Abstract
Rationale
The use and misuse of prescription opiates in adolescent populations, and in particular, adolescent female populations, has increased dramatically in the past two decades. Given the significant role that opioids play in neuroendocrine function, exposure to opiates during this critical developmental period could have significant consequences for the female, as well as her offspring.
Objectives
In the current set of studies, we utilized the female rat to model the transgenerational impact of adolescent opiate exposure.
Methods
We examined locomotor sensitization in response to the dopamine D2/D3 receptor agonist quinpirole in the adult male progeny (F1 and F2 generation) of females exposed to morphine during adolescence. All females were drug-free for at least 3 weeks prior to conception, eliminating the possibility of direct fetal exposure to morphine.
Results
Both F1 and F2 progeny of morphine-exposed females demonstrated attenuated locomotor sensitization following repeated quinpirole administration. These behavioral effects were coupled with increased quinpirole-induced corticosterone secretion, and up-regulated kappa opioid receptor (KOR) and dopamine D2 receptor (D2R) gene expression within the NAc.
Conclusions
These results suggest significant modifications in response to repeated D2R activation in the progeny of females exposed to opiates during adolescence. Given the significant role that the D2R plays in psychopathology, adolescent opiate exposure could shift the vulnerability of future offspring to psychological disorders, including addiction. Moreover, that effects are also observed in the F2 generation suggests that adolescent opiate exposure can trigger transgenerational epigenetic modifications impacting systems critical for motivated behavior.
Keywords: Opiate, transgenerational, epigenetics, quinpirole, sensitization, kappa receptor, drug abuse, dopamine
Introduction
Recent surveys indicate that the use of prescription narcotics has dramatically increased in adolescent populations over the past decade (SAMHSA 2011). Unlike adult use patterns, which typically demonstrate higher drug use in males (Becker and Hu 2008; Fattore et al. 2008), misuse of prescription drugs during adolescence is similar in girls and boys (Frese and Eiden 2011; SAMHSA 2011). Some studies even suggest higher rates of opiate use in young females (Frese and Eiden 2011). Thus, adolescent girls now represent a population increasingly at risk for exposure to opiates (Frese and Eiden 2011). Because adolescence is a period of significant neural and endocrine maturation, it represents a distinct developmental period which may be particularly vulnerable to the long-term effects of opiates. Few studies, however, have examined the consequences of opiate exposure during this period that may be unique to the female user, including effects that may extend to future generations.
Previous studies utilizing the rat to model the long-term effects of female adolescent exposure to the prototypical opiate morphine, observed significant effects of prior drug exposure in both the exposed female (Byrnes 2005a; 2008) as well as in her future offspring (Byrnes 2005b; Byrnes et al. 2011; Johnson et al. 2011). These offspring effects were not due to fetal exposure to morphine as all animals were abstinent for at least 3 weeks prior to mating. Thus, transgenerational effects of morphine exposure occurring prior to mating were observed, suggesting that maternal drug history can influence future offspring development. Interestingly, the male offspring of morphine exposed mothers (MORF1) demonstrated enhanced locomotor sensitization in response to repeated morphine when compared to the male offspring of saline exposed mother's (SALF1), while similar effects were not observed in F1 females (Byrnes 2005b). It is unclear whether the effects on locomotor sensitization in these offspring are specific to the effects of morphine or whether similar effects might be observed in response to other drugs.
In addition to morphine-induced locomotor sensitization, locomotor sensitization in response to a number of direct and indirect dopamine agonists has been well-documented (Steketee and Kalivas 2011; Vanderschuren and Kalivas 2000; Wise and Leeb 1993). Locomotor sensitization in response to the D2/D3 dopamine receptor agonist, quinpirole, is one such model (Szumlinski et al. 2000; Wise and Leeb 1993). The advantage of the quinpirole-induced model of locomotor sensitization is that the effects are due in part to activation of post-synaptic D2/D3 receptors in the nucleus accumbens (NAc) (Carpenter et al. 2003; Koeltzow et al. 2003). Thus, by examining locomotor sensitization in response to repeated quinpirole, we can examine transgenerational effects on processes mediated by D2-like receptors in the NAc. As activation of D2/D3 activity in the NAc stimulates corticosterone secretion (Ikemoto and Goeders 1998), plasma corticosterone levels can provide an additional readout for changes in NAc function in the offspring of opiate-exposed adolescent females.
While differences in MORF1 and SALF1 males observed in previous studies suggest that maternal drug history, even in the absence of in utero exposure, can alter the development of future offspring, it is possible that direct effects of adolescent morphine exposure on gametes may play a role in this phenomenon. Thus, any observed effects in the F1 generation may not be considered purely transgenerational. Therefore, the current set of studies examined the effects in both F1 and F2 generations. Specifically, locomotor responses to acute and repeated quinpirole treatment, and associated corticosterone secretion, were studied in F1 and F2 males derived from females exposed to morphine during adolescence. In addition, expression of kappa opioid receptor (KOR) and D2 dopamine receptor (D2R) mRNA within the NAc were also studied, as both of these receptor subtypes have been implicated in the development of quinpirole-induced locomotor sensitization (Perreault et al. 2006; Perreault et al. 2007a; Perreault et al. 2007b; Rowlett et al. 1995). Overall, the current findings demonstrate significant multigenerational effects of female adolescent morphine exposure which appear to impact both stress- and reward-related processes mediated by the NAc.
Materials and Methods
Animals
Female Sprague-Dawley rats [Crl:CD(SD)BR] were purchased from Charles River Breeding Laboratories at 22 days of age. Animals were housed 3-4 per cage in light-(700-1900h; all testing conducting during light phase) and temperature-(21-240 C) controlled rooms, and provided with food and water ad libitum.
Adolescent Morphine Exposure
Beginning at 30 days of age, females were injected daily (s.c.) with morphine sulfate (15 mg/ml, Injectable; Butler-Schein Animal Health, Dublin, OH) for a total of 10 days using an increasing dosing regimen. Thus, the drug was administered every other day, with volumes adjusted to yield increasing dosages for each treatment (i.e. 5, 10, 15, 20, 25 mg/kg). Age-matched control animals received saline injections (s.c.) with volumes adjusted to match those of drug-treated animals.
