Abstract
Pyrogallol (CAS No. 87-66-1), a benzenetriol used historically as a hair dye and currently in a number of industrial applications, was nominated to the National Toxicology Program (NTP) for testing based on lack of toxicity and carcinogenicity data. Three-month and two-year toxicity studies to determine the toxicity and carcinogenicity of pyrogallol when applied to naïve skin (i.e. dermal administration) were conducted in both sexes of F344/N rats and B6C3F1/N mice. In the three-month studies, adult rodents were administered pyrogallol in 95% ethanol 5 days per week for 3 months at doses of up to 150 mg /kg body weight (rats) or 600 mg/kg (mice), Based on the subchronic studies, the doses for the 2-year studies in rats and mice were 5, 20 and 75 mg/kg of pyrogallol. All mice and most rats survived until the end of the three-month study and body weights were comparable to controls. During the 2-year study, survival of dosed rats and male mice was comparable to controls; however survival of 75 mg/kg female mice was significantly decreased compared to controls. The incidences of microscopic non-neoplastic lesions at the site of application were significantly higher in all dosed groups of rats and mice and in both the 3 months and 2-year studies. In the 2-year study, hyperplasia, hyperkeratosis and inflammation tended to be more severe in mice than in rats, and in the mice they tended to be more severe in females than in males. The incidence of squamous cell carcinoma at the site of application (SOA) in 75 mg/kg female mice and SOA squamous cell papillomas in 75 mg/kg male mice were greater than controls. Pyrogallol was carcinogenic in female mice and may have caused tumors in male mice.
Keywords: pyrogallol, hyperkeratosis, hyperplasia, squamous cell carcinoma, squamous papilloma
Introduction
Pyrogallol is a benzenetriol produced when carbon dioxide is split from gallic acid by heat [1]; it can be found in nature as a product of the decomposition of plant tannins and is produced commercially from gallic acid [2, 3]. Historically, pyrogallol has been used as a hair dye, leather and wool stain and photographic developer. However, current main commercial applications in the United States include corrosion-inhibition (to protect metals during processing or cleaning) and the manufacture of other chemicals [2]. During the late 1980s and early 1990s, hair dyes sold in the United States contained 0.1% to 5.0% pyrogallol by weight [4, 5], however, pyrogallol-based hair dyes are not currently available to the public (R.L. Bronaugh, FDA; personal communication). Manufacture of hair products containing pyrogallol in South America has been reported as recently as 2005 [6].
Ingestion of plant materials high in pyrogallol is poisonous to ruminants [7, 8] and oral LD50 values in the Sprague-Dawley rat range from 800 mg/kg to 1,800 mg/kg [5]. While pyrogallol ingestion or excessive skin application have been associated with severe acute toxicity in humans [9, 10, 11], most human exposure occurs by skin contact at concentrations much lower than those associated with acute poisoning. A number of human studies in individuals exposed to hairdressing chemicals showed that pyrogallol is a contact sensitizer [12, 13, 14, 15, 16, 17]. Pyrogallol was also identified as a weak skin sensitizer in experimental animal models [5, 18]. Dermal application to laboratory animals caused skin irritation but had no effect on survival [5, 19, 20, 21].
Pyrogallol was nominated to the National Toxicology Program (NTP) for subchronic and chronic toxicity and carcinogenicity evaluation based on its frequent occurrence in natural and manufactured products, including hair dyes, and lack of carcinogenicity data. Studies were conducted in both sexes of F344/N rats and B6C3F1/N mice via the dermal route because that is the primary route of exposure for humans. This paper describes the major study findings including the nonneoplastic and neoplastic skin lesions observed at the site of application.
Methods
Chemicals
Pyrogallol (CAS No. 87-66-1; 1,2,3-benzenetriol; 2,3-dihydroxyphenol; gallamine; pyrogallic acid; 1,2,3-trihydroxybenzene) was obtained from Aceto Corporation (Lake Success, NY; lot number 010326). Purity was determined by high-performance liquid chromatography with ultraviolet detection to be greater than 99%. Dose formulations were prepared by mixing pyrogallol and95% ethanol to give the required concentrations. Dose formulations were analyzed three times during the subchronic study and every three months during the chronic study and were within 10% of target pyrogallol concentrations.
