Abstract
Background
Preclinical studies demonstrated antiproliferative synergy of 1,25-D3 (calcitriol) with cisplatin. The goals of this phase I/II study were to determine the recommended phase II dose (RP2D) of 1,25-D3 with cisplatin and docetaxel and its efficacy in metastatic non-small-cell lung cancer.
Methods
Patients were ≥18 years, PS 0–1 with normal organ function. In the phase I portion, patients received escalating doses of 1,25-D3 intravenously every 21 days prior to docetaxel 75 mg/m2 and cisplatin 75 mg/m2 using standard 3 + 3 design, targeting dose-limiting toxicity (DLT) rate <33 %. Dose levels of 1,25-D3 were 30, 45, 60, and 80 mcg/m2. A two-stage design was employed for phase II portion. We correlated CYP24A1 tag SNPs with clinical outcome and 1,25-D3 pharmacokinetics (PK).
Results
34 patients were enrolled. At 80 mcg/m2, 2/4 patients had DLTs of grade 4 neutropenia. Hypercalcemia was not observed. The RP2D of 1,25-D3 was 60 mcg/m2. Among 20 evaluable phase II patients, there were 2 confirmed, 4 unconfirmed partial responses (PR), and 9 stable disease (SD). Median time to progression was 5.8 months (95 % CI 3.4, 6.5), and median overall survival 8.7 months (95 % CI 7.6, 39.4). CYP24A1 SNP rs3787554 (C > T) correlated with disease progression (P = 0.03) and CYP24A1 SNP rs2762939 (C > G) trended toward PR/SD (P = 0.08). There was no association between 1,25-D3 PK and CYP24A1 SNPs.
Conclusions
The RP2D of 1,25-D3 with docetaxel and cisplatin was 60 mcg/m2 every 21 days. Pre-specified endpoint of 50 % confirmed RR was not met in the phase II study. Functional SNPs in CYP24A1 may inform future studies individualizing 1,25-D3.
Keywords: Calcitriol, Lung cancer, CYP24A1-SNPs
Introduction
The current standard of care for initial therapy for metastatic NSCLC is the use of platinum doublet in patients with a good performance status (PS) whose tumors lack EGFR or ALK mutation [1]. Response rates for doublet therapies are between 25 and 30 %, with a median survival time of about eight months [2]. While adding biologics, such as bevacizumab to platinum doublets, increases median survival to about 12 months in a subgroup of nonsquamous NSCLC patients [3], advanced lung cancer remains a uniformly fatal disease. 1,25-D3 has antiproliferative activity in many solid tumors and synergistically enhanced cisplatin cytotoxicity in multiple cell lines [4]. Pre-treatment of human squamous cell carcinoma cells with 1,25-D3 reduces their capacity to repair cisplatin-damaged DNA [5]. 1,25-D3 also decreases cellular expression of p53 and p21, in both in vitro and in vivo models, enhancing cytotoxicity of cisplatin [6]. The addition of 1,25-D3 to cisplatin in dogs with spontaneous solid tumors increases the response rate compared to cisplatin alone. Three out of seven dogs treated with 1,25-D3 and cisplatin exhibited a complete clinical response [7]. At supraphysiological doses, 1,25-D3 may lead to hypercalcemia [8]. It has also been demonstrated that use of dexamethasone increases the cytotoxic activity of 1,25-D3 and mitigates 1,25-D3 induced hypercalcemia [9]. We hypothesized that 1,25-D3 may improve response rates to cisplatin- based chemotherapy in patients with advanced NCSLC. The phase I study tested the hypothesis that 1,25-D3 can be safely combined with standard platinum-based therapy in patients with NSCLC at doses required to generate biologically effective blood concentrations observed in mice and dogs. The efficacy of 1,25-D3 in combination with cisplatin/dexamethasone/docetaxel at the maximum tolerated dose (MTD) (determined during phase I) was assessed by enrolling patients in a phase II study. We also hypothesized that single nucleotide polymorphisms (SNPs) in the CYP24A1 gene could alter expression of the 24-hydroxylase enzyme and impact the rate at which an individual can metabolize 1–25-D3, and therefore the activity of 1,25-D3.
Our goals in this study were to (a) establish the MTD and dose-limiting toxicities (DLT) of intravenous (I.V.) 1,25-D3 in combination with cisplatin and docetaxel (phase I), (b) to assess the efficacy of combining 1,25-D3 with cisplatin and docetaxel at the recommended phase II dose (RP2D) (phase II), (c) to perform pharmacokinetic (PK) studies of I.V 1,25-D3 in the phase II study, using a limited sampling technique and explore a correlation between PKs of 1,25-D3 and SNPs in the CYP24A1 gene.
Materials and methods
Patient criteria
Key inclusion criteria included histologically or cytologically confirmed metastatic (pleural metastasis or IV) non-small-cell lung cancer (NSCLC) in patients >18 years who were chemotherapy naïve with evaluable or measurable disease (for phase II) defined by the Response Evaluation Criteria in Solid Tumors (RECIST), Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 to 1, with adequate bone marrow function (neutrophils > 1,500/µL, hemoglobin >10 g/dL and platelets > 100,000/µL), adequate hepatic function (total bilirubin within the upper limit of the institutional normal range, transaminases (SGOT or SGPT) ≤1.5 times the upper limit of the institutional normal range), adequate renal function (creatinine within the upper limit of the institutional normal range; creatinine clearance ≥50 mL/min). Patients had to have normal cardiac function or compensated heart disease with no history of unstable angina or CHF in the 6 weeks prior to study entry. Palliative radiation was permitted as long as the amount of bone marrow exposed was not >10 %. Palliative radiation to brain metastasis was allowed as long as 2 weeks had elapsed following whole brain radiation; no wait was required for stereotactic radiosurgery. Women could not be pregnant or lactating. The expected life expectancy at study enrollment of least 3 months was required. Prior adjuvant chemotherapy use was permitted.
Key exclusion criteria included presence of any grade ≥2 peripheral neuropathy or history of severe hypersensitivity reaction to 1,25-D3, cisplatin or docetaxel or other drugs formulated with polysorbate 80, hypercalcemia (serum albumin corrected calcium >10.7 mg/dL), history of renal/bladder stones in the previous 10 years, single kidney or uncontrolled heart disease. Concurrent use of calcium supplements, thiazides, digoxin, glucocorticoids, phenytoin, barbiturates, rifampin, carbamazepine, phenobarbital or St John’s Wort was not permitted. All patients gave written informed consent, and the protocol was approved by the Institutional Review Boards (IRBs) of Roswell Park Cancer Institute, the University of Michigan, St. Joseph’s Hospital, Ann Arbor, and the Ann Arbor Veterans Adminstration Hospital. The study was conducted in accordance with the Good Clinical Practice Guidelines as issued by the International Conference on Harmonization and the Declaration of Helsinki.
