Abstract
Metastasis is the major cause of breast cancer mortality. We recently reported that aberrant G-protein coupled receptor (GPCR) signaling promotes breast cancer metastasis by enhancing cancer cell migration and invasion. Phosphatidylinositol 3-kinase γ (PI3Kγ) is specifically activated by GPCRs. The goal of the present study was to determine the role of PI3Kγ in breast cancer cell migration and invasion. Immunohistochemical staining showed that the expression of PI3Kγ protein was significantly increased in invasive human breast carcinoma when compared to adjacent benign breast tissue or ductal carcinoma in situ. PI3Kγ was also detected in metastatic breast cancer cells, but not in normal breast epithelial cell line or in non-metastatic breast cancer cells. In contrast, PI3K isoforms α, β and δ were ubiquitously expressed in these cell lines. Overexpression of recombinant PI3Kγ enhanced the metastatic ability of non-metastatic breast cancer cells. Conversely, migration and invasion of metastatic breast cancer cells were inhibited by a PI3Kγ inhibitor or by siRNA knockdown of PI3Kγ but not by inhibitors or siRNAs of PI3Kα or PI3Kβ. Lamellipodia formation is a key step in cancer metastasis, and PI3Kγ blockade disrupted lamellipodia formation induced by the activation of GPCRs such as CXC chemokine receptor 4 and protease-activated receptor 1, but not by the epidermal growth factor tyrosine kinase receptor. Taken together, these results indicate that upregulated PI3Kγ conveys the metastatic signal initiated by GPCRs in breast cancer cells, and suggest that PI3Kγ may be a novel therapeutic target for development of chemotherapeutic agents to prevent breast cancer metastasis.
Keywords: PI3Kγ, breast cancer, metastasis, migration and invasion, lamellipodia
1. Introduction
Recent preclinical and clinical studies have demonstrated that specific G-protein coupled receptor (GPCR) systems are excessively activated in malignant breast cancer due to over-expression of receptors [1–4], abnormally elevated levels of ligands for GPCRs [4–6] and/or down-regulation of their regulators [7], which contributes to the progression and spread of breast cancer [8]. For example, signaling initiated by CXC chemokine receptor 4 (CXCR4) and protease-activated receptors (PARs) on breast cancer cells drives cancer cells to migrate and invade through surrounding tissues and spread to distant organs [5,9]. Unfortunately, clinical trials with drugs inhibiting specific GPCR activation show limited efficacy, presumably because metastasis could be driven by several different classes of GPCR simultaneously, thereby generating metastatic signal redundancy.
GPCRs convey signals via heterotrimeric G-proteins (classified into Gs, Gi, Gq, G12) in the form of activated Gα-GTP and Gβγ subunits. We recently demonstrated that Gβγ released from Gi-proteins promotes migration and invasion of metastatic breast cancer cells by generating the lamellipodia protrusions at the leading edge of migrating cancer cells [10], suggesting that blockade of Gβγ could attenuate breast cancer metastasis. However, Gβγ cannot be blocked indiscriminately because of its diverse physiological roles. Therefore, the challenge is to target Gβγ effectors that are vital to breast cancer metastasis but inconsequential for physiologically normal cells.
The most studied type I phosphatidylinositol 3-kinases (PI3Ks), including α, β, γ and δ, play a pivotal role in numerous cellular functions [11]. PI3Kγ is especially intriguing because it is normally expressed primarily in hematopoietic cells [12], which have a physiological need to migrate. In addition, PI3Kγ is only activated by Gβγ following stimulation of GPCRs, whereas PI3Kα, β and δ are stimulated by receptor tyrosine kinases [11, 13]. In fact, GPCR-dependent activation of PI3Kγ in neutrophils causes its accumulation at the leading edge of migrating cells, which is a critical determinant of cell migration [12,14]. Although somatic mutations of PI3Kα are very common in cancers [15,16], and may promote cancer cell growth and invasion in colorectal and breast cancer [17,18], the contribution of PI3Kγ to human cancer is much less clear with different studies showing conflicting results [19–22]. Brazzatti et al. [22] recently reported that knockdown of PI3Kγ inhibited lung colonization of human breast cancer MDA-MB-231 cells in xenografts and suppressed primary tumor growth, metastases and lung colonization caused by mouse 4T1.2 mammary carcinoma allografts. While this suggests an important role for PI3Kγ in breast cancer tumor growth and metastasis, these studies did not explore the molecular mechanisms associated with PI3Kγ signaling or whether PI3Kγ protein levels were correlated with the metastatic potential of various human breast cancer cell lines or human breast cancer specimens.
In the present study, we show that PI3Kγ is aberrantly expressed in invasive human breast carcinoma and its expression level correlates with the metastatic potential of established breast cancer cell lines. Most importantly, we show that silencing PI3Kγ with its siRNA or treatment with a PI3Kγ-selective inhibitor, but not with inhibitors or siRNAs of PI3Kα and β, attenuates lamellipodia formation and suppresses migration and invasion of breast cancer cells. Thus, targeting dysregulated PI3Kγ may provide a novel strategy for development of chemotherapeutic agents to suppress breast cancer metastasis.
2. Materials and Methods
2.1. Cell lines, reagents and plasmids
MCF-10A, MCF-7, T47D, MDA-MB-231 and MDA-MB-436 cells were purchased from the American Type Culture Collection (ATCC) (Manassas, VA). MDA-MB-231 and -436 cells were cultured in DMEM with 10% fetal bovine serum (FBS). MCF-7 cells were cultured in IMEM and 10% FBS with 10 μg/ml insulin. MCF-10A cells were grown in MEBM with additives (ATCC), and T47D cells were maintained in RPMI 1640 with 10% FBS.