Animals
Male and female F344/N rats and B6C3F1/N mice were obtained from Taconic Farms, Inc. (Germantown, NY) and quarantined for 11–14 days. At study start, rats were 6–8 weeks old and mice were 6–7 weeks old. Irradiated NTP-2000 wafer feed (Zeigler Brothers, Inc., Gardners, PA) and tap water (Columbus, OH, municipal supply) were available ad libitum. Rats and mice were housed individually.
Dosing volumes were 0.5 mL/kg body weight for rats and 2.0 mL/kg for mice. Pyrogallol was administered over the application site with a Corning Lambda (Corning, Inc., Corning, NY) single channel pipetter with a disposable polyethylene tip. An area slightly larger than the interscapular application site was clipped 24 hours before the first dose and weekly thereafter. Animal care and use were in accordance with the Laboratory Animal Welfare Act of 1966 (P.L. 89–544) as amended and the Public Health Service Policy on Humane Care and Use of Animals. Animals were treated humanely and with regard for alleviation of pain and distress. All animals were housed in facilities accredited by the Association for Assessment and Accreditation of Laboratory Animal Care and all procedures were approved by Battelle's Institutional Animal Care and Use Committee. These studies were conducted in compliance with FDA Good Laboratory Practice for nonclinical laboratory studies (21 CFR 58).
Three-month study
Groups of 10 male and 10 female rats and mice received dermal applications (i.e. to naïve skin) of pyrogallol in 95% ethanol at doses of 9.5, 18.75, 37.5, 75, or 150 mg pyrogallol/kg body weight (rats) or 38, 75, 150, 300, or 600 mg/kg (mice), or ethanol vehicle control alone, 5 days per week for 14 weeks. Body weights and clinical signs were recorded weekly. Dose was limited by the maximum solubility of pyrogallol in the 95% ethanol vehicle (300 mg/mL) and the fixed dosing volumes used (0.5 mL/kg rats, 2 mL/kg mice). Weights of major organs were recorded at necropsy, including heart, liver, kidney, lung, testis, uterus, thymus and thyroid gland.
Two-year study
Groups of 50 male and 50 female rats and mice received dermal applications of pyrogallol in 95% ethanol at doses of 5, 20, or 75 mg/kg, or ethanol vehicle control alone, 5 days per week for up to 104 (rats) or 105 (mice) weeks. Body weights were recorded weekly for 13 weeks and monthly thereafter.
Clinical and pathology examinations
Animals were observed twice daily and clinical findings were recorded monthly beginning at week 5. The animals were euthanized by carbon dioxide asphyxiation. Necropsies were performed on all animals. All organs were fixed in 10% neutral buffered formalin, trimmed and processed, sectioned to a thickness of 4 to 6 μm, and stained with hematoxylin and eosin (H & E). Complete histopathologic examination of control skin and the skin at the site of application (SOA) was performed on all rats and mice. Severity grades for non-neoplastic skin lesions were assigned based on a qualitative assessment of the lesions using a four-tier scoring system of severity grading expressed as numbers where 1 = minimal, 2 = mild, 3 = moderate, 4 = marked. In addition to the skin, all other organs were examined histopathologically according to current NTP specifications for the conduct of toxicity and carcinogenicity studies. Additional study details and findings can be found in Technical Report #574 online at http://ntp.niehs.nih.gov/go/14366.
Statistical analysis
To determine statistical differences between incidences, Fisher exact test and poly-3 test were used where applicable. Organ and body weight data were analyzed with the parametric multiple comparison procedures of Dunnett [22] and Williams [23, 24]. Analysis of effects on survival used Cox's [25] method for testing two groups for equality and Tarone's [26] life table test to identify dose-related trends.
Results
Three-month study
All rats and mice survived until the end of the study except for one vehicle control female rat that died of chylothorax (unrelated to treatment) on day 43 of the study. The mean body weight gain of 150 mg/kg female rats was 13% less than that of the vehicle controls; otherwise, the final body weights relative to controls and body weight gains of dosed groups of rats and mice were similar to those of the vehicle controls (Table 1). There were no significant changes in the absolute or relative organ weights at necropsy (data not shown). Chemical-related clinical findings included brown staining and irritation of the skin at the SOA; at study termination, most of the pyrogallol-treated rats and mice had yellow-brown staining and irritation at the SOA.
Table 1.
