Abstract
Objective
To determine the effects of weight-bearing (WB) versus nonweight-bearing (NWB) exercise for persons with diabetes mellitus (DM) and peripheral neuropathy (PN).
Design
Randomized controlled trial with evaluations at baseline and after intervention.
Setting
University-based physical therapy research clinic.
Participants
Participants with DM and PN (N=29) (mean age ± SD, 64.5±12.5y; mean body mass index [kg/m2] ± SD, 35.5±7.3) were randomly assigned to WB (n=15) and NWB (n=14) exercise groups. All participants (100%) completed the intervention and follow-up evaluations.
Interventions
Group-specific progressive balance, flexibility, strengthening, and aerobic exercise conducted sitting or lying (NWB) or standing and walking (WB) occurred 3 times a week for 12 weeks.
Main Outcome Measures
Measures included the 6-minute walk distance (6MWD) and daily step counts. Secondary outcome measures represented domains across the International Classification of Functioning, Disability and Health.
Results
The WB group showed greater gains than the NWB group over time on the 6MWD and average daily step count (P<.05). The mean and 95% confidence intervals (CIs) between-group difference over time was 29m (95% CI, 6–51) for the 6MWD and 1178 (95% CI, 150–2205) steps for the average daily step count. The NWB group showed greater improvements than the WB group over time in hemoglobin A1c values (P<.05).
Conclusions
The results of this study indicate the ability of this population with chronic disease to increase 6MWD and daily step count with a WB exercise program compared with an NWB exercise program.
Keywords: Diabetes mellitus, Exercise, Rehabilitation
Persons with diabetes mellitus (DM) and lower-extremity pathology, such as peripheral neuropathy (PN), have an almost 3-fold increase in risk of limited mobility compared with those having neither.1 The most frequently reported mobility limitations are related to an inability to walk a quarter mile and to climb 10 steps without resting.1 Gregg,2 Volpato,3 and colleagues report substantial functional limitations, especially in weight-bearing (WB) activities (ie, limitations in walking 2–3 blocks) in women with DM, and relate this limitation to PN.
Although considerable research has documented the benefits of moderately intense physical activity (ie, brisk walking) for those with DM,4-6 little research has been conducted investigating the effects of exercise among people with DM and PN, perhaps because of investigator concerns regarding exercise-related injury to participants’ insensitive feet and skepticism that exercise could be beneficial. The most common contributor for diabetic plantar ulcers is high plantar stresses in the presence of sensory neuropathy and foot deformity.7,8 Historically, people with DM and PN have been advised to avoid WB activity,9 but inactivity may contribute to the deconditioning of the skin and lowering tolerance for WB activities.10 Several studies provide evidence to support the hypothesis that people with DM and PN who are less active are at greater risk for skin breakdown than those who are more active.11-13 In addition, the Feet First randomized controlled trial demonstrated that people with DM and PN in a community-based, relatively low-intensity intervention, can increase bout-related daily steps (14% after 6mo) without an increase in skin breakdown.14
The current study provided a more intensive and progressive intervention than the Feet First14 program using supervised WB (eg, treadmill walking) and nonweight-bearing (NWB) (eg, stationary bicycle ergometer) exercise approaches. The purpose of this prospective randomized controlled trial was to determine the effect of a WB exercise program compared with an NWB exercise program on the primary outcome measures of the 6-minute walk distance (6MWD) and daily step counts (steps/d). Secondary outcome measures represented domains across the International Classification of Functioning, Disability and Health. We hypothesized that the WB exercise would show greater improvements in primary outcomes compared with the NWB exercise group.
Methods
Informed consent was obtained from all participants who agreed to participate with a form approved by the institutional review board.
Inclusion criteria
Participants were required to have type 2 DM, PN (inability to sense the 5.07 Semmes-Weinstein monofilament on at least 1 spot on the plantar foot and inability to sense vibration at the plantar great toe from a biothesiometer at <25V), have a step count 2000 to 9000 steps per day, currently exercising <3 times per week, <20 minutes per session, and have approval of their primary physician to participate in the study.
Exclusion criteria
Participants were excluded who weighed more than 136 kilograms (scanner weight limit used in a different portion of study), had a severe foot deformity that would require custom therapeutic footwear, or had a comorbidity or took a medication that would interfere with ability to exercise according to the current American Diabetes Association guidelines.9
Sample size and recruitment
Recruitment began in 2009 and was terminated in 2011. Because the natural tendency in this population is for walking ability to decline,14 we thought a 20% increase in average daily step count would be meaningful. Armstrong et al15 reported that this population takes 4548±779 steps per day. Assuming the NWB group would not show a difference in average daily step count, a 20% (910 steps) between-group difference would result in an effect size equal to 1.15 SD units. With an estimated alpha of .05, power of .80, and an effect size of 1.15 SD units, an a priori power analysis estimated a recruitment sample size of 14 in each of the 2 exercise groups for the primary outcome variables. Although the a priori estimated sample size needed for average daily step count was 14 in each group, we had planned to recruit 32 subjects in each group because of possible attrition and smaller estimated effect sizes for secondary outcome variables. Attrition was low, but recruiting participants who met the criteria and were willing to exercise was challenging (fig 1), and we stopped recruitment with the number of subjects described in this study.
Fig 1.
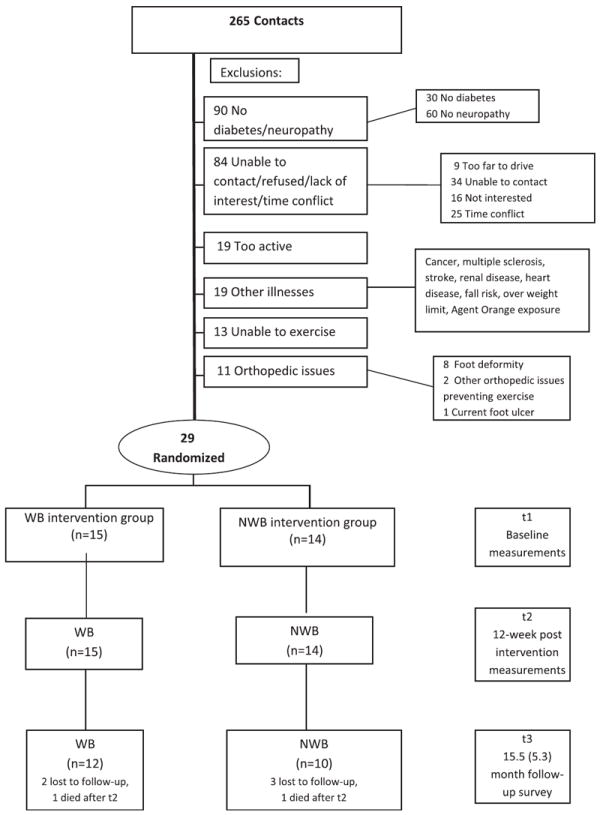
Flowchart diagram.