N-((1E)-(6-bromoimidazo-[1,2-a]pyridin-3-yl)methylene)-N′-methyl-N″-(2-methyl-5-nitrobenzene)sulfonohydrazide, HCl (PI3Kα inhibitor VIII or PIK-75, IC50 = 5.8, 1300, and 78 nM for lipid kinase activity of PI3Kα, β and γ isoforms, respectively) [23], 7-methyl-2-(morpholin-4-yl)-9-(1-phenylaminoethyl)-pyrido[1,2-a]-pyrimidin-4-one (PI3Kβ inhibitor VI or TGX-221, IC50 = 0.005, 5, and ≥3.5 μM for PI3Kβ, α and γ isoforms, respectively) [24], 5-(2,2-difluoro-benzo[1,3]dioxol-5-ylmethylene)-thiazolidine-2,4-dione (PI3Kγ inhibitor II or AS-604850, IC50 = 0.25, 4.5, and > 20 μM for PI3Kγ, α and β) [25], and a broad spectrum PI3K inhibitor 2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (Ly294002) were obtained from EMD Biosciences (San Diego, CA). Rabbit PI3Kα antibody and PI3Kγ antibody were from Cell Signaling Technology (Danvers, MA) and IRDye800-labeled anti-rabbit IgG was from LI-COR Bioscience. Rabbit PI3Kβ antibody and mouse PI3Kδ antibody were purchased from Millipore (Billerica, MA) and Santa Cruz (Santa Cruz, CA), respectively. ON-TARGETplus SMARTpool siRNAs targeting human PI3Kα, PI3Kβ or PI3Kγ, were purchased from Thermo Scientific Dharmacon (Lafayette, CO). Epidermal growth factor (EGF) was from BD Biosciences (San Jose, CA). CXCL12 was from R&D Systems (Minneapolis, MN) and protease activated receptor 1 (PAR1) agonist TFLLR was from Peptides International (Louisville, KY). Unless indicated otherwise, remaining reagents were purchased from either Sigma-Aldrich (St. Louis, MO) or Thermo Fisher Scientific (Waltham, MA).
The pEYFP-PI3Kγ-CAAX plasmid encoding YFP-tagged PI3Kγ with the CAAX motif for constitutive association with the plasma membrane was a gift from Dr. Bernd Nürnberg (University of Tübingen, Germany) [26]. PI3Kγ consists of the catalytic subunit p110γ and two regulatory subunits, p101 and p84 [27–29]. A constitutively membrane targeted PI3Kγ (p110γ-CAAX) could compensate for the lack of its regulatory subunits [26].
2.2. Conventional RT-PCR and quantitative real-time RT-PCR analysis
The procedures for conventional RT-PCR and real-time RT-PCR were described previously [30]. β-actin was used as the internal control. The PI3Kγ primers resulting in a 98-bp product are: 5′-ttgtgatgggaacttctgga-3′, 5′-ggtttgtgtgat gacgaagg-3′. The conventional RT PCR cycling conditions were 1 cycle at 94°C for 3 min; 35 cycles of 94°C for 30 sec, 58°C for 30 sec, 72°C for 30 sec, followed by 1 cycle of 72°C for 7 min.
2.3. Western blot analysis
Protein was extracted from cultured cells using 1x RIPA lysis buffer (Santa Cruz). Samples (40 μg) were electrophoresed and subjected to Western blot using β-actin and PI3K antibodies as we previously described [31]. Images were captured with an Odyssey (LI-COR Biosciences).
2.4. Immunofluorescence staining
MDA-MB-231 cells and MCF-7 cells transfected with control vector or vector encoding PK3Kγ were seeded on coverslips overnight, and then fixed and stained with PI3Kγ antibody as previously described [31]. To investigate the effects of different inhibitors on lamellipodia formation, serum-starved MDA-MB-231 cells seeded on coverslips were pretreated with PI3Kα and PI3Kβ inhibitors or PI3Kγ inhibitor for 1 h, and then stimulated without or with TFLLR (25 μM), CXCL12 (50 ng/ml) or EGF (5 ng/ml) for 30 min at 37°C. F-actin was visualized with rhodamine-labeled phalloidin (Cytoskeleton, Denver, CO) as previously described [7,10]. Lamellipodia, the flattened F-actin-rich leading edge of migrating cells, were outlined and measured in length using the Image-Pro Plus software from Media Cybernetics, Inc. (Rockville, MD). The summed length of lamellipodia was expressed as a percentage of total cell circumferences [7].
2.5. Immunohistochemistry analysis of human breast tissues
Archived formalin-fixed, paraffin-embedded human breast tissue blocks were from the Creighton University Department of Pathology as approved by the Creighton University Institutional Review Board. Immunohistochemistry was performed as we previously reported [7], using a rabbit PI3Kγ antibody (Cell Signaling Technology). The negative control used non-immune rabbit IgG as the primary antibody. Expression levels of PI3Kγ protein were graded using a four-tier system from 0 (negative) to 3 (high) based on overall staining intensity by two pathologists, independently. A total of 40 sets of pair-matched patient samples were included.
2.6. RNA interference and overexpression of PI3Kγ
MDA-MB-231 cells (1 × 106 in 100 μl) were electroporated with 300 nM of control siRNA (negative control #1 siRNA, Ambion) or ON-TARGETplus SMARTpool siRNA for human PI3Kα, PI3Kβ or PI3Kγ using Nucleofector kits with an Amaxa Nucleofector System (Lonza) (Allendale, NJ). Cells were seeded on 6-well plates. The following day, adherent MDA-MB-231 cells were re-transfected with the same siRNA using Lipofectamine 2000 as previously described [32]. Two days later, cells were harvested for Western blot analysis of PI3Kα, PI3Kβ or PI3Kγ expression or migration and invasion assays.
MCF-7 cells (2 × 106) electroporated with 4 μg of plasmid encoding YFP or YFP-tagged PI3Kγ were seeded on 6-well plates. Stable MCF-7 cell lines were established with standard methods, and selected with G-418 (800 μg/ml) for three weeks as we previously described [7]. Positive clones were amplified and verified by Western blotting for PI3Kγ expression.
2.7. Transwell invasion and migration assays
Matrigel invasion assays were carried out at 37°C for 16h using 24-well Transwell inserts (Corning) (Tewksbury, MA) coated with 30 μg of Matrigel (BD Biosciences) as we previously described [7,10]. Transwell cell migration assays were performed similarly without Matrigel, but only for 5h for MDA-MB-231 and MDA-MB-436 cells or 12 h for MCF-7 cells. Conditioned medium (CM) from NIH-3T3 cells was collected and used as a chemoattractant as previously reported [5,7].
2.8. 3D invasion assay
A collagen solution was prepared by mixing NIH 3T3 CM and collagen (2.5 mg/ml) at pH 7.3. Collagen gels were prepared by pipetting 100 μl of this solution into Transwell insets, allowing it to polymerize, and then equilibrating at 37 °C with 5% CO2. MCF-7 cells (50,000/well) were added to the upper chamber in 200 μl serum free DMEM while the lower chamber received 0.6 ml of NIH-3T3 CM. After 48 h, cells were fixed in 3% glutaraldehyde in PBS, stained with 0.1% toluidine blue in 30% methanol and destained. Thin cross-sections were photographed using an upright Nikon Eclipse 80i microscope. Three random fields at 100x magnification were selected and the number of invading cells per high power field (HPF) was counted manually. Data are presented as average numbers of invading cells per HPF.
