Abstract
Rationale
Chronic food restriction (FR) increases rewarding effects of abused drugs and persistence of a cocaine-conditioned place preference (CPP). When there is a single daily meal, circadian rhythms are correspondingly entrained, and pre- and postprandial periods are accompanied by different circulating levels of metabolic hormones that modulate brain dopamine function.
Objectives
The present study assessed whether rewarding effects of d-amphetamine, cocaine, and persistence of cocaine CPP differ between FR subjects tested in the pre- and postprandial period.
Materials and methods
Rats were stereotaxically implanted with intracerebral microinjection cannulae and an electrode in lateral hypothalamus. Rewarding effects of d-amphetamine and cocaine were assessed using electrical self-stimulation in rats tested 1-4 or 18-21 hrs after the daily meal. Non-implanted subjects acquired a cocaine CPP while ad libitum fed, then were switched to FR and tested for CPP at these same times.
Results
Rewarding effects of intra-nucleus accumbens (NAc) d-amphetamine, intraventricular cocaine, and persistence of cocaine CPP did not differ between rats tested 18-21 hrs food deprived, when ghrelin and insulin levels were at peak and nadir, respectively, and those tested 1-4 hrs after feeding. Rats that expressed a persistent CPP had elevated levels of p-ERK1, GluA1, and p-Ser845-GluA1 in NAc core, and the latter correlated with CPP expression.
Conclusions
Psychostimulant reward and persistence of CPP in FR rats are unaffected by time of testing relative to the daily meal. Further, NAc biochemical responses previously associated with enhanced drug responsiveness in FR rats are associated with persistent CPP expression.
Keywords: food restriction, nucleus accumbens, reward, self-stimulation, d-amphetamine, cocaine, conditioned place preference, ghrelin, insulin, ERK 1/2, GluA1
The hypothesis that drugs with abuse liability target the neural substrates for appetitively motivated behavior is supported by the high comorbidity of disordered eating and drug abuse (Krahn et al. 1992; Pisetsky et al. 2008; Root et al. 2010; Seo and Jiang 2009; Wiederman and Pryor 1996), the diminished prevalence of substance use among obese individuals (Simon et al. 2006; Warren et al. 2005), and the increased vulnerability to use, relapse, and drug-induced psychopathology among those who are dieting or have low body mass index (Austin and Gortmaker 2001; Cheskin et al. 2005; French et al. 1994; Rosse et al. 2005). In animal models, high energy diets decrease, and chronic food restriction (FR) increases the rewarding effects of psychostimulants and other abused drugs (Bell et al. 1997; Cabeza de Vaca and Carr 1998; Carr et al. 2000; Carroll and Meisch 1984; Davis et al. 2008; Wellman et al. 2007). A variety of pre- and postsynaptic neuroadaptations have been identified in the mesoaccumbens pathway that are consistent with behavioral hypersensitivity to drugs of abuse in FR rats (Carr et al. 2010; Carr et al. 2003; Deroche et al. 1995; Haberny et al. 2004; Pothos et al. 1995; Stamp et al. 2008; Zhen et al. 2006). However, there is evidence that FR regimens that deliver equivalent caloric and macronutrient content, and induce similar body weight loss, are differentially effective in potentiating amphetamine-induced locomotor activity based on the number, size, and patterning of meals (Marinkovic et al. 2007; Sharpe et al. 2012). Thus, it is not clear to what extent the potentiating effects of FR on drug reward are mediated by relatively stable neuroadaptations versus dynamic physiological processes (Diaz-Munoz et al. 2000; Drazen et al. 2006; Krieger 1974).
Sensitivity of free feeding rats to the reinforcing effect of cocaine can vary according to time of day (Baird and Gauvin 2000). Moreover, when subjects are allowed only one scheduled opportunity to eat each day, circadian rhythms uncouple from the light-dark cycle and become entrained by time of feeding (Davidson and Stephan 1999; Escobar et al. 1998). Among the physiological processes subject to meal entrainment are corticosteroid secretion, brain monoaminergic activity, and levels of neuronal activity and clock gene expression in nucleus accumbens (Angeles-Castellanos et al. 2007; Diaz-Munoz et al. 2000; Krieger 1974). Further, peak levels of ghrelin and corticosterone, and nadir levels of insulin, precede scheduled feeding, with opposite extremes prevailing in the postprandial period (Diaz-Munoz et al. 2000; Drazen et al. 2006). These transient but dramatic differences in metabolic hormone levels may modulate behavioral responsiveness to drugs that target the brain dopamine system (Ambroggi et al. 2009; Barrot et al. 2000; Davis et al. 2007; Daws et al. 2011; Dickson et al. 2011).
In all prior behavioral and biochemical studies of this laboratory, FR subjects were fed a single meal each day (Carr 2007; 2011). The meal was provided at approximately 1700h and testing took place between 1000 and 1500h. Consequently, subjects were acutely food-deprived (~15-20 hrs) in addition to being chronically FR and 20% below their pre-FR body weight. The primary purpose of the present study was to assess whether two key behavioral effects of FR – increased drug reward magnitude (Cabeza de Vaca and Carr 1998) and persistent expression of a cocaine conditioned place preference (Zheng et al. 2012) – differ between FR subjects tested 1-4 versus 18-21 hrs after completion of the daily meal. Thus, in Experiments 1 and 2, the reward magnitude of intracerebrally injected d-amphetamine and cocaine were assessed in FR subjects tested between 1000 and 1500h using the intracranial electrical self-stimulation paradigm. One subgroup of subjects in each experiment received their daily meal at 0800h (AM-fed) and a second subgroup received their meal at 1700h (PM-fed). Circulating levels of ghrelin, insulin, and corticosterone were measured at a time corresponding to the midpoint of the behavioral testing period. In Experiment 3, subjects underwent cocaine place preference conditioning while in the ad libitum fed state. This was followed by a switch to FR with one subgroup AM-fed and the other PM-fed. Three weeks after initiation of FR, 15 daily tests of conditioned place preference (CPP) were conducted. After the final CPP test, nucleus accumbens shell and core were obtained and biochemical correlates of CPP expression were assessed.
Materials and methods
Subjects and Surgical Procedures
Subjects were mature male Sprague-Dawley rats (Taconic Farms, Germantown, NY) initially weighing 375-425 g. Food and water were available ad libitum except as noted. Animals were individually housed in clear plastic cages with bedding under a 12 h light:dark photoperiod with lights on at 0700 h. Several days after arrival in the central animal facility, rats in Experiment 1 were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and stereotaxically implanted with a 0.25 mm diameter monopolar stimulating electrode (Plastics One, Roanoke, VA) in the lateral hypothalamic medial forebrain bundle (skull flat coordinates: 3.0 mm posterior to bregma, 1.6 mm lateral to the sagittal suture, and 8.5 mm ventral to skull surface). An anterior ipsilateral stainless steel skull screw served as ground. Rats were also implanted with two chronically indwelling guide cannulae (26 ga) that were placed bilaterally 2.0 mm dorsal to injection sites in the NAc medial shell (1.6 mm anterior to bregma; 2.1 mm lateral to the sagittal suture, tips angled 8o toward the midline, 5.8 mm ventral to skull surface). The electrode, ground, cannulae, and three additional mounting screws were then permanently secured to the skull by flowing dental acrylic around them. Rats in Experiment 2 were implanted with a stimulating electrode in the lateral hypothalamus and a single contralateral cannula, 1.0 mm dorsal to a lateral ventricular injection site, using the coordinates: 1.0 mm posterior to bregma, 1.6 mm lateral to the sagittal suture, and 3.4 mm ventral to skull surface. Experimental procedures were approved by the Institutional Animal Care and Use Committee at the New York University School of Medicine and were consistent with the Principles of Laboratory Animal Care (NIH Publication no. 85-23).