Participants were recruited from our database of previous participants, the Washington University School of Medicine Research Participant Registry, cable television commercials, a newspaper story, and recruitment posters displayed in a Diabetes Treatment Center and on area commuter trains. Participants were given $10 in cash at the completion of every visit to cover travel expenses and serve as an incentive for attendance, and an additional $50 was given for completing final testing.
Design and randomization
Participants were randomized into 2 groups (WB, NWB) using a prearranged schedule generated by the statistician (M.JS.) using a computer program. Allocation was concealed to all except the research coordinator who entered subjects into the study. Participant characteristics are summarized in table 1; there were 15 and 14 participants in the WB and NWB groups, respectively. There were no significant differences between groups in any of the characteristics (P>.05).
Table 1.
Participant characteristics
| Characteristics | WB Group | NWB Group |
|---|---|---|
| No. of participants | 15 | 14 |
| Male/female | 10/5 | 7/7 |
| Age (y) | 65.2±12.8 | 63.9±12.5 |
| Duration of DM (y) | 11.4±8.1 | 13.4±5.4 |
| Body mass index (kg/m2) | 36.8±6.3 | 33.1±7.3 |
| Neuropathy–biothesiometer (V) | 44.1±8.6 | 45.0±8.7 |
| No. of comorbidities | 2.3±1.7 | 1.7±1.2 |
| Cardiac procedures/conditions | 11 | 6 |
| Hypertension | 11 | 11 |
| History of cancer | 4 | 3 |
| History of foot ulcer | 2 | 2 |
NOTE. Values are mean ± SD or as otherwise indicated. There was no difference found between groups in any measures (P>.05).
Interventions
All participants exercised, as able, in 1-hour group sessions (1–4 participants per group), 3 times per week for 12 weeks, which were supervised by a physical therapist and an assistant. Duration and intensity were matched between groups as closely as possible. Target heart rate was intended to be 60% to 70% of the age-predicted maximum, and activity was adjusted to stay within those limits using a heart rate monitor and a rating of perceived exertion between 11 to 13 on a 6 to 20 scale.9 Intensity for all exercises was individualized with the intent to exceed their routine physical stress level (based on daily community-based step counts), and therefore incur positive adaptations to physical stress, but not exceed their estimated intensity for injury.10,14,16-19 Exercise participation was modified, postponed, or stopped based on the current guidelines of the American Diabetes Association.9 The exercise sessions began with 20 minutes of group-specific flexibility and stretching exercises (appendix 1) followed by strengthening exercises (appendix 2) and aerobic exercise (appendix 3).
To help avoid skin injury, all exercises included in this study, except for the heel rise, had peak plantar pressures that were less than or equal to those during level walking.20 Furthermore, the physical therapist and the participant each performed a visual inspection of the participant’s feet and footwear, and recorded foot skin temperature using a handheld infrared thermometera before and after each session, as described previously.21 Initially, participants were not allowed to continue exercising if pretest temperature differences were >2.2°C when compared across feet,21 but because there was a high rate (20% on first 26 participants) of false positives (ie, temperature differences of >2.2°C despite no visible lesion, redness, or progression of lesion regardless of activity level), the study data safety monitoring committee agreed to discontinue use of the temperature monitoring as part of required precautions. Participants wore their own athletic or walking shoes that passed a screen for excessive wear, fit (length and width), accommodation of bony deformities, and areas of high pressure.22 Participants with footwear that did not meet the criteria were helped to select appropriately fitting shoes.
WB exercise program
Baseline duration of walking was individually calculated based on participants’ own average daily step count collected over 7 days using the StepWatch Activity Monitor.b Participants were instructed to increase their center-based step count every 2 weeks by 24% on the 3 days that they participated in the exercise program, thus resulting in an average increase in their daily step count by 10% during that 2-week period (see appendix 3). The WB group conducted most exercises in a standing position, used body weight for resistance exercises (ie, sit to stand, stair climbing), and a treadmill or walking around a large circular hallway for aerobic exercise.
NWB exercise program
The NWB group conducted all exercises in a sitting or lying position. They used elastic resistance bandsc with increasing stiffness for load resistance and a stationary upright or recumbent cycle ergometer for aerobic exercise. Duration of stationary bicycle time started with the time predicted from the participants’ average daily step counts and was increased every 2 weeks in a similar fashion to the WB group (see appendix 3).
Outcome measurements
Full testing occurred immediately before and after the 12-week intervention period. All outcome measures were collected and analyzed by a tester blinded to group assignment, except for the posttreatment 6MWD, which was conducted by a physical therapist who also provided some treatment. All measures were collected in a physical therapy laboratory except the blood draws for hemoglobin A1c, which were collected at a hospital outpatient laboratory.
Six-minute walk distance
The 6MWD was performed as a measure of physical function and walking endurance. The participants walked in a hallway and were told that the goal was to walk as far as possible in 6 minutes. The test has been validated in obese adults.23 A meaningful change in score is considered to be greater than 20m (65.6ft).24
Step activity monitoring
Average daily step count was estimated using the StepWatch Activity Monitor, an accelerometer attached to the participant’s ankle, which provides a time stamped recording of strides (1 stride equals 2 steps). We used an average steps per day for a 7-day period collected over 14 days, a reliable and valid measure of overall activity levels.11 For a day to be included, the activity had to be apparent for at least 8 hours a day, and at least 1 weekend day was included in the 7-day average.