Study design and treatment plan
The trial design was an open label, multi-institutional, nonrandomized, phase I/II study of the investigational agent 1,25-D3 in combination with standard chemotherapy consisting of cisplatin (75 mg/m2) and docetaxel (75 mg/m2) every 3 weeks. Patients received 1,25-D3 I.V over 60 min once every 21 days immediately prior to docetaxel infusion except for cycle 1 in the phase II portion wherein 1,25-D3 was administered 24 h prior to docetaxel infusion to allow for PK studies. 1,25-D3 was supplied as 1mcg vials. The starting dose of 1,25-D3 for this trial was 30 mcg/m2 I.V q 3 weeks. This dose was based on earlier dog studies [7]. Subsequent dose levels were 45, 60, and 80 mcg/m2. Three patients were entered at each dose level. In the absence of dose-limiting toxicity (DLT), the next dose level of 1,25-D3 was explored. If DLT was seen in one patient, three further patients were added at that dose level. If no additional DLT was seen, escalation to the next dose level occurred. MTD was the highest dose level that resulted in DLT in not more than 1 out of 6 patients. MTD was based on the first cycle of therapy administered; to be evaluable, a patient must have completed 1 cycle of therapy. The recommended phase II dose (RP2D) was defined as the MTD derived from the phase I study. Intrapatient dose escalation was not permitted. Cisplatin/docetaxel was administered immediately following 1,25-D3, I.V every 3 weeks. To prevent allergic reactions to docetaxel and to mitigate 1,25-D3 associated hypercalcemia, dexamethasone 4 mg orally, twice daily was administered starting the day before chemotherapy for 3 days.
A DLT was any of the following toxicities that were attributable to study treatment on cycle 1: Hypercalcemia, defined as an increase in corrected serum calcium ≥12 mg/ dL persisting >7 days or <7 days if symptomatic, any corrected calcium ≥13 mg/dL (symptomatic or asymptomatic) or development of nephrolithiasis, symptomatic or radiographic. Hypophosphatemia was defined as a DLT if phosphate <1.5 mg/dL persisting for >10 days or phosphate <1.5 mg/dL associated with symptoms unequivocally related to hypophosphatemia: profound weakness, ventilatory compromise or serum phosphate <0.75 mg/dL. Sustained increases (>72 h) in creatinine of more than double the baseline and >2.5 mg/dL were also a DLT. Any grade 3 or greater non-hematological toxicity that could be attributed to cisplatin/docetaxel alone, dose delays of greater than 2 weeks, and any grade 4 hematological toxicity that lasted for greater than 7 days. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria of Adverse Events version 3.0. The DLT definition was expanded at the start of dose level 3 (60 mcg/m2) to include grade 4 hematological toxicity lasting greater than 7 days, all grade 5 events (except disease progression), any hospitalization/removal from study related to toxicities or adverse events from the study drugs and grade 3 or greater non-hematological toxicity other than alopecia and nausea/vomiting that is not maximally treated.
Standard dose modifications were defined for both docetaxel and cisplatin for both hematological and non-hematological toxicities. For Grade 3 and 4 toxicities, treat-ment had to be held until the toxicity resolved to Grade 1 or less, then reinstituted (if medically appropriate) at a 25 % dose reduction. If treatment was withheld for longer than three weeks due to Grade 3/4 toxicity, the patient was withdrawn from the study. No dose modifications were allowed for DLTs related to 1,25-D3, in which case patients had to be discontinued from study. Primary use of growth factors was not permitted in accordance with ASCO guidelines. Patients were continued on treatment unless they had unacceptable toxicities, evidence of progressive disease, up to a total of four cycles in the case of stable/responding disease, at the discretion of the investigator.
Clinical evaluation at baseline and follow-up
A complete medical history, physical examination, and pregnancy test for women with reproductive potential, complete blood count, and comprehensive chemistry profile were obtained within 1 week before treatment initiation. Baseline computed tomography (CT) scans of the chest and abdomen were obtained within 4 weeks before initiation of treatment. Complete blood count and comprehensive chemistry were repeated on a weekly basis. Calcium, phosphorous, and creatinine levels were measured before as well as 3 days after each 1,25-D3 infusion. Medical history, physical examination, and toxicity assessment as per National Cancer Institute Common Toxicity Criteria 3.0 were done on days 1 and 15 of cycle 1 and on day 1 of each subsequent cycle. Corrected calcium was used to grade hypercalcemia [corrected calcium = serum calcium + (Normal albumin − patient albumin) × 0.8]. CT scans were repeated every two cycles to assess response. Responses were categorized according to the Response Evaluation Criteria in Solid Tumors. Responding patients in the phase II study were required to undergo a confirmatory CT scan in 30 days. Date of last follow-up on this study was February 2012.
Pharmacokinetics
Blood sampling and serum 1,25-D3 measurements: 1,25-D3 PK blood samples (7 mL) were obtained on the first day (day 1) of treatment of cycle 1 of the phase II portion of the study when only 1,25-D3 was administered, thus eliminating the influence by docetaxel, cisplatin, and related premedications on serum 1,25-D3 PK. Samples were collected before, at the end of the 1 h IV infusion, and 2 and 4 h after 1,25-D3 administration. Serum was immediately separated by centrifugation at 1,000 × g at 4 °C for 10 min, protected from direct light and stored in 1–2 mL aliquots at −70 °C until assayed for 1,25-D3. Serum 1,25-D3 concentrations were determined using 1,25-dihydroxyvitamin D3-[I125] RIA kit from DiaSorin Co. (Stillwater, MN) as previously described [11, 12]. Serum samples were diluted to fall within the range of the calibration curve (5–230 pg/mL) using 1,25-D3-free serum solution provided in the RIA kit. Limited sampling techniques [10] for pharmacokinetic (PK) sampling were utilized for 1,25-D3 levels in the phase II portion of the study. The PK data were correlated with presence/absence of CYP24A1 polymorphisms in the phase II part of the study.