Body weight changes, body weight relative to controls and incidence of non-neoplastic lesions at the site of application in F344/N rats and B6C3F1/N mice treated dermally for 3 months with pyrogallol.
| Number of Animals with Lesion (severity) | ||||||
|---|---|---|---|---|---|---|
| Dose (mg/kg) | Mean change in body weight (g) | Final weight relative to controls (%) | Squamous hyperplasia | Hyperkeratosis | Chronic active inflammation | Ulcer |
| Male F344/N rats | ||||||
| 0 | 224 ± 5 | 1 (1.0) | 0 | 0 | 0 | |
| 9.5 | 225 ± 9 | 100 | 10** (1.0) | 10** (1.1) | 9** (1.1) | 0 |
| 18.75 | 231 ± 6 | 102 | 10** (1.0) | 10** (1.3) | 9** (1.2) | 0 |
| 37.5 | 226 ± 6 | 101 | 10** (1.0) | 10** (1.7) | 10** (1.0) | 0 |
| 75 | 215 ± 6 | 97 | 10** (1.5) | 10** (2.2) | 10** (1.8) | 0 |
| 150 | 215 ± 5 | 97 | 10** (1.4) | 10** (2.0) | 10** (1.9) | 1 (2.0) |
| Female F344/N rats | ||||||
| 0 | 103 ± 4 | 0 | 0 | 0 | 0 | |
| 9.5 | 105 ± 3 | 102 | 10** (1.0) | 10** (1.0) | 8** (1.0) | 0 |
| 18.75 | 102 ± 3 | 100 | 10** (1.0) | 10** (1.4) | 10** (1.5) | 0 |
| 37.5 | 95 ± 4 | 97 | 10** (1.0) | 10** (1.4) | 9** (1.8) | 0 |
| 75 | 97 ± 4 | 98 | 10** (1.3) | 10** (1.9) | 10** (2.0) | 0 |
| 150 | 90 ± 3* | 95 | 10** (1.1) | 10** (2.1) | 10** (1.8) | 0 |
| Male B6C3F1/N mice | ||||||
| 0 | 12.3 ± 0.6 | 0 | 0 | 0 | 0 | |
| 38 | 12.7 ± 0.6 | 103 | 10** (1.0) | 10** (1.0) | 10** (1.3) | 0 |
| 75 | 11.7 ± 0.6 | 99 | 10** (1.0) | 10** (1.0) | 10** (1.4) | 0 |
| 150 | 12.1 ± 0.6 | 102 | 10** (1.1) | 10** (1.0) | 10** (1.5) | 0 |
| 300 | 10.3 ± 0.9 | 96 | 10** (1.6) | 10** (1.2) | 10** (1.7) | 1 (2.0) |
| 600 | 10.5 ± 0.6 | 96 | 10** (2.2) | 10** (1.5) | 10** (2.2) | 2 (2.0) |
| Female B6C3F1/N mice | ||||||
| 0 | 11.3 ± 0.6 | 0 | 0 | 0 | 0 | |
| 38 | 12.7 ± 1.1 | 103 | 10** (1.0) | 10** (1.0) | 10** (1.9) | 0 |
| 75 | 12.0 ± 0.7 | 102 | 10** (1.1) | 10** (1.0) | 10** (2.1) | 0 |
| 150 | 11.3 ± 0.6 | 98 | 10** (1.0) | 10** (1.0) | 10** (2.0) | 0 |
| 300 | 9.4 ± 0.7 | 93 | 10** (1.4) | 10** (1.1) | 10** (2.1) | 0 |
| 600 | 9.8 ± 0.6 | 94 | 10** (1.8) | 10** (1.4) | 10** (2.1) | 3 (2.0) |
N = 10. Average severity grades indicated in parenthesis: 1=minimal, 2=mild, 3=moderate, 4=marked.
Significant differences from vehicle control group:
p ≤ 0.05 by Williams' test;
p ≤ 0.01 by Fisher exact test.
