Abstract
Background
Emergency department (ED) dosing of vancomycin and its effect on outcomes has not been examined.
Study objective
To describe current vancomycin dosing practices for ED patients, focusing on patient factors associated with administration, dosing accuracy based on patient body weight, and clinical outcomes.
Methods
Single center, retrospective cohort study of vancomycin administered in the ED over 18 months in an academic, tertiary care ED. Data were collected on 4,656 patients. Data were analyzed using a generalized estimating equations GEE) model to account for multiple doses being administered to the same patient.
Results
The ED dose was continued, unchanged, in 2,560 admitted patients (83.8%). The correct dose was given 980 times (22.1%), 3,143 doses (70.7%) were under dosed, and 318 were overdosed (7.2%). Increasing weight was associated with under dosing (adjusted odds ratio 1.52 per 10 kg body weight, p < 0.001). Doses of vancomycin >20 mg/kg had longer hospital length of stay, p = 0.005, were more likely to spend ≥ 3 days in the hospital, OR 1.49 (1.12, 1.98, p = 0.006), and to die, OR 1.88 (1.22, 2.90, p = 0.004).
Conclusion
In this largest study to date examining ED vancomycin dosing, vancomycin was commonly given. Dosing outside the recommended range was frequent, and especially prevalent in patients with a higher bodyweight. The ED dose of vancomycin was frequently continued as an inpatient, regardless of dosing accuracy. There is significant room for improvement in dosing accuracy and indication. Vancomycin dosing in the ED may also affect clinical outcomes.
Keywords: Vancomycin, emergency department, dosing, outcomes
INTRODUCTION
Antibiotic resistance is a major public health concern, and is developing at a rate that out paces new antimicrobial therapies [1]. Infections secondary to methicillin-resistant S. aureus (MRSA) are increasing in frequency [2–5], and are a major problem in both the community and hospital settings. Furthermore, multi-drug resistant pathogens are a frequent cause of inappropriate antimicrobial therapy, which is associated with worse outcomes in a variety of infectious conditions [6–12].
The administration of vancomycin is appropriate in the setting of known or suspected MRSA infection [13], or in the setting of severe systemic illness with high risk of mortality [9]. Unfortunately, while MRSA infections are on the rise, resistance to vancomycin is as well, and resistance could be attributed to both overuse, as well as an inappropriate dosing regimen [13, 14]. The emergence of vancomycin intermediate S. aureus (VISA) and vancomycin resistant S. aureus (VRSA) has been described since the mid-1990s [14]. These pathogens are almost uniformly associated with prior vancomycin exposure, and inappropriately sub therapeutic dosing [13]. Additionally, worse outcomes have been observed for patients infected with MRSA isolates having higher bacterial minimum inhibitory concentrations (MIC) [15]. Some data support a relationship between serum concentration of vancomycin and treatment success, although this data is not consistent [13, 16].
For these reasons, recent recommendations for vancomycin use include using a dosing scheme that is based on actual body weight (even in obese patients) in order to help achieve the goal pharmacokinetic:pharmacodynamic target [13]. In all patients, especially those at high risk for MRSA infections, vancomycin must be dosed appropriately, with consideration of patient characteristics and presumed infectious source in order to achieve adequate concentrations at the site of infection. Previous data indicate that up to 40% of vancomycin given in the Emergency Department (ED) is inappropriate based on patient selection [14]. With the likely increasing incidence of vancomycin dosing in the ED, as well as increasing emergence of vancomycin resistant organisms, the ED could play a significant role in not only reducing mortality in severe sepsis[17], but also in contributing to antibiotic resistance. Characterizing the dosing of this antibiotic is the first step toward better tailoring this therapy to maximize therapeutic potential, limiting individual and population side effects, and providing guidance with respect to an empiric dosing strategy.
This study was designed to describe the frequency of vancomycin use in the ED, the dosing practices, and to examine if ED dosing has long-term effects on outcome. We hypothesized that the administration of vancomycin was common, for a wide variety of indications, and that dosing would be largely inaccurate. We also hypothesized that in those patients admitted to the hospital, ED dosing would be influential on subsequent inpatient dosing (dose administered), and under dosing would be associated with sub-therapeutic levels, and worse outcomes.
MATERIALS AND METHODS
This analysis was a single-center retrospective cohort study. The protocol was approved by the Human Research Protection Office of the primary investigator’s institution.
The study was conducted in the ED of an urban, academic tertiary care institution, with an annual census of >90,000 patients. The subjects were consecutive adult ED patients administered intravenous vancomycin over an 18 month period (December 2008 to June 2010). Data were collected on patients identified by query of the ED automated medication dispensing system linked to ED and inpatient electronic medical records.