2.9. Statistical analysis
Tissue immunohistochemistry staining scoring was analyzed with a Kruskal–Wallis test and Dunn posttest. Other results are mean ± S.E. of at least three determinations, with statistical comparisons done by Student’s t-test with p < 0.05 considered to be significant.
3. Results
3.1. PI3Kγ is upregulated in human breast tumors
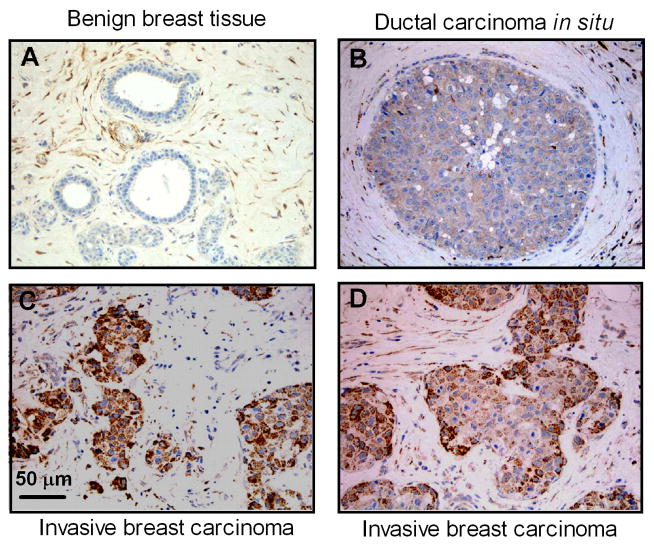
We first performed immunohistochemistry staining for PI3Kγ protein in histologically benign and neoplastic cells of 40 archival human breast tissue blocks using a PI3Kγ specific antibody. PI3Kγ protein expression was elevated in ductal carcinoma in situ and invasive breast carcinoma when compared to adjacent benign mammary tissue (Fig. 1). Expression levels of PI3Kγ were graded from 0–3 based on overall staining intensity. Table 1 shows that average PI3Kγ staining intensities in ductal carcinoma in situ were increased compared to normal or adjacent normal breast tissues (0.73 ± 0.17 vs. 0.19 ± 0.08, p < 0.01). PI3Kγ protein expression in invasive breast carcinoma was significantly higher than that in ductal carcinoma in situ (1.60 ± 0.18 vs. 0.73 ± 0.17, p < 0.001). These data show that up-regulation of PI3Kγ protein is correlated with the degree of tumor invasiveness, and suggest possible relevance to aberrant migration and invasion of breast cancer cells.
Fig. 1.
Upregulation of PI3Kγ protein expression in human invasive breast carcinomas. Sections of formalin-fixed, paraffin embedded breast tissue were immunohistochemically stained for PI3Kγ protein as described in “Materials and Methods”. Images shown are representative of 40 matched breast cancer specimens. (A) Benign breast tissue, (B) Ductal carcinoma in situ. (C and D) Invasive breast carcinoma.
Table 1.
PI3Kγ expression by immunohistochemistry staining in human breast cancer specimens
| Breast specimens | n | Staining intensity
|
Average± S.E. | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Normal/Adjacent Normal | 37 | 32 | 3 | 2 | 0 | 0.19 ± 0.08 |
| Ductal Carcinoma in Situ | 26 | 13 | 8 | 4 | 1 | 0.73 ± 0.17* |
| Invasive Carcinoma | 40 | 9 | 9 | 11 | 11 | 1.60 ± 0.18**,# |
Statistical significance was determined using a Kruskal-Wallis test and Dunn posttest.
p<0.01,
p<0.001 vs. Normal/Adjacent Normal
p<0.001 vs. Ductal carcinoma in Situ
3.2. PI3Kγ is overexpressed in metastatic breast cancer cells
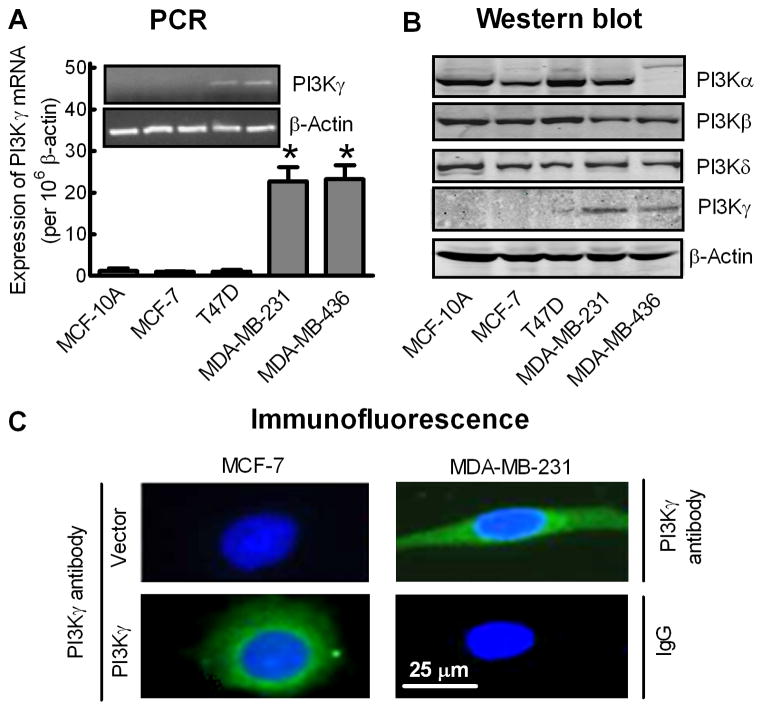
Quantitative real-time PCR and Western blot analysis were further performed to investigate the levels of PI3Kγ mRNA and protein in established human breast cancer cell lines. As shown in Fig. 2A and 2B, PI3Kγ mRNA and protein were almost undetectable in an immortalized human breast epithelial cell line (MCF-10A) or in non-metastatic breast cancer MCF-7 and T47D cells, but were significantly increased in metastatic breast cancer MDA-MB-231 and MDA-MB-436 cells. Immunofluorescent staining also showed expression of PI3Kγ protein in the cytosol of MDA-MB-231 cells but not in MCF-7 cells unless the MCF-7 cells were transfected with a recombinant PI3Kγ plasmid (Fig. 2C). In contrast, other type I PI3Ks were ubiquitously expressed in these breast cell lines, except that PI3Kα was not detected in MDA-MB-436 cells (Fig. 2B).