Feeding regimens
During the second week of training in the ICSS protocol, rats in Experiments 1 and 2 were switched from unlimited access to food (pelleted LabDiet 5001) to a single 10-g meal each day, representing ~40% of the ad libitum intake. Half of the subjects in each experiment received the daily meal in the home cage at 0800h (AM-fed) and the remaining half received the daily meal at 1700h (PM-fed). The meal was consumed within 1 hour. This food restriction regimen continued until body weights decreased by 20% (approximately two weeks). ICSS training continued throughout this period. For the remainder of each experiment, daily feeding was titrated to maintain body weights at 80% of the initial value, typically requiring an upward adjustment to 12-16-gram meals. Drug testing did not begin until rats had been stabilized for one week at their target body weight (i.e. 80% of pre-restriction body weight). Rats in Experiment 3 remained ad libitum fed until place preference conditioning was completed and the first post-conditioning test of place preference was conducted. Subsequently, half of the rats were placed on the AM-fed and half on the PM-fed FR regimen described above. CPP testing did not resume until subjects had achieved the 20% decrease from pre-restriction body weight and stabilization at that value for one additional week (about 3 weeks total).
Self-stimulation (ICSS) apparatus
Brain stimulation training and testing were conducted in eight standard test chambers (26 × 26 × 21 cm) placed within sound attenuating cubicles. Each chamber had a retractable lever mounted on one wall and a house light mounted on the opposite wall. Four constant current stimulators (PHM-152B/2; Med-Associates, Georgia, VT) with dual outputs were used to deliver trains of 0.1 ms cathodal pulses, which were conducted to implanted electrodes by way of commutators and flexible cables. Electrical stimulation, contingencies, and data recording were controlled through Dell XPS R400 computers and interface (Med-Associates).
Self-stimulation procedures
After one week of postsurgical recovery, rats were trained to lever press for 0.5 s trains of electrical stimulation at a frequency of 100 pulses per second (pps). The initial stimulation intensity of 120 μA was systematically varied to locate, for each rat, the lowest intensity that maintained vigorous lever pressing. Each subsequent training session consisted of twenty four 60-s trials. Extension of the lever and a 2-s train of ‘priming’ stimulation initiated each trial. Each trial was terminated by retraction of the lever and followed by a 10-s intertrial interval. Each lever press produced a 1-s train of stimulation, except for those presses emitted during the stimulation train, which did not increase reinforcement density. The number of lever presses and reinforcements were recorded for each trial.
Initial training was followed by rate-frequency training, which continued for approximately two weeks. Rate-frequency curves were generated by presenting 12 trials in which the frequency of brain stimulation decreased over successive trials (approximately 0.05 log units each trial) from an initial frequency of 100 pps to a terminal frequency of 28 pps. At least two such series were presented in each training session. During the second week of training, subjects in Experiments 1 and 2 were divided into two groups matched for body weight and M-50 (the brain stimulation frequency that supported 50% of the maximum reinforcement rate) and assigned to either the AM-fed or PM-fed regimen of FR. Training and mock microinjection test sessions continued, at least twice per week, for all rats, during the ensuing ~3 week period during which groups achieved and then stabilized at the target body weight.
Intracerebral microinjection procedures
For NAc microinjections, solutions were loaded into two 30 cm lengths of PE-50 tubing attached at one end to 5-μl Hamilton syringes filled with distilled water and at the other end to 31-gauge injector cannulae, which extended 2.0 mm beyond the implanted guides. The 0.5 μl injection volumes were delivered manually over a period of 90 sec at a rate of 0.05 μl/10 sec. One minute following completion of the microinjection, injector needles were removed from guides, stylets were replaced, and animals were returned to test chambers for an additional 4-min prior to behavioral testing. For lateral ventricular injections, solutions were loaded into a 30 cm length of PE-50 tubing attached at one end to a 250-l Hamilton syringe filled with distilled water and at the other end to a 33-gauge injector cannula which extended 1 mm beyond the implanted guide. The syringe was mounted on a Harvard 2272 microliter syringe pump which delivered the 5.0 μl injection volume over a period of 95 s. One minute following injections, injector cannulae were removed, stylets replaced, and animals were returned to the test chambers where post-injection tests were initiated 10 m after completion of the injection. The accuracy of intraventricular cannula placements had been verified two weeks prior to drug testing by demonstrating a vigorous and short latency (i.e., <60 s) drinking response to 50 ng angiotensin II.
ICSS testing
All testing was conducted between 1000h and 1400h with subjects from the AM-fed group tested during the first ~2 hrs and subjects from the PM-fed group tested during the final ~2 hrs. Each test session began with a pre-injection test consisting of three rate-frequency series (42 min). The first series in a session was considered a ‘warm-up’ and data were excluded. This was followed by intracerebral microinjections which were followed, in 5 or 10 min, by a post-injection test consisting of two rate-frequency series (28 min). For each rate-frequency series, the number of reinforcements obtained as a function of brain stimulation frequency was recorded. For each rat, the two series from each test were averaged to yield a single rate-frequency function per test.
Place Preference Apparatus
Behavioral conditioning and testing were conducted in a three-compartment apparatus. Each Lucite test chamber (61 × 30.5 × 30.5 cm) consisted of two large side compartments (25.4 × 30.5 × 30.5 cm) separated by a small center area (10.2 × 30.5 × 30.5 cm). One of the large compartments had black walls with horizontal white stripes and a white grid floor composed of parallel stainless steel rods (0.2 cm diameter mounted 1.0 cm apart), whereas the other had white walls and a black wire mesh floor (1.3 × 1.3 cm squares). The small center compartment had white walls and a smooth ceramic floor. Removable partitions matching the compartment walls were used to isolate rats within specific compartments during conditioning. During CPP test sessions, the partitions were removed to allow rats to freely access the entire apparatus. Automated data collection was accomplished through 24 infrared photo-beam detectors along the length of the test chamber; the number and location of beam interruptions was scanned at 100 times per second. Information about beam status was stored and later transformed into a complete record of activity during a session (VersaMax system, Accuscan, Columbus, OH). The dependent measure was time spent in each compartment. During preconditioning test sessions (one per experiment) rats displayed no unconditioned preference for one side of the apparatus over the other.
Place Conditioning and Testing Procedures
Prior to experiments, rats were habituated to transport and handling on at least five occasions. The first day of each experiment was a pre-conditioning test session in which each rat was placed in the center compartment of the CPP apparatus with partitions removed and was allowed to move freely for 20 min. Time spent in each compartment was recorded. Based on the absence of initial preference for either conditioning compartment, rats were randomly assigned to receive cocaine in one of the two larger compartments.
Each rat underwent eight conditioning sessions, each of 20 min duration, over eight consecutive days. On alternate days, rats were injected with cocaine HCl immediately before being confined to the cocaine-paired compartment. On intervening days, rats received saline-vehicle injections before being confined to the opposite compartment.
The first CPP test was conducted two days after the eighth conditioning session. During this test, no injection was administered before placing each rat in the center compartment with partitions in place for 15 seconds. Partitions were subsequently removed and rats were allowed to move freely in the apparatus for 20 min. After the first CPP test, rats were semirandomly divided into two groups matched for CPP test performance. All subjects were then placed on FR regimens (either AM or PM fed). Three weeks later, CPP testing resumed. These CPP tests were conducted once per day for a total of 15 tests over a 17 day period. All testing was conducted between 1000h and 1400h with subjects in the AM-fed group tested during the first ~2 hrs and subjects in the PM-fed group tested during the final ~2 hrs.
Measurement of plasma hormone levels by ELISA
Three days after the final ICSS test in Experiment 1, six AM-fed and six PM-fed subjects were sacrificed at a time corresponding to their scheduled behavioral testing and trunk blood was collected. Blood was also collected from a group of six age-matched ad libitum fed rats that had undergone the same ICSS procedures as Experiment 1.
To prepare plasma samples for assay of insulin and corticosterone, whole blood was immediately transferred to chilled polypropylene tubes spray-coated with K2EDTA as anticoagulant and followed by centrifugation at 3000 × g for 15 min at 4°C. Samples were then aliquotted and stored at −20°C. For assay of ghrelin (active), AEBSF (Sigma-Aldrich, St. Louis, MO) was added prior to centrifugation, to a final concentration of 1 mg/ml. Following centrifugation, samples were acidified with HCl to a final concentration of 0.05 N, and then aliquotted and stored at −20°C.
Levels of insulin and corticosterone were determined using Insulin Ultrasensitive and Corticosterone ELISA Kits (ALPCO Diagnostics, Salem, NH), and levels of active ghrelin were determined using the Rat/Mouse Ghrelin (Active) ELISA Kit (Millipore, Billerica, MA). All ELISAs were performed according to manufacturers’ protocols.