The investigator performing the primary data collection (CM) was blinded to all study hypotheses. Doses of vancomycin administered in the ED were obtained via query of the ED automated medication dispensing system. ED electronic medical records were used to establish demographics, as well as ED chief complaint, diagnoses, co-administration of other antibiotics, and disposition from the ED. These records were linked electronically to inpatient records to capture any other important data (i.e. missing weights). A master database was then created from the above sources, cross checking for accuracy prior to statistical analysis. Variables were defined prior to data extraction and placed in a standardized format during the data collection process.
We first sought to describe how vancomycin is dosed with respect to patient characteristics such as weight, renal function, age, diagnoses, and site of infection. We then sought to determine dosing accuracy. We defined “correct” dose as 15–20mg/kg of the actual body weight based on guideline recommendations [13]. For this reason, we restricted the analysis of dosing accuracy to the patients with a weight available in the ED at time of presentation. We then examined whether ED vancomycin was continued after hospital admission, and whether these doses differed from that administered in the ED. Finally, we examined clinically relevant outcomes associated with vancomycin use in the ED.
Descriptive statistics were used to explore dosing practices with respect to patient demographic factors, as well as baseline clinical variables. Patients are not routinely weighed in the ED, and the weight available to the treating physician is either an estimation of weight at the time of presentation, or based upon a documented weight from a previous hospitalization. For this reason, and to assess accuracy of ED weight estimation, Pearson’s correlation was used to describe the correlation between ED weight and inpatient weight, where patients are routinely weighed after admission. The ED electronic medical record system allows for up to four diagnoses to be assigned to each patient at time of ED discharge. Based on frequency, we divided these ED diagnoses into 9 diagnostic categories. Analysis with a generalized logit model, fit by maximum likelihood where the variances are adjusted using the cluster structure of the data, was used to account for the relationship between repeated vancomycin doses being given to the same patient. When the overall model was significant, specific contrasts were used to compare the probability of correct vs. overdose and correct vs. under dose. Odds ratios and 95% confidence intervals were reported for significant contrasts, and a p-value < 0.05 was considered significant. Multivariable models were created to examine the patient characteristics predictive of dosing correctness, as well as dosing associated with clinically relevant outcomes. The predictors in the multivariable model included statistically significant predictors from the univariable model, as well as clinically important factors. The data analysis was generated using SAS software, version 9.1 of the SAS System for Linux (SAS Institute Inc., Cary, NC, USA). The statistical analysis was completed in consultation with a biostatistician.
RESULTS
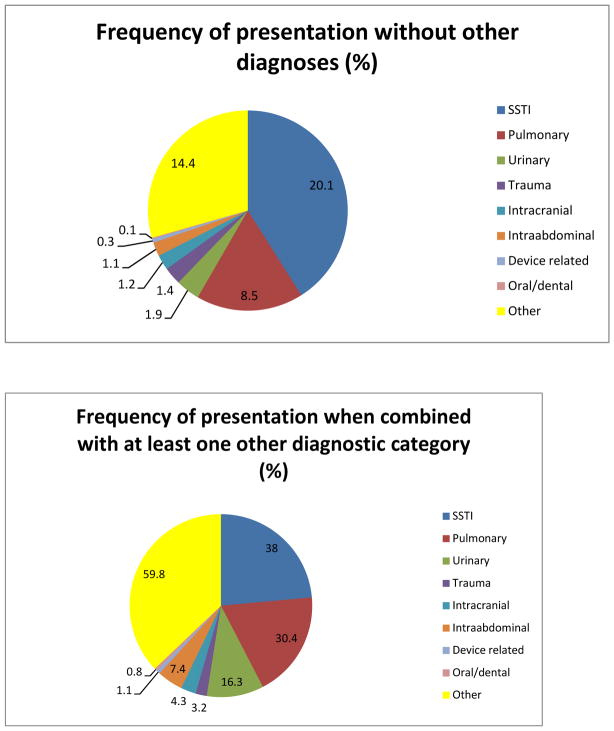
Vancomycin was administered in the ED to 4,656 patients over the 18 month period. Baseline characteristics for the subjects are shown in Table 1. Of the 4,656 patients given vancomycin, a weight was available to the emergency physician and documented on the ED record for 4,057 (87.1%) patients on which to base dosing amount and accuracy. Pearson correlation was used to assess the association between ED weight and inpatient weight, and the correlation was found to be very high (r = 0.92, p < 0.001). There were 1,095 unique working diagnoses amongst the patient cohort, and the ED diagnoses were divided into 9 diagnostic categories. We measured the association of each infectious diagnostic category with vancomycin use, both independently and combined with up to 3 other diagnostic categories (Figure 1). Most patients dosed with vancomycin were assigned to multiple infectious diagnostic categories based on ED diagnoses at time of disposition.
Table 1.