Fig. 2.
Aberrant expression of PI3Kγ in human metastatic breast cancer cell lines. (A) Analysis of PI3Kγ mRNA expression in breast cell lines by quantitative real-time PCR. Bars represent the mean ± S.E. of PI3Kγ mRNA levels, normalized by β-actin expression levels (n=4). *p<0.01 compared to MCF-10A cells. Inset: Conventional PCR for PI3Kγ (35 cycles) and β-actin (25 cycles). (B) Western blot analysis of PI3Kα, PI3Kβ, PI3Kγ and PI3Kδ protein expression levels in breast cell lines. β-actin was used as an internal control. Images shown are representative of four separate experiments. (C) Immunofluorescence staining of PI3Kγ protein (green) with DAPI staining of the nuclei (blue) in MDA-MB-231 cells and MCF-7 cells transfected with vector or PK3Kγ. The negative control used non-immune rabbit IgG as the primary antibody. Images shown are representative of at least 50 cells from three separate experiments.
3.3. Blocking PI3Kγ activity attenuates metastatic breast cancer cell migration and invasion
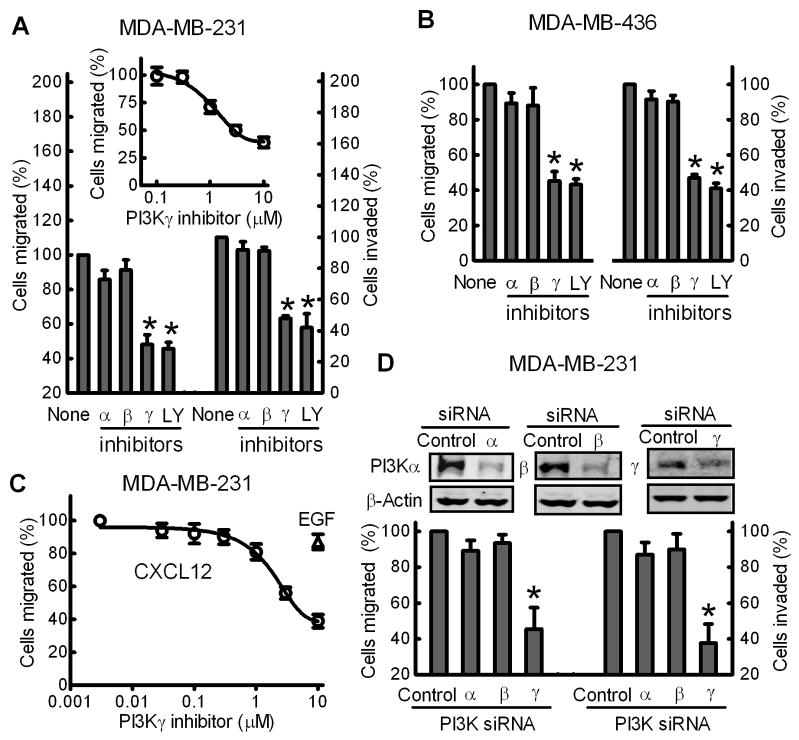
Directed cell migration and invasion are critical steps in the tumor metastasis cascade [5]. NIH-3T3 fibroblast CM contains numerous chemokines and growth factors, and is widely used for inducing cancer cell migration and invasion in Transwell assays [5,7]. Thus, the effect of PI3K inhibitors on the NIH-3T3 CM-stimulated migration and invasion of metastatic breast cancer MDA-MB-231 and MDA-MB-436 cells was examined (Fig. 3). PI3Kγ-selective inhibitor attenuated MDA-MB-231 cell migration in a dose-dependent manner with a maximum inhibition of 60% and IC50 of 1.2 ± 0.3 μM (Fig. 3A, inset). 3 μM PI3Kγ-selective inhibitor or 10 μM Ly294002 (LY), a broad-spectrum PI3K inhibitor [33], inhibited migration and invasion of both MDA-MB-231 and -436 cells by 50–60% (Fig. 3A and 3B). In contrast, inhibitors of PI3Kα or β at concentrations 5-fold or 20-fold higher than the IC50 reported for their primary targets in vitro [23,24] had no significant effect.
Fig. 3.
PI3Kγ blockade attenuates migration and invasion of metastatic breast cancer cells. MDA-MB-231 (A) and MDA-MB-436 (B) cells were pre-treated with PI3Kα inhibitor (30 nM), β inhibitor (100 nM), γ inhibitor (3 μM), or the general PI3K inhibitor LY (10 μM) for 15 min prior to Transwell migration and invasion assays in response to NIH-3T3 CM. Data are the % of cells migrated or invaded compared to untreated cells (None). Bars show the mean± S.E. (n=3) with *p<0.01 compared to untreated cells. Inset, migration assay of MDA-MB-231 cells pre-treated with various concentrations of PI3Kγ inhibitor (n=3). (C) PI3Kγ inhibitor attenuated CXCR4-dependent migration of MDA-MB-231 cells. Cells were treated with various concentrations of PI3Kγ inhibitor prior to Transwell migration assays in response to 50 ng of CXCL12 (○) or EGF (Δ). Data shown are the mean± S.E. (n=3). (D) MDA-MB-231 cells were dual-transfected with ON-TARGETplus SMARTpool PI3Kα, PI3Kβ or PI3Kγ siRNA or control siRNA as described in “Materials and Methods”. Cells were harvested for Western blot analysis of PI3Kα, PI3Kβ or PI3Kγ expression (upper panel) or migration and invasion assays in response to NIH-3T3 CM. Bars show the mean± S.E. (n=4) with *p<0.01 compared to cells transfected with control siRNA.
CXC chemokine receptor 4 (CXCR4) plays an important role in breast cancer metastasis [7,34,35], and initiates signaling through Gi-proteins when its ligand, CXCL12, is bound [36]. As shown in Fig. 3C, the PI3Kγ inhibitor attenuated CXCL12-stimulated cell migration in a dose-dependent manner with a maximum inhibition of 80% and IC50 of 2.7 ± 0.3 μM. In contrast, the PI3Kγ inhibitor only had a very modest effect (<20%) on cell migration stimulated by epidermal growth factor (EGF), a process dependent on the EGF receptor tyrosine kinase. These data suggested that upregulated PI3Kγ has an important role in GPCR-stimulated migration and invasion of metastatic breast cancer cells.
