/255111a0. [DOI] [PubMed] [Google Scholar]
- 4.Imamura M, et al. Targeted gene expression using the GAL4/UAS system in the silkworm Bombyx mori. Genetics. 2003;165(3):1329–1340. doi: 10.1093/genetics/165.3.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato T, Kajikawa M, Maenaka K, Park EY. Silkworm expression system as a platform technology in life science. Appl Microbiol Biotechnol. 2010;85(3):459–470. doi: 10.1007/s00253-009-2267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamura T, et al. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat Biotechnol. 2000;18(1):81–84. doi: 10.1038/71978. [DOI] [PubMed] [Google Scholar]
- 7.Tomita M, et al. Transgenic silkworms produce recombinant human type III procollagen in cocoons. Nat Biotechnol. 2003;21(1):52–56. doi: 10.1038/nbt771. [DOI] [PubMed] [Google Scholar]
- 8.Heinrich JC, Scott MJ. A repressible female-specific lethal genetic system for making transgenic insect strains suitable for a sterile-release program. Proc Natl Acad Sci USA. 2000;97(15):8229–8232. doi: 10.1073/pnas.140142697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas DD, Donnelly CA, Wood RJ, Alphey LS. Insect population control using a dominant, repressible, lethal genetic system. Science. 2000;287(5462):2474–2476. doi: 10.1126/science.287.5462.2474. [DOI] [PubMed] [Google Scholar]
- 10.Catteruccia F, Benton JP, Crisanti A. An Anopheles transgenic sexing strain for vector control. Nat Biotechnol. 2005;23(11):1414–1417. doi: 10.1038/nbt1152. [DOI] [PubMed] [Google Scholar]
- 11.Fu G, et al. Female-specific insect lethality engineered using alternative splicing. Nat Biotechnol. 2007;25(3):353–357. doi: 10.1038/nbt1283. [DOI] [PubMed] [Google Scholar]
- 12.Fu G, et al. Female-specific flightless phenotype for mosquito control. Proc Natl Acad Sci USA. 2010;107(10):4550–4554. doi: 10.1073/pnas.1000251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin L, et al. Engineered female-specific lethality for control of pest Lepidoptera. ACS Synth Biol. 2013;2(3):160–166. doi: 10.1021/sb300123m. [DOI] [PubMed] [Google Scholar]
- 14.Papathanos PA, et al. Sex separation strategies: Past experience and new approaches. Malar J. 2009;8(Suppl 2):S5. doi: 10.1186/1475-2875-8-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alphey L, Nimmo D, O’Connell S, Alphey N. Insect population suppression using engineered insects. Adv Exp Med Biol. 2008;627:93–103. doi: 10.1007/978-0-387-78225-6_8. [DOI] [PubMed] [Google Scholar]
- 16.Alphey L. Engineering insects for the sterile insect technique. In: Vreysen M, Robinson A, Hendrichs J, editors. Area-Wide Control of Insect Pests: From Research to Field Implementation. Dordrecht, Netherlands: Springer; 2007. pp. 51–60. [Google Scholar]
- 17.Ant T, et al. Control of the olive fruit fly using genetics-enhanced sterile insect technique. BMC Biol. 2012;10:51. doi: 10.1186/1741-7007-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bargielowski I, Nimmo D, Alphey L, Koella JC. Comparison of life history characteristics of the genetically modified OX513A line and a wild type strain of Aedes aegypti. PLoS ONE. 2011;6(6):e20699. doi: 10.1371/journal.pone.0020699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris AF, et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat Biotechnol. 2012;30(9):828–830. doi: 10.1038/nbt.2350. [DOI] [PubMed] [Google Scholar]
- 20.Harris AF, et al. Field performance of engineered male mosquitoes. Nat Biotechnol. 2011;29(11):1034–1037. doi: 10.1038/nbt.2019. [DOI] [PubMed] [Google Scholar]