Baseline characteristics of patients given vancomycin in the Emergency Department
| Subject, No. | 4,656 |
|
| |
| Sex, No. (%) | |
| Male | 2,429 (52.2) |
|
| |
| Age, y, median (IQR) | 54.5 (41.4–67.4) |
|
| |
| Race, No. (%) | |
| Black | 2,327 (50) |
| Caucasian | 2,008 (43.1) |
| Other | 321 (6.9) |
|
| |
| End stage renal disease, No. (%) | 340 (7.3) |
|
| |
| Vasopressors, No. (%) | 468 (10.1) |
|
| |
| Serum creatinine (mg/dl), mean (SD) | 1.62 (2.07) |
|
| |
| Estimated creatinine clearance (ml/min), mean (SD)* | 64.4 (14.1) |
|
| |
| Height (cm), mean (SD) | 170.5 (9.3) |
|
| |
| Weight (kg), mean (SD) | 83.4 (28.2) |
|
| |
| BMI (kg/m2), mean (SD)# | 30.4 (9.1) |
By Cockcroft-Gault equation
Body mass index
Figure 1.
ED diagnostic categories at time of disposition
*There were 1,095 unique diagnoses. These working diagnoses were at the time of ED disposition and reflect the potential infectious source (s) for which vancomycin was given.
The majority of patients were admitted to the hospital (87.4%), but 533 patients (11.4%) were discharged from the ED after administration of vancomycin. Table 2 shows the antibiotic profile associated with vancomycin administration. The other antimicrobials most frequently administered were for gram-negative pathogens.
Table 2.
Antibiotic profile of vancomycin administration
| Administered alone, No. (%) | 1090 (23.4) |
|
| |
| Multiple doses given in ED, No. (%) | 372 (8.0) |
|
| |
| 1000mg administered, No. (%) | 4,293 (92.2) |
|
| |
| Co-administered with other abx., No. (%) | 3566 (76.6) |
|
| |
| Co-administered with another antibiotic with primarily gram + coverage, No. (%) | 60 (1.3) |
|
| |
| Co-administered in association with double gram− coverage, No. (%) | 412 (8.8) |
|
| |
| Antibiotics co-administered for single gram− coverage, No. (%) | |
| Cefepime | 1657 (46.7) |
| Piperacillin/tazobactam | 708 (19.9) |
| Ceftriaxone | 387 (10.9) |
| Ciprofloxacin | 152 (4.3) |
| Meropenem | 75 (2.1) |
| Moxifloxocin | 48 (1.4) |
| Gentamicin | 43 (1.2) |
| Aztreonam | 25 (0.7) |
Vancomycin was administered in combination with 9 other antibiotics total
There were 4,441 doses of vancomycin given to the patients with a weight measurement available in the ED at the time of presentation (n = 4,057). Vancomycin was dosed correctly 22.1% of the time (n = 980 doses), and those patients receiving the recommended dose had a mean weight of 61.6 kg. Seventy-one percent (3,143 doses) of patients were dosed below the recommended dose, and had a mean weight of 93.9 kg.
There were several patient characteristics which influenced dosing accuracy in the univariable model (Table 3). However, after creation of the multivariable model, only patient weight remained a significant influence on dosing accuracy. Specifically, ED creatinine had no influence on dosing accuracy (p = 0.58), nor did age (p = 0.45). General logit modeling comparing correct vs. overdose and correct vs. under dose for each 10 kg increase in patient weight, yielded odds ratios (95% confidence interval) of 0.16 (0.11,0.25, p <0.0001) and 7.66 (5.74, 10.2, p <0.0001). Each 10kg increase in patient weight was associated with nearly an eightfold increase in the likelihood of being under dosed as compared to correctly dosed.
Table 3.
Patient characteristics predicting dosing correctness, univariable comparison
| ED dose given | Correct (n = 980) | Overdose (n = 318) | Under dose (n = 3143) | p |
|---|---|---|---|---|
| Age (y), mean(SD) | 53.8 (19.9) | 55.4 (19.8) | 52.8 (17.2) | 0.044 |
| Weight (kg), mean (SD)* | 61.6 (10.2) | 48.8 (11.6) | 93.9 (26.3) | <0.001 |
| Serum creatinine (mg/dl), median (SD) | 0.83 (1.99) | 0.85 (1.96) | 0.95 (1.99) | 0.005 |
| Caucasian race, n (%) | 408 (41.6) | 102 (32.1) | 1405 (44.7) | <0.001 |
| Male sex, n (%) | 418 (42.7) | 108 (34.0) | 1822 (58.0) | <0.001 |
| Vasopressors in ED, n (%) | 106 (10.8) | 45 (14.1) | 279 (8.9) | 0.008 |
| SSTI**, n (%) | 328 (33.5) | 59 (18.6) | 1468 (46.7) | <0.001 |
| Pulmonary source, n (%) | 337 (34.4) | 134 (42.1) | 752 (23.9) | <0.001 |
| Intra abdominal source, n (%) | 90 (9.2) | 29 (9.1) | 199 (6.3) | 0.006 |
| Urinary source, n (%) | 156 (15.9) | 68 (21.4) | 424 (13.5) | 0.012 |
Only significant predictor of dosing accuracy after multivariable comparison
Skin and soft tissue infection
Table 4 shows the effect of ED dosing accuracy on clinical outcomes, with p values representing the significance of the overall model. Specific contrasts within the overall model revealed under dosing was significantly associated with sub therapeutic vancomycin levels, OR 0.60 (0.44, 0.83, p = 0.002). Specific contrasts within the overall model also revealed that patients receiving doses of vancomycin >20 mg/kg in the ED had longer hospital length of stay, p = 0.005, were more likely to spend ≥ 3 days in the hospital, OR 1.49 (1.12, 1.98, p = 0.006), and to die, OR 1.88 (1.22, 2.90, p = 0.004).