3.4. siRNA-mediated knockdown of PI3Kγ, but not PI3Kα or PI3Kβ, attenuates metastatic breast cancer cell migration and invasion
To exclude any non-specific effects of PI3Kγ inhibitor, siRNA was used to individually silence the endogenous PI3Kα, PI3Kβ or PI3Kγ expression in metastatic breast cancer cells (Fig 3D, top panel). Dual-transfection of a PI3Kγ-specific siRNA into MDA-MB-231 cells reduced PI3Kγ protein levels by 70 ± 11% when compared to a negative control siRNA. Knockdown of endogenous PI3Kγ attenuated migration and invasion of MDA-MB-231 cells in response to NIH-3T3 CM by about 60% (Fig. 3D). In contrast, 80% reduction of endogenous PI3Kα or PI3Kβ by their specific siRNAs (Fig. 3D, top panel) had no significant effect on migration and invasion of MDA-MB-231 cells.
3.5. PI3Kγ blockade attenuates GPCR-dependent lamellipodia formation of metastatic breast cancer cells
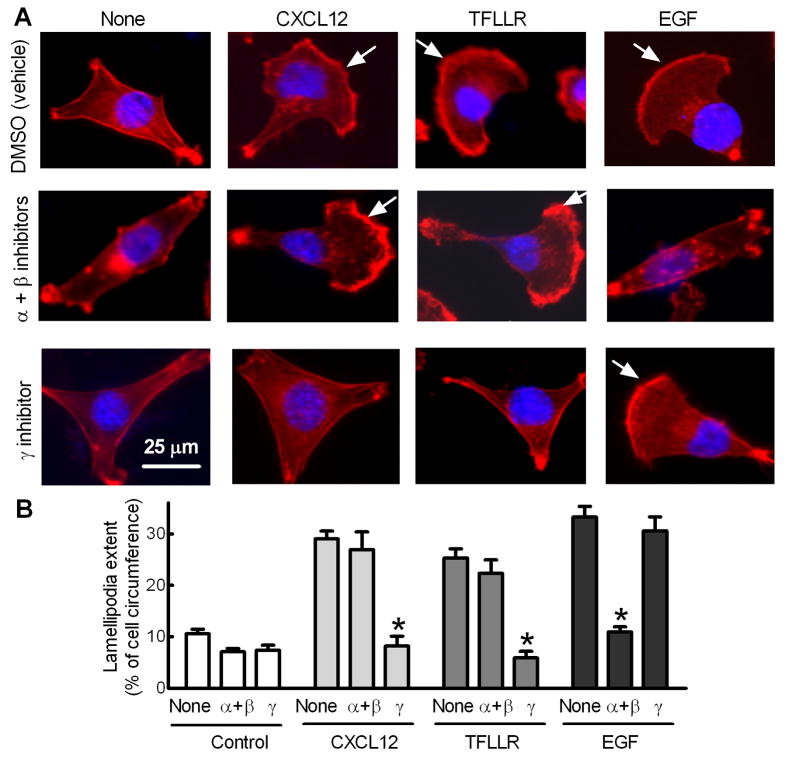
Formation of lamellipodia, the flattened F-actin-rich leading edge of migrating cells, is required for cancer cell migration and invasion during metastasis [37]. The effect of PI3Kγ blockade on CXCR4-dependent lamellipodia formation was investigated. As shown in Fig. 4, CXCL12 increased lamellipodia extent by 2.7-fold, which was blocked by the PI3Kγ inhibitor but not by inhibitors of PI3Kα and β. In addition, the PI3Kγ inhibitor, but not inhibitors of PI3Kα and β, also blocked lamellipodia formation induced by TFLLR, an agonist of G-protein coupled protease-activated receptor 1 (PAR1) known to promote breast cancer cell invasion [5,7]. In contrast, the 3-fold increase in lamellipodia formation in response to EGF was blocked by inhibitors of PI3Kα and β but not by the PI3Kγ inhibitor. These data suggest that PI3Kγ functions as a key molecule linking GPCRs to lamellipodia formation in metastatic breast cancer cells.
Fig. 4.
Blocking PI3Kγ activity attenuates GRCR-dependent lamellipodia formation of breast cancer cells. MDA-MB-231 cells cultured in serum-free medium were treated with inhibitors of PI3Kα (30 nM) and β (100 nM) or γ (3 μM) 1 h prior to 30 min incubation with CXCL12 (50 ng/ml), TFLLR (25 μM) or EGF (5 ng/ml). Cells were then fixed and subjected to F-actin staining using rhodamine-labeled phalloidin. (A) Images shown are representative of at least 50 cells. Arrows indicates lamellipodial regions. (B) Lamellipodial extent at cell leading edges was quantified as a fraction of cell circumference on 50 randomly selected cells in each group. Columns, means; bars, S.E., *p<0.001 compared to cells without inhibitor treatment (None).
3.6. PI3Kγ overexpression enhances MCF-7 cell migration and invasion in 3D collagen matrices
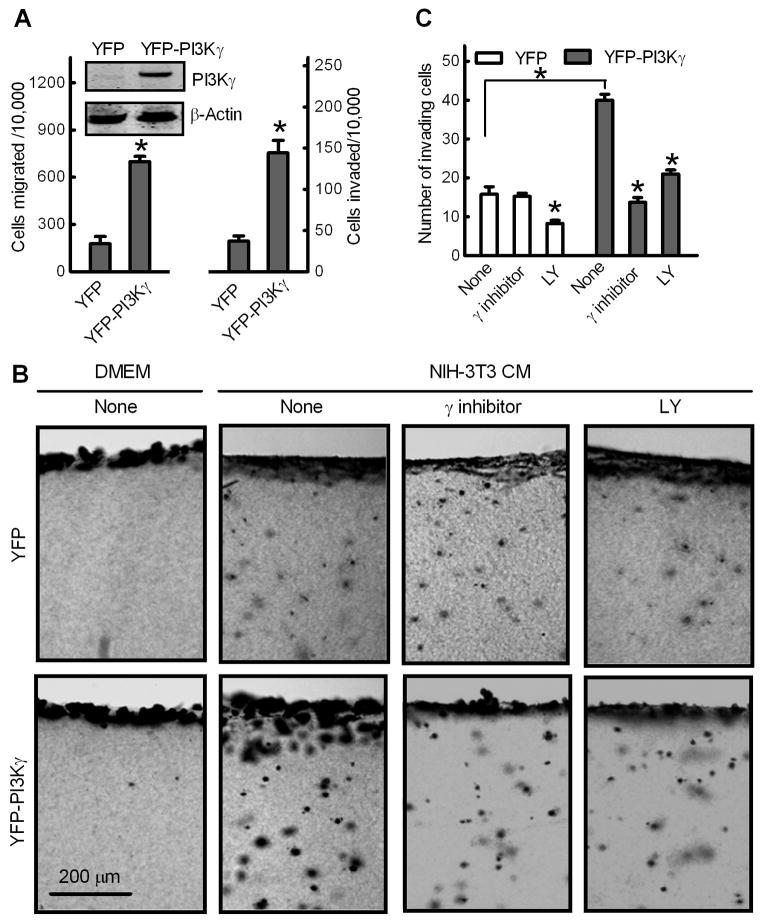
Non-metastatic breast cancer MCF-7 cells were further used as an in vitro model to investigate the effect of exogenously expressed PI3Kγ on breast cancer metastasis. MCF-7 cells were stably transfected with vector encoding YFP or YFP-tagged, membrane-targeted PI3Kγ protein. Transwell assays with these cells indicated that PI3Kγ overexpression increased migration and invasion of MCF-7 cells by about 3-fold (Fig. 5A).