Secondary outcome measures
The Foot and Ankle Ability Measure is a self-report measure of physical function and investigates the participant’s perception of 26 activities of daily living (ie, walking on even ground and up hills). We report the participant’s overall perception (0%–100%) of foot and ankle ability.25 The Beck Depression Inventory-II was used to assess impact of the exercise program on negative affect.26 Higher scores correspond to higher levels of depression. A 9-item Physical Performance Test was used to measure functional limitations.27 Hemoglobin A1c was used as an indicator of blood glucose control, while fat free mass was measured using dual-energy x-ray absorptiometryd as an indicator of body composition.28 Right plantar flexion peak torque was measured while participants were sitting using a Biodex isokinetic dynamometere with an angular velocity of 60° per second as an indicator of ankle muscle strength impairment. Right dorsiflexion range of motion was measured prone with the knee extended as a measure of ankle joint impairments.29,30
Skin lesions on the lower leg were monitored to document the safety of the interventions. All surfaces of the foot were photographed before and after treatment using a digital camera and stored electronically. If the treating therapist observed any break in the skin, they completed a wound documentation form describing size (width, length, depth), location, apparent reason for the wound, and the action taken. Pictures and forms were sent to 2 blinded adjudicators (and a third if there was disagreement). Wounds were graded as a lesion (superficial injury, eg, abrasion, laceration, blister, or maceration) or an ulcer (full thickness skin wound through the dermis).
A follow-up survey was sent to participants a mean time ± SD of 15.5±5.3 months after they completed participation in their intervention to understand better their perspective of the value of the exercise program and their current exercise/skin monitoring habits.
Data analysis
Statistical analysis on an intention-to-treat basis was performed using the Statistical Package for the Social Sciences softwaref; alpha was set to .05. A 2 group (WB, NWB) × 2 time (pre- and posttesting) repeated-measures analysis of variance was used.31 Analyses focused on between-group differences over time, that is, whether the repeated-measures analysis of variance for group × time interaction was significant. Mean between- and within-group differences over time with a 95% confidence interval (CI) are reported.
Results
All 29 participants completed the 12-week intervention. The WB and NWB groups attended mean ± SD 83.4%±11.0%, and 83.3%±10.8% of total exercise sessions, respectively. Results are presented in table 2.
Table 2.
Summary of results of outcome variables
| Variable | Group | Pretest Value Mean ± SD | Posttest Value Mean ± SD | Mean Within-Group Time Difference (95% CI) | Mean Between-Group Difference, Change Over Time (95% CI) | Group × Time Interaction P |
|---|---|---|---|---|---|---|
| Primary variables | ||||||
| 6MWD (m) | WB | 378±72 | 404±78 | 27 (11 to 42) | ||
| NWB | 418±106 | 417±112 | −2 (−18 to 14) | 29 (6 to 51) | .014 | |
| Average daily step | WB | 4909±1398 | 5593±1449 | 685 (−29 to 1399) | ||
| count (steps) | NWB | 6571±2186 | 6078±2023 | −493 (−1232 to 246) | 1178 (150 to 2205) | .026 |
| Secondary variables | ||||||
| Overall perception, | WB | 73.0±21.6 | 83.7±12.5 | 10.7 (1.8 to 19.5) | ||
| FAAM (0–100) (%) | NWB | 79.5±16.8 | 85.2±13.7 | 5.7 (−3.8 to 15.2) | 5 (−8 to 17.9) | NS |
| Beck Depression | WB | 7.7±5.8 | 5.8±4.8 | −1.9 (−4.1 to 0.3) | ||
| Inventory (0−63) | NWB | 7.9±7.1 | 5.3±3.8 | −2.6 (−4.9 to −0.4) | 0.8 (−2.4 to 4.0) | NS |
| Physical Performance | WB | 28.1±4.6 | 29.5±4.9 | 1.4 (0.04 to 2.8) | ||
| Test (9 items; 36 max) | NWB | 27.1±4.6 | 28.7±4.2 | 1.6 (0.2 to 3.0) | −0.2 (−2.1 to 1.8) | NS |
| Glycated hemoglobin | WB | 6.9±1.3 | 7.0±1.3 | 0.1 (−0.2 to 0.4) | ||
| (HbA1c) (%) | NWB | 7.8±2.1 | 7.4±1.6 | −0.4 (−0.8 to −0.1) | 0.5 (0.03 to 0.96) | .037 |
| Fat free mass | WB | 63.5±11.6 | 63.3±11.5 | −0.2 (−1.2 to 0.8) | ||
| DXA (kg) | NWB | 57.3±11.6 | 57.9±11.9 | 0.6 (−0.5 to 1.6) | −0.8 (−2.2 to 0.6) | NS |
| Plantar flexion | WB | 38.0±20.3 | 42.8±24.2 | 4.8 (−2.6 to 12.1) | ||
| Peak torque (Nm) | NWB | 38.4±12.6 | 39.1±12.1 | 0.7 (−6.9 to 8.2) | 4.1 (−6.5 to 14.6) | NS |
| Dorsiflexion | WB | 3.6±6.9 | 7.7±4.2 | 4.1 (1.7 to 6.5) | ||
| Range of motion (deg) | NWB | 3.1±4.7 | 5.5±5.2 | 2.4 (−0.1 to 4.9) | 1.7 (−1.8 to 5.2) | NS |
Abbreviations: DXA, dual-energy x-ray absorptiometry; FAAM, Foot and Ankle Ability Measure; max, maximum; NS, not significant.
The WB group showed greater gains than the NWB group over time (significant interactions) in the primary outcomes of the 6MWD and average daily step count (P<.05). The mean between-group difference over time was 29m (95% CI, 6–51) for the 6MWD and 1178 steps (95% CI, 150–2205) for the average daily step count.
The NWB group showed greater improvements than the WB group over time (significant interaction) in hemoglobin A1c values (P<.05). The mean between-group difference over time was .50% (95% CI, .03–.96). There were no other between-group over time differences in outcome measures.
Adverse events
There were a total of 13 lesions and 4 ulcers observed during the study (table 3). One person in the WB group had a calf strain during treadmill walking, but was able to continue to exercise with a lower intensity (shorter time on treadmill, fewer heel raises), and the strain resolved within 1 week. Three of 14 participants in the NWB group modified their stationary cycle aerobic activity a total of 3 occasions, and 6 of the 15 participants in the WB group modified (12 occasions) or deferred (8 occasions) their treadmill aerobic training because of pain.