Whole cell homogenates prepared from NAc for biochemical measurements
Immediately after the final CPP test in Experiment 3a, subjects were briefly exposed to CO2 and decapitated by guillotine, and brains were extracted and rapidly frozen in powdered dry ice. A series of four 500-m sections were cut using an IEC Minotome cryostat, and NAc core and shell were dissected using a combination of micropunch and microknife under an Olympus dissecting microscope. Tissue samples were then homogenized in 10 volumes of 50 mM Tris-HCl, pH 7.4, 300 mM NaCl, 1% Nonidet P-40, 10% glycerol, 1 mM EDTA, and manufacturer recommended concentrations of Phosphatase Inhibitor Cocktails I and II and Mammalian Protease Inhibitor Cocktail (all from Sigma). The homogenates were centrifuged at 30,000 × g for 10 min at 4°C, and the non-solubilized fraction was discarded. Lysates were then mixed with 2x Laemmli Sample Buffer (Biorad) mixed with β-mercaptoethanol and boiled for 5 minutes. Unused denatured lysate was stored in sample buffer in aliquots at −80°C. The protein content was determined by the Bradford assay using bovine serum albumin as standard.
Western blotting
Proteins were separated by electrophoresis on precast 4-12% sodium dodecyl sulfate polyacrylamide gels (Lonza, Rockland, ME). Dual colored protein standards (Bio-Rad, Hercules, CA) were loaded to estimate the size of target proteins and to assure complete transfer of proteins from gel to membrane. Proteins were electrophoretically transferred to nitrocellulose membranes and blocked for 60 minutes with 5% nonfat milk in tris buffered saline with 0.05% Tween-20 (TBST) with shaking at room temperature, then probed overnight at 4 °C usi ng primary antibodies for target proteins or the protein loading control, α-tubulin.
Antibodies used included mouse monoclonal anti-GluA1 (1:1000; MAB2263, Millipore, Temecula, CA), rabbit polyclonal antiphospho-Ser845-GluA1 (1: 1500; AB5849, Millipore, Temecula, CA), mouse monoclonal anti-phospho-(Thr202/Tyr204)-p44/42 ERK1/2 (1:2000; Cell Signaling, Beverly, MA), rabbit polyclonal anti-p42/44 ERK1/2 (1:5000; Cell Signaling, Beverly, MA), and mouse monoclonal anti-α-tubulin (1: 10,000; T6199, Sigma-Aldrich, St. Louis, MO). After probing with primary antibodies and washing with TBS-T buffer (3×5 min), membranes were incubated with horseradish peroxidase conjugated anti-mouse/rabbit IgG (1:10,000; Cell Signaling, Beverly, MA). Proteins were visualized using a chemiluminescence ECL kit (Pierce). Densitometric analysis of the bands was performed using the NIH Image J software.
Data analysis
For each ICSS test, the rate-frequency function was used to derive three parameters. The maximum reinforcement rate, described by a line that parallels the x-axis, was defined as the mean of all consecutive values within 10% of the highest rate for the curve. All remaining values comprised the descending portion of the curve, with the lowest point being at the highest frequency to produce fewer than 2.5 reinforcements per minute. Regression analysis of the descending portion of the curve was used to calculate the M-50 and x-axis intercept measures of reward threshold which are defined as the log pulse frequency sustaining half the maximum reinforcement rate and x-axis intercept of the regression line, respectively. For threshold parameters, antilog transformations were applied and natural frequencies were used to calculate the percentage change occurring in post-injection tests relative to a pre-injection test. Changes in the reinforcing efficacy of stimulation produced by drugs of abuse are reflected as parallel leftward shifts in the rate-frequency curve with similar effects on the two threshold measures. Results for each parameter were analyzed by 2-way mixed design ANOVA with time of feeding as the between subjects factor and drug dose as the within subjects factor.
In the CPP experiment, results obtained in the pre-conditioning test were analyzed by t-test to confirm absence of an unconditioned preference for one of the two conditioning compartments. Results obtained in the first CPP test were analyzed as time spent, in seconds, on the cocaine-paired side versus the saline-paired side by t-test. For CPP tests that followed the three week dietary manipulation, results were analyzed by 3-way mixed design ANOVA with time of feeding as the between subjects factor and side and day as the within subjects factors.
Results for plasma hormone levels were analyzed by 1-way ANOVA followed by Fisher LSD tests.
Results for p-ERK 1, p-ERK 2, GluA1, and p-S845-GluA1 were analyzed separately for NAc core and shell. Given the lack of difference in CPP expression between AM- and PM-fed groups, subjects were pooled for analysis. Subjects were separated into two groups based on presence or absence of CPP in the test that immediately preceded sacrifice. Presence was defined as more time spent on the cocaine-paired vs the saline-paired side with a lower limit of + 100 sec. CPP was considered absent in all remaining subjects. Levels of phospho-proteins were compared between the two groups by t-test. As follow-up, in the group expressing a CPP, the relationship between phospho-protein levels and time spent on the cocaine-paired side was assessed by calculating the Pearson product moment coefficient.
Histology
Upon completion of all behavioral testing, rats in Experiments 1 and 2 were euthanized with CO2 and decapitated. Brains were removed and fixed in 10% buffered formalin for at least 48 hr. Frozen coronal sections, 40 m thick, were cut on a Reichert-Jung Cryostat, thaw-mounted on gelatin-coated glass slides and stained with cresyl violet. Electrode placements and injection sites were determined by visual inspection of sections under an Olympus SZ40 microscope. In order for a rat’s behavioral data to be included in the analysis, cannula placements in Experiment 1 were required to be judged as bilaterally accurate within the NAc medial shell (with some unilaterally placed on the shell/core and shell/olfactory tubercle borders included).
Drugs
D-amphetamine (Sigma-Aldrich) was dissolved in sterile 0.9% saline and microinjected bilaterally in a dose of 0.0, 5.0, and 12.5 μg. Cocaine HCl (NIDA via Research Triangle Institute, Research Triangle Park, NC) was dissolved in sterile 0.9% saline. In Experiment 2 cocaine was microinjected in a dose of 0.0, 50.0, and 100.0 μg. In Experiment 3 the conditioning dose of cocaine was 12.0 mg/kg (i.p.).
Experiment 1 Intra-NAc shell microinjection of d-amphetamine in AM- and PM-fed FR rats
This experiment was based on our prior observation that d-amphetamine microinjected in NAc shell exerted a markedly greater rewarding effect in FR relative to ad libitum fed rats (Carr et al. 2009). This result recapitulated the effect of systemically and intracerebroventricularly administered d-amphetamine (Cabeza de Vaca and Carr, 1998). The virtue of central administration is that possible effects of diet or recency of intake on peripheral drug handling are superseded and treatment effects can be attributed to changes in sensitivity of a neural substrate. Experiment 1 was methodologically similar to the previous experiment with the exception that all subjects were FR; however, 13 were AM-fed and 11 were PM-fed. Test sessions were conducted 3-4 days apart and dose order was counterbalanced and matched between groups
Experiment 2 Intracerebroventricular microinjection of cocaine in AM- and PM-fed FR rats
Direct microinjection of cocaine into brain tissue can produce local anesthetic effects that obscure the psychostimulant effect (e.g., Gong et al., 1996). This experiment was therefore based on our prior observation that cocaine microinjected into the lateral ventricle produced a dose-related rewarding effect that was markedly greater in FR relative to ad libitum fed rats (Carr et al. 2000). Given that Experiment 3 examined behavioral responsiveness of subjects to a cocaine-paired environment, it was of interest to determine whether time of feeding differentially alters the modulatory effect of FR on the primary reward stimulus vs a conditioned stimulus paired with that reward. Experiment 2 was methodologically similar to the previous experiment with the exception that all subjects were FR; however, 8 were AM-fed and 8 were PM-fed. Test sessions were conducted 3-4 days apart and dose order was counterbalanced and matched between groups.
Experiment 3
-
Persistence of cocaine CPP expression in AM- and PM-fed FR rats
This experiment was based on our prior observation that when a cocaine CPP was induced in ad libitum fed rats and half were switched to FR for 3 weeks prior to resumption of testing, CPP extinguished by the 5th test in rats that remained ad libitum fed, while a robust CPP persisted in rats that had been switched to FR (Zheng et al. 2012). Experiment 3 was methodologically similar to the previous experiment with the exception that all subjects were switched to FR after the first CPP test; however, 13 were AM-fed and 13 were PM-fed. Subjects were tested in 15 sessions, conducted once per day, over a period of 17 days.