Fig. 5.
Overexpression of PI3Kγ promotes MCF-7 cell migration and invasion. Non-metastatic breast cancer MCF-7 cells were stably transfected with YFP or constitutively membrane-targeted YFP-PI3Kγ. (A) Transwell migration and invasion assays. Data shown are numbers of cells migrated or invaded per 10,000 loaded cells. Bars show the mean ± S.E. (n=5) with *p<0.01 compared to cells transfected with YFP. Inset: Western blot of PI3Kγ and β-actin in MCF-7 cell lines. (B and C) PI3Kγ promotes breast cancer MCF-7 cell invasion of 3D collagen matrices. MCF-7 cells were incubated with type I collagen gels for 48 h in the presence of DMEM or NIH-3T3 CM without (none) or with 10 μM PI3Kγ inhibitor or LY. Gels were fixed, stained and cross-section images were photographed. (B) Images of representative fields, showing the cell monolayer and the underlying collagen matrix containing invading cancer cells. (C) Quantitation of MCF-7 cell invasion. Data are expressed as mean numbers of invading cells per field ± S.E. (n=4) under 10X magnification from ten fields. *p<0.01 compared to cells transfected with YFP or cells in the absence of inhibitors (None).
Because tumor cell invasion depends on proteolysis and dispersion through 3D extracellular matrix barriers largely composed of type I collagen [38], invasive abilities of these MCF-7 stable cell lines were compared in an in vitro 3D invasion assay [39]. Cells were seeded on top of a gel composed of type I collagen. The cells first adhered, forming a monolayer, and then invaded the 3D gel in response to chemoattractants. As shown in Fig. 5B and 5C, NIH-3T3 CM stimulated invasion of MCF-7 control cells expressing YFP into 3D collagen matrices, which was inhibited by a broad-spectrum PI3K inhibitor LY but not by the PI3Kγ inhibitor. Compared to MCF-7 control cells, the number of invaded MCF-7/YFP-PI3Kγ cells was 2.5-fold higher, and this increase was largely abolished in the presence of the PI3Kγ inhibitor or LY.
4. Discussion
Although several lines of evidence indicate a role of PI3Kγ in pancreatic cancer and breast cancer [19–22], our results provide the first detailed evidence that expression levels of PI3Kγ are correlated with the metastatic potential of established human breast cancer cell lines. In contrast, PI3Kα, β and δ were ubiquitously expressed in these breast cell lines. More importantly, our data showed that PI3Kγ protein expression was modestly increased in noninvasive ductal carcinoma in situ as compared to normal breast tissues but was markedly increased in invasive breast carcinoma. These results suggest that PI3Kγ upregulation may be an early event in the development of an invasive breast cancer phenotype.
PI3Kγ is well established as a focal point for activation when chemokine sensors are stimulated, and thus it plays a key role in the directional migration of leukocytes toward chemoattractants [40]. A lack of PI3Kγ has been shown to block the ability of neutrophils and macrophages to respond to stimuli from several GPCRs [41]. Similarly, chemokines and their receptors are involved in cancer metastasis [1], raising the possibility that mechanisms underlying cancer epithelial cell migration and invasion are similar to those underlying leukocyte trafficking. Thus, several breast cancer cell lines were selected as a model system to establish the role of PI3Kγ in breast cancer metastasis. Our data showed that blocking endogenous PI3Kγ in metastatic MDA-MB-231 and MDA-MB-436, either with the selective PI3Kγ inhibitor or by siRNA-mediated knockdown, attenuated migration and invasion abilities of these cells. Conversely, overexpression of recombinant PI3Kγin non-metastatic breast cancer MCF-7 cells that lacks endogenous PI3Kγ expression, significantly increased cell migration and invasion in transwell assays. Similar results were observed from a 3D invasion assay demonstrating that overexpression of PI3Kγ enhanced cellular invasion of MCF-7 cells into collagen matrices. It should be noted that the PI3Kγ-selective inhibitor used in our studies attenuated metastatic breast cancer cell migration with an IC50 of 1.2 ± 0.3 μM. Loss of metastatic abilities of breast cancer cells after treatment with 3 μM of the PI3Kγ inhibitor might also be due to partial inhibitory effects on PI3Kα (in vitro IC50 = 4.5 μM). However, selective inhibitors of PI3Kα or β at concentrations much higher than the IC50 reported for their primary targets in vitro had no significant effects. More importantly, siRNA knockdown of PI3Kα or β did not attenuate metastatic abilities of breast cancer cells. Thus, these data together suggest that upregulated PI3Kγ, but not PI3Kα or β, plays an important role in breast cancer invasiveness and metastasis.