CYP24A1 genotype analysis
Five ml of whole blood for CYP24A1 genotyping was collected before treatment in the phase II arm of the study. DNA was extracted from the blood using a Q1Aamp Blood kit. (QIAGEN, Hilden, Germany). Using the Tagger algorithm implemented in the Haploview program and dense genotyping data from the HapMap database, we identified linkage disequilibrium (LD) tagging SNPs to capture variation with R2 greater than 0.8. Selection was restricted to those with a minor allele frequency of greater than 5 % in the reference panel. Hardy–Weinberg equilibrium tests for each of the SNPs were performed using the Pearson goodness of fit test using a pre-determined cutoff of P value ≤.001. Listed in Table 4 are the SNPs studied.
Table 4.
CYP24A1 SNPs according to location
| # of SNPs | SNP location | dbSNP id |
|---|---|---|
| 1 | 3UTR(2293)G > A | – |
| 2 | 3UTR(2393)C > T | – |
| 3 | Ex11(1529)C > T | rs116065115 |
| 4 | Ex3(475)C > T; R159 W | – |
| 5 | IVS3(81)A > T | rs111675277 |
| 6 | 3UTR (2801_2802_insAT) | rs10623012 |
| 7 | 3UTR(2417)C > T | rs11907350 |
| 8 | IVS3(−179)A > G | rs2245153 |
| 9 | IVS1(118)G > C | rs2248137 |
| 10 | IVS2(−105)A > G | rs2259735 |
| 11 | IVS8(−180)G > A | rs2274131 |
| 12 | IVS8(−200)C > T | rs2274132 |
| 13 | Ex4(553)C > T; A184 | rs2296241 |
| 14 | 3UTR(1595)C > T | rs2762934 |
| 15 | IVS5(−149)C > G | rs2762939 |
| 16 | 5’FR(−668)A > C | rs2762943 |
| 17 | IVS1(165)C > T | rs35792925 |
| 18 | IVS2(−850G > T | rs36106327 |
| 19 | IVS4(−308)C > T | rs3787554 |
| 20 | IVS(147)C > T | rs455539538 |
| 21 | IVS4(−66)A > C | rs4809958 |
| 22 | IVS4(58)A > G | rs4809960 |
| 23 | IVS5(−162)A > G | rs6013905 |
| 24 | Ex8(1120)T > C; M374T | rs6022990 |
| 25 | IVS3(103)T > C | rs6022999 |
| 26 | IVS1(21)C > T | rs602300 |
| 27 | Ex6(744)G > A; T248 | rs6068816 |
| 28 | IVS7(46)C > A | rs6091828 |
Statistical analysis
Study Design/Endpoints: The design of the Phase I trial utilized a standard 3 + 3 dose-finding scheme. Dose of 1,25-D3 was escalated using cohorts of 3 patients as outlined above. The MTD was to be determined for 1,25-D3 in combination with fixed doses of cisplatin. The phase II portion of this study was to be initiated using the MTD obtained from the phase I portion of the trial. All patients enrolled and receiving any therapy were evaluable for toxicity. The goal of the phase II study was to assess activity of 1,25-D3, cisplatin, and docetaxel in a two-stage sequential design. The endpoint was confirmed response rates (CR + PR). The phase II portion of the trial was based on the hypothesis: Ho: H0 : PR ≤ 0.25Ha : PR ≥0.45 where PR is the probability of response. The test statistic was simply the number of responses among the number of patients evaluable for response. The null hypothesis was the range of probabilities that are associated with an “inactive” therapeutic effect, and the alternative hypothesis was the range of probabilities associated with an “active” therapeutic effect. Previous studies that utilized docetaxel and cisplatin have shown response rates of about 30 % in advanced NSCLC. The two-stage design [13] was used. A maximum of 39 patients were needed for the phase II portion. In the first stage, 20 patients receiving the recommended phase II dose (RP2D) were evaluated for response (this includes the MTD cohort of response evaluable patients in the phase I portion); if the number of confirmed responses were less than or equal to 3, then the study was to be closed and the treatment deemed ineffective for this population. If 4 or more responses were observed, the study would go on to step 2 to enroll an additional 19 patients. If there were 14 or more responses observed in the enrolled 39 evaluable patients, then the regimen would be declared active. In addition to response assessment, for exploratory purpose, we utilized Kaplan– Meier estimates and the confidence intervals for time to progression (TTP) and overall survival (OS). Previous studies utilizing cisplatin and docetaxel as initial therapy for advanced NSCLC have yielded response rates of 31–35 % with a TTP ranging from 4–11 months [14, 15]. CYP24A1 SNP data were analyzed to determine whether there was a relationship between any single polymorphism or combinations of polymorphisms and serum 1,25-D3 exposure and response (any PR vs SD vs PD). We used logistic models to analyze CYP24A1 associations with AUC and Cmax separately. The Jonckheere-Terpstra test was used to examine CYP24A1 polymorphism associations with response. All polymorphism analyses were exploratory and hypothesis generating, so multiple comparison adjustments were not made.
Results
Patient characteristics
Thirty-four patients were enrolled (18 in phase I and 16 in phase II) with a median age of 54 (range 34–79) years. The male/female ratio was 22:12. All but two patients were white. There were 21 patients with adenocarcinoma, 12 with squamous cell carcinomas, and one not specified NSCLC. There were 4 patients with pleural metastasis and 30 with distant metastasis. There were 29 smokers (current and former) and 2 never smokers. The patient characteristics are outlined in Table 1.
Table 1.
Patient characteristics
| Patient characteristics (N = 34) | |
|---|---|
| Gender (male/female) | 22/12 |
| Age (median/range), years | 54 (34–79) |
| Stage IIIB/IV | 4/30 |
| Histology | |
| Adenocarcinoma | 21 |
| Squamous carcinoma | 12 |
| NSCLC NOS | 1 |
| Performance status (PS) | |
| PS 0 | 16 |
| PS 1 | 18 |
| Prior radiation | 16 |
| Smoking status | |
| Never smoker | 2 |
| Ever smoker | 29 |
| Unknown | 3 |
Phase I study
Eighteen patients were enrolled, and 16 were evaluable for toxicity assessments. Two patients were not evaluable as they progressed prior to completion of cycle 1. Dose levels of 1,25-D3 evaluated in combination with docetaxel and cisplatin were 30, 45, 60, and 80 mcg/m2. No DLTs were noted at dose level 1, 2 and in the initial 3 patients at the 60 mcg/m2 dose level. At dose level 4, (80 mcg/m2), 2/4 patients developed grade 4 neutropenia lasting more than 7 days. Even though this neutropenia was attributed to cisplatin– docetaxel, a contributory effect of 1,25-D3 could not be excluded. The dose escalation was discontinued, and the dose level 60 mcg/m2 was expanded to include a total 6 patients. One of these six patients developed grade 4 neutropenia lasting greater than 7 days. Dose level 3 (60 mcg/m2) was chosen as the RP2D.