-
Biochemical correlates of CPP expression in NAc shell and core of FR rats
This experiment was conducted based on our prior observations that administration of a D-1 DA agonist (SKF-82958) produced markedly greater phosphorylation of ERK 1/2 and Ser845-GluA1 in NAc of FR relative to ad libitum fed rats, correlating with the enhanced rewarding effect of the drug in the FR group (Carr et al. 2010; Haberny et al. 2004). Further, GluA1 in NAc shell has been implicated in the enhanced rewarding effect of D-1 DA agonist in FR rats (Carr et al. 2010). The purpose of Experiment 3b was to test the prediction that persistent expression of a CPP by FR rats is accompanied by increases in these NAc biochemical responses. Immediately following the final CPP test, subjects were sacrificed and brains were harvested.
Results
Experiment 1 Intra-NAc shell microinjection of d-amphetamine
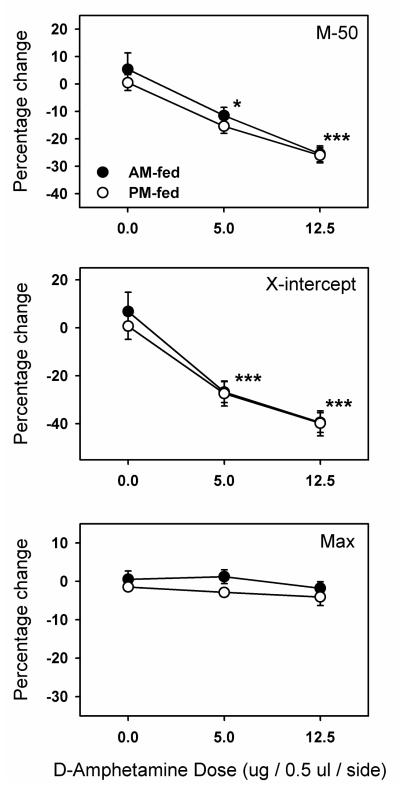
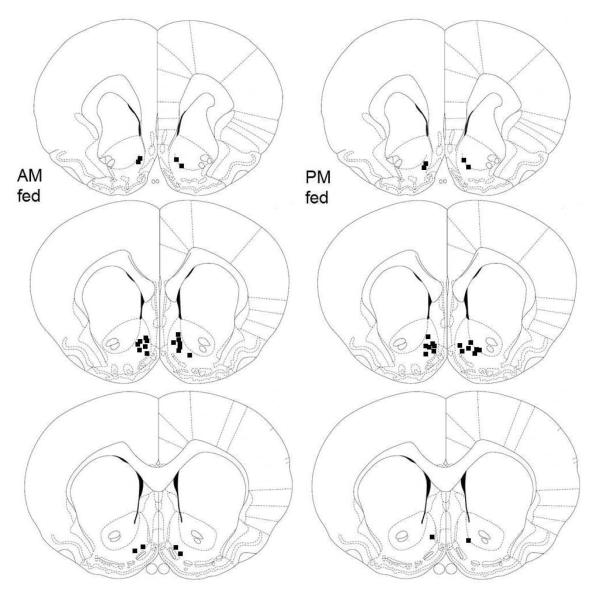
Confirming prior observations, d-amphetamine microinjected bilaterally in NAc shell produced a left shift in the ICSS rate-frequency curve as indicated by a lowering of both the M-50 and x-axis intercept measures of reward threshold (Figure 1). Both measures reflected a significant dose effect (M-50: F2,44=38.1, p <.0001; x-intercept: F2,44=47.6, p <.0001), but no effect of time of feeding, and no interaction between dose and time of feeding. There were no treatment-induced changes in maximum reinforcement rate. Microinjections sites were confirmed, histologically, to lie within the NAc medial shell or its border with the lateral shell, core or olfactory tubercle (Figure 2).
Figure 1. Intra-NAc shell microinjection of d-amphetamine in AM and PM fed rats.
D-amphetamine was microinjected bilaterally in the NAc shell of AM-fed (filled circles) and PM-fed (open circles) food-restricted rats. Shown are mean ±SEM percent change in two measures of reward threshold, M-50 (top panel) and X-intercept (middle panel), as a function of dose. Percent change in the maximum reinforcement rate (bottom panel) is also shown. *p<0.05, ***p<0.001 compared to the 0.0 (vehicle) dose.
Figure 2. NAc microinjection sites in AM and PM fed rats.
Schematic representation of bilateral NAc microinjection sites in AM fed (left) and PM fed (right) food-restricted rats. Adapted from the atlas of Paxinos and Watson (1997).
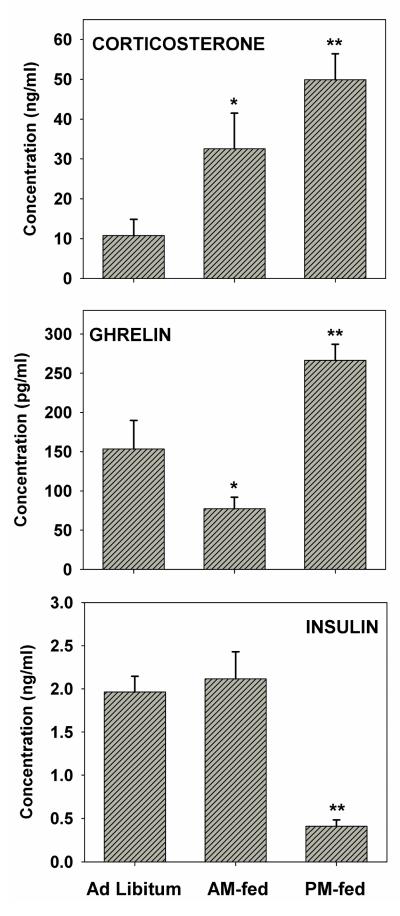
Trunk blood taken several days after the final behavioral test, but at the time of day when behavioral testing had been conducted, revealed that corticosterone varied among feeding conditions (F2,12=8.3, p <.005) with elevated levels in both AM-fed (p<.05) and PM-fed (p<.01) relative to age-matched ad libitum fed controls (Figure 3). However, the difference between AM-fed and PM-fed rats was not significant. In contrast, ghrelin levels varied among feeding conditions (F2,15=13.9, p<.001) with levels in AM-fed subjects lower than in controls (p<.05) and PM-fed subjects (p<.01), and levels in PM-fed subjects higher than in AM-fed and control subjects (p<.01). Insulin levels varied among feeding conditions (F2,15=19.6, p<.001) with levels in PM-fed subjects markedly lower than in AM-fed subjects and controls (p<.01) but no difference between AM-fed subjects and controls.
Figure 3. Hormone levels at the time of day when subjects underwent behavioral testing.
Plasma levels of corticosterone (top), ghrelin (middle), and insulin (bottom). Corticosterone: *p<0.05, **p<0.01 relative to ad libitum fed; Ghrelin: *p<0.05, relative to ad libitum fed, **p<0.01 relative to AM-fed and ad libitum fed; Insulin: **p<0.01 relative to AM-fed and ad libitum fed.