Table 3.
Characterizations of skin breakdown: lesions and ulcers
| Group | Lesions by Group and Location on Foot (13 lesions in 12 participants)
|
|||
|---|---|---|---|---|
| Total No. of Lesions | No. of Participants With a Lesion | No. on WB Surface of Foot | No. on NWB Surface of Foot | |
| WB | 7 | 7 | 2 | 5 |
| NWB | 6 | 5 | 0 | 6 |
|
| ||||
| Ulcers by Group and Location on Foot (4 ulcers on 3 participants)
|
||||
| Group | Total No. of Ulcers/ Participants | No. of Participants With an Ulcer | No. on WB Surface of Foot | No. on NWB Surface of Foot |
|
| ||||
| WB | 1 | 1 | 1 | 0 |
| NWB | 3 | 2 | 3 | 0 |
NOTE. All lesions were superficial (ie, not full thickness wound) (2–5mm), except for 3 superficial scratches. Average time to heal was 8.8±7.2 days. Average size of the 4 ulcers was 12.5×16×2-mm deep. Average time to heal was 20.7±15.8 days except for 1 ulcer, which was not healed at the end of the intervention. The data are for descriptive purposes, because the study was not powered to detect differences in lesions or ulcers between groups.
Follow-up questionnaire
We received 22 completed surveys a mean time ± SD of 15.5±5.3 months after completion of their intervention (table 4). During this follow-up period, 1 participant had died in each group unrelated to the study and the 5 others did not respond to mailings or phone calls. In brief, 86% reported feeling better as a result of their participation in the exercise program, and 41% reported they were still exercising 3 to 7 days a week.
Table 4.
Follow-up questionnaire (percent answered per questionnaires returned)
| Questions | NWB (n=10) |
WB (n=12) |
Total (N=22) |
|---|---|---|---|
| Overall, do you think you feel better, worse, or about the same because of your participation in the exercise program? | |||
| (1) Better | 90 | 83 | 86 |
| (2) Worse | 0 | 8 | 5 |
| (3) No different | 10 | 8 | 9 |
| In your opinion, how strenuous was the exercise program? | |||
| (1) Too easy | 20 | 17 | 18 |
| (2) Just right | 80 | 83 | 82 |
| (3) Too difficult | 0 | 0 | 0 |
| What were your thoughts of the exercise program in this study? (circle all that apply) | |||
| (1) Too far away | 0 | 8 | 5 |
| (2) Fun | 50 | 92 | 73 |
| (3) Time consuming (tedious) | 0 | 0 | 0 |
| (4) Just the right amount of time | 60 | 58 | 59 |
| (5) Exercise times were convenient | 80 | 92 | 86 |
| (6) Exercise times not convenient | 0 | 0 | 0 |
| (7) Confidence building | 60 | 83 | 73 |
| (8) Positive lifestyle changes | 50 | 58 | 55 |
| Would you participate in another exercise program? | |||
| (1) Yes | 100 | 58 | 77 |
| (2) No | 0 | 0 | 0 |
| (3) Not sure | 0 | 42 | 23 |
| How often are you exercising? | |||
| (1) 7d/wk | 20 | 8 | 14 |
| (2) 3–6d/wk | 20 | 33 | 27 |
| (3) 1–3d/wk | 40 | 33 | 36 |
| (4) <1d/wk | 10 | 0 | 5 |
| (5) I never exercise for at least 20 minutes at a time | 10 | 25 | 18 |
| How often do you check your feet? | |||
| (1) 7d/wk | 40 | 67 | 55 |
| (2) 3–6d/wk | 30 | 25 | 27 |
| (3) 1–3d/wk | 20 | 8 | 14 |
| (4) I never check my feet | 10 | 0 | 5 |
| Do you check your feet more, less, or about the same amount compared with before you were in the study? | |||
| (1) More | 60 | 58 | 59 |
| (2) Less | 10 | 0 | 5 |
| (3) Same | 30 | 33 | 32 |
| Since your participation, have you had any skin breakdown or injuries on your feet? | |||
| (1) Yes | 0 | 8* | 5 |
| (2) No | 100 | 92 | 95 |
Participant reports burning skin on feet from soaking feet in water that was too hot.
Discussion
Consistent with our hypothesis, the WB exercise group showed greater gains over time compared with the NWB exercise group in the primary outcomes of the 6MWD and average daily step count (see table 2). While one would expect WB exercise to have a greater impact on walking ability than NWB exercise, it is only recently that this population has been encouraged to walk,5,9 and the effects of a progressive walking program are mostly unknown. These improvements are somewhat greater than those achieved by the Feet First study intervention, which reported no change in the 6MWD, no change in total daily steps, and a 14% increase in average daily steps in 30 minutes after the 6-month community intervention program.14 The methods and exercise intervention in the current study were more intensive (3 times per week supervised by a physical therapist vs 8 supervised sessions combined with home exercise 3 times per week) but over a shorter duration (12wk vs 6mo) than those used in the Feet First study. While the overall activity level is still low, these improvements are important given the natural tendency for activity in this group to decline (13% decrease in daily step count over 1y in the Feet First control group).14
There were benefits observed in the NWB group that were not observed in the WB group. The NWB group showed an improvement in hemoglobin A1c values, similar to another recent study investigating the effect of exercise on people with DM and PN.32 Post hoc analysis on actual time spent performing aerobic exercise indicated that the NWB group started at a higher duration (14.4±3.9min vs 11.4±2.9min, P=.027) and ended at a higher duration (26.6±6.5min vs 18.7±4.9min, P=.032) of aerobic exercise. This increased volume of exercise may have been enough to help improve hemoglobin A1c values. Those in the NWB group also had fewer complaints of lower-extremity musculoskeletal pain during aerobic exercise than the WB group. Consistent with other recent recommendations,5,14,32 we believe people with DM and PN who do not have severe foot deformity or open ulcers should be given the choice to exercise in a WB or NWB capacity, and that exercise should be tailored to match their personal goals.