Phase II component efficacy
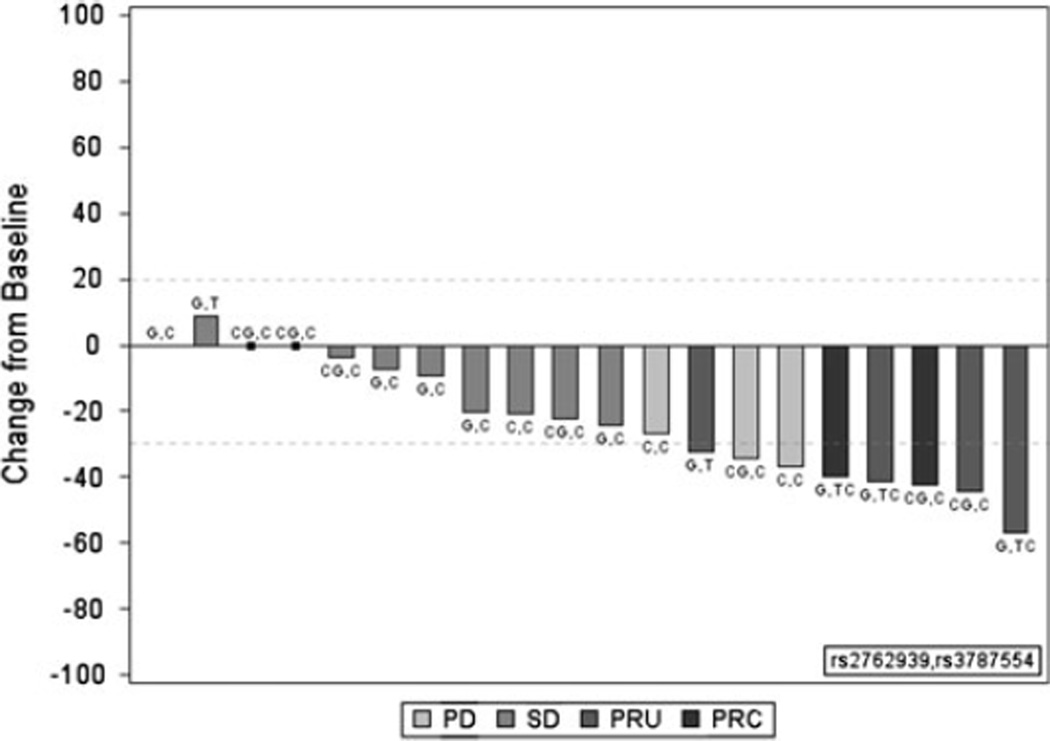
Twenty-two patients were treated at the RP2D, including five response evaluable phase I patients who received the RP2D during the phase I component. One patient in the phase II study went to another therapy prior to the 30 day window for a confirmatory scan and was thus excluded from analysis. Among 5 response evaluable Phase I patients, and 15 evaluable phase II patients, there were 2 confirmed partial responses (PR), 4 unconfirmed PRs, and 9 patients with stable disease (SD). There were 5 pts who progressed. Unconfirmed PRs were related to the fact that there was no requirement in the phase I portion (five patients at the MTD were counted in the phase II portion) for a confirmatory scan. Of the 2 confirmed partial responses, one was in a man with adenocarcinoma; the second patient was in a woman with squamous cell carcinoma with a durable (12 months) response. Another patient had stable disease, but multiple bone metastases increased in density, suggesting a responsive or healing effect on bone; this patient did not require further pain medications. The responses are depicted in a waterfall plot in Fig. 1. The required number of confirmed partial or complete responses needed to continue as per the phase II design was 4 patients. Since this was not reached at interim analysis, the protocol was closed to accrual.
Fig. 1.
Correlation of response to CYP24A1 single nucleotide polymorphisms (SNPs) in n = 20 patients. Waterfall plot depicting change in size of target lesion to the combination of 1–25D3 in combination with docetaxel and cisplatin in patients with metastatic NSCLC. RECIST method was used to judge partial response and stable disease. The specific genotype for the CYP24A1 SNPs 2762939 and 3787554 are depicted for each patient who demonstrated progressive disease (PD), stable disease (SD), unconfirmed partial response (UPR) and conformed partial response (CPR)
Safety
Hematological toxicities
The combination therapy of 1,25-D3 and cisplatin and docetaxel was associated with myelosuppression. Of note, nearly half the patients had received palliative radiation prior to enrollment. Eight patients (45 %) in the phase I portion of the study exhibited grade 3 or greater neutropenia with cycle 1. Grade 4 neutropenia was the DLT. One patient with febrile neutropenia died as a result of infection. Table 2 demonstrates toxicities ≥grade 2 with cycle 1 as well as all cycles in the phase I portion of the study. In the phase II portion of the study at the 60 mcg/m2, there were 9 patients (50 %) with grade 3/4 neutropenia in any cycle (Supplementary Table 1).
Table 2.