Experiment 2 Intracerebroventricular injection of cocaine
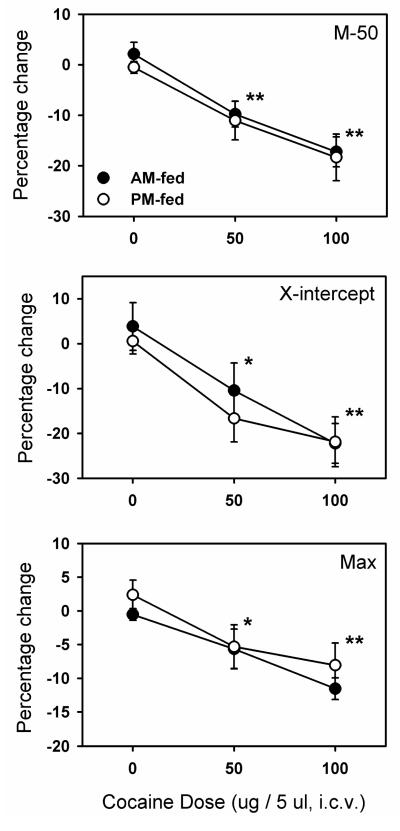
Confirming prior observations, cocaine microinjected into the lateral ventricle produced a left shift in the ICSS rate-frequency curve as indicated by a lowering of both the M-50 and x-axis intercept measures of reward threshold (Figure 4). Both measures reflected a significant dose effect (M-50: F2,28=26.1, p <.001; x-intercept: F2,28=13.0, p <.001), but no effect of time of feeding and no interaction between dose and time of feeding. There was also a main effect of dose on maximum reinforcement rate (F2,28=14.3, p <.001), with no effect of feeding or interaction between dose and time of feeding. The small but significant decrease in maximum reinforcement rate, not seen in Experiment 1 where d-amphetamine was microinjected into NAc shell, may reflect competition between lever pressing and hyperactivity resulting from diffusion of intraventricular cocaine into multiple striatal regions.
Figure 4. Intracerebroventricular microinjection of cocaine in AM and PM fed rats.
Cocaine was microinjected into the lateral ventricle of AM-fed (filled circles) and PM-fed (open circles) food-restricted rats. Shown are mean ±SEM percent change in two measures of reward threshold, M-50 (top panel) and X-intercept (middle panel), and maximum reinforcement rate (bottom panel) as a function of dose. *p<0.05, ***p<0.001 compared to the 0.0 (vehicle) dose.
Experiment 3 Cocaine conditioned place preference
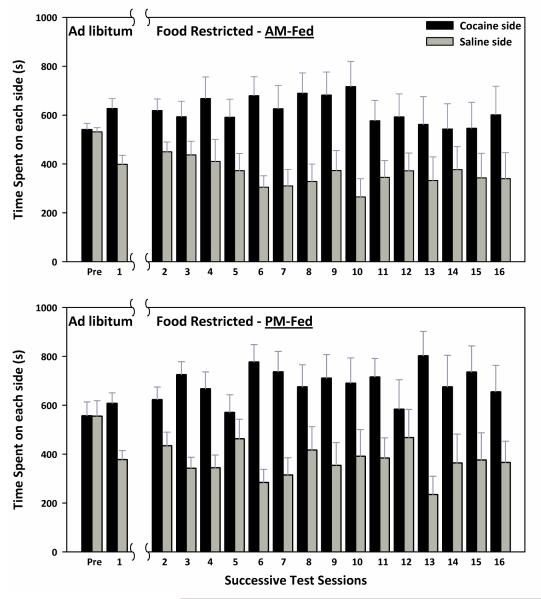
On CPP test 1, after conditioning and prior to diet manipulation, the 26 rats displayed a strong preference for the cocaine-paired side of the chamber (t(25)=3.81, p<.001). Using the results of test 1, two groups were matched on CPP expression and side of chamber associated with cocaine. The resulting groups displayed CPP (AM-fed: t(12)=2.62, p<.025; PM-fed: t(12)=2.66, p<.025). Beginning three weeks following the switch to FR, CPP was expressed across the next 15 test sessions as indicated by a main effect of side (F1,22=13.3, p <.001), with no effect of time of feeding, no interaction between time of feeding and side, no interaction between time of feeding and day, and no interaction between time of feeding, side, and day (Figure 5).
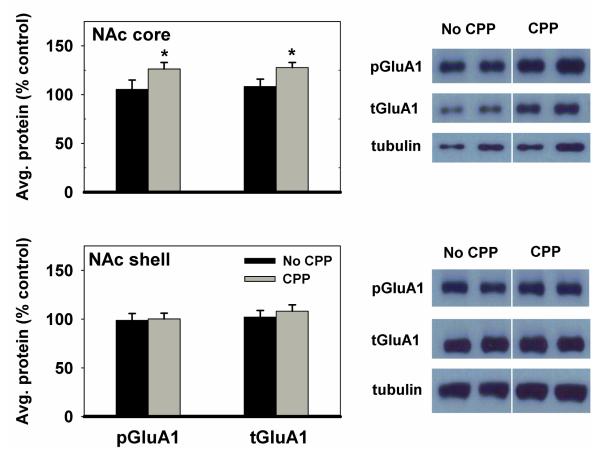
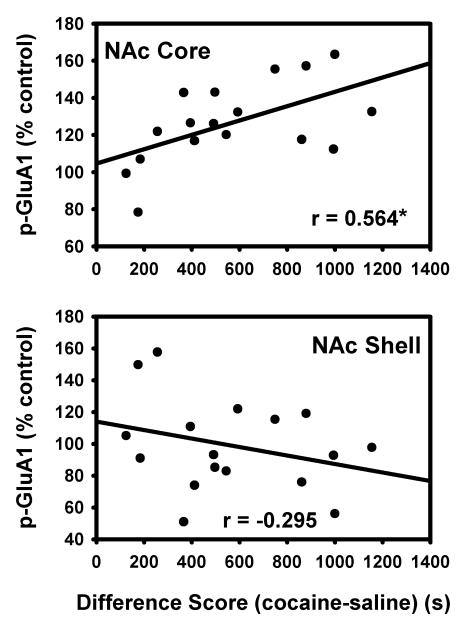
Because the two feeding groups (AM and PM) did not differ in CPP expression, their biochemical data were combined during Western analysis. Immediately following the final CPP test, animals were sacrificed and brains were harvested for Western blot analysis. In the NAc core, animals that displayed a CPP expressed higher levels of p-Ser845-GluA1 (t(24)1-tailed=2.2, p<0.025) and total GluA1 (tGluA1) (t(24)1-tailed=1.9, p<0.05) than animals that did not display a CPP. Animals that displayed a CPP and those that did not display a CPP expressed similar levels of p-Ser845-GluA1 and tGluA1 in the NAc shell (Figure 6). Additionally, a regression analysis of the individual subjects’ difference scores (time spent in the cocaine-paired compartment – time spent in the saline-paired compartment) showed a correlation with the average phosphorylation on Ser845 of GluA1 in the NAc core (r=0.564, F(1,15)=7.0, p<.05). The weak negative correlation between difference scores and phosphorylation of GluA1 in the shell was not significant (Figure 7). Inspection of CPP performance of subjects categorized as not displaying CPP on the day of brain harvesting revealed that eight of the nine subjects could be assigned to one of two profiles: (i) those with a consistent aversion for the cocaine-paired side, including the final test, and (ii) those with a weak or inconsistent preference for the cocaine-paired side, throughout. Values for p-Ser845-GluA1, relative to the mean of the group, for those in the first category were 92, 121, 124 and 146%. Values for those in the second category were 57, 61, 75 and 114%. These limited data suggest that expression of a learned response, whether it be preference for the cocaine- or saline-paired side, may be associated with increased phosphorylation of GluA1 in NAc core. This possibility warrants further investigation and militates against inclusion of data from these animals in the correlational analysis depicted in Figure 7.
Figure 5. CPP expression in AM and PM fed rats.
Rats were all ad libitum (AL) fed during the pre-conditioning session (pre) and conditioning with a 12.0 mg/kg (i.p.) dose of cocaine. After the first test session (1), all rats were food-restricted for three weeks. Testing then resumed daily for fifteen days (2-16). Mean (±SEM) time spent (s) on the cocaine- and saline-paired sides of the CPP apparatus during each test session are shown for AM fed (top) and PM fed (bottom) rats. CPP was expressed across sessions (p <.001), with no effect of time of feeding, and no interactions between factors.
Figure 6. p-Ser845-GluA1 and tGluA1 levels in the nucleus accumbens (NAc) of CPP vs. non-CPP expressing rats.
Results obtained in Nac core (top) and shell (bottom) (mean ±SEM) for subjects expressing CPP in the 16th test session are expressed in comparison to the normalized control, which is defined as the non-CPP expressing group. Data from AM and PM fed groups were combined because of the lack of behavioral difference between groups. *p<0.05
Figure 7. Correlation between difference scores of CPP-expressing rats and p-Ser845-GluA1 in the nucleus accumbens.