Mating
Between 3-4 weeks after their final injection, adolescent-exposed females (F0) were mated with males from our breeding colony. On postnatal day 1 (PND1) litters were culled to ten pups (5 males: 5 females). Litters were weaned on PND21. Male offspring from these first litters were not used in the current study; however, the female offspring were used to generate F2 offspring (i.e. adult F1 females mated with unrelated colony males). Three weeks after their first litter was weaned, SALF0 and MORF0 females were re-mated. These second litters were also culled to 10 (5 males: 5 females) on PND1 and weaned on PND21. Male offspring were housed with their siblings, and were used for study as adults. Thus, all of the F1 data presented below were obtained from the second litters of SALF0 and MORF0, which will herein be referred to as SALF1 and MORF1, respectively. All F2 animals were generated from first litter SALF1 and MORF1 females mated to drug-free males and will herein be referred to as SALF2 and MORF2, respectively. Three separate adolescent exposure cohorts were used in the current set of studies with a total of 44 litters represented (21 MOR litters; 23 SAL litters). All F1 and F2 animals were tested as adults and were run in age-matched groups. At no point in the study were differences in offspring bodyweight as a function of maternal adolescent exposure observed (all animals were weighed PND1, PND21 and throughout adult behavioral testing). In all of the reported findings, only 1-2 offspring per litter were used in any experimental condition.
Acute Response to Quinpirole
Adult F1 and F2 males were administered quinpirole (0.5 mg/kg, sc) or its 0.9% saline vehicle (1 ml/kg, sc). Animals were immediately placed in automated infrared activity chambers (SmartFrames®, Kinder Scientific, Poway, CA) and locomotor behavior (beam breaks) was monitored for 90 minutes. Animals were then euthanized using brief CO2 (<30 sec) followed by rapid decapitation. Trunk blood was collected (and processed to plasma), and brains flash- frozen in -20°C methylbutane. Plasma was stored at -20°C prior to processing for corticosterone; brains were stored at -80°C prior to preparation for gene expression analysis.
Quinpirole Sensitization
Adult F1 and F2 males were administered with either quinpirole (0.5 mg/kg, sc) or saline and their locomotor behavior was monitored for 90 minutes as described above. Animals were then returned to their home cage. This procedure was repeated every 3-4 days for a total of 8 exposures. At the end of 8th exposure, animals were euthanized, with blood and brains collected and stored as described above.
Corticosterone Analysis
Plasma corticosterone was determined using a standard radioimmunoassay (Corticosterone Coat-a-Count, Seimens, CA). All samples were run in duplicate.
Quantitative PCR
Brains were cryostat-mounted, and bilateral tissue punches (1 mm3) were taken from the NAc. Total RNA was extracted using RNeasy (Qiagen) followed by cDNA conversion using RETROscript®. Real time PCR was performed on an ABI Prism 7700 (Applied Biosystems, Foster City, CA). Taqman® primers were purchased from Applied Biosystems (Dopamine D2 receptor - Rn00561126_m1; Opioid Kappa receptor - Rn01448892_m1; S16 – Rn01476520_g1). Final quantification of mRNA was obtained using the comparative cycle threshold (CT) method (Pfaffl 2001).
Statistical Analyses
Acute response to quinpirole was analyzed with a three-way repeated measures Analysis of Variance (ANOVA), with time post-injection interval as the within-subject factor, and maternal history (i.e. adolescent MOR or SAL) and drug treatment (saline or quinpirole) as the between subject factors. Quinpirole sensitization was analyzed using three-way repeated measures ANOVA with day of exposure as the within-subject factor, and maternal history and drug treatment as the between-subject factors. Both corticosterone and NAc gene expression were examined using two-way ANOVA's with maternal history and drug treatment as the factors. Significant main effects were followed by post hoc analyses using the Tukey's Test and significance was defined as p < 0.05.
Results
Locomotor Response Following Acute Quinpirole
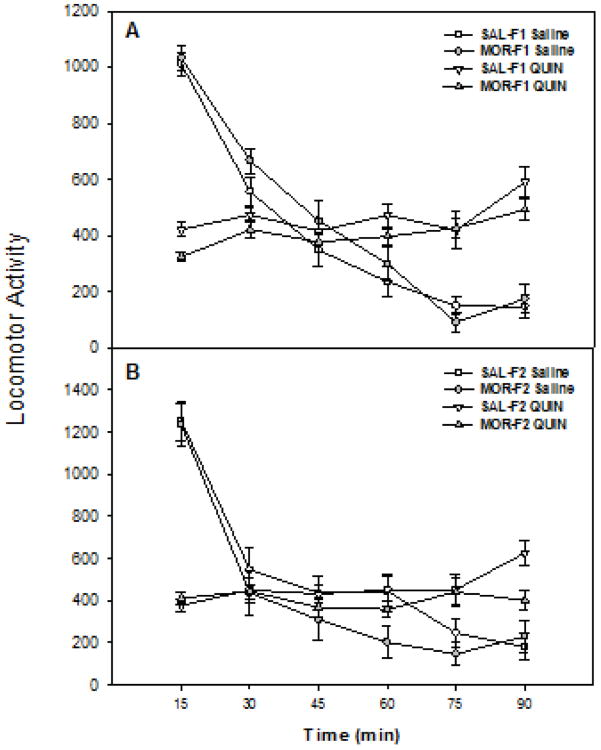
The acute effects of quinpirole on locomotor activity in F1 males are shown in Figure 1 (panel A). There was a significant main effect of time (F[5,255] = 68.5, p < 0.001) and a drug × time interaction (F[5,255] = 101.9, p < 0.001). However, there was no time × maternal history (p=0.62) or time × drug × maternal history interaction (p=0.27). Due to the biphasic nature of the quinpirole, there was also no main effect of drug (p=.8). Moreover, there was no maternal history effect (p=0.8) and no drug × maternal history interaction (p = 0.09). Similar effects were observed in the F2 generation (Figure 1, panels B). Thus, in F2 females, there was a significant main effect of time (F[5,215] = 43.9, p < 0.001) and a drug × time interaction (F[5,215] = 31.6, p < 0.001), but no time × maternal history (p=.38) and no time × drug × maternal history interaction (p=0.1). No other main effects or interactions were observed.