Table 4.
Dosing correctness and clinical outcomes
| ED dose given | Under dose | Correct | Overdose | p |
|---|---|---|---|---|
| Therapeutic Vancomycin Level, No. (%)* | 176 (14.1) | 79 (21.3) | 25 (19.7) | 0.004 |
| HLOS (days), Mean (SD) | 5.9 (8.9) | 6.1 (8.8) | 7.5 (14.7) | 0.001 |
| HLOS ≥ 3 days, No. (%) | 1825 (58.1) | 585 (59.7) | 219 (68.9) | 0.002 |
| Death, No. (%) | 172 (5.5) | 64 (6.5) | 37 (11.6) | 0.002 |
Median (IQR) vancomycin level in entire cohort was 12.0 (7.8–17.8) mcg/ml.
P value comparison between correct vs. under dose and overdose
In a subset of 662 patients admitted to the intensive care unit (ICU), weight was also the only influence on dosing accuracy in the multivariable model. General logit modeling comparing correct vs. overdose and correct vs. under dose for each 10 kg increase in patient weight, yielded odds ratios (95% confidence interval) of 0.12 (0.03,0.41, p =0.0007) and 9.65 (4.91, 19.0, p <0.0001). Dosing accuracy had no influence on clinical outcomes in patients admitted to the ICU, including death (p = 0.876).
The median vancomycin level of admitted patients was 12.0 mcg/ml. Levels were obtained at a mean of 48.3 hours, and represented 1,559 trough levels (81.4%) and 356 random levels (18.6%). Table 5 shows the effect of vancomycin levels on clinical outcomes, with p values representing the significance of the overall model. Specific contrasts within the overall model revealed that supratherapeutic levels were more likely to have a hospital stay ≥ 3 days, OR 1.87 (1.07, 3.27, p = 0.03), higher peak creatinine levels, OR 1.34 (1.15, 1.56, p = 0.0002), and more than twice as likely to die, OR 2.06 (1.28, 3.32, p = 0.003).
Table 5.
Association Between Vancomycin Levels* and Outcomes
| Subtherapeutic | Therapeutic | Supratherapeutic | p | |
|---|---|---|---|---|
| HLOS ≥ 3 days, No. (%) | 1070 (86.8) | 287 (89.1) | 338 (93.9) | 0.001 |
| Peak Creatinine, mg/dl, Mean (SD) | 1.88 | 2.72 | 3.47 | < 0.001 |
| Death, No. (%) | 69 (5.6) | 28 (8.7) | 59 (16.4) | < 0.001 |
Data represents 1,915 patients with non-peak vancomycin levels checked. “Therapeutic” level is defined as 15–20 mcg/ml.
P value comparison between therapeutic vs. subtherapeutic and supratherapeutic
ED vancomycin administration also seemed to influence subsequent inpatient treatment. Of the 4,070 ED patients given vancomycin and subsequently admitted, the drug was continued in 3,056 patients (75.1%). Using inpatient weight, most inpatient vancomycin dosing was also incorrect (66.8%) and the majority of patients (83.8%) were given a dose unchanged from the dose administered in the ED.
DISCUSSION
Knowledge of patient characteristics, infectious sources, and antibiotic pharmacokinetics can aid in the choice of antibiotic and also the optimal dose to be administered. As the ED is the first treatment site for many infections, the role the ED plays in adequately treating infections, properly dosing antibiotics to attain therapeutic levels, and contributing to antibiotic resistance is vital.
Our study, which represents the largest study to date describing vancomycin dosing in the ED, was designed not only to simply describe what is occurring in the ED with respect to this drug, but also to examine potential outcomes associated with ED dosing. Vancomycin was given most commonly for skin and soft tissue infections (SSTI). The majority of these infections may represent simple cutaneous abscesses, which generally do not require antibiotic therapy, but rather incision and drainage alone, as demonstrated by multiple trials [3, 18–22]. Furthermore, many of these infections may be due to community acquired MRSA (CA-MRSA), which is more often susceptible to a greater number of antibiotic classes than is healthcare associated MRSA (HA-MRSA), and may therefore obviate the need for vancomcyin in many cases [2, 23].