The lesions that occurred during this study generally were small, healed quickly (see table 3), and were consistent with recent studies of those with DM and PN, showing minimal training related adverse events.14,32 Importantly, 3 of the 4 ulcers occurred in the 5 participants with history of a previous ulcer. Reports on annual population-based incidence (new onset) of diabetic foot ulcers range between 1.0% and 4.1%,33 but in those with a history of skin breakdown, ulcers reoccur at a rate of 20% to 70% a year.34,35 Additional research is needed to determine the value and safety of WB and NWB exercise for people with history of ulcers and for those with severe foot deformity.36 Research also is needed to determine if these positive results can be translated into community settings.
We believe there were a number of reasons for the low dropout rate and high adherence rate in this study. Participants were provided with $10 at each visit to cover transportation expenses and provide an incentive for adherence. While not consistent with clinical care, this approach appeared to motivate adherence substantially. In addition, each person’s exercise program was individually tailored to their current ability and activity level. The overall exercise program was considered moderate, and participants generally (82%) thought this intensity level was just right (see table 4). Furthermore, participants were under close supervision of their skin and vital signs using a small group (1–4) approach, which seemed to foster a sense of safety, community, and accountability.
Study limitations
The study had a small number of participants and was not powered adequately to determine group differences in secondary outcomes. Between-group differences over time for the primary variables, although significant, had a wide 95% CI with the potential for a low treatment effect. We believe there is potential for greater improvement with a higher exercise intensity and/or duration. The aerobic exercise duration, especially for the WB group, was not as much as we had hoped. We underestimated the number of additional steps needed for a 10% increase each week, because we based the increase on time duration of walking at a step rate of 100 steps per minute (see appendix 3), but participants walked slower than that.37 This study also had limited follow-up. We focused on the controlled, short-term effects of moderate exercise in an understudied, high-risk population, but longer-term follow-up with a larger sample size and greater exercise duration is needed. Furthermore, we used a blinded tester for most measures, but we should have used a blinded tester for the 6MWD. We acknowledge this limitation but contend that any bias was minimized by using highly consistent and standardized instructions. Finally, these participants were selected from a much broader range of people with DM and PN (see fig 1), and results can be generalized only to those meeting the inclusion and exclusion criteria of this study.
Conclusions
People in the WB exercise group showed greater gains in daily step count and 6MWD compared with those in the NWB exercise group, while those in the NWB group showed greater improvements in hemoglobin A1c values compared with those in the WB group. Additional research is required to determine whether higher intensity/duration and a combination of WB and NWB exercise would improve outcomes further, without compromising safety, and if results can be translated to a community setting.
Suppliers
Xilas Medical Inc. 3819 Harry Wurzbach Rd, San Antonio, TX 78209.
Orthocare Innovations, 840 Research Pkwy, Ste 200, Oklahoma City, OK 73104.
Theraband; Hygenic Corp, 1245 Home Ave, Akron, OH 44310.
Hologic, Waltham, 250 Campus Dr, Marlborough, MA 02451.
Biodex Medical Systems, 20 Ramsey Rd, Shirley, NY 11967.
SPSS version 16.0; SPSS Inc, 233 S Wacker Dr, 11th Fl, Chicago, IL 60606.
Acknowledgments
We thank Cindi Inman, Kay Bohnert, Kshamata Shah, Ellen Frye, Erin McKeague, and Molly Burns for providing valuable assistance with participant testing, interventions, data entry, and data analysis.
From the National Institutes of Health (grant nos. NCMRR R21 HD058938, T32 HD007434-17 NSMRC R24HD650837, NIH UL1 RR024992), Diabetes Research Training Center (grant no. 5 P60 DK20579), and scholarships from the Foundation for Physical Therapy.
List of abbreviations
- CI
confidence interval
- DM
diabetes mellitus
- NWB
nonweight bearing
- PN
peripheral neuropathy
- 6MWD
6-minute walk distance
- WB
weight bearing
Appendix 1 Exercise Program
Flexibility and stretching exercises
Knee flexion, face lying (both exercise groups)
Lie face down with your legs straight and relatively close together
Bend your right knee
Don’t let your back move as you bend your knee
Hold for 30 seconds then return your leg to starting position
Repeat with left leg
Repeat entire sequence 3 times
Hands and knees rocking back (both exercise groups)
Get on your hands and knees
Rock back toward your heels, keeping your back straight
Return to start position
Repeat 3 times
Hamstring stretch (both exercise groups)
Lie on your back
Clasp your right thigh and pull it toward you
Extend your knee, keeping your back and thigh still, until you feel a gentle stretch in the back of your right thigh
Hold stretch 30 seconds, return to start position
Repeat with left leg
Repeat entire sequence 3 times
Sitting calf stretch (NWB group)
Sit in a chair with your knee extended
Place a towel around the bottom of your right foot
Pull the towel toward you till you feel a stretch in your calf
Hold stretch 30 seconds, return to start position
Repeat with left leg
Repeat entire sequence 3 times
Standing calf stretch (WB group)
Stand facing the wall
Lean to the wall and place right foot forward
Make sure your foot is facing straight forward, not turned out to the side
Keeping your back heel on the ground, lean forward till gentle stretch is felt in your calf
Hold stretch 30 seconds, return to start position
Repeat with left leg
Repeat entire sequence 3 times
Toe stretch (both exercise groups)
Sitting, cross right foot up onto thigh
Grasp toes of right foot with your hand and curl the toes down