Figure 1.
Upper Panel: Acute locomotor effects of quinpirole in F1 male offspring of females exposed to morphine or saline during adolescence. Adult F1 males were tested for locomotor activity following treatment with quinpirole (0.5 mg/kg, sc) or its 0.9% saline vehicle. Data are mean (± SEM) locomotor activity (beam breaks) over 90 min of testing for groups of 6-10 animals (p<0.001 for main effects of time and a drug × time interaction). Lower Panel: Acute locomotor effects of quinpirole in F2 male offspring of females exposed to morphine or saline during adolescence. Adult F2 males were tested for locomotor activity following treatment with quinpirole (0.5 mg/kg, sc) or its 0.9% saline vehicle. Data are mean (± SEM) locomotor activity (beam breaks) for groups of 9-14 animals (p<0.001 for main effects of time and a drug × time interaction).
Locomotor Response Following Repeated Quinpirole
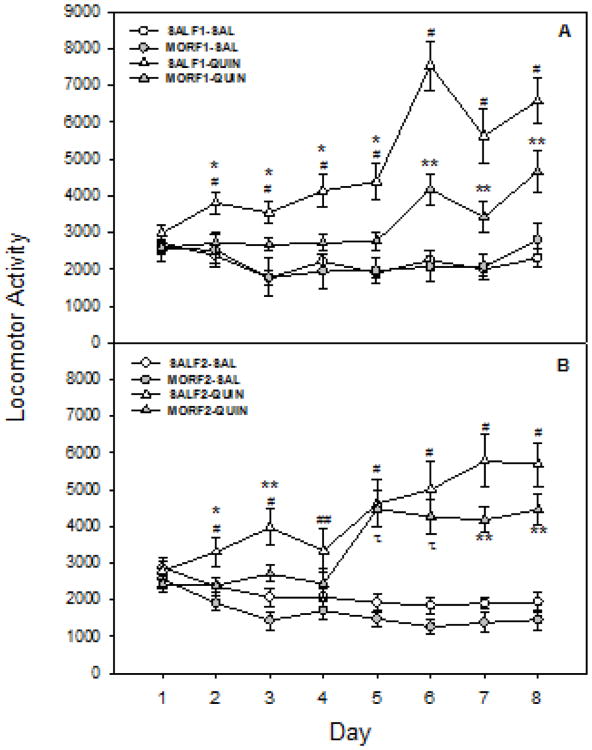
Effects of maternal history emerged following repeated quinpirole treatment. Moreover, these effects were similar in both the F1 and F2 generation. Data from the F1 generation (Figure 2, panel A) demonstrate that that the increased locomotor response to quinpirole over the 8 days of exposure is attenuated in MOR-F1 males. Statistically, there was a significant main effect quinpirole exposure day (F[7,203] = 22.7, p < 0.001), a drug × day interaction (F[7,203] = 20.5, p < 0.001), a maternal history × day interaction (F[7,203] = 3.2, p < 0.01), and a drug × maternal history × day interaction (F[7,203] = 3.3, p < 0.01). There were also significant main effects of drug (F[1,25] = 37.2, p < 0.001) and maternal history (F[1,25] = 7.1, p < 0.02), as well as a drug × maternal history interaction (F[1,25] = 7.5, p < 0.02). Post hoc analyses indicate that there were no differences between SALF1 and MORF1 in response to repeated saline, but that significant differences in response to repeated quinpirole were observed as early as the second day of exposure. In F1 males, quinpirole-treated animals had increased locomotor activity when compared to saline-treated animals; however, these effects developed more slowly in MORF1 males and overall were attenuated when compared to SALF1 animals.
Figure 2.
Locomotor effects of repeated quinpirole in F1 and F2 male offspring of females exposed to morphine or saline during adolescence. Adult males were tested for locomotor activity following daily treatment with quinpirole (0.5 mg/kg, sc) or its 0.9% saline vehicle. Data represent mean locomotor activity (beam breaks/90 min) ± SEM across 8 days. Upper Panel: Locomotor activity in F1 males (n = 6-10/group). Lower Panel: Locomotor in F2 males (n = 9-14). * vs SAL-quin; **p<0.05 vs SAL-sal and MOR-sal; *** vs all other groups: all p's < 0.05.
Significant differences based on adolescent exposure were also observed in F2 animals (Figure 2; panel B), and once again differences in quinpirole responding between SALF2 and MORF2 were observed as early as the second exposure. There was a significant main effect of day of exposure (F[7,301] = 11.2, p < 0.001) and a drug × day interaction (F[7,301] = 32.9, p < 0.001), although there was no maternal history × day interaction (p=0.2) or drug × maternal history × day interaction (p = 0.5). However, there were significant main effects of both drug (F[1,39] = 41.9, p < 0.001) and maternal history (F[1,39] = 5.0, p < 0.05), with no drug × maternal history interaction (p = 0.5). As observed in the F1 generation, post hoc analyses indicate that no differences were observed between SALF2 and MORF2 groups at any time after saline treatment. Repeated quinpirole treatment increased locomotor activity in both SALF2 and MORF2 males, however, the effects were dampened in MORF2 males. As in the F1 generation, these differences in the response to quinpirole were observed as early as exposure day 2. Thus, in both generations, all quinpirole-treated animals demonstrated locomotor sensitization by day 8, but the magnitude of the effect was diminished in the F1 offspring of female exposed to morphine during adolescent development and continued to be muted in the F2 generation as well.