Hematological and non-hematological toxicities grade 2 and higher (cycle 1 and all cycles) at least possibly related to therapy: phase I part of study
| AE_NAME | DL 30 mcg/m2 (n = 5) |
DL 45 mcg/m2 (n = 3) |
DL 60 mcg/m2 (n = 6) |
DL 80 mcg/m2 (n = 4) |
|---|---|---|---|---|
| Cycle 1 | ||||
| Death | 1 | 0 | 0 | 0 |
| Dehydration grade 3/4 | 1 | 0 | 0 | 1 |
| Diarrhea grade 2/3 | 0 | 0 | 2 | 1 |
| Fatigue grade 2 | 0 | 0 | 3 | 1 |
| Fatigue grade 3 | 0 | 0 | 0 | 1 |
| Hypocalcemia grade 2/3 | 1 | 0 | 2 | 0 |
| Hyponatremia grade 3 | 1 | 0 | 1 | 0 |
| Hypophosphatemia grade 3 | 0 | 0 | 1 | 0 |
| Hypotension grade 2/3 | 0 | 1 | 1 | 0 |
| Hypotension grade 4 | 1 | 0 | 0 | 0 |
| Muscle weakness grade 3 | 0 | 0 | 0 | 1 |
| Neutropenia grade 2 | 2 | 2 | 0 | 0 |
| Neutropenia grade 3 | 1 | 0 | 1 | 0 |
| Neutropenia grade 4 | 1 | 1 | 1 | 2 |
| Febrile neutropenia | 1 | 0 | 0 | 0 |
| Thrombosis grade 3 | 0 | 0 | 1 | 2 |
| All cycles | ||||
| Dehydration grade 2/3 | 1 | 0 | 1 | 2 |
| Dehydration grade 4 | 1 | 0 | 0 | 0 |
| Diarrhea grade 2/3 | 0 | 0 | 2 | 1 |
| Fatigue grade 2/3 | 0 | 1 | 5 | 2 |
| Hypocalcemia grade 2/3 | 1 | 0 | 2 | 0 |
| Hyponatremia grade 3 | 1 | 0 | 1 | 0 |
| Hypophosphatemia grade 2 | 0 | 1 | 1 | 0 |
| Hypophosphatemia grade 3 | 0 | 0 | 1 | 0 |
| Hypotension grade 3 | 0 | 0 | 2 | 0 |
| Infection grade 3 | 0 | 0 | 1 | 0 |
| Infection grade 5 | 1 | 0 | 0 | 0 |
| Nausea grade 3 | 0 | 0 | 0 | 1 |
| Neutropenia grade 2 | 2 | 2 | 1 | 0 |
| Neutropenia grade 3 | 2 | 0 | 1 | 0 |
| Neutropenia grade 4 | 1 | 1 | 1 | 2 |
| Febrile neutropenia | 2 | 1 | 0 | 0 |
| Neuropathy grade 2 | 1 | 0 | 1 | 0 |
| Pain grade 2 | 1 | 0 | 0 | 1 |
| Pulmonary embolus grade 4 | 1 | 0 | 0 | 0 |
| Thrombosis grade 3 | 0 | 0 | 0 | 1 |
| Vomiting grade 3 | 0 | 0 | 1 | 0 |
Non-hematological toxicities
The most frequent grade 1–2 toxicities encountered, based on percentage of cycles delivered, were fatigue (50 %) and anemia (29 %). The most common non-hematological adverse events ≥grade 3 were fatigue, diarrhea, nausea, and dehydration. There was no hypercalcemia noted. One patient developed renal failure as a result of infection in the setting of febrile neutropenia. Supplementary Table 1 lists the most frequently observed adverse events ≥grade 3 considered possibly, probably, or definitely related to study therapy for the 22 patients treated in the phase II cohort at RP2D.
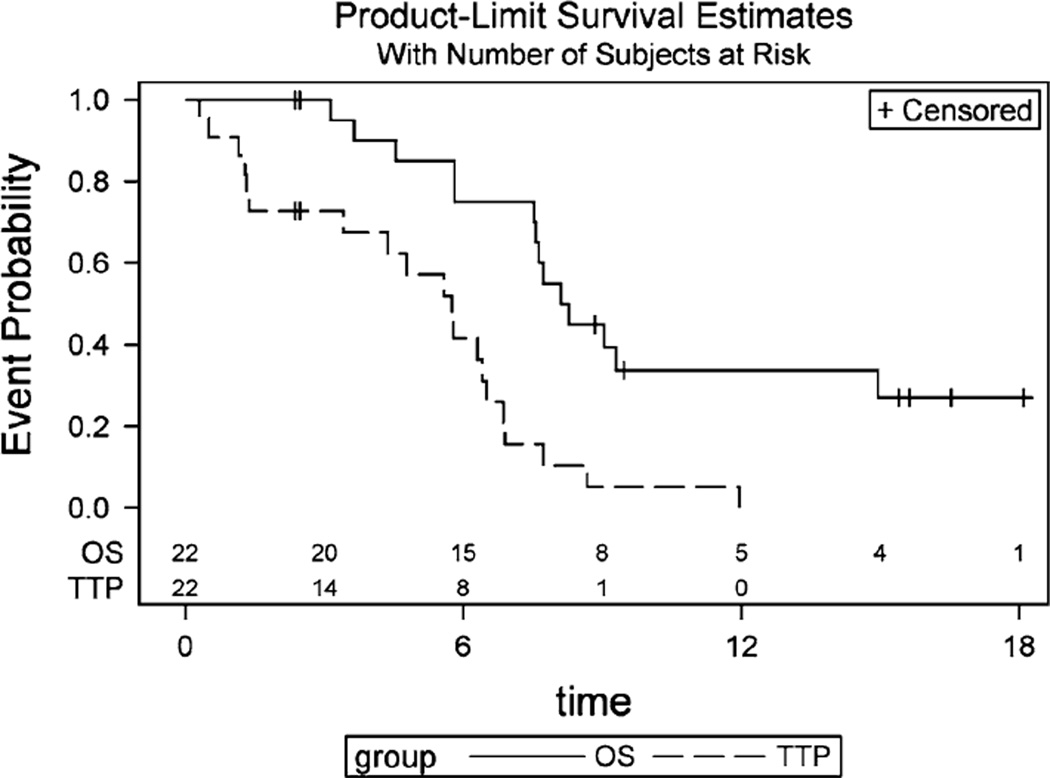
Time to progression and overall survival
The median time to progression was 5.8 months (95 % CI 3.4, 6.5), and the median overall survival was 8.7 months (95 % CI 7.6, 39.4) (Fig. 2). In a univariate analysis, greater TTP correlated with female sex having a 72 % decreased hazard of progression than males and adenocarcinoma histology having a 88 % decreased hazard of progression compared to squamous carcinoma. Interestingly, baseline calcitriol serum concentrations > 50 pg/mL corresponded with improved overall survival compared to concentrations <50 pg/mL (Table 3).
Fig. 2.
Kaplan–Meier plot for progression free and overall survival
Table 3.