Microscopically, the incidences of squamous hyperplasia, hyperkeratosis, and chronic active inflammation of the skin at the SOA were significantly increased in all groups dosed with pyrogallol; these lesions occurred in nearly all of the treated animals (Table 1) and were morphologically similar in rats and mice. Squamous hyperplasia consisted of an increase in the thickness of the epidermis from the normal one to two layers of epithelial cells to three to five layers (minimal) or six to eight layers (mild). Hyperkeratosis was characterized by pronounced thickening of the stratum corneum layer. Chronic active inflammation consisted of variable numbers of lymphocytes and macrophages (with fewer neutrophils) diffusely infiltrating the superficial dermis. The severities of these lesions ranged from minimal to moderate, and in general, increased with increasing dose. Ulcers (graded as mild) at the SOA occurred in one 150 mg/kg male rat, one 300 mg/kg male mice, two 600 mg/kg male mice, and three 600 mg/kg female mice. One 600 mg/kg female mouse had minimal epidermal necrosis at the SOA (data not shown).
The stratum corneum layer of the skin at the SOA in dosed rats often had a yellow-brown discoloration. This discoloration was attributed to absorption of pyrogallol and was most evident at higher doses.
Two-year studies
In the two-year studies, animals were administered pyrogallol at 5, 20 and 75 mg/kg. Survival and mean body weights of dosed groups of male and female rats and male mice were similar to that of the vehicle control groups (Table 2). However, survival of 75 mg/kg female mice was significantly decreased, as 23 female mice were euthanized before study end due to the presence of ulcers at the SOA. In addition, mean body weights of female mice were generally more than 10% less than vehicle controls on study weeks 50 to 90 but were within 10% of controls by the end of the study. Irritation and/or ulceration of the skin at the site of application were the only chemical-related clinical findings and occurred predominantly in the 20 and 75 mg/kg male and female groups.
Table 2.
Survival and mean body at termination of F344/N rats and B6C3F1/N mice treated dermally for 2 years with pyrogallol.
| Dose (mg/kg) | Survival to end of studya | Mean body weight at termination in grams (% relative to controls) | ||
|---|---|---|---|---|
| F344/N rats | Male | Female | Male | Female |
| 0 | 23 | 29 | 503.5 ± 10.3 | 347.5 ± 6.4 |
| 5 | 28b | 33 | 489.4 ± 9.4 (97) | 348.6 ± 6.4 (100) |
| 20 | 28 | 26b | 479.2 ± 10.5 (95) | 355.4 ± 7.4 (102) |
| 75 | 28 | 31 | 479.9 ± 7.0 (95) | 332.6 ± 6.7 (96) |
| B6C3F1/N mice | Male | Female | Male | Female |
| 0 | 37 | 33 | 52.3 ± 1.5 | 61.7 ± 1.8 |
| 5 | 36 | 30 | 52.2 ± 1.5 (98) | 59.1 ± 2.0 (97) |
| 20 | 34 | 36 | 53.1 ± 1.5 (96) | 59.3 ± 1.5 (95) |
| 75 | 31 | 16*** | 47.7 ± 1.5 (93) | 54.5 ± 2.4 (92) |
Number of animals out of N = 50.
Includes 2 males and one female that died during the last week of the study.
p= 0.001 pairwise comparison to control.
Lesions at the SOA were morphologically similar to those observed in the 3-month study. The incidences of hyperplasia and hyperkeratosis were significantly greater than those in the vehicle control groups in most dosed groups of rats and mice; incidences of inflammation were significantly increased in several groups treated with 20 or 75 mg/kg (Table 3). In addition to lesions already seen in the 3-months study, sebaceous gland hyperplasia was significantly increased in rats and mice; mice developed pigmentation, fibrosis and ulcers (Table 3).
Table 3.
Incidence and severity of non-neoplastic lesions at the site of application in F344/N rats and B6C3F1/N mice treated dermally for 2 years with pyrogallol.