Corticosterone Secretion Following Acute and Repeated Quinpirole
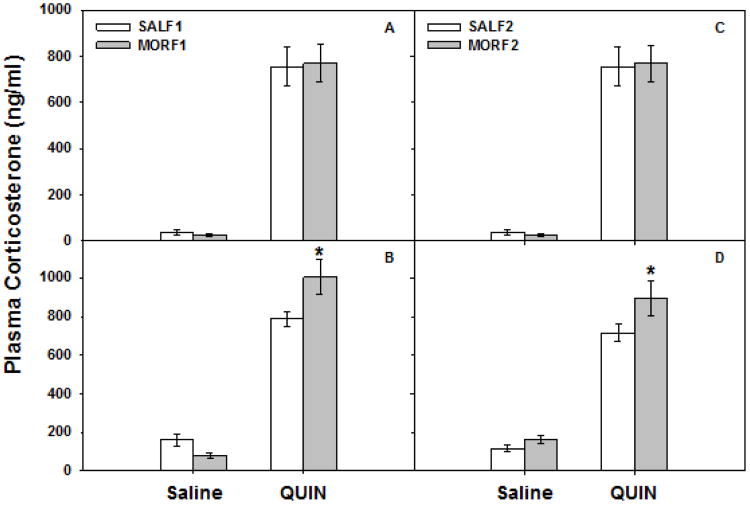
As measured immediately after testing on exposure day 1, quinpirole-treatment significantly increased circulating corticosterone. As shown in Figure 3 (panel A), both SALF1 and MORF1 animals had a robust response to quinpirole, with a main effect of drug (F[1,18] =152.9, p < 0.001), but no effect of maternal history (p=0.98), and no drug × maternal history interaction (p=0.83). However, after 8 days quinpirole treatment, significant differences between SALF1 and MORF1 were observed. As illustrated in Figure 3 (panel B), there continued to be a main effect of drug (F[1,29] =213.5, p < 0.001). In addition, while there was no main effect of maternal history (p=0.22), there was a significant drug × maternal history interaction (F[1,29] =8.1, p < 0.01). Post hoc analyses indicate that on exposure day 8, quinpirole-treated MORF1 males demonstrated a more robust corticosterone response than quinpirole-treated SALF1 males (p<0.01). No differences were observed between saline-treated groups.
Figure 3.
Panels A and B: Plasma corticosterone in F1 offspring of females exposed to morphine during adolescence. Adult male F1 offspring were treated acutely (once) or repeatedly (8 times) with quinpirole (0.5 mg/kg, sc) or its 0.9% saline vehicle. Corticosterone was determined in plasma collected 90 min after the acute (panel A) or repeated (panel B) treatment. Data are corticosterone (ng/ml) ± SEM for groups of 5-10 animals. Panels C and D. Plasma corticosterone in F2 offspring of females exposed to morphine during adolescence. Adult male F2 offspring were treated acutely (once) or repeatedly (8 times) with quinpirole (0.5 mg/kg, sc) or its 0.9% saline vehicle. Corticosterone was determined in plasma collected 90 min after the acute (panel C) or repeated (panel D) treatments. Data are corticosterone (ng/ml) ± SEM for groups of 5-7 animals. *p<0.05 vs SAL-quin.
Similar, although less robust differences in corticosterone secretion were observed in the F2 generation, as displayed in Figure 3 (panels C and D). Once again, following their initial exposure to quinpirole, both SALF1 and MORF1 males had significantly increased levels of corticosterone when compared to saline-treated groups (main effect of drug (F[1,19] =93.9, p < 0.001; see Figure 3, panel C). As in the F1 generation, no main effects of maternal history (p=0.46) or drug × maternal history interaction (p=0.94) were observed. However, on exposure day 8 (Figure 3, panel D), a similar augmentation of quinpirole-induced corticosterone secretion was observed in MORF2 animals. Statistically, there were main effects of drug (F[1,20] =145.05, p < 0.001) and maternal history (F[1,20] =5.6, p < 0.05), although there was no drug × maternal history interaction (p=0.2). Based on a priori predictions derived from the results of our F1 studies, we conducted post hoc analyses on day 8 corticosterone secretion. Once again, significant differences in corticosterone secretion between SALF2 and MORF2 animals exposed to repeated quinpirole were observed (p < 0.05), while no differences were detected in saline-treated animals (p=0.34). Collectively, these results suggest that differences in activation of the HPA axis in response to repeated quinpirole exposure can be discerned in both the F1 and F2 generation as a function of maternal exposure to morphine during adolescence. Thus, MORF1 males secrete significantly more corticosterone following repeated exposure to quinpirole than their SALF1 counterparts and a similar, although less robust effect can be discerned in the F2 generation as well.
KOR and D2 Gene Expression in NAc Following Acute or Repeated Quinpirole
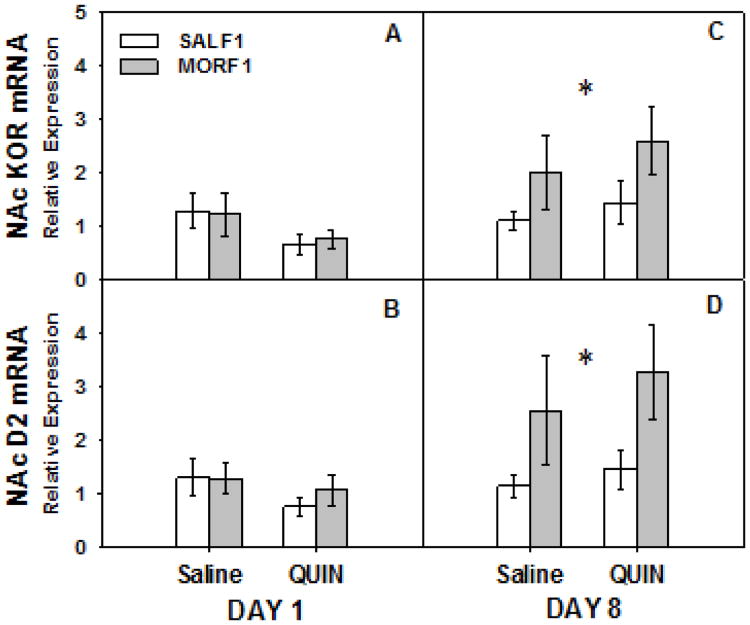
Differences in the expression of both KOR and D2R mRNA in the NAc were examined in F1 animals sacrificed after their initial exposure to either saline or quinpirole. These data are illustrated in Figure 4 (panels A and B). For KOR expression, while there was a modest trend for a main effect of drug (F[1,18]= 3.97, p = 0.07), no main effect of maternal history (p=0.94), and no drug by maternal history interaction (p=0.78). For D2R expression, no main effects (both p's > 0.2) or interaction (p=0.59) were observed. Thus, on the first day of exposure, there were no discernible differences in KOR or D2R mRNA expression in the NAc between SALF1 and MORF1 males. However, after 8 days of treatment, maternal history effects on NAc KOR and D2R mRNA were observed (Figure 4, panels C and D). Statistically, there was a main effect of maternal history on both KOR (F[1,27]= 4.5, p = 0.04) and D2R (F[1,27]= 6.2, p = 0.02) expression, which was due to a significant increase in KOR and D2R gene expression in both saline- and quinpirole-treated MORF1 males. No main effects of drug (both p's > 0.4) or interactions (both p's > 0.8) were observed.