Univariate cox regression models
| Overall survival |
Progression-free survival |
|||
|---|---|---|---|---|
| HR (95 % CI) | P value | HR (95 % CI) | P value | |
| Gender | ||||
| Female versus male | 0.40 (0.1, 1.5) | 0.17 | 0.28 (0.09, 0.90) | 0.033 |
| Smoking status | ||||
| Non-smoker versus ever-smoker | 0.30 (0.04, 2.4) | 0.25 | 0.45 (0.10, 2.0) | 0.30 |
| Stage | ||||
| IIIB versus IV | 1.00 (0.27, 3.8) | 0.98 | 1.23 (0.38, 4.0) | 0.73 |
| Histology | ||||
| Adeno versus squamous | 0.13 (0.03, 0.65) | 0.01 | 0.12 (0.02, 0.59) | 0.009 |
| Calcitriol Cmax | ||||
| ≤3,369 versus >3,369 pg/mL | 1.18 (0.28, 4.9) | 0.82 | 2.25 (0.6, 8.3) | 0.22 |
| Baseline calcitriol | ||||
| <50 versus ≥50 pg/mL | 12.1 (1.4, 105) | 0.02 | 2.9 (0.79, 11.0) | 0.11 |
| SNP rs2762939 G versus (C or CG) | 1.9 (0.6, 6.4) | 0.29 | 1.21 (0.46, 3.2) | 0.70 |
| SNP rs3787554 C versus(TC or T) | 4.8 (0.6, 37.4) | 0.13 | 1.96 (0.6, 6.9) | 0.30 |
HR hazard ratio, 95 % CI 95 % confidence Interval of the hazard ratio
Pharmacokinetics
Baseline serum 1,25-D3 levels (C0) were from the pretreatment serum samples, and peak serum 1,25-D3 concentrations (Cmax) were from the samples obtained at the end of the infusion. A limited sampling strategy [16] using the 2 and 4-h post serum 1,25-D3 concentration was used to calculate the serum 1,25-D3 AUC0–12h using the equation: Log AUC0–12h = 1.309 + 0.3960 × log (1,25-D3) at 2 h + 0.4643 × log (1,25-D3) at 4 h. The AUC0–24h values were calculated from the AUC0–12h versus AUC0–24h relationship given by linear equation (r2 = 0.83): Y = mx + b, where slope (m) = 2.109 ± 0.2166 and the Y-intercept (b) = −2015 ± 4.787. Individual patient serum 1,25-D3 baseline (C0), Cmax, AUC0–12h and AUC0–24h are shown in supplementary Table 2. The baseline serum 1,25-D3 levels (C0) observed in the NSCLC patients enrolled in this study were with the normal range (16–80 pg/mL). Despite the differences in the 1,25-D3 dose administered-fixed dose versus 1,25-D3 dosing based on body surface, the calculated serum 1,25-D3 AUC0–12h and AUC0–24h values of this study were not significantly (P = 0.0623) from the similarly calculated AUC0–12h and AUC0–24h values for patients [8, 16] receiving similar total 1,25-D3 dose (96–125 µg).
Pharmacogenomic analysis
Twenty-two patients had genotype data and 20 were evaluable for Response (9 SD, 2 confirmed PR and 4 unconfirmed PR and 5 PD). The GG allele in rs 2762939 correlated with both SD and PR (unconfirmed and confirmed PR combined) (P = 0.078). This SNP is in linkage disequilibrium with rs 6022999 (D′ = 0.89, LOD = 5.4, r2 = 0.52). When we examined the relationship between this variant and the 1,25-D3 pharmacokinetics, there was no association between presence of the GG allele and Cmax or AUC of 1,25-D3. We also found that the rs3787554 C > T variant (IVS4 (−308)C > T)correlated with progressive disease (PD) (P = 0.010). Again there was no correlation between this SNP and Cmax or AUC of 1,25-D3. The SNPs are outlined in Table 4.
Discussion
1,25-D3 has antitumor and differentiating activities in pre-clinical models of cancer. Specifically, synergy for activity between 1,25-D3 and cisplatin has been demonstrated both in in vitro and in vivo studies [5, 7]. Thus, we hypothesized that 1,25-D3 would improve the response rate to cisplatin-based therapy in metastatic NCSLC. Previous pre-clinical data indicated that antitumor activity is 1,25-D3 concentration dependent—antitumor activity increases as concentrations are escalated from 1 to 100 nmol/L [17]. The maximal antitumor effects are seen with pharmacological doses of 1,25-D3 and that such exposure can be safely achieved in animals using a high dose, intermittent schedule of administration. AUC and C (max) 1,25-D3 concentrations of 32 ng h/ml and 9.2 ng/ml are associated with striking antitumor effects in a murine squamous cell carcinoma model [9]. In dog studies, the MTD was 3.75 µg/kg (1DLT consisting of severe hypercalcemia). At 2.25 µg/kg, which generates a mean Cmax of 16 nM, there were no DLTs noted [7]. Using a conversion factor described by Freireich et al. [18], we believed that a SD of 30 µg/m2 was safe.
This phase I clinical trial is the first to investigate use of I.V. 1,25-D3 in advanced metastatic NSCLC. The present study was designed to identify the MTD dose of 1,25-D3 in combination with cisplatin and docetaxel in this disease. The MTD of I.V. 1,25-D3 in combination with cisplatin and docetaxel was 60 mcg/m2 (equivalent to about 120 mcg) administered every 3 weeks. DLTs, in the form of grade 4 neutropenia, were noted. The DLTs we observed in the form of grade 4 neutropenia could have been due to the chemotherapy alone; however, the effect of 1,25-D3 on this cannot be excluded. It is to be noted that the definition of DLT was expanded to include grade 4 hematological toxicity at the start of dose level 3. Previous studies of 1,25-D3 PKs in combination with paclitaxel or carboplatin did not reveal any alteration of PK parameters of the chemotherapeutic drugs. Additionally, a large phase III study of cisplatin docetaxel in advanced NSCLC revealed a myelosuppression rate of 41 %, not dissimilar to what we noted [14]. It is of interest that there were no dose-limiting toxicities which could be attributable to 1,25-D3 (e.g., hypercalcemia). This may have been due to concurrent administration of pre-chemotherapy dexamethasone. We demonstrated that high doses of I.V. 1,25-D3 up to 120 mcg in an intermittent schedule every 3 weeks could be given safely in advanced NSCLC patients. Previous studies have demonstrated that weekly doses ranging from 74 to 120 mcg are safe [8, 19]. The dosing regimen employed in the present trial achieved mean AUC and Cmax 1,25-D3 concentrations of 32 ng h/ml and 3.5 ng/ml (concentrations associated with antiproliferative effects in mice) [20]. Despite these serum concentrations, we did not see an increase in response rate to cisplatin-based chemotherapy or an improvement in progression-free or overall survival compared to studies of cisplatin/docetaxel alone. We believe that this may relate to lung cancer heterogeneity and varying degrees of CYP24A1 expression by the tumor. In fact, we have recently demonstrated that increased CYP24A1 mRNA expression, the major vitamin D3 catabolizing enzyme, in tumor tissues of NSCLC patients is associated with poor prognosis [17]. We also demonstrated that antiproliferative effects of 1,25-D3 are proportional to CYP24A1 mRNA in various lung cancer cell lines. Given our recent data that CYP24A1 is a potential predictive marker of 1,25-D3 activity in NSCLC, individualized treatments based on tumor genotype merit exploration.