Magnitude of CPP expression, measured as time spent in the cocaine-paired side - time spent in the saline-paired side (difference score), is correlated with average phosphorylation of Ser845-GluA1 in the NAc core (top) and shell (bottom) of subjects that expressed a CPP. *p<.05
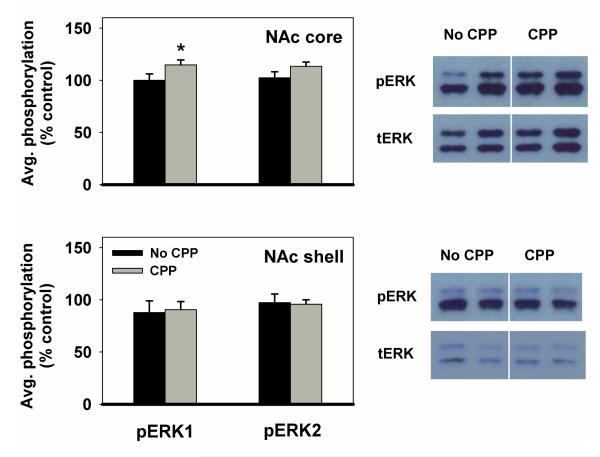
ERK1 and ERK2 were analyzed separately because the bands corresponding to them on the immunoblot were well-separated, and the two isoforms may be differentially involved in regulating cocaine effects (Ferguson et al. 2006). In the NAc core, animals that displayed a CPP expressed higher levels of pERK1 than animals that did not display CPP (t(24)1-tailed=1.7, p<0.05). pERK2 displayed a trend in the same direction as pERK1. In the NAc shell, there was no difference between groups in the amount of pERK1 or pERK2 expressed (Figure 8).
Figure 8. pERK1 and pERK2 levels in the nucleus accumbens of CPP vs. non-CPP expressing rats.
Results obtained in NAc core (top) and shell (bottom) (mean ±SEM) are expressed in comparison to the normalized control, which is defined as the non-CPP expressing group. Data from AM and PM fed groups were combined because of the lack of behavioral difference between groups. *p<0.05
Discussion
The purpose of this study was to determine whether the rewarding effects of d-amphetamine, cocaine, and persistence of cocaine CPP are dependent upon the relationship between time of testing and time of feeding in FR rats. It was reasoned that if neurobehavioral and hormonal rhythms entrained by time of feeding contribute to the enhanced responsiveness of FR subjects to drugs and a drug-paired environment, then results obtained in the pre- and postprandial test periods would differ. In Experiment 1, the reward-potentiating effect of d-amphetamine microinjected into NAc shell was assessed in an ICSS protocol. In Experiment 2, the reward-potentiating effect of cocaine microinjected into the lateral ventricle was similarly assessed. Results from both experiments indicate that although there are dramatic differences in the levels of plasma insulin and ghrelin in groups tested pre-(PM-fed) and postprandially (AM-fed), there are no differences between groups in the reward magnitude of d-amphetamine and cocaine. In Experiment 3a, preference for a cocaine-paired environment was tested daily. Both the AM- and PM-fed groups displayed a persistent CPP that did not extinguish within 15 test sessions. This contrasts with CPP expression of animals conditioned in an identical fashion but maintained on ad libitum feeding throughout; ad libitum fed subjects displayed extinction of CPP by the 5th test session while subjects switched to FR persisted as in the present study (Zheng et al., 2012). The persistent CPP in FR subjects does not appear to result from impairment of extinction learning because a similar experiment was conducted examining conditioned place aversion induced by LiCl and opposite results were obtained; animals switched to FR displayed extinction of the CPA within several sessions while ad libitum fed rats persisted (Zheng and Carr, unpublished). It is therefore proposed that the persistent cocaine CPP in FR rats reflects an enhanced sensitivity to the incentive-motivating effect of cocaine-paired contextual stimuli, paralleling the enhanced sensitivity to cocaine itself. In Experiment 3b, NAc biochemical correlates of cocaine CPP expression were assessed. Subjects that displayed a CPP had higher levels of GluA1, p-Ser845-GluA1 and pERK1 in the NAc core than those that did not display a CPP. In addition, a strong positive correlation was observed between CPP expression and p-Ser845-GluA1 in the NAc core. Thus, the different levels of insulin and ghrelin associated with time of testing confirm that the two groups of FR subjects were in significantly different physiological states, but results point to stable incentive effects of psychostimulants and a psychostimulant-paired context, and suggest stability of neuroadaptations that underlie the enhancing effects of FR.
A behavioral phenomenon that provided rationale for evaluating responsiveness of FR subjects to drugs and a drug-paired environment in the pre- and postprandial periods is food anticipatory activity (Mistlberger 2011). As one component of the entrainment of circadian rhythms by time of feeding, FR rats fed a single scheduled meal each day display hyperactivity during the several hours preceding the meal. This behavior is accompanied by neural activation of nucleus accumbens core and is decreased by excitotoxic lesions of this structure (Angeles-Castellanos et al. 2007; Mendoza et al. 2005). Further, anticipatory hyperactivity correlates with rising plasma ghrelin levels (Verhagen et al. 2011) and is decreased by ghrelin receptor knock out (Blum et al. 2009; Verhagen et al. 2011). Taken together, the known involvement of NAc in drug reward and CPP expression, evidence of homology between locomotor activation and psychostimulant reward (Wise and Bozarth 1987), and the enhancing effect of peripheral ghrelin injection on cocaine-induced behavior (Davis et al. 2007; Wellman et al. 2008), established plausibility of the hypothesis that behavioral effects measured in the present study would be greater in the pre- than in the postprandial period. Yet, rewarding effects of d-amphetamine and cocaine, and persistence of cocaine-CPP did not differ between the AM-fed and PM-fed groups. There are numerous potential explanations of the discrepant effects of acute bolus injection of ghrelin in ad libitum fed rats and the preprandial ghrelin surge in FR rats. The most obvious is the use of different behavioral paradigms in the present and previous studies; effects of ghrelin on psychostimulant reward in the ICSS protocol have not been examined, nor have effects on expression of a previously acquired CPP. As another possibility, the facilitatory effect of ghrelin on ventral tegmental dopamine neuronal firing rate, mediated via the cholinergic link from laterodorsal tegmental nucleus (Jerlhag et al., 2012), may be blunted in FR rats which display decreased dopamine neuronal excitability (Pan et al., 2011). It is also possible that pre- and postsynaptic neuroadaptations in NAc of chronically FR rats, affecting both dopamine and glutamate transmission (e.g., Carr et al., 2003; Haberny et al., 2004; Zhen et al., 2006; Stamp et al., 2008; Carr et al., 2010), overshadow any modulatory effect of fluctuating ghrelin levels. Another consideration is that the sustained increase in average circulating levels in FR subjects may induce central ghrelin resistance as has been observed in human patients with anorexia nervosa (Miljic et al., 2006).
The increased levels of p-ERK1, GluA1 and p-Ser845-GluA1 in NAc core of FR subjects that continued to express CPP after 15 test sessions, relative to those that no longer did, provide potential clues to neuroadaptations that mediate the enhancing effect of FR on incentive effects of psychostimulants and associated environments. Previously, it was observed that D-1 dopamine receptor stimulation with SKF-82958 induced markedly greater phosphorylation of ERK 1/2 (Haberny et al. 2004) and Ser845-GluA1 (Carr et al. 2010) in NAc of FR relative to ad libitum fed rats. The behavioral significance of increased drug-induced NAc ERK phosphorylation in FR rats was assessed by injecting inhibitors of the kinase upstream of ERK, systemically or directly into NAc. MEK inhibitor treatments had no effect on the rewarding or locomotor-activating effects of SKF-82958 or d-amphetamine but did block SKF-82958-induced phosphorylation of ERK, CREB, and c-fos expression (Carr et al. 2009; Haberny et al. 2004), suggesting involvement in synaptic plasticity. In light of evidence that NAc core ERK 1/2 phosphorylation is necessary for the acquisition, expression, and reconsolidation of cocaine CPP (Miller and Marshall 2005), it is possible that the enhanced phosphorylation of ERK observed in response to D-1 agonist administration in FR rats extends to physiological D-1 DA receptor stimulation during exposure to a cocaine-paired environment and thereby contributes to the persistent cocaine CPP in FR rats.