Figure 4.
NAc KOR and D2 mRNA following acute (Panels A and B) and repeated (panels C and D) quinpirole treatment in F1 offspring of females exposed to morphine during adolescence. Adult male F1 offspring were treated acutely (once) or repeatedly (8 times) with quinpirole (0.5 mg/kg, sc) or its 0.9% saline vehicle. The NAc was collected and prepared for quantitative PCR 90 min after the acute or repeated treatments. Data are relative expression of NAc KOR (panels A and C) and D2 mRNA (Panels B and D) for groups of 5-9 animals. *p < 0.05 main effect of maternal drug history.
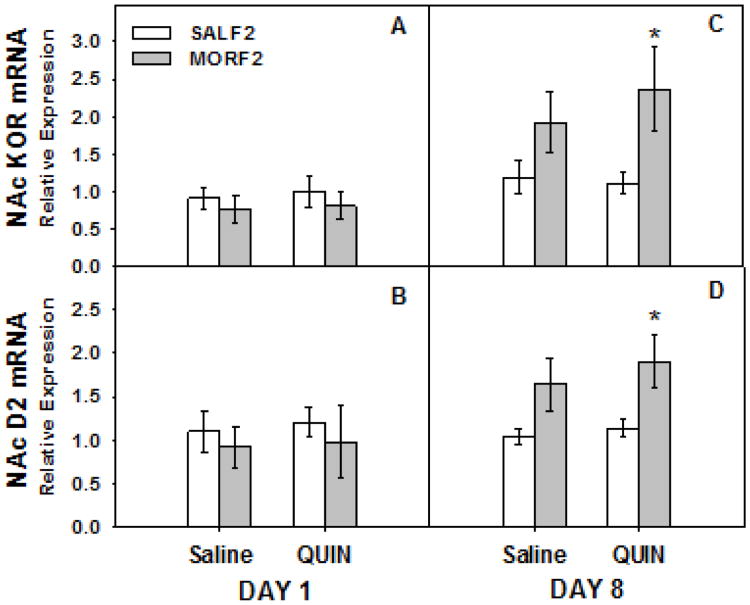
Similar effects were found in the F2 generation. These data are illustrated in Figure 5. Once again, on the first day of treatment (Figure 5; panels A and B), no significant effects drug (all p's >0.05) were observed. On day 8 (Figure 5; panels C and D), there were significant main effects of maternal history on both KOR (F[1,43]= 5.9, p = 0.02) and D2R (F[1,43]= 8.6, p < 0.01) expression. As in F1 males, these effects were due to a significant increase in KOR and D2R mRNA expression in both saline- and quinpirole-treated MORF2 animals. Again, no main effects of drug (both p's > 0.4) or drug × maternal history interactions (both p's > 0.5) were observed.
Figure 5.
NAc KOR and D2 mRNA following acute and repeated quinpirole treatment in F2 offspring of females exposed to morphine during adolescence. Adult male F2 offspring were treated acutely (once) or repeatedly (8 times) with quinpirole (0.5 mg/kg, sc) or its 0.9% saline vehicle. The NAc was collected and prepared for quantitative PCR 90 min after the acute or repeated treatments. Data are relative expression of NAc KOR (panels A and C) and D2 mRNA (panels B and D) for groups of 6-14 animals. *p<0.05 main effect of maternal drug history.
Discussion
The current findings demonstrate multigenerational consequences of adolescent morphine exposure in systems regulating reward and stress. Differences in sensitization to the locomotor effects of the D2/D3 agonist quinpirole as well as the effects of repeated quinpirole administration on corticosterone secretion were observed in the first generation (MORF1 as compared to SALF1), with similar effects also observed in the second generation (MORF2). Increased expression of KOR and D2 mRNA within the NAc was also observed following quinpirole sensitization in both MORF1 and MORF2 males. Overall, these data demonstrate functional neuroadaptations within the NAc that are transmitted across two generations following adolescent exposure to opiates. As alterations in mesoaccumbal dopamine are implicated in numerous psychopathological conditions (e.g. mood disorders, addiction), these findings may have broad implications regarding the role of prior maternal drug use (i.e. use occurring prior to conception), on the developmental trajectory of future generations.
The choice of the D2/D3 agonist quinpirole to assess changes in NAc D2-receptor function was based on the unique properties of this direct agonist. Acutely, the biphasic locomotor response to quinpirole administration allows for the examination of two distinct D2 receptor-mediated effects that are primarily regulated within the NAc. First, the initial hypoactivity observed in response to quinpirole administration is due to activation of release-regulating presynaptic D2 receptors (i.e. autoreceptors), which leads to decreased dopamine release in the NAc (de Haas et al. 2011). Indeed, there is evidence that following repeated quinpirole administration these D2 autoreceptors become subsensitive, attenuating the effects of quinpirole on dopamine release and thereby eliminating this initial phase of hypoactivity (de Haas et al. 2011). In contrast, the subsequent locomotor activating effects observed in response to acute quinpirole are due to direct activation of postsynaptic D2 receptors in the NAc (Dreher and Jackson 1989; Phillips et al. 1995; Wu et al. 1993). Thus, locomotor activity following acute quinpirole provides insight into differences in the sensitivity of both pre- and postsynaptic NAc D2 receptors. In addition to effects on locomotor activity, systemic quinpirole increases corticosterone secretion, an effect mediated predominantly by D2 receptors in the NAc (Ikemoto and Goeders 1998). Thus, examination of quinpirole-induced corticosterone secretion provides a preliminary assessment of the interplay between outputs from the NAc and the regulation of the stress axis.