We also tested our hypothesis that various SNPs in the CYP24A1 gene may correlate with systemic exposure to 1,25-D3 and therefore response. CYP24A1 plays a pivotal role in maintaining vitamin D homeostasis. CYP24A1 SNPs or haplotypes have also been associated with other diseases in humans, including coronary artery disease and asthma [21, 22]. Our group previously demonstrated that splice variants of CYP24A1 resulting from certain SNPs can influence the activity of 1–25D3 in prostate cancers [23]. Roff et al. demonstrated that a functional SNP in the VDRE of CYP24A1 promoter led to decreased protein binding and transactivation in vitro, and reduced expression of CYP24A1 in human lymphocytes; importantly, this functional SNP was noted in African Americans [24]. While we could not find such a statistically important relationship between CYP24A1 SNPs and PK parameters, we found a negative predictive value of CYP24A1 genotype in patients who may not respond to exogenous 1,25-D3. Despite the fact that previous transgenic rodent studies have supported the role of the CYP24A1 gene on stability of plasma levels of 1,25-D3 [25], the current study did not establish any association between rs2762939 and 1,25-D3 serum concentrations. It remains possible that the identified SNP may influence 1,25-D3 locally and, consequently NSCLC, without affecting vitamin D stores, as reflected by 25(OH)D levels or peak concentrations of 1,25-D3 in the serum. Further functional characterizations of these SNPs and their relationship to vitamin D metabolism need to be performed. Future planned studies may include a prospective study of a combination of tumor genotypes (high/low CYP24A1 mRNA) and patient phenotype (specific CYP24A1 SNPs) to identify patients who may benefit from 1,25-D3.
Supplementary Material
Translational relevance.
1α,25-Dihydroxyvitamin D3 (1,25-D3), the active form of vitamin D in supra-physiological doses exhibits antiproliferative and differentiation-inducing effects in several cancers. Pre-clinical studies have demonstrated synergy for antitumor activity with cisplatin and other chemotherapy. Here, we demonstrate that we can safely combine high doses of 1,25-D3 with cisplatin and docetaxel in patients with advanced lung cancer. These doses achieve “antiproliferative” serum concentrations of 1,25-D3 associated with antitumor activity in pre-clinical models. Pre-specified endpoint of confirmed response rate of 50 % using 1,25-D3 with cisplatin and docetaxel was not met in the phase II study. Specific SNPs in the CYP24A1 gene (encodes for CYP24A1, rate limiting enzyme in 1,25-D3 metabolism) were associated with response outcomes, albeit in a small number of patients. We have previously demonstrated that antiproliferative effects of 1,25-D3 are proportional to tumor CYP24A1 mRNA. Future studies may necessitate taking into consideration tumor genotype and patient phenotype in studies involving 1,25-D3.
Acknowledgments
Supported by grants from the NIHR21CA 128193-01-A, VA Merit I01CX000333-02 to NR, UL1RR024986 and University of Michigan’s Cancer Center Support Grant (5 P30 CA46592), by the use of the Cancer Center Clinical Trials Office Core.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00280-013-2109-x) contains supplementary material, which is available to authorized users.
Conflict of interest None.
Contributor Information
N. Ramnath, Email: nithyar@umich.edu, Division of Medical Oncology, Department of Internal Medicine, University of Michigan Comprehensive Cancer Center, 1500 East Medical Center Drive, Ann Arbor, MI 48109, USA; Veterans Administration Ann Arbor Healthcare System, Ann Arbor, MI, USA.
S. Daignault-Newton, Biostatistics Core, Comprehensive Cancer Center, University of Michigan, Ann Arbor, MI, USA
G. K. Dy, Departments of Medicine and Pharmacology, Roswell Park Cancer Institute, Buffalo, NY, USA
J. R. Muindi, Departments of Medicine and Pharmacology, Roswell Park Cancer Institute, Buffalo, NY, USA
A. Adjei, Departments of Medicine and Pharmacology, Roswell Park Cancer Institute, Buffalo, NY, USA
V. L. Elingrod, College of Pharmacy, University of Michigan, Ann Arbor, MI, USA
G. P. Kalemkerian, Division of Medical Oncology, Department of Internal Medicine, University of Michigan Comprehensive Cancer Center, 1500 East Medical Center Drive, Ann Arbor, MI 48109, USA
K. B. Cease, Division of Medical Oncology, Department of Internal Medicine, University of Michigan Comprehensive Cancer Center, 1500 East Medical Center Drive, Ann Arbor, MI 48109, USA Veterans Administration Ann Arbor Healthcare System, Ann Arbor, MI, USA.
P. J. Stella, St. Joseph’s Hospital, Ann Arbor, MI, USA
D. E. Brenner, Division of Medical Oncology, Department of Internal Medicine, University of Michigan Comprehensive Cancer Center, 1500 East Medical Center Drive, Ann Arbor, MI 48109, USA Veterans Administration Ann Arbor Healthcare System, Ann Arbor, MI, USA.