Then point ankle and foot down (in the direction you push on the gas pedal)
Hold 30 seconds
Repeat with left leg
Repeat entire sequence 3 times
Appendix 2
Balance and strengthening program
| Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | Level 6 |
|---|---|---|---|---|---|
| NWB group balance and strengthening program | |||||
| 1. Toe crunches (10 reps, 2 sets) | 1. Toe crunches (15 reps, 2 sets) | 1. Toe crunches (20 reps, 2 sets) | 1. Toe crunches (25 reps, 2 sets) | 1. Toe crunches (30 reps, 2 sets) | 1. Toe crunches (35 reps, 2 sets) |
| 2.Knee lifts while seated, back unsupported, arms supporting at side (10 reps, 2 sets) | 2. Knee lifts while seated, back unsupported, arms across chest (10 reps, 2 sets) | 2. Sit on inflatable ball, knee lifts with 2-hand support (10 reps, 2 sets) | 2. Sit on inflatable ball, knee lifts, arms supporting at side (10 reps, 2 sets) | 2. Sit on inflatable exercise ball, march, arms across chest (10 reps, 2 sets) | 2. Sit on inflatable exercise ball, march, with alternating arm raises (10 reps, 2 sets) |
| 3. Sit on an inflatable ball, arms to side (10 seconds, 2 reps) | 3. Sit on an inflatable ball, arms across chest (10 seconds, 2 rep) | 3. Sit on inflatable ball, use legs to reach toward objects on the floor (10 objects, 2 times) | 3. Sit on inflatable ball, reach for objects just beyond arm length away (10 objects, 2 times) | 3. Sit on inflatable exercise ball, catch a beach ball (10 seconds, 2 reps) | 3. Sit on inflatable exercise ball, catch a weighted ball (10 seconds, 2 reps) |
| 4. Sitting Theraband (yellow) around knees, abduct hip (10 reps, 2 sets) | 4. Lying on side, feet together, open knee (10 reps, 2 sets) | 4. Lying on side, feet together, open knee and lift leg (10 reps, 2 sets) | 4. Lying on side, leg lift with progressive weight (10 reps, 2 sets) | 4. Lying on side, leg lift with progressive weight (10 reps, 2 sets) | 4. Lying on side, leg lift with progressive weight (10 reps, 2 sets) |
| 5. Sitting Theraband (yellow) single leg press, each side (10 reps, 2 sets) | 5. Sitting Theraband (red) single leg press, each side (10 reps, 2 sets) | 5. Sitting Theraband (green) single leg press, each side (10 reps, 2 sets) | 5. Sitting Theraband (blue) single leg press, each side (10 reps, 2 sets) | 5. Sitting Theraband (black) single leg press, each side (10 reps, 2 sets) | 5. Sitting Theraband (gray) single leg press, each side (10 reps, 2 sets) |
| 6. Theraband (yellow) resisted dorsiflexion (10 reps, 2 sets) | 6. Theraband (red) resisted dorsiflexion (10 reps, 2 sets) | 6. Theraband (green) resisted dorsiflexion (10 reps, 2 sets) | 6. Theraband (blue) resisted dorsiflexion (10 reps, 2 sets) | 6. Theraband (black) resisted dorsiflexion (10 reps, 2 sets) | 6. Theraband (gray) resisted dorsiflexion (10 reps, 2 sets) |
| 7. Theraband (yellow) resisted plantarflexion (10 reps, 2 sets) | 7. Theraband (red) resisted plantarflexion (10 reps, 2 sets) | 7. Theraband (green) resisted plantarflexion (10 reps, 2 sets) | 7. Theraband (blue) resisted plantarflexion (10 reps, 2 sets) | 7. Theraband (black) resisted plantarflexion (10 reps, 2 sets) | 7. Theraband (gray) resisted plantarflexion (10 reps, 2 sets) |
| WB group balancing and strengthening program | |||||
| 1. Toe crunches (10 reps, 2 sets) | 1. Toe crunches (15 reps, 2 sets) | 1. Toe crunches (20 reps, 2 sets) | 1. Toe crunches (25 reps, 2 sets) | 1. Toe crunches (30 reps, 2 sets) | 1. Toe crunches (35 reps, 2 sets) |
| 2. 1-leg stand with bilateral hand support (30 seconds, 2 times) | 2. 1-leg stand with 1-hand support (30 seconds, 2 times) | 2. 1-leg stand, no hand support (30 seconds, 2 times) | 2. Stand with 2 feet on balance disk, bilateral hand support (30 seconds, 2 times) | 2. Stand with 2 feet on balance disk, no hand support (30 seconds, 2 times) | 2. 1-leg stand on balance disk, hand support as needed (30 seconds, 2 times) |
| 3. Step sideways and then step backwards with 1-hand support (10 steps, 2 times) | 3. Step sideways and then step backwards with no hand support (10 steps, 2 times) | 3. Step sideways and then backwards on exercise mat, 1-hand support (10 steps, 2 times) | 3. Step sideways and then backwards on exercise mat, no hand support (10 steps, 2 times) | 3. Step over objects, no hand support (5 objects, 3 times) | 3. Step over objects, no hand support (10 objects, 3 times) |
| 4. 2-leg heel stand (toes up), back against wall (5 reps, 2 sets) | 4. 2-leg heel stand (toes up), back against wall (10 reps, 2 sets) | 4. 2-leg heel stand (toes up), back against wall (15 reps, 2 sets) | 4. 2-leg heel stand (toes up), back against wall (20 reps, 2 sets) | 4. Single-leg heel stand (toes up), back against wall (10 reps, 2 sets) | 4. Single-leg Heel Stand (toes up), back against wall (15 reps, 2 sets) |
| 5. 2-leg heel raises (5 reps, 2 sets) | 5. 2-leg heel raises (10 reps, 2 sets) | 5. 2-leg heel raises (15 reps, 2 sets) | 5. 2-leg heel raises (20 reps, 2 sets) | 5. Single-leg heel raises (5 reps, 2 sets) each side | 5. Single-leg heel raises (10 reps, 2 sets) each side |
| 6. Sit to stand (3 reps, 2 sets) | 6. Sit to stand (5 reps, 2 sets) | 6. Sit to stand (7 reps, 2 sets) | 6. Sit to stand (10 reps, 2 sets) | 6. Sit to stand (12 reps, 2 sets) | 6. Sit to stand (15 reps, 2 sets) |
| 7. Step ups, with 2-hand support (10 reps, 2 sets) | 7. Step ups, with 1-hand support (10 reps, 2 sets) | 7. Step ups, with no hand support (10 reps, 2 sets) | 7. Stair climbing, with 1-hand support (up and down 5 stairs, 2 times) | 7. Stair climbing, with 1-hand support (up and down 1 flight, 2 times) | 7. Stair climbing, hand support as needed (up and down up to 4 flights, 2 times) |
Appendix 3 Aerobic Exercise Intervention for Both Groups
Progressive walking program for the WB group
The goal was to increase daily average step count by about 10% every 2 weeks. Participants were instructed and supervised to increase their daily step count by 24% on the 3 days that they participated in the exercise program according to the subsequent schedule. Increasing average step count by about 24% on exercise days while maintaining their usual step rate on off days would result in a 10% increase in average daily step count, which was progressed another 10% every 2 weeks.