No significant effects of maternal drug history were observed when offspring (F1 or F2) were examined on day 1 of treatment. In contrast, significant effects of maternal drug history on all dependent measures were observed following repeated quinpirole administration. Females exposed to morphine during adolescent development produced male offspring with attenuated locomotor sensitization in response to quinpirole, an effect that emerged as soon as the second day of drug administration and persisted throughout testing. The locomotor response to saline administration, however, was similar between SALF1 and MORF1 animals, with all animals demonstrating a reduction in locomotor activity across days.
Contrary to the blunted locomotor sensitization, quinpirole-stimulated corticosterone secretion was increased in MORF1 animals compared to their SALF1 counterparts on the final day of testing. The interaction between the HPA axis and locomotor sensitization has been explored in a number of studies (de Jong et al. 2009; Ortiz et al. 1995; Prasad et al. 1996; Schmidt et al. 1999). Locomotor sensitization to psychostimulants is enhanced when corticosterone levels are increased, an effect that appears to be mediated by glucocorticoid receptors within the NAc shell (Marinelli and Piazza 2002; Steketee et al. 1992). However, quinpirole sensitization is not enhanced by glucocorticoids, with hypophysectomy having no significant effect on quinpirole locomotor sensitization (Culver and Szechtman 2004). Indeed, significant reductions in locomotor activity in response to quinpirole are observed in the presence of the synthetic glucocorticoid dexamethasone (Wrobel et al. 2005). Thus, differential activation of the HPA axis and subsequent activation of glucocorticoid receptors within the NAc in MORF1 males could play a role in the attenuated expression of locomotor sensitization observed in response to quinpirole. Overall, our behavioral and corticosterone measures indicate that there is an alteration in limbic regulation of the HPA axis in the male offspring of females exposed to morphine during adolescence. Moreover, these effects continue to be observed in the F2 generation as well.
When examined after the final drug exposure, a significant increase in the expression of both KOR and D2R mRNA was observed in the NAc of MORF1 animals. While the effects were more robust in quinpirole-treated MORF1 animals, a tendency toward similar effects was also observed in saline-treated MORF1 males. Activation of KOR in the NAc inhibits D1R-mediated activity (Steiner and Gerfen 1996). Of relevance to the current findings, increased D1R activity is a key mechanism underlying the development and expression of locomotor sensitization (Henry et al. 1998; Hummel and Unterwald 2002; Taepavarapruk et al. 2000). Thus, compensatory changes in KOR expression could negatively modulate D1R-mediated output and thereby decrease the locomotor response to repeated quinpirole administration in MORF1 animals.
SALF1 sensitized animals did not express increased D2R mRNA within the NAc when compared to saline controls. These results are in agreement with other studies in the literature that have failed to detect any alterations in D2R mRNA in quinpirole-sensitized animals (Perreault et al. 2007a). Increased D2R expression was observed in MORF1 animals. However, similar (though less robust) alterations appear to occur in saline-treated MORF1 animals. These results suggest the response to repeated testing may be modified in MORF1 animals. Such underlying modifications could then allow for the emergence of differential drug effects. In that regard, it should be noted that locomotor sensitization to quinpirole is context-dependent (Einat et al. 1996; Szechtman et al. 1993; Szumlinski et al. 1997). To what extent the differences in MORF1 animals reflects alterations in context-dependent learning, a process that itself involves adaptations within the NAc, remains to be determined.
The most notable aspect of these findings is the possibility of persisting effects in the F2 generation. Indeed, differences with regard to the behavioral, endocrine, and molecular changes in response to repeated quinpirole administration were observed in MORF2 males. These data decrease the likelihood that F1 effects simply reflect direct effects of morphine exposure on gametes present in the adolescent females during the exposure regimen. Moreover, these findings strongly suggest an epigenetic modification that is maintained across at least two generations. The mechanisms underlying such multigenerational effects remain undetermined, although a recent examination of maternal behavior in adolescent-morphine exposed females does not implicate deficient maternal care (Johnson et al. 2011). One possibility is a persistent modification in the prenatal environment impacting early neurodevelopment. Indeed, the endogenous opioid system plays a critical role in both early embryonic as well as later prenatal development (Meriney et al. 1991; Vernadakis et al. 1990; Zagon et al. 1997; Zagon et al. 1999). Such alterations in the prenatal environment may be induced by the exposure to morphine during adolescent development and then propagated by persistent modifications impacting the development of subsequent generations. Indeed, such multigenerational epigenetic effects in response to endocrine disruptors have been documented previously (Crews et al. 2007; Cummings et al. 2010; Ho and Burggren 2010), although the mechanism underlying forward transmission of these effects has yet to be elucidated.
To what extent these findings relate to patterns of substance use and mental health disorders observed in families is unknown. However, given the critical role that D2R function in the NAc plays in psychopathology and stress-related disorders, the current findings support the hypothesis that female adolescent opiate exposure may serve as a multigenerational trigger for neurodevelopmental alterations that increase the risk for mental health and/or stress-related disorders, both of which may increase vulnerability for substance abuse. Whether similar effects are observed when opiate exposure occurs in adulthood remains to be determined. It is also unknown whether similar effects would be observed in female offspring. Our previous findings demonstrated enhanced locomotor sensitization in response to morphine in MORF1 males, but not MORF1 females. Future studies could be conducted to determine whether transgenerational alterations in quinpirole-induced locomotor sensitization demonstrate a similar sexually dimorphic profile. Finally, future studies will be needed to determine the mechanisms underlying multigenerational transmission of neural and endocrine adaptations induced by exposure to opiates. Given the significant increase in the use of prescription opiates, especially in younger, reproductively active populations, the current findings raise significant concerns regarding the potential long-term impact of these drugs on future generations.