S. Troeschel, Veterans Administration Ann Arbor Healthcare System, Ann Arbor, MI, USA
C. S. Johnson, Departments of Medicine and Pharmacology, Roswell Park Cancer Institute, Buffalo, NY, USA
D. L. Trump, Departments of Medicine and Pharmacology, Roswell Park Cancer Institute, Buffalo, NY, USA
References
- 1.Azzoli CG, Temin S, Aliff T, Baker S, Jr, Brahmer J, Johnson DH, et al. 2011 Focused Update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung Cancer. J Clin Oncol. 2011;29(28):3825–3831. doi: 10.1200/JCO.2010.34.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 3.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 4.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 5.Hershberger PA, McGuire TF, Yu WD, Zuhowski EG, Schellens JH, Egorin MJ, et al. Cisplatin potentiates 1,25-dihydroxyvitamin D3-induced apoptosis in association with increased mitogen-activated protein kinase kinase kinase 1 (MEKK-1) expression. Mol Cancer Ther. 2002;1(10):821–829. [PubMed] [Google Scholar]
- 6.Liu M, Lee MH, Cohen M, Bommakanti M, Freedman LP. Transcriptional activation of the Cdk inhibitor p21 by vitamin D3 leads to the induced differentiation of the myelomonocytic cell line U937. Genes Dev. 1996;10(2):142–153. doi: 10.1101/gad.10.2.142. [DOI] [PubMed] [Google Scholar]
- 7.Rassnick KM, Muindi JR, Johnson CS, Balkman CE, Ramnath N, Yu WD, et al. In vitro and in vivo evaluation of combined calcitriol and cisplatin in dogs with spontaneously occurring tumors. Cancer Chemother Pharmacol. 2008;62(5):881–891. doi: 10.1007/s00280-008-0678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fakih MG, Trump DL, Muindi JR, Black JD, Bernardi RJ, Creaven PJ, et al. A phase I pharmacokinetic and pharmacodynamic study of intravenous calcitriol in combination with oral gefitinib in patients with advanced solid tumors. Clin Cancer Res. 2007;13(4):1216–1223. doi: 10.1158/1078-0432.CCR-06-1165. [DOI] [PubMed] [Google Scholar]
- 9.Trump DL, Hershberger PA, Bernardi RJ, Ahmed S, Muindi J, Fakih M, et al. Anti-tumor activity of calcitriol: pre-clinical and clinical studies. J Steroid Biochem Mol Biol. 2004;89–90(1–5):519–526. doi: 10.1016/j.jsbmb.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 10.Muindi JR, Wilson JW, Peng Y, Capozolli MJ, Johnson CS, Trump DL. A limited sampling method for the estimation of serum calcitriol area under the curve in cancer patients. J Clin Pharmacol. 2003;43(8):894–900. doi: 10.1177/0091270003255925. [DOI] [PubMed] [Google Scholar]
- 11.Smith DC, Johnson CS, Freeman CC, Muindi J, Wilson JW, Trump DL. A Phase I trial of calcitriol (1,25-dihydroxycholecalciferol) in patients with advanced malignancy. Clin Cancer Res. 1999;5(6):1339–1345. [PubMed] [Google Scholar]
- 12.Muindi JR, Peng Y, Potter DM, Hershberger PA, Tauch JS, Capozzoli MJ, et al. Pharmacokinetics of high-dose oral calcitriol: results from a phase 1 trial of calcitriol and paclitaxel. Clin Pharmacol Ther. 2002;72(6):648–659. doi: 10.1067/mcp.2002.129305. [DOI] [PubMed] [Google Scholar]
- 13.Kepner JL, Chang MN. Samples of exact k-stage group sequential designs for Phase II and Pilot studies. Control Clin Trials. 2004;25(3):326–333. doi: 10.1016/j.cct.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Fossella F, Pereira JR, von Pawel J, Pluzanska A, Gorbounova V, Kaukel E, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol. 2003;21(16):3016–3024. doi: 10.1200/JCO.2003.12.046. [DOI] [PubMed] [Google Scholar]
- 15.Kubota K, Watanabe K, Kunitoh H, Noda K, Ichinose Y, Katakami N, et al. Phase III randomized trial of docetaxel plus cisplatin versus vindesine plus cisplatin in patients with stage IV non-small-cell lung cancer: the Japanese Taxotere Lung Cancer Study Group. J Clin Oncol. 2004;22(2):254–261. doi: 10.1200/JCO.2004.06.114. [DOI] [PubMed] [Google Scholar]
- 16.Muindi JR, Johnson CS, Trump DL, Christy R, Engler KL, Fakih MG. A phase I and pharmacokinetics study of intravenous calcitriol in combination with oral dexamethasone and gefitinib in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2009;65(1):33–40. doi: 10.1007/s00280-009-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G, Kim SH, King AN, Zhao L, Simpson RU, Christensen PJ, et al. CYP24A1 is an independent prognostic marker of survival in patients with lung adenocarcinoma. Clin Cancer Res. 2011;17(4):817–826. doi: 10.1158/1078-0432.CCR-10-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50(4):219–244. [PubMed] [Google Scholar]
- 19.Chadha MK, Tian L, Mashtare T, Payne V, Silliman C, Levine E, et al. Phase 2 trial of weekly intravenous 1,25 dihydroxy cholecalciferol (calcitriol) in combination with dexamethasone for castration-resistant prostate cancer. Cancer. 2010;116(9):2132–2139. doi: 10.1002/cncr.24973. [DOI] [PubMed] [Google Scholar]
- 20.Trump DL, Muindi J, Fakih M, Yu WD, Johnson CS. Vitamin D compounds: clinical development as cancer therapy and prevention agents. Anticancer Res. 2006;26(4A):2551–2556. [PubMed] [Google Scholar]
- 21.Shen H, Bielak LF, Ferguson JF, Streeten EA, Yerges-Armstrong LM, Liu J, et al. Association of the vitamin D metabolism gene CYP24A1 with coronary artery calcification. Arterioscler Thromb Vasc Biol. 2010;30(12):2648–2654. doi: 10.1161/ATVBAHA.110.211805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wjst M, Heimbeck I, Kutschke D, Pukelsheim K. Epigenetic regulation of vitamin D converting enzymes. J Steroid Biochem Mol Biol. 2010;121(1–2):80–83. doi: 10.1016/j.jsbmb.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 23.Muindi JR, Nganga A, Engler KL, Coignet LJ, Johnson CS, Trump DL. CYP24 splicing variants are associated with different patterns of constitutive and calcitriol-inducible CYP24 activity in human prostate cancer cell lines. J Steroid Biochem Mol Biol. 2007;103(3–5):334–337. doi: 10.1016/j.jsbmb.2006.12.060. [DOI] [PubMed] [Google Scholar]
- 24.Roff A, Wilson RT. A novel SNP in a vitamin D response element of the CYP24A1 promoter reduces protein binding, transactivation, and gene expression. J Steroid Biochem Mol Biol. 2008;112(1–3):47–54. doi: 10.1016/j.jsbmb.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St-Arnaud R, Arabian A, Travers R, Barletta F, Raval-Pandya M, Chapin K, et al. Deficient mineralization of intramembranous bone in vitamin D-24-hydroxylase-ablated mice is due to elevated 1,25-dihydroxyvitamin D and not to the absence of 24,25-dihydroxyvitamin D. Endocrinology. 2000;141(7):2658–2666. doi: 10.1210/endo.141.7.7579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.