The molecular mechanism underlying PI3Kγ-facilitated breast cancer cell migration and invasion was also characterized. Lamellipodia are a key structure required for cancer cell migration and invasion during metastatic progression [37], and chemoattractant activation of receptors triggers reorganization of the actin cytoskeleton to form lamellipodia [7,42]. The PI3Kγ inhibitor, but not PI3Kα and β inhibitors, blocked lamellipodia formation of MDA-MB-231 cells induced by agonists of G-protein coupled CXCR4 and PAR1. In contrast, the PI3Kγ inhibitor had little effect on lamellipodia formation and cell migration induced by EGF, a process not dependent on G-proteins, but mediated by EGF receptor tyrosine kinase. It is well established that the small G-protein Rac controls lamellipodia formation in cells. Aberrant Rac activity is found in some human cancers and mediates cancer metastasis [43,44] in part, by generating lamellipodia protrusions at the leading edge of migrating cancer cells. Inhibition of Rac activity blocked breast cancer cell migration and invasion [44]. Rac acts as a molecular switch and can cycle between an inactive GDP-bound form and an active GTP-bound form catalyzed by guanine nucleotide exchange factors (GEF) [45]. Interestingly, in many cell types, PI3K activity is necessary and sufficient for receptor-driven Rac activation via RacGEFs [46]. Thus, our data suggest that PI3Kγ may function as a key molecule specifically linking GPCRs to Rac-dependent lamellipodia formation to promote breast cancer cell migration and invasion.
In summary, our data are the first to establish a correlation between PI3Kγ expression and the metastatic potential of human breast cancer cell lines and breast cancer specimens. More importantly, either blockade or knockdown of PI3Kγ was sufficient to inhibit the migration and invasion of metastatic breast cancer cells, whereas overexpression of recombinant PI3Kγ in non-metastatic breast cancer cells increased their migratory and invasive abilities. These findings support a crucial role for upregulated PI3Kγ in breast cancer metastasis. We recently demonstrated that Gβγ released from G-proteins following stimulation of GPCRs promotes migration and invasion of breast cancer cells. However, Gβγ cannot be blocked indiscriminately because of its vital physiological role. Since PI3Kγ is only activated by Gβγ and was not expressed in normal breast tissue but upregulated in invasive breast cancer, PI3Kγ may provide a desirable target for blocking metastatic GPCR signals in breast cancer cells. A further study of in vivo effects of PI3Kγ inhibitors on breast cancer metastasis will potentially lead to new avenues for cancer drug design. In addition, PI3Kγ protein expression in human breast cancer specimens varied significantly, even within the same category. Since this may be related to breast cancer sub-type, we are currently examining the expression patterns of ER, PR and HER2 to precisely classify these breast cancer specimens. If PI3Kγ over-expression is more common in a particular sub-type of breast cancer, this may become an important marker for planning treatment and developing new therapies.
Acknowledgments
We gratefully acknowledge Dr Bernd Nürnberg from University of Tübingen for providing the pEYFP-PI3Kγ-CAAX plasmid. The authors thank Chuu-Yun A. Wong for technical support. This work was supported by the National Institutes of Health (CA125661 and P20-RR018759), Nebraska State LB595 and LB692 research program.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokinereceptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 2.Wülfing P, Kersting C, Tio J, Fischer RJ, Wülfing C, Poremba C, et al. Endothelin-1-, endothelin-A- and endothelin-B-receptor expression is correlated with vascular endothelial growth factor expression and angiogenesis in breast cancer. Clin Cancer Res. 2004;10:2393–400. doi: 10.1158/1078-0432.ccr-03-0115. [DOI] [PubMed] [Google Scholar]
- 3.Hoey RP, Sanderson C, Iddon J, Brady G, Bundred NJ, Anderson NG. The parathyroid hormone- related protein receptor is expressed in breast cancer bone metastases and promotes autocrine proliferation in breast carcinoma cells. Br J Cancer. 2003;88:567–73. doi: 10.1038/sj.bjc.6600757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Even-Ram S, Uziely B, Cohen P, Grisaru-Granovsky S, Maoz M, Ginzburg Y, et al. Thrombin receptor overexpression in malignant and physiological invasion processes. Nat Med. 1998;4:909–14. doi: 10.1038/nm0898-909. [DOI] [PubMed] [Google Scholar]
- 5.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120:303–13. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Guise TA, Yin JJ, Mohammad KS. Role of endothelin-1 in osteoblastic bone metastases. Cancer. 2003;97:779–84. doi: 10.1002/cncr.11129. [DOI] [PubMed] [Google Scholar]
- 7.Xie Y, Wolff DW, Wei TT, Wang B, Deng C, Kirui J, et al. Breast cancer migration and invasion depends on proteasome degradation of regulator of G protein signaling 4 (RGS4) Cancer Res. 2009;69:5743–51. doi: 10.1158/0008-5472.CAN-08-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 9.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–67. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 10.Kirui JK, Xie Y, Wolff DW, Jiang H, Abel PW, Tu Y. Gβγ signaling promotes breast cancer cell migration and invasion. J Pharmacol Exp Ther. 2010;333:393–403. doi: 10.1124/jpet.109.164814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–54. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, et al. Function of P13Kγ in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287:1040–6. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 13.Leopoldt D, Hanck T, Exner T, Maier U, Wetzker R, Nürnberg B. Gβγ stimulates phosphoinositide 3-kinase-gamma by direct interaction with two domains of the catalytic p110 subunit. J Biol Chem. 1998;273:7024–9. doi: 10.1074/jbc.273.12.7024. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson GJ, Milne L, Kulkarni S, Sasaki T, Walker S, Andrews S, et al. PI3Kγ has an important context- dependent role in neutrophil chemokinesis. Nat Cell Biol. 2007;9:86–91. doi: 10.1038/ncb1517. [DOI] [PubMed] [Google Scholar]
- 15.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: Variations on a theme. Oncogene. 2008;27:5497–510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 2006;94:455–9. doi: 10.1038/sj.bjc.6602970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuels Y, Diaz LA, Schmidt-kittler O, Cummins JM, Delong L, Cheong I, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–73. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Liu G, Dziubinski M, Yang Z, Ethier SP, Wu G. Comprehensive analysis of oncogenic effects of PIK3CA mutations in human mammary epithelial cells. Breast Cancer Research and treatment. 2008;112:217–27. doi: 10.1007/s10549-007-9847-6. [DOI] [PubMed] [Google Scholar]