A dose of 1 gram of vancomycin is administered to most patients receiving the drug (92.2%), despite that dose being outside the recommended weight-based dose in the great majority of patients. This leads to inappropriate dosing in 77.9% of patients, with the majority of patients being under dosed. Weight-based dosing is recommended [13] and at least one study demonstrates that a 1 gram dosing regimen is inadequate to attain therapeutic levels [24]. There are likely multiple factors associated with choosing a 1 gram-based dosing strategy in the ED. Given limited time, computer order entry and the preformulation of vancomycin in 1 gram bags, “clicking” the order for a 1 gram dose is quicker and more convenient in an otherwise hectic ED setting. Fear of nephrotoxicity may also be influential, as some studies do show nephrotoxicity associated with vancomycin [25, 26]. Our data suggests that while serum creatinine did not influence dosing accuracy, patients with supratherapeutic vancomcyin levels did have higher peak creatinine levels. However the role that vancomycin plays in contributing to nephrotoxicity is debated. Most data suggest that unless vancomycin is administered with other nephrotoxic agents, the incidence of acute kidney injury is very low [13, 15, 27].
Patient weight was the only significant predictor of dosing inaccuracy, and was highly influential. Each 10kg increase in patient weight was associated with a nearly eightfold increase in the likelihood of being under dosed. Physiological changes associated with obesity, such as increased volume of distribution and drug clearance, make the dosing of antimicrobials challenging in this patient cohort. Our study shows that increasing weight causes substantial dosing inaccuracy and should be studied further, as this is an area with significant room for improvement.
This study also shows that the ED dosing of vancomycin has potential effects on patient outcome. Patients dosed in the ED with more than 20mg/kg of vancomycin spent more time in the hospital, and were almost twice as likely to die before hospital discharge. ED dosing was also highly influential on subsequent vancomycin levels, which also contributed to patient outcome, as patients dosed with vancomycin in the ED with supratherapeutic vancomycin levels had worse outcomes with respect to hospital length of stay, peak creatinine, and death.
We hypothesized that patients receiving (1) lower doses of vancomycin and (2) with lower levels would have worse outcomes. Our results did not support these hypotheses. This may reflect the discord between vancomycin dosing guidelines and empiric dosing in the ED. The vancomycin guidelines are intended for patients with known or highly suspected MRSA infections, and are based mainly on non-randomized, observational data [13]. No empiric dosing guidelines exist, and whether current guidelines can be applied to the ED with any validity is unknown. While the ED dosing of vancomycin matters, only a small fraction of patients given vancomycin in the ED have a MRSA infection, and even a smaller fraction have serious infections with higher MICs for which higher dosing would be clinically important. While our data shows the great majority of patients in the ED are dosed outside the recommended range, this likely does not represent a majority of patients receiving ineffective treatment. It does however, reveal that patients without MRSA infections are likely over exposed to vancomycin, and real-time diagnostic tests are needed to identify patients with MRSA infections, which could assist the emergency physician in the decision to dose with vancomycin or not.
Finally, the majority of patients given vancomycin in the ED will be given vancomycin after admission to the hospital at a dose unchanged from the ED dose. Care in the ED impacts long term outcome across multiple clinical arenas [17, 28–33]. This is not a new finding. The fact that most vancomycin dosing was continued unchanged after admission to the hospital, despite being administered outside of recommended range in the majority of patients, again highlights the fact that ED treatment is highly influential on subsequent inpatient care. This also likely influenced the sub therapeutic vancomycin levels seen in the majority of the patients in this study. Interventions to improve dosing indication and accuracy could have a significant impact on hospital practice.
The increase in vancomycin use is likely multi factorial. The more immediate concern in the ED may be treatment failure more than antibiotic resistance. This may influence a low threshold to dose vancomycin in patients with little or no risk factors for MRSA. MRSA infections are known to be increasing, and an increasing number of ED visits are due to MRSA SSTIs [5, 19]. There is also an increased incidence of severe sepsis in the United States, and clinicians are increasingly aware of the benefits of early, appropriate antimicrobial therapy for the critically ill, infected patient [9, 34]. With an ED mortality rate of only 0.3% and an inpatient mortality of 7.4%, we believe this to play a minor role in the increased vancomycin use at our institution. Vancomycin use can be limited in the ED given the existing literature that suggests either vancomycin is unnecessary when treating uncomplicated SSTI [3, 18–22], and MRSA is unlikely to be involved in intra abdominal infections [35], and urinary tract infections [36, 37]. Vancomycin exhibits time-dependent killing, requiring an adequate area under the concentration curve (AUC) divided by the MIC, to achieve efficacy. Based on these pharmacokinetic properties, its use in patients who are discharged from the ED (533 in this study) after only one dose should be strongly discouraged as well.