Progressive stationary bike program for the NWB group
Subjects in this group participated in the same duration of extra minutes of activity, but they exercised on the stationary bike rather than walking. They were encouraged to maintain their usual activity level (ie, steps per day) outside of the treatment time.
| Starting Average Daily Step Count (SAM data) | Goal for Next 2wk | 24% Step Increase During Training (3x/wk) | Extra Minutes of Walking or Biking (100 steps/min) Per Tx Session | Average Step Per Day Per Week Given 3d Train 4d Regular |
|---|---|---|---|---|
| 3000 | 3300 | 720 | 7 | 3309 |
| 3309 | 3639 | 794 | 8 | 3649 |
| 3649 | 4014 | 876 | 9 | 4024 |
| 4024 | 4427 | 966 | 10 | 4438 |
| 4438 | 4882 | 1065 | 11 | 4895 |
| 4895 | 5384 | 1175 | 12 | 5398 |
| 5398 | 5938 | 1296 | 13 | 5953 |
| 5953 | 6549 | 1429 | 14 | 6566* |
| 6566 | 7222 | 1576 | 16 | 7241 |
| 7241 | 7965 | 1738 | 17 | 7986 |
| 7986 | 8784 | 1917 | 19 | 8807 |
| 8807 | 9688 | 2114 | 21 | 9713 |
| 9713 | 10,684 | 2331 | 23 | 10,712 |
| 10,712 | 11,783 | 2571 | 26 | 11,814 |
| 11,814 | 12,995 | 2835 | 28 | 13,029 |
| 13,029 | 14,332 | 3127 | 31 | 14,369 |
| 14,369 | 15,806 | 3449 | 34 | 15,847 |
| 15,847 | 17,432 | 3803 | 38 | 17,477 |
| 17,477 | 19,225 | 4194 | 42 | 19,275 |
| 19,275 | 21,202 | 4626 | 46 | 21,257 |
| 21,257 | 23,383 | 5102 | 51 | 23,444 |
| 23,444 | 25,788 | 5626 | 56 | 25,855 |
Abbreviations: SAM, Step Activity Monitor; Tx, treatment.
Example: a subject in the WB exercise program would start with an average daily walking duration of 5900 steps per day and increase 10% to 6565 average steps per day at 2 weeks by walking an additional 14 minutes at about 100 steps per minute each of the 3 exercise days. Subjects would walk the prescribed amount on the 3 exercise days. On off days, they would walk their regular self-selected amount, but their step count would be carefully monitored using the SAM. After 3 months, this subject potentially could walk 10,700 steps per day.
A subject with a beginning average step rate of 5900 steps per day in the NWB exercise program would be assigned a beginning stationary bike duration of 14 minutes and increase every 2 weeks by the time previously indicated. After 3 months, the subject would exercise on the stationary bicycle for 23 minutes, a comparable time with that of the subject walking in the WB exercise program.
Footnotes
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit on the authors or on any organization with which the authors are associated.
References
- 1.Centers for Disease Control and Prevention (CDC) Mobility limitation among persons aged > or =40 years with and without diagnosed diabetes and lower extremity disease–United States, 1999-2002. MMWR Morb Mortal Wkly Rep. 2005;54:1183–6. [PubMed] [Google Scholar]
- 2.Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care. 2002;25:61–7. doi: 10.2337/diacare.25.1.61. [DOI] [PubMed] [Google Scholar]
- 3.Volpato S, Blaum C, Resnick H, Ferrucci L, Fried LP, Guralnik JM. Comorbidities and impairments explaining the association between diabetes and lower extremity disability: The Women’s Health and Aging Study. Diabetes Care. 2002;25:678–83. doi: 10.2337/diacare.25.4.678. [DOI] [PubMed] [Google Scholar]
- 4.Gregg EW, Gerzoff RB, Caspersen CJ, Williamson DF, Narayan KM. Relationship of walking to mortality among US adults with diabetes. Arch Intern Med. 2003;163:1440–7. doi: 10.1001/archinte.163.12.1440. [DOI] [PubMed] [Google Scholar]
- 5.Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33:e147–67. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snowling NJ, Hopkins WG. Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care. 2006;29:2518–27. doi: 10.2337/dc06-1317. [DOI] [PubMed] [Google Scholar]
- 7.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990;13:513–21. doi: 10.2337/diacare.13.5.513. [DOI] [PubMed] [Google Scholar]
- 8.Veves A, Murray HJ, Young MJ, Boulton AJ. The risk of foot ulceration in diabetic patients with high foot pressure: a prospective study. Diabetologia. 1992;35:660–3. doi: 10.1007/BF00400259. [DOI] [PubMed] [Google Scholar]
- 9.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C, White RD. Physical activity/exercise and type 2 diabetes: a consensus statement from the American diabetes association. Diabetes Care. 2006;29:1433–8. doi: 10.2337/dc06-9910. [DOI] [PubMed] [Google Scholar]
- 10.Mueller MJ, Maluf KS. Tissue adaptation to physical stress: a proposed “Physical Stress Theory” to guide physical therapist practice, education, and research. Phys Ther. 2002;82:383–403. [PubMed] [Google Scholar]
- 11.Maluf KS, Mueller MJ. Comparison of physical activity and cumulative plantar tissue stress among subjects with and without diabetes mellitus and a history of recurrent plantar ulcers. Clin Biomech. 2003;18:567–75. doi: 10.1016/s0268-0033(03)00118-9. [DOI] [PubMed] [Google Scholar]
- 12.LeMaster JW, Reiber GE, Smith DG, Heagerty PJ, Wallace C. Daily weight-bearing activity does not increase the risk of diabetic foot ulcers. Med Sci Sports Exerc. 2003;35:1093–9. doi: 10.1249/01.MSS.0000074459.41029.75. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong DG, Lavery LA, Holtz-Neiderer K, et al. Variability in activity may precede diabetic foot ulceration. Diabetes Care. 2004;27:1980–4. doi: 10.2337/diacare.27.8.1980. [DOI] [PubMed] [Google Scholar]