It was also previously observed that, in addition to SKF-82958, cocaine (Liu et al. 2011) induced greater phosphorylation of Ser845-GluA1 in NAc of FR relative to ad libitum fed rats, that synaptic abundance of GluA1 is greater in NAc of FR than ad libitum fed rats (Peng et al. 2011), and that a GluA1 antagonist microinjected into NAc selectively reversed the enhanced rewarding effect of SKF-82958 in FR rats (Carr et al. 2010). GluA1 phosphorylation on Ser845 increases channel open probability, protects the receptor from lysosomal degradation, and facilitates synaptic insertion (Ehlers et al. 2007; Esteban et al. 2003; He et al. 2009; Man et al. 2007; Oh et al. 2006). The idea that increased excitability of D-1 DA receptor-expressing medium spiny neurons in NAc, resulting from increased phosphorylation and synaptic incorporation of GluA1, would enhance reward is consistent with recent evidence obtained using optogenetic stimulation in D1- and D2-Cre BAC transgenic mice (Lobo et al. 2010). Consequently, the increased levels of GluA1 protein, and correlation between p-Ser845-GluA1 and CPP may reflect a key neuroadaptation induced by FR that contributes to the enhanced behavioral responsiveness of subjects to psychostimulants and associated environments.
The absence of dynamic variation in behavioral responsiveness to drugs and drug-paired context in relation to time of feeding and hormone levels does not rule out a role for average changes in hormone levels over the course of FR in development of underlying neuroadaptations. For example, hypoinsulinemia would be expected to decrease expression and function of the ventral tegmental somatodendritic dopamine transporter (Daws et al. 2011), elevate local extracellular DA concentrations and dampen firing, leading to compensatory upregulation of postsynaptic receptor signaling in NAc, as has been observed (Carr 2007). Another example, supported by previous evidence, concerns elevated corticosterone. Corticosterone is generally elevated during FR (Marinkovic et al. 2007; Sharpe et al. 2012; Stamp et al. 2008), has been shown to correlate with and/or be required for enhanced psychostimulant-induced hyperactivity in FR rats, and increases psychostimulant-induced extracellular DA in NAc (Barrot et al. 2000; Deroche et al. 1995). In addition, corticosterone increases the synaptic delivery of AMPA receptors (Groc et al., 2008; Krugers et al. 2010; Yuen et al., 2011). Although adrenalectomy was previously found to have no effect on cocaine reward in the ICSS paradigm, this was not studied in FR rats (Abrahamsen and Carr 1997). In light of the known association between corticosterone and drug sensitivity in FR subjects, and recent evidence of AMPA receptor trafficking regulation by corticosterone, it will be of interest to investigate linkage between plasma corticosterone, AMPA receptors in NAc, and the enhanced responsiveness of FR rats to psychostimulant drugs and environmental contexts they have been paired with.
Acknowledgments
This research was supported by DA03956 from NIDA/NIH.
References
- Abrahamsen GC, Carr KD. Effect of adrenalectomy on cocaine facilitation of lateral hypothalamic self-stimulation. Brain Res. 1997;755:156–61. doi: 10.1016/s0006-8993(97)00187-x. [DOI] [PubMed] [Google Scholar]
- Ambroggi F, Turiault M, Milet A, Deroche-Gamonet V, Parnaudeau S, Balado E, Barik J, van der Veen R, Maroteaux G, Lemberger T, Schutz G, Lazar M, Marinelli M, Piazza PV, Tronche F. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat Neurosci. 2009;12:247–9. doi: 10.1038/nn.2282. [DOI] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Mendoza J, Escobar C. Restricted feeding schedules phase shift daily rhythms of c-Fos and protein Per1 immunoreactivity in corticolimbic regions in rats. Neuroscience. 2007;144:344–55. doi: 10.1016/j.neuroscience.2006.08.064. [DOI] [PubMed] [Google Scholar]
- Austin SB, Gortmaker SL. Dieting and smoking initiation in early adolescent girls and boys: a prospective study. Am J Public Health. 2001;91:446–50. doi: 10.2105/ajph.91.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird TJ, Gauvin D. Characterization of cocaine self-administration and pharmacokinetics as a function of time of day in the rat. Pharmacol Biochem Behav. 2000;65:289–99. doi: 10.1016/s0091-3057(99)00207-5. [DOI] [PubMed] [Google Scholar]
- Barrot M, Marinelli M, Abrous DN, Rouge-Pont F, Le Moal M, Piazza PV. The dopaminergic hyper-responsiveness of the shell of the nucleus accumbens is hormone-dependent. Eur J Neurosci. 2000;12:973–9. doi: 10.1046/j.1460-9568.2000.00996.x. [DOI] [PubMed] [Google Scholar]
- Bell SM, Stewart RB, Thompson SC, Meisch RA. Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacology (Berl) 1997;131:1–8. doi: 10.1007/s002130050258. [DOI] [PubMed] [Google Scholar]
- Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL, Abizaid A. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience. 2009;164:351–9. doi: 10.1016/j.neuroscience.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18:7502–10. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD. Chronic food restriction: enhancing effects on drug reward and striatal cell signaling. Physiol Behav. 2007;91:459–72. doi: 10.1016/j.physbeh.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Carr KD. Food scarcity, neuroadaptations, and the pathogenic potential of dieting in an unnatural ecology: binge eating and drug abuse. Physiol Behav. 2011;104:162–7. doi: 10.1016/j.physbeh.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Cabeza de Vaca S, Sun Y, Chau LS. Reward-potentiating effects of D-1 dopamine receptor agonist and AMPAR GluR1 antagonist in nucleus accumbens shell and their modulation by food restriction. Psychopharmacology (Berl) 2009;202:731–43. doi: 10.1007/s00213-008-1355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Chau LS, Cabeza de Vaca S, Gustafson K, Stouffer M, Tukey DS, Restituito S, Ziff EB. AMPA receptor subunit GluR1 downstream of D-1 dopamine receptor stimulation in nucleus accumbens shell mediates increased drug reward magnitude in food-restricted rats. Neuroscience. 2010;165:1074–86. doi: 10.1016/j.neuroscience.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Kim GY, Cabeza de Vaca S. Chronic food restriction in rats augments the central rewarding effect of cocaine and the delta1 opioid agonist, DPDPE, but not the delta2 agonist, deltorphin-II. Psychopharmacology (Berl) 2000;152:200–7. doi: 10.1007/s002130000523. [DOI] [PubMed] [Google Scholar]
- Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience. 2003;119:1157–67. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Meisch RA. Increased drug-reinforced behavior due to food-deprivation. Advances in Behavioral Pharmacology. 1984;4:47–88. [Google Scholar]
- Cheskin LJ, Hess JM, Henningfield J, Gorelick DA. Calorie restriction increases cigarette use in adult smokers. Psychopharmacology (Berl) 2005;179:430–6. doi: 10.1007/s00213-004-2037-x. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Stephan FK. Plasma glucagon, glucose, insulin, and motilin in rats anticipating daily meals. Physiol Behav. 1999;66:309–15. doi: 10.1016/s0031-9384(98)00308-4. [DOI] [PubMed] [Google Scholar]
- Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ, Benoit SC. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci. 2008;122:1257–63. doi: 10.1037/a0013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KW, Wellman PJ, Clifford PS. Augmented cocaine conditioned place preference in rats pretreated with systemic ghrelin. Regul Pept. 2007;140:148–52. doi: 10.1016/j.regpep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Avison MJ, Robertson SD, Niswender KD, Galli A, Saunders C. Insulin signaling and addiction. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Maccari S, Le Moal M, Simon H, Piazza PV. Stress-induced sensitization and glucocorticoids. I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci. 1995;15:7181–8. doi: 10.1523/JNEUROSCI.15-11-07181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Munoz M, Vazquez-Martinez O, Aguilar-Roblero R, Escobar C. Anticipatory changes in liver metabolism and entrainment of insulin, glucagon, and corticosterone in food-restricted rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2048–56. doi: 10.1152/ajpregu.2000.279.6.R2048. [DOI] [PubMed] [Google Scholar]
- Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E. The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol. 2011;340:80–7. doi: 10.1016/j.mce.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 2006;147:23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–60. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar C, Diaz-Munoz M, Encinas F, Aguilar-Roblero R. Persistence of metabolic rhythmicity during fasting and its entrainment by restricted feeding schedules in rats. Am J Physiol. 1998;274:R1309–16. doi: 10.1152/ajpregu.1998.274.5.R1309. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–43. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology. 2006;31:2660–8. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- French SA, Perry CL, Leon GR, Fulkerson JA. Weight concerns, dieting behavior, and smoking initiation among adolescents: a prospective study. Am J Public Health. 1994;84:1818–20. doi: 10.2105/ajph.84.11.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W, Neill D, Justice JB., Jr Conditioned place preference and locomotor activation produced by injection of psychostimulants into ventral pallidum. Brain Res. 1996;707:64–74. doi: 10.1016/0006-8993(95)01222-2. [DOI] [PubMed] [Google Scholar]
- Groc L, Choquet D, Chaouloff F. The stress hormone corticosterone conditions AMPAR surface trafficking and synaptic potentiation. Nat Neurosci. 2008;11:868–70. doi: 10.1038/nn.2150. [DOI] [PubMed] [Google Scholar]
- Haberny SL, Berman Y, Meller E, Carr KD. Chronic food restriction increases D-1 dopamine receptor agonist-induced phosphorylation of extracellular signal-regulated kinase 1/2 and cyclic AMP response element-binding protein in caudate-putamen and nucleus accumbens. Neuroscience. 2004;125:289–98. doi: 10.1016/j.neuroscience.2004.01.037. [DOI] [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci U S A. 2009;106:20033–8. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Janson A, Waters S, Engel JA. Concomitant release of ventral tegmental acetylcholine and accumbal dopamine by ghrelin in rats. PLoS One. 2012;7:e49557. doi: 10.1371/journal.pone.0049557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn D, Kurth C, Demitrack M, Drewnowski A. The relationship of dieting severity and bulimic behaviors to alcohol and other drug use in young women. J Subst Abuse. 1992;4:341–53. doi: 10.1016/0899-3289(92)90041-u. [DOI] [PubMed] [Google Scholar]
- Krieger DT. Food and water restriction shifts corticosterone, temperature, activity and brain amine periodicity. Endocrinology. 1974;95:1195–201. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- Krugers HJ, Hoogenraad CC, Groc L. Stress hormones and AMPA receptor trafficking in synaptic plasticity and memory. Nat Rev Neurosci. 2010;11:675–81. doi: 10.1038/nrn2913. [DOI] [PubMed] [Google Scholar]
- Liu S, Zheng D, Peng XX, Cabeza de Vaca S, Carr KD. Enhanced cocaine-conditioned place preference and associated brain regional levels of BDNF, p-ERK1/2 and p-Ser845-GluA1 in food-restricted rats. Brain Res. 2011;1400:31–41. doi: 10.1016/j.brainres.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–90. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci U S A. 2007;104:3579–84. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic P, Pesic V, Loncarevic N, Smiljanic K, Kanazir S, Ruzdijic S. Behavioral and biochemical effects of various food-restriction regimens in the rats. Physiol Behav. 2007;92:492–9. doi: 10.1016/j.physbeh.2007.04.023. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, Escobar C. Entrainment by a palatable meal induces food-anticipatory activity and c-Fos expression in reward-related areas of the brain. Neuroscience. 2005;133:293–303. doi: 10.1016/j.neuroscience.2005.01.064. [DOI] [PubMed] [Google Scholar]
- Miljic D, Pekic S, Djurovic M, Doknic M, Milic N, Casanueva FF, Ghatei M, Popovic V. ghrelin has partial or no effect on appetite, growth hormone, prolactin, and cortisol release in patients with anorexia nervosa. J Clin Endocrinol Metab. 91:1491–95. doi: 10.1210/jc.2005-2304. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–84. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Neurobiology of food anticipatory circadian rhythms. Physiol Behav. 2011;104:535–45. doi: 10.1016/j.physbeh.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–8. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Pan Y, Chau L, Liu S, Avshalumov MV, Rice ME, Carr KD. A food restriction protocol that increases drug reward decreases tropomyosin receptor kinase B in the ventral tegmental area, with no effect on brain-derived neurotrophic factor or tropomyosin receptor kinase B protein levels in dopaminergic forebrain regions. Neuroscience. 2011;197:330–8. doi: 10.1016/j.neuroscience.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3rd edition Academic Press; San Diego: 1997. [Google Scholar]
- Peng XX, Ziff EB, Carr KD. Effects of food restriction and sucrose intake on synaptic delivery of AMPA receptors in nucleus accumbens. Synapse. 2011 doi: 10.1002/syn.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisetsky EM, Chao YM, Dierker LC, May AM, Striegel-Moore RH. Disordered eating and substance use in high-school students: results from the Youth Risk Behavior Surveillance System. Int J Eat Disord. 2008;41:464–70. doi: 10.1002/eat.20520. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Creese I, Hoebel BG. Restricted eating with weight loss selectively decreases extracellular dopamine in the nucleus accumbens and alters dopamine response to amphetamine, morphine, and food intake. J Neurosci. 1995;15:6640–50. doi: 10.1523/JNEUROSCI.15-10-06640.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root TL, Pinheiro AP, Thornton L, Strober M, Fernandez-Aranda F, Brandt H, Crawford S, Fichter MM, Halmi KA, Johnson C, Kaplan AS, Klump KL, La Via M, Mitchell J, Woodside DB, Rotondo A, Berrettini WH, Kaye WH, Bulik CM. Substance use disorders in women with anorexia nervosa. Int J Eat Disord. 2010;43:14–21. doi: 10.1002/eat.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosse R, Deutsch S, Chilton M. Cocaine addicts prone to cocaine-induced psychosis have lower body mass index than cocaine addicts resistant to cocaine-induced psychosis--Implications for the cocaine model of psychosis proneness. Isr J Psychiatry Relat Sci. 2005;42:45–50. [PubMed] [Google Scholar]
- Seo DC, Jiang N. Associations between smoking and extreme dieting among adolescents. J Youth Adolesc. 2009;38:1364–73. doi: 10.1007/s10964-009-9421-0. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Klaus JD, Beckstead MJ. Meal schedule influences food restriction-induced locomotor sensitization to methamphetamine. Psychopharmacology (Berl) 2012;219:795–803. doi: 10.1007/s00213-011-2401-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, Kessler RC. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–30. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamp JA, Mashoodh R, van Kampen JM, Robertson HA. Food restriction enhances peak corticosterone levels, cocaine-induced locomotor activity, and DeltaFosB expression in the nucleus accumbens of the rat. Brain Res. 2008;1204:94–101. doi: 10.1016/j.brainres.2008.02.019. [DOI] [PubMed] [Google Scholar]
- Verhagen LA, Egecioglu E, Luijendijk MC, Hillebrand JJ, Adan RA, Dickson SL. Acute and chronic suppression of the central ghrelin signaling system reveals a role in food anticipatory activity. Eur Neuropsychopharmacol. 2011;21:384–92. doi: 10.1016/j.euroneuro.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Warren M, Frost-Pineda K, Gold M. Body mass index and marijuana use. J Addict Dis. 2005;24:95–100. doi: 10.1300/J069v24n03_08. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Hollas CN, Elliott AE. Systemic ghrelin sensitizes cocaine-induced hyperlocomotion in rats. Regul Pept. 2008;146:33–7. doi: 10.1016/j.regpep.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman PJ, Nation JR, Davis KW. Impairment of acquisition of cocaine self-administration in rats maintained on a high-fat diet. Pharmacol Biochem Behav. 2007;88:89–93. doi: 10.1016/j.pbb.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederman MW, Pryor T. Substance use and impulsive behaviors among adolescents with eating disorders. Addict Behav. 1996;21:269–72. doi: 10.1016/0306-4603(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–92. [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011;16:156–70. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen J, Reith ME, Carr KD. Chronic food restriction and dopamine transporter function in rat striatum. Brain Res. 2006;1082:98–101. doi: 10.1016/j.brainres.2006.01.094. [DOI] [PubMed] [Google Scholar]
- Zheng D, Cabeza de Vaca S, Carr KD. Food restriction increases acquisition, persistence and drug prime-induced expression of a cocaine-conditioned place preference in rats. Pharmacol Biochem Behav. 2012;100:538–44. doi: 10.1016/j.pbb.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]