LIMITATIONS
This study was a carefully controlled analysis of retrospective data, but several limitations affect the interpretation of our findings. First, the retrospective nature of the data collection can be criticized, but we carefully selected robust measurements (i.e., weight, drug administration dose) that would have reflected data available to the treating clinician. Further, data were electronically abstracted from medical records systems and validated across repeated measurements to minimize data entry error. Although these data are robust, we have not elucidated patient or disease factors which may have contributed to non-recommended dosing regimens. There is no way to capture all of the factors associated with patient care that may have influenced the decision to not only give vancomycin, but also at a particular dose.
The division of ED diagnoses into diagnostic categories was somewhat arbitrary. Given the myriad of diagnoses generated by the electronic medical record, there was no logical way to analyze them all separately in a meaningful fashion without a grouping strategy. The definition of “dosing accuracy” can be debated as well. Although some clinicians disagree on vancomycin dosing, based on guideline recommendations, we believe our definition of 15 – 20 mg/kg to be the correct accepted dosing. Similarly, we defined a “therapeutic” vancomycin level as as 15–20 mcg/ml. This more aggressive level is generally recommended to improve clinical outcomes for complicated infections [13]. We thought this cutoff appropriate, based on the changing susceptibility pattern, MIC breakpoints, and local guidelines at our institution. It is possible though, that with a median vancomycin level of 12.0, some patients labeled as subtherapeutic with respect to level, were indeed adequately treated. Vancomycin dosing has changed over the last 25 years as MRSA has become more prevalent and the MIC of isolates has increased. Some of these trends are somewhat geographic, so the single-center nature of our study may reduce the external validity to similar centers with similar antibiograms. The generalizability of this study may not be able to be extended to centers where vancomycin use is less and MRSA less prevalent.
This study included all patients to whom vancomycin was given empirically in the ED. This does not take into consideration indication or appropriateness, as many patients without a MRSA infection could have been dosed with vancomycin. While this may make the dose prescribed a less critical issue, we believe it is important to give a real world account of how vancomycin is actually being used in the ED.
This study is also limited by a lack of microbiological data, and measurements of severity of illness. Across a broad cohort of ED patients treated empirically, perhaps guideline dosing recommendations cannot be applied. It is possible that in patients with MRSA infections, that under dosing and sub therapeutic levels would have been associated with worse outcomes, secondary to lack of adequate antimicrobial therapy. Our data suggest that most of the adverse effects associated with ED vancomycin use are secondary to a higher dosing strategy. This may reflect patients without MRSA infections who could only be harmed by an aggressive dosing strategy, and saw no benefit to vancomycin use, given a lack of a MRSA infection. It is also possible that physicians opted for higher doses due to a higher severity of illness or different clinical infectious sources in those patients. However, on multivariable analysis, vasopressor use and pulmonary source were not influential on dosing accuracy, and dosing accuracy exerted no influence on outcome in patients admitted to the ICU. There are many confounders, including severity of illness, which may have contributed to these clinical outcomes, and a cause-effect relationship with vancomycin dosing cannot be established based on these data. Therefore, the results should be interpreted in context of these limitations.
CONCLUSIONS
This study found vancomycin administration in the ED is very common, for a wide variety of indications, frequently dosed incorrectly, and most often continued after hospital admission. Furthermore, our data suggests a possible association with overdosing of vancomycin and worse clinical outcomes, to include hospital length of stay, renal function, and death. Further studies are needed to assess the applicability of vancomycin dosing guidelines to the ED population, and better diagnostic tools are needed to identify at risk patients who actually have a MRSA infection. The potential for the ED to be a common ground for antibiotic treatment success, failure, and emergence of resistance could be significant. The contribution of ED vancomycin dosing on patient outcome needs further studied in prospective trials.
ARTICLE SUMMARY.
-
Why is this topic important?
Despite increasing rates of MRSA infections and increased need for vancomycin, the dosing characteristics of this drug have not been examined in the ED. The prevalence and accuracy of its use are unknown, and the effect of ED dosing of vancomycin on clinical outcomes is unknown.
-
What does this study attempt to show?
This study attempts to first characterize how the drug is being used in the ED, and also to demonstrate any clinical outcomes associated with its use.
-
What are the key findings?
Vancomycin is given frequently in the ED. Dosing inaccuracy is common and seems to be driven by increasing patient weight. Doses in excess of 20mg/kg are associated with increased length of stay, as well as higher mortality.
-
How is patient care impacted?
This is the largest ED-based vancomycin study to date and is the first attempt to characterize the use of the drug in the ED. It is possible that the frequency of its use is unnecessary and dosing inaccuracy contributes to adverse patient outcomes. Future trials should aim to better characterize the patient most likely to benefit from its use, limiting potential unnecessary exposure when appropriate.