- 19.Attoub S, De Wever O, Bruyneel E, Mareel M, Gespach C. The transforming functions of PI3-Kinase-γ are linked to disruption of intercellular adhesion and promotion of cancer cell invasion. Ann NY Acad Sci. 2008;1138:204–13. doi: 10.1196/annals.1414.027. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki T, Irie-Sasaki J, Horie Y, Bachmaier K, Fata JE, Li M, et al. Colorectal carcinomas in mice lacking the catalytic subunit PI3Kγ. Nature. 2000;406:897–902. doi: 10.1038/35022585. [DOI] [PubMed] [Google Scholar]
- 21.Edling CE, Selvaggi F, Buus R, Maffucci T, Di Sebastiano P, Friess H, et al. Key role of Phosphoinositide 3-Kinase Class IB in pancreatic cancer. Clin Cancer Res. 2010;16:4928–37. doi: 10.1158/1078-0432.CCR-10-1210. [DOI] [PubMed] [Google Scholar]
- 22.Brazzatti JA, Klingler-Hoffmann M, Haylock-Jacobs S, Harata-Lee Y, Niu M, Higgins MD, et al. Differential roles for the p101 and p84 regulatory subunits of PI3Kγ in tumor growth and metastasis. Oncogene. 2012;31:2350–61. doi: 10.1038/onc.2011.414. [DOI] [PubMed] [Google Scholar]
- 23.Knight ZA, Gonzalez B, Feldman ME, Zunder ER, Goldenberg DD, Williams O, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–47. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson SP, Schoenwaelder SM, Goncalves I, Nesbitt WS, Yap CL, Wright CE, et al. PI 3-kinase p110β: a new target for antithrombotic therapy. Nat Med. 2005;11:507–14. doi: 10.1038/nm1232. [DOI] [PubMed] [Google Scholar]
- 25.Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, et al. Blockade of PI3Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med. 2005;11:936–43. doi: 10.1038/nm1284. [DOI] [PubMed] [Google Scholar]
- 26.Brock C, Schaefer M, Reusch HP, Czupalla C, Michalke M, Spicher K, et al. Roles of G beta gamma in membrane recruitment and activation of p110γ/p101 phosphoinositide 3-kinase γ. J Cell Biol. 2003;160:89–99. doi: 10.1083/jcb.200210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoyanov B, Volinia S, Hanck T, Rubio I, Loubtchenkov M, Malek D, et al. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science. 1995;269:690–3. doi: 10.1126/science.7624799. [DOI] [PubMed] [Google Scholar]
- 28.Stephens LR, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, et al. The G beta gamma sensitivity of a PI3K is dependent upon a tightly associated adaptor, p101. Cell. 1997;89:105–14. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 29.Suire S, Coadwell J, Ferguson GJ, Davidson K, Hawkins P, Stephens LR. p84, a new Gbetagammaactivated regulatory subunit of the type IB phosphoinositide 3-kinase p110γ. Curr Biol. 2005;15:566–70. doi: 10.1016/j.cub.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 30.Cao X, Qin J, Xie Y, et al. Regulator of G-protein signaling 2 (RGS2) inhibits androgen-independent activation of androgen receptor in prostate cancer cells. Oncogene. 2006;25:3719–34. doi: 10.1038/sj.onc.1209408. [DOI] [PubMed] [Google Scholar]
- 31.Jiang H, Abel PW, Toews ML, Deng C, Casale TB, Xie Y, et al. Phosphoinositide 3-kinase gamma regulates airway smooth muscle contraction by modulating calcium oscillations. J Pharmacol Exp Ther. 2010;334:703–9. doi: 10.1124/jpet.110.168518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang H, Xie Y, Abel PW, Toews ML, Townley RG, Casale TB, et al. Targeting phosphoinositide 3-kinase γ in airway smooth muscle cells to suppress interleukin-13-induced mouse airway hyperresponsiveness. J Pharmacol Exp Ther. 2012;342:305–11. doi: 10.1124/jpet.111.189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith LD, Hickman ES, Parry RV, Westwick J, Ward SG. PI3Kγ is the dominant isoform involved in migratory responses of human T lymphocytes: Effects ex vivo maintenance and limitations of non-viral delivery of siRNA. Cellular signaling. 2007;19:2528–39. doi: 10.1016/j.cellsig.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Li YM, Pan Y, Wei Y, Cheng XY, Zhou BP, Tan M, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–69. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 35.Holland JD, Kochetkova M, Akekawatchai C, Dottore M, Lopez A, McColl SR. Differential functional activation of chemokine receptor CXCR4 is mediated by G proteins in breast cancer cells. Cancer Res. 2006;66:4117–24. doi: 10.1158/0008-5472.CAN-05-1631. [DOI] [PubMed] [Google Scholar]
- 36.Monterrubio M, Mellado M, Carrera AC, Rodríguez-Frade JM. PI3Kgamma activation by CXCL12 regulates tumor cell adhesion and invasion. Biochem Biophys Res Commun. 2009;388:199–204. doi: 10.1016/j.bbrc.2009.07.153. [DOI] [PubMed] [Google Scholar]
- 37.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell Migration: Integrating Signals from Front to Back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 38.MacDougall JR, Matrisian LM. Contributions of tumor and stromal matrix metalloproteinases to tumor progression, invasion and metastasis. Cancer Metastasis Rev. 1995;14:351–62. doi: 10.1007/BF00690603. [DOI] [PubMed] [Google Scholar]
- 39.Fisher KE, Pop A, Koh W, Anthis NJ, Saunders WB, Davis GE. Tumor cell invasion of collagen matrices requires coordinate lipid agonist-induced G-protein and membrane- type matrix metalloproteinase-1-dependent signaling. Mol Cancer. 2006;5:69. doi: 10.1186/1476-4598-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss-Haljiti C, Pasquali C, Ji H, Gillieron C, Chabert C, Curchod ML, et al. Involvement of phosphoinositide 3-kinase, Rac, and PAK signaling in chemokine-induced macrophage migration. J Biol Chem. 2004;279:43273–84. doi: 10.1074/jbc.M402924200. [DOI] [PubMed] [Google Scholar]
- 41.Barberis L, Hirsch E. Targeting phosphoinositide 3-kinase γ to fight inflammation and more. Thromb Haemost. 2008;99:279–285. doi: 10.1160/TH07-10-0632. [DOI] [PubMed] [Google Scholar]
- 42.Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, et al. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291:1800–3. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchia Hideki, Condeelis John. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochimica et Biophysica Acta. 2007;1773:642–52. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baugher PJ, Krishnamoorthy L, Price JE, Dharmawardhane SF. Rac1 and Rac3 isoform activation is involved in the invasive and metastatic phenotype of human breast cancer cells. Breast Cancer Research. 2005;7:965–74. doi: 10.1186/bcr1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bottner B, Van Aelst L. Rac and Cdc42 effectors. Prog Mol Subcell Biol. 1999;22:136–58. doi: 10.1007/978-3-642-58591-3_7. [DOI] [PubMed] [Google Scholar]
- 46.Hawkins PT, Eguinoa A, Qiu RG, Stokoet D, Cooke FT, Walters R, et al. PDGF stimulates an increase in GTP-Rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]