- 21.Marrelli MT, Li C, Rasgon JL, Jacobs-Lorena M. Transgenic malaria-resistant mosquitoes have a fitness advantage when feeding on Plasmodium-infected blood. Proc Natl Acad Sci USA. 2007;104(13):5580–5583. doi: 10.1073/pnas.0609809104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simmons GS, et al. Field performance of a genetically engineered strain of pink bollworm. PLoS ONE. 2011;6(9):e24110. doi: 10.1371/journal.pone.0024110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wise de Valdez MR, et al. Genetic elimination of dengue vector mosquitoes. Proc Natl Acad Sci USA. 2011;108(12):4772–4775. doi: 10.1073/pnas.1019295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dafa’alla T, Fu G, Alphey L. Use of a regulatory mechanism of sex determination in pest insect control. J Genet. 2010;89(3):301–305. doi: 10.1007/s12041-010-0041-y. [DOI] [PubMed] [Google Scholar]
- 25.Gong P, et al. A dominant lethal genetic system for autocidal control of the Mediterranean fruitfly. Nat Biotechnol. 2005;23(4):453–456. doi: 10.1038/nbt1071. [DOI] [PubMed] [Google Scholar]
- 26.Phuc HK, et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol. 2007;5:11. doi: 10.1186/1741-7007-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan A, Tanaka H, Tamura T, Shiotsuki T. Precocious metamorphosis in transgenic silkworms overexpressing juvenile hormone esterase. Proc Natl Acad Sci USA. 2005;102(33):11751–11756. doi: 10.1073/pnas.0500954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bello B, Resendez-Perez D, Gehring WJ. Spatial and temporal targeting of gene expression in Drosophila by means of a tetracycline-dependent transactivator system. Development. 1998;125(12):2193–2202. doi: 10.1242/dev.125.12.2193. [DOI] [PubMed] [Google Scholar]
- 29.Morrison NI, et al. Engineered repressible lethality for controlling the pink bollworm, a lepidopteran pest of cotton. PLoS ONE. 2012;7(12):e50922. doi: 10.1371/journal.pone.0050922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beech C, Koukidou M, Morrison NI, Alphey L. Genetically Modified Insects: Science, Use, Status and Regulation. ICGEB Collection Biosafety Rev. 2012;6:66–124. [Google Scholar]
- 31. Beech C, et al. (2009) Risk analysis of a hypothetical open field release of a self-limiting transgenic Aedes aegypti mosquito strain to combat dengue. As Pac J Mol Biol Biotech 17(3):97–108.
- 32.Suzuki MG, Ohbayashi F, Mita K, Shimada T. The mechanism of sex-specific splicing at the doublesex gene is different between Drosophila melanogaster and Bombyx mori. Insect Biochem Mol Biol. 2001;31(12):1201–1211. doi: 10.1016/s0965-1748(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 33.Labbé GM, Nimmo DD, Alphey L. piggybac- and PhiC31-mediated genetic transformation of the Asian tiger mosquito, Aedes albopictus (Skuse) PLoS Negl Trop Dis. 2010;4(8):e788. doi: 10.1371/journal.pntd.0000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martins S, et al. Germline transformation of the diamondback moth, Plutella xylostella L., using the piggyBac transposable element. Insect Mol Biol. 2012;21(4):414–421. doi: 10.1111/j.1365-2583.2012.01146.x. [DOI] [PubMed] [Google Scholar]
- 35.Shukla JN, Jadhav S, Nagaraju J. Novel female-specific splice form of dsx in the silkworm, Bombyx mori. Genetica. 2011;139(1):23–31. doi: 10.1007/s10709-010-9479-3. [DOI] [PubMed] [Google Scholar]
- 36.Duan J, et al. Novel female-specific trans-spliced and alternative splice forms of dsx in the silkworm Bombyx mori. Biochem Biophys Res Commun. 2013;431(3):630–635. doi: 10.1016/j.bbrc.2012.12.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.