Acknowledgments
This project was supported by NIH/NCRR Washington University-ICTS Grant Number UL1 RR024992, as part of a K30 Postdoctoral Program intramural grant awarded to BF. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
The authors would like to acknowledge Karen Steger-May, MA, from the Division of Biostatistics for her assistance with the statistical analysis of these data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Brian M. Fuller, Division of Emergency Medicine, Department of Anesthesiology, Division of Critical Care, Washington University School of Medicine, St. Louis, MO, USA.
Nicholas Mohr, Department of Emergency Medicine, Department of Anesthesia, Division of Critical Care, Roy J. and Lucille A. Carver College of Medicine, University of Iowa, Iowa City, IA, USA.
Lee Skrupky, Department of Pharmacy, Barnes Jewish Hospital, St. Louis, MO, USA.
Kristen Mueller, Division of Emergency Medicine, Washington University School of Medicine, St. Louis, MO, USA.
Craig McCammon, Department of Pharmacy, Barnes Jewish Hospital, St. Louis, MO, USA.
References
- 1.Norrby SR, Nord CE, Finch R. Lack of development of new antimicrobial drugs: a potential serious threat to public health. The Lancet infectious diseases. 2005;5 (2):115–119. doi: 10.1016/S1473-3099(05)01283-1. [DOI] [PubMed] [Google Scholar]
- 2.Achiam CC, Fernandes CM, McLeod SL, Salvadori MI, John M, Seabrook JA, Theakston KD, Milburn S, Hussain Z. Methicillin-resistant Staphylococcus aureus in skin and soft tissue infections presenting to the Emergency Department of a Canadian Academic Health Care Center. European journal of emergency medicine : official journal of the European Society for Emergency Medicine. 2010 doi: 10.1097/MEJ.0b013e328337901a. [DOI] [PubMed] [Google Scholar]
- 3.May L, Harter K, Yadav K, Strauss R, Abualenain J, Keim A, Schmitz G. Practice patterns and management strategies for purulent skin and soft-tissue infections in an urban academic ED. The American journal of emergency medicine. 2011 doi: 10.1016/j.ajem.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 4.Odell CA. Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) skin infections. Current opinion in pediatrics. 2010;22(3):273–277. doi: 10.1097/MOP.0b013e328339421b. [DOI] [PubMed] [Google Scholar]
- 5.Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA., Jr Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Annals of emergency medicine. 2008;51(3):291–298. doi: 10.1016/j.annemergmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Lerma F. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. Intensive care medicine. 1996;22 (5):387–394. doi: 10.1007/BF01712153. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The Influence of Inadequate Antimicrobial Treatment of Bloodstream Infections on Patient Outcomes in the ICU Setting*. Chest. 2000;118(1):146. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 8.Kollef MH. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clinical infectious diseases. 2000;31(Supplement 4):S131. doi: 10.1086/314079. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, Suppes R, Feinstein D, Zanotti S, Taiberg L. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock*. Critical care medicine. 2006;34(6):1589. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 10.Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik S. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. JOURNAL OF INTERNAL MEDICINE-OXFORD- 1998;244:379–386. doi: 10.1046/j.1365-2796.1998.00379.x. [DOI] [PubMed] [Google Scholar]
- 11.Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clinical infectious diseases. 2003;36(11):1418. doi: 10.1086/375057. [DOI] [PubMed] [Google Scholar]
- 12.Mueller EW, Hanes SD, Croce MA, Wood GC, Boucher BA, Fabian TC. Effect from multiple episodes of inadequate empiric antibiotic therapy for ventilator-associated pneumonia on morbidity and mortality among critically ill trauma patients. The Journal of trauma. 2005;58(1):94. doi: 10.1097/01.ta.0000141890.29032.9a. [DOI] [PubMed] [Google Scholar]
- 13.Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, Dalovisio JR, Levine DP. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49(3):325–327. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 14.Wright S. Appropriateness of Vancomycin Use in the Emergency Department. Annals of Emergency Medicine. 1998;32:531–536. doi: 10.1016/s0196-0644(98)70030-7. [DOI] [PubMed] [Google Scholar]
- 15.Hidayat L. High dose vancomycin therapy for MRSA infections efficacy and toxicity. Arch Intern Med. 2006;166:2138–2144. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 16.Jeffres MN, Isakow W, Doherty JA, McKinnon PS, Ritchie DJ, Micek ST, Kollef MH. Predictors of mortality for methicillin-resistant Staphylococcus aureus health-care-associated pneumonia: specific evaluation of vancomycin pharmacokinetic indices. CHEST. 2006;130(4):947–955. doi: 10.1378/chest.130.4.947. [DOI] [PubMed] [Google Scholar]