- 14.LeMaster JW, Mueller MJ, Reiber GE, Mehr DR, Madsen RW, Conn VS. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: feet first randomized controlled trial. Phys Ther. 2008;88:1385–98. doi: 10.2522/ptj.20080019. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong DG, Abu-Rumman PL, Nixon BP, Boulton AJM. Continuous activity monitoring in persons at high risk for diabetes related lower-extremity amputation. J Am Podiatr Med Assoc. 2001;91:451–5. doi: 10.7547/87507315-91-9-451. [DOI] [PubMed] [Google Scholar]
- 16.Brown M, Sinacore DR, Ehsani AA, Binder EF, Holloszy JO, Kohrt WM. Low-intensity exercise as a modifier of physical frailty in older adults. Arch Phys Med Rehabil. 2000;81:960–5. doi: 10.1053/apmr.2000.4425. [DOI] [PubMed] [Google Scholar]
- 17.Buchner DM, Hornbrook MC, Kutner NG, et al. Development of the common data base for the FICSIT trials. J Am Geriatr Soc. 1993;41:297–308. doi: 10.1111/j.1532-5415.1993.tb06708.x. [DOI] [PubMed] [Google Scholar]
- 18.Richardson JK, Sandman D, Vela S. A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Arch Phys Med Rehabil. 2001;82:205–9. doi: 10.1053/apmr.2001.19742. [DOI] [PubMed] [Google Scholar]
- 19.Gardner MM, Buchner DM, Robertson MC, Campbell AJ. Practical implementation of an exercise-based falls prevention programme. Age Ageing. 2001;30:77–83. doi: 10.1093/ageing/30.1.77. [DOI] [PubMed] [Google Scholar]
- 20.Shah KM, Mueller MJ. Effect of selected exercises on in-shoe plantar pressures in people with diabetes and peripheral neuropathy. Foot (Edinb) 2012;22:130–4. doi: 10.1016/j.foot.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lavery LA, Higgins KR, Lanctot DR, et al. Home monitoring of foot skin temperatures to-prevent ulceration. Diabetes Care. 2004;27:2642–7. doi: 10.2337/diacare.27.11.2642. [DOI] [PubMed] [Google Scholar]
- 22.Boulton AJ, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment. A report of the Task Force of the Foot Care Interest Group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Phys Ther. 2008;88:1436–43. doi: 10.1093/ptj/88.11.1436. [DOI] [PubMed] [Google Scholar]
- 23.Beriault K, Carpentier AC, Gagnon C, et al. Reproducibility of the 6-minute walk test in obese adults. Int J Sports Med. 2009;30:725–7. doi: 10.1055/s-0029-1231043. [DOI] [PubMed] [Google Scholar]
- 24.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–9. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 25.Martin RR, Irrgang JJ, Burdett RG, Conti SF, Van Swearingen JM. Evidence of validity for the Foot and Ankle Ability Measure (FAAM) Foot Ankle Int. 2005;26:968–83. doi: 10.1177/107110070502601113. [DOI] [PubMed] [Google Scholar]
- 26.Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 27.Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients. The Physical Performance Test. J Am Geriatr Soc. 1990;38:1105–12. doi: 10.1111/j.1532-5415.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 28.Villareal DT, Binder EF, Yarasheski KE, et al. Effects of exercise training added to ongoing hormone replacement therapy on bone mineral density in frail elderly women. J Am Geriatr Soc. 2003;51:985–90. doi: 10.1046/j.1365-2389.2003.51312.x. [DOI] [PubMed] [Google Scholar]
- 29.Diamond JE, Mueller MJ, Delitto A, Sinacore DR. Reliability of a diabetic foot evaluation. Phys Ther. 1989;69:797–802. doi: 10.1093/ptj/69.10.797. [DOI] [PubMed] [Google Scholar]
- 30.Mueller MJ, Diamond JE, Delitto A, Sinacore DR. Insensitivity, limited joint mobility, and plantar ulcers in patients with diabetes mellitus. Phys Ther. 1989;69:453–9. doi: 10.1093/ptj/69.6.453. [DOI] [PubMed] [Google Scholar]
- 31.Keppel G. Design and analysis: a researcher’s handbook. 3. Edgewood Cliffs: Prentice Hall; 1991. [Google Scholar]
- 32.Otterman NM, van Schie CH, van der Schaaf M, van Bon AC, Busch-Westbroek TE, Nollet F. An exercise programme for patients with diabetic complications: a study on feasibility and preliminary effectiveness. Diabet Med. 2011;28:212–7. doi: 10.1111/j.1464-5491.2010.03128.x. [DOI] [PubMed] [Google Scholar]
- 33.Reiber GE. Epidemiology of foot ulcers and amputations in the diabetic foot. In: Bowker JH, Pfeifer MA, editors. Levin and O’Neal’s the diabetic foot. 6. St Louis: Mosby; 2001. pp. 13–32. [Google Scholar]
- 34.Sinacore DR. Total contact casting for diabetic neuropathic ulcers. Phys Ther. 1996;76:296–301. doi: 10.1093/ptj/76.3.296. [DOI] [PubMed] [Google Scholar]
- 35.Apelqvist J, Larsson J, Agardh CD. Long-term prognosis for diabetic patients with foot ulcers. J Intern Med. 1993;233:485–91. doi: 10.1111/j.1365-2796.1993.tb01003.x. [DOI] [PubMed] [Google Scholar]
- 36.Kanade RV, van Deursen RW, Harding K, Price P. Walking performance in people with diabetic neuropathy: benefits and threats. Diabetologia. 2006;49:1747–54. doi: 10.1007/s00125-006-0309-1. [DOI] [PubMed] [Google Scholar]
- 37.Tuttle LJ, Hastings MK, Mueller MJ. A moderate-intensity weight-bearing exercise program for a person with type 2 diabetes and peripheral neuropathy. Phys Ther. 2012;92:133–41. doi: 10.2522/ptj.20110048. [DOI] [PMC free article] [PubMed] [Google Scholar]