Acknowledgments
This work was supported by NIH R01DA25674 (EMB).
Footnotes
All authors declare that, except for income received from our primary employer, no financial support or compensation has been received from any individual or corporate entity for this research or related professional service. There are no personal financial holdings that could be perceived as constituting a potential conflict of interest. The authors have full control of all primary data and that they agree to allow the journal to review their data if requested.
References
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes EM. Chronic morphine exposure during puberty decreases postpartum prolactin secretion in adult female rats. Pharmacol Biochem Behav. 2005a;80:445–51. doi: 10.1016/j.pbb.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Byrnes EM. Transgenerational consequences of adolescent morphine exposure in female rats: effects on anxiety-like behaviors and morphine sensitization in adult offspring. Psychopharmacology (Berl) 2005b;182:537–44. doi: 10.1007/s00213-005-0122-4. [DOI] [PubMed] [Google Scholar]
- Byrnes EM. Chronic morphine exposure during puberty induces long-lasting changes in opioid-related mRNA expression in the mediobasal hypothalamus. Brain Res. 2008;1190:186–92. doi: 10.1016/j.brainres.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Byrnes JJ, Babb JA, Scanlan VF, Byrnes EM. Adolescent opioid exposure in female rats: transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring. Behav Brain Res. 2011;218:200–5. doi: 10.1016/j.bbr.2010.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter TL, Pazdernik TL, Levant B. Differences in quinpirole-induced local cerebral glucose utilization between naive and sensitized rats. Brain Res. 2003;964:295–301. doi: 10.1016/s0006-8993(02)04115-x. [DOI] [PubMed] [Google Scholar]
- Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci U S A. 2007;104:5942–6. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver KE, Szechtman H. Hypophysectomy does not block sensitization to the dopamine agonist quinpirole or its modulation by the MAOI clorgyline. Horm Behav. 2004;45:23–30. doi: 10.1016/j.yhbeh.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Clemens LG, Nunez AA. Mother counts: how effects of environmental contaminants on maternal care could affect the offspring and future generations. Front Neuroendocrinol. 2010;31:440–51. doi: 10.1016/j.yfrne.2010.05.004. [DOI] [PubMed] [Google Scholar]
- de Haas R, Nijdam A, Westra TA, Kas MJ, Westenberg HG. Behavioral pattern analysis and dopamine release in quinpirole-induced repetitive behavior in rats. J Psychopharmacol. 2011;25:1712–9. doi: 10.1177/0269881110389093. [DOI] [PubMed] [Google Scholar]
- de Jong IE, Steenbergen PJ, de Kloet ER. Behavioral sensitization to cocaine: cooperation between glucocorticoids and epinephrine. Psychopharmacology (Berl) 2009;204:693–703. doi: 10.1007/s00213-009-1498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreher JK, Jackson DM. Role of D1 and D2 dopamine receptors in mediating locomotor activity elicited from the nucleus accumbens of rats. Brain Res. 1989;487:267–77. doi: 10.1016/0006-8993(89)90831-7. [DOI] [PubMed] [Google Scholar]
- Einat H, Einat D, Allan M, Talangbayan H, Tsafnat T, Szechtman H. Associational and nonassociational mechanisms in locomotor sensitization to the dopamine agonist quinpirole. Psychopharmacology (Berl) 1996;127:95–101. doi: 10.1007/BF02805980. [DOI] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W. Sex differences in drug addiction: a review of animal and human studies. Womens Health (Lond Engl) 2008;4:51–65. doi: 10.2217/17455057.4.1.51. [DOI] [PubMed] [Google Scholar]
- Frese WA, Eiden K. Opioids: nonmedical use and abuse in older children. Pediatr Rev. 2011;32:e44–52. doi: 10.1542/pir.32-4-e44. [DOI] [PubMed] [Google Scholar]
- Henry DJ, Hu XT, White FJ. Adaptations in the mesoaccumbens dopamine system resulting from repeated administration of dopamine D1 and D2 receptor-selective agonists: relevance to cocaine sensitization. Psychopharmacology (Berl) 1998;140:233–42. doi: 10.1007/s002130050762. [DOI] [PubMed] [Google Scholar]
- Ho DH, Burggren WW. Epigenetics and transgenerational transfer: a physiological perspective. J Exp Biol. 2010;213:3–16. doi: 10.1242/jeb.019752. [DOI] [PubMed] [Google Scholar]
- Hummel M, Unterwald EM. D1 dopamine receptor: a putative neurochemical and behavioral link to cocaine action. J Cell Physiol. 2002;191:17–27. doi: 10.1002/jcp.10078. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Goeders NE. Microinjections of dopamine agonists and cocaine elevate plasma corticosterone: dissociation effects among the ventral and dorsal striatum and medial prefrontal cortex. Brain Res. 1998;814:171–8. doi: 10.1016/s0006-8993(98)01070-1. [DOI] [PubMed] [Google Scholar]
- Johnson NL, Carini L, Schenk ME, Stewart M, Byrnes EM. Adolescent opiate exposure in the female rat induces subtle alterations in maternal care and transgenerational effects on play behavior. Front Psychiatry. 2011;2:29. doi: 10.3389/fpsyt.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeltzow TE, Austin JD, Vezina P. Behavioral sensitization to quinpirole is not associated with increased nucleus accumbens dopamine overflow. Neuropharmacology. 2003;44:102–10. doi: 10.1016/s0028-3908(02)00328-3. [DOI] [PubMed] [Google Scholar]
- Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–94. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- Meriney SD, Ford MJ, Oliva D, Pilar G. Endogenous opioids modulate neuronal survival in the developing avian ciliary ganglion. J Neurosci. 1991;11:3705–17. doi: 10.1523/JNEUROSCI.11-12-03705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, DeCaprio JL, Kosten TA, Nestler EJ. Strain-selective effects of corticosterone on locomotor sensitization to cocaine and on levels of tyrosine hydroxylase and glucocorticoid receptor in the ventral tegmental area. Neuroscience. 1995;67:383–97. doi: 10.1016/0306-4522(95)00018-e. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Graham D, Bisnaire L, Simms J, Hayton S, Szechtman H. Kappa-opioid agonist U69593 potentiates locomotor sensitization to the D2/D3 agonist quinpirole: pre-and postsynaptic mechanisms. Neuropsychopharmacology. 2006;31:1967–81. doi: 10.1038/sj.npp.1300938. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Graham D, Scattolon S, Wang Y, Szechtman H, Foster JA. Cotreatment with the kappa opioid agonist U69593 enhances locomotor sensitization to the D2/D3 dopamine agonist quinpirole and alters dopamine D2 receptor and prodynorphin mRNA expression in rats. Psychopharmacology (Berl) 2007a;194:485–96. doi: 10.1007/s00213-007-0855-3. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Seeman P, Szechtman H. Kappa-opioid receptor stimulation quickens pathogenesis of compulsive checking in the quinpirole sensitization model of obsessive-compulsive disorder (OCD) Behav Neurosci. 2007b;121:976–91. doi: 10.1037/0735-7044.121.5.976. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GD, Howes SR, Whitelaw RB, Robbins TW, Everitt BJ. Analysis of the effects of intra-accumbens SKF-38393 and LY-171555 upon the behavioural satiety sequence. Psychopharmacology (Berl) 1995;117:82–90. doi: 10.1007/BF02245102. [DOI] [PubMed] [Google Scholar]
- Prasad BM, Ulibarri C, Kalivas PW, Sorg BA. Effect of adrenalectomy on the initiation and expression of cocaine-induced sensitization. Psychopharmacology (Berl) 1996;125:265–73. doi: 10.1007/BF02247338. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Mattingly BA, Bardo MT. Repeated quinpirole treatment: locomotor activity, dopamine synthesis, and effects of selective dopamine antagonists. Synapse. 1995;20:209–16. doi: 10.1002/syn.890200304. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings, Substance Abuse and Mental Health Services Administration. NSDUH Series H-41 HHS Publication No (SMA) 2011:11–4658. [Google Scholar]
- Schmidt ED, Tilders FJ, Binnekade R, Schoffelmeer AN, De Vries TJ. Stressor- or drug-induced sensitization of the corticosterone response is not critically involved in the long-term expression of behavioural sensitization to amphetamine. Neuroscience. 1999;92:343–52. doi: 10.1016/s0306-4522(98)00725-8. [DOI] [PubMed] [Google Scholar]
- Steiner H, Gerfen CR. Dynorphin regulates D1 dopamine receptor-mediated responses in the striatum: relative contributions of pre- and postsynaptic mechanisms in dorsal and ventral striatum demonstrated by altered immediate-early gene induction. J Comp Neurol. 1996;376:530–41. doi: 10.1002/(SICI)1096-9861(19961223)376:4<530::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drugseeking behavior. Pharmacol Rev. 2011;63:348–65. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Sorg BA, Kalivas PW. The role of the nucleus accumbens in sensitization to drugs of abuse. Prog Neuropsychopharmacol Biol Psychiatry. 1992;16:237–46. doi: 10.1016/0278-5846(92)90075-p. [DOI] [PubMed] [Google Scholar]
- Szechtman H, Talangbayan H, Eilam D. Environmental and behavioral components of sensitization induced by the dopamine agonist quinpirole. Behav Pharmacol. 1993;4:405–410. [PubMed] [Google Scholar]
- Szumlinski KK, Allan M, Talangbayan H, Tracey A, Szechtman H. Locomotor sensitization to quinpirole: environment-modulated increase in efficacy and context-dependent increase in potency. Psychopharmacology (Berl) 1997;134:193–200. doi: 10.1007/s002130050442. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Goodwill AM, Szechtman H. Locomotor sensitization to quinpirole in rats: effects of drug abstinence and sex. Psychopharmacology (Berl) 2000;152:304–11. doi: 10.1007/s002130000538. [DOI] [PubMed] [Google Scholar]
- Taepavarapruk P, Floresco SB, Phillips AG. Hyperlocomotion and increased dopamine efflux in the rat nucleus accumbens evoked by electrical stimulation of the ventral subiculum: role of ionotropic glutamate and dopamine D1 receptors. Psychopharmacology (Berl) 2000;151:242–51. doi: 10.1007/s002130000376. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vernadakis A, Sakellaridis N, Geladopoulos T, Mangoura D. Function of opioids early in embryogenesis. Ann N Y Acad Sci. 1990;579:109–22. doi: 10.1111/j.1749-6632.1990.tb48354.x. [DOI] [PubMed] [Google Scholar]
- Wise RA, Leeb K. Psychomotor-stimulant sensitization: a unitary phenomenon? Behav Pharmacol. 1993;4:339–349. [PubMed] [Google Scholar]
- Wrobel A, Zebrowska-Lupina I, Wielosz M. Dexamethasone reduces locomotor stimulation induced by dopamine agonists in mice. Pharmacol Rep. 2005;57:451–7. [PubMed] [Google Scholar]
- Wu M, Brudzynski SM, Mogenson GJ. Differential effects of quinpirole in the nucleus accumbens depending on the initial level of locomotor activity. Brain Res Bull. 1993;32:395–8. doi: 10.1016/0361-9230(93)90206-q. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Tobias SW, McLaughlin PJ. Endogenous opioids and prenatal determinants of neuroplasticity. Adv Exp Med Biol. 1997;429:289–303. doi: 10.1007/978-1-4757-9551-6_21. [DOI] [PubMed] [Google Scholar]
- Zagon IS, Wu Y, McLaughlin PJ. Opioid growth factor and organ development in rat and human embryos. Brain Res. 1999;839:313–22. doi: 10.1016/s0006-8993(99)01753-9. [DOI] [PubMed] [Google Scholar]