- 17.Rivers ENB, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 18.Duong M, Markwell S, Peter J, Barenkamp S. Randomized, controlled trial of antibiotics in the management of community-acquired skin abscesses in the pediatric patient. Annals of emergency medicine. 2010;55(5):401–407. doi: 10.1016/j.annemergmed.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 19.Moran G. Methicillin-Resistant S. aureus Infections among patients in the emergency department. N Engl J Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 20.Llera JL, Levy RC. Treatment of cutaneous abscess: a double-blind clinical study. Annals of emergency medicine. 1985;14(1):15–19. doi: 10.1016/s0196-0644(85)80727-7. [DOI] [PubMed] [Google Scholar]
- 21.Rajendran PM, Young D, Maurer T, Chambers H, Perdreau-Remington F, Ro P, Harris H. Randomized, double-blind, placebo-controlled trial of cephalexin for treatment of uncomplicated skin abscesses in a population at risk for community-acquired methicillin-resistant Staphylococcus aureus infection. Antimicrobial agents and chemotherapy. 2007;51(11):4044. doi: 10.1128/AAC.00377-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MC, Rios AM, Aten MF, Mejias A, Cavuoti D, Mccracken GH, Jr, HARDY R. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. The Pediatric infectious disease journal. 2004;23(2):123. doi: 10.1097/01.inf.0000109288.06912.21. [DOI] [PubMed] [Google Scholar]
- 23.Hasty MB, Klasner A, Kness S, Denmark TK, Ellis D, Herman MI, Brown L. Cutaneous community-associated methicillin-resistant staphylococcus aureus among all skin and soft-tissue infections in two geographically distant pediatric emergency departments. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2007;14(1):35–40. doi: 10.1197/j.aem.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Patanwala AE, Norris CJ, Nix DE, Kopp BJ, Erstad BL. Vancomycin dosing for pneumonia in critically ill trauma patients. The Journal of trauma. 2009;67(4):802–804. doi: 10.1097/TA.0b013e31818e90d2. [DOI] [PubMed] [Google Scholar]
- 25.Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clinical infectious diseases. 2009;49(4):507. doi: 10.1086/600884. [DOI] [PubMed] [Google Scholar]
- 26.Hazlewood KA, Brouse SD, Pitcher WD, Hall RG. Vancomycin-associated nephrotoxicity: grave concern or death by character assassination? The American journal of medicine. 2010;123(2):182. e181–182. e187. doi: 10.1016/j.amjmed.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong-Beringer A, Joo J, Tse E, Beringer P. Vancomycin-associated nephrotoxicity: a critical appraisal of risk with high-dose therapy. International journal of antimicrobial agents. 2011;37(2):95–101. doi: 10.1016/j.ijantimicag.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Pollack C, Jr, Braunwald E. update to the ACC. AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: implications for emergency department practice. Ann Emerg Med. 2008;51:591–606. doi: 10.1016/j.annemergmed.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Group. TNIoNDaSr-PSS. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 30.Kumar A, Roberts D, Wood K, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. CRITICAL CARE MEDICINE-BALTIMORE. 2006;34:1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 31.Wood K. Major Pulmonary Embolism: Review of a Pathophysiologic Approach to the Golden Hour of Hemodynamically Significant Pulmonary Embolsim. CHEST. 2002;121:877–905. doi: 10.1378/chest.121.3.877. [DOI] [PubMed] [Google Scholar]
- 32.Boersma E, Maas A, Deckers JW, et al. Early thrombolytic treatment in acute myocardial infarction: reappraisal of the golden hour. The Lancet infectious diseases. 1996;348(9030):771–775. doi: 10.1016/S0140-6736(96)02514-7. [DOI] [PubMed] [Google Scholar]
- 33.Blow O, Magliore L, Claridge J, et al. The Golden Hour and the Silver Day: Detection and Correction of Occult Hypoperfusion within 24 Hours Improves Outcomes from Major Trauma. Journal of Trauma- Injury, Infection, & Critical Care. 1999;47(5):964. doi: 10.1097/00005373-199911000-00028. [DOI] [PubMed] [Google Scholar]
- 34.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. CRITICAL CARE MEDICINE-BALTIMORE- 2001;29 (7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJC, Baron EJ, O’Neill PJ, Chow AW, Dellinger EP, Eachempati SR. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surgical infections. 2010;11(1):79–109. doi: 10.1089/sur.2009.9930. [DOI] [PubMed] [Google Scholar]
- 36.Daza R, Gutiérrez J, Piédrola G. Antibiotic susceptibility of bacterial strains isolated from patients with community-acquired urinary tract infections. International journal of antimicrobial agents. 2001;18(3):211–215. doi: 10.1016/s0924-8579(01)00389-2. [DOI] [PubMed] [Google Scholar]
- 37.Muder RR, Brennen C, Rihs JD, Wagener MM, Obman A, Stout JE, Yu VL. Isolation of Staphylococcus aureus from the urinary tract: association of isolation with symptomatic urinary tract infection and subsequent staphylococcal bacteremia. Clinical infectious diseases. 2006;42(1):46. doi: 10.1086/498518. [DOI] [PubMed] [Google Scholar]