| Dose (mg/kg) | Hyperplasia | Hyperkerat osis | Inflammation | Fibrosis | Pigmentation | Sebaceous gland hyperplasia | Ulcer |
|---|---|---|---|---|---|---|---|
| Male F344/N rats | |||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 6* (1.0) | 2 (1.0) | 0 | 0 | 0 | 0 | 0 |
| 20 | 20** (1.0) | 21** (1.0) | 0 | 0 | 0 | 12** (1.0) | 0 |
| 75 | 50** (1.2) | 48** (1.6) | 46** (1.3) | 0 | 0 | 48** (1.1) | 0 |
| Female F344/N rats | |||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 9** (1.0) | 6* (1.0) | 3 (1.0) | 0 | 0 | 0 | 0 |
| 20 | 11** (1.0) | 22** (1.0) | 6* (1.0) | 0 | 0 | 5* (1.0) | 0 |
| 75 | 49** (1.1) | 49** (1.8) | 49** (1.4) | 0 | 0 | 41** (1.7) | 0 |
| Male B6C3F1/N mice | |||||||
| 0 | 8 (1.3) | 11 (1.5) | 2 (1.5) | 3 (2.0) | 0 | 1 (2.0) | 1 (2.0) |
| 5 | 24** (1.2) | 43** (1.3) | 6 (1.7) | 6 (1.7) | 0 | 6 (1.3) | 1 (3.0) |
| 20 | 47** (1.6) | 50** (1.9) | 37** (1.2) | 28** (1.4) | 9** (1.0) | 4 (1.3) | 2 (2.5) |
| 75 | 50** (2.5) | 50** (2.6) | 44** (2.4) | 47** (2.5) | 39** (1.5) | 24** (1.5) | 23** (3.0) |
| Female B6C3F1/N mice | |||||||
| 0 | 20 (1.4) | 24 (1.5) | 12 (1.3) | 5 (1.8) | 0 | 1 (1.1) | 2 (3.0) |
| 5 | 31* (1.3) | 38** (1.4) | 14 (1.1) | 6 (1.3) | 0 | 2 (1.0) | 0 |
| 20 | 49** (1.9) | 49** (2.7) | 42** (1.2) | 31** (1.3) | 35** (1.0) | 6 (1.2) | 3 (1.3) |
| 75 | 49** (3.3) | 49** (3.4) | 48** (3.0) | 49** (3.1) | 40** (1.8) | 34** (1.5) | 33** (3.1) |
N = 50. Average severity grades indicated in parenthesis: 1=minimal, 2=mild, 3=moderate, 4=marked.
Significant differences from vehicle control group:
p ≤ 0.01 by Fisher exact test.
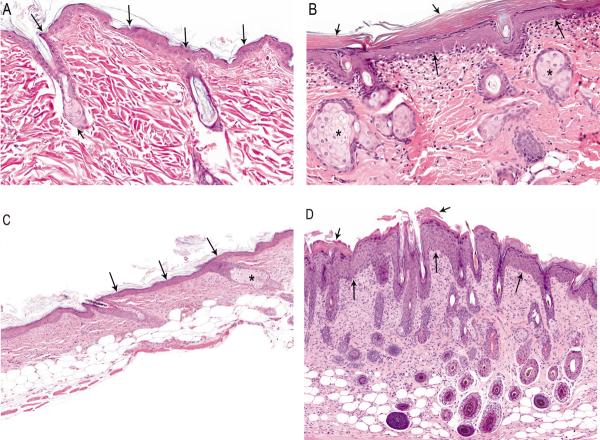
While rats had only minimal (3–4 cell layers) to mild (5–6 cell layers) hyperplasia (Figure 1A and 1B), mice also had moderate (7–8 layers) and marked (> 8 layers) hyperplasia (Figure 1C and 1D). Hyperplasia was usually accompanied by varying degrees of hyperkeratosis, which was characterized by increased layers of keratin overlying the epidermis (Figures 1B and 1D). Hyperkeratosis was considered minimal if the keratin overlying the stratum granulosum was thin and loosely packed and mild when there was a thick, dense, compact band of keratin above the stratum granulosum. In the mice, the thickening of the stratum corneum occurred as both orthokeratotic and, to a lesser extent, parakeratotic hyperkeratosis (Figure 1D). Minimal to mild inflammation in the rat was characterized by scattered infiltrates of lymphocytes, macrophages, plasma cells, and neutrophils in the superficial dermis (Figure 1B). In addition to the features seen in rats, mice also displayed marked inflammation with low numbers of mast cells in the dermis and occasional infiltration into the subcutis or epidermis (Figure 1D). Sebaceous gland hyperplasia was of minimal to mild severity in both rats and mice, and was characterized by increased incidences with increased doses (Table 3) and increased size of the sebaceous glands (Figures 1B and 1D); in mice this was concomitant with more frequent hair follicles (Figure 1D).
Figure 1.
(A) Normal skin, control male rat. The epidermis consists of a single layer of epithelial cells (long arrows). Note the relative absence of a superficial layer of keratin and the sebaceous gland (short arrow) associated with a hair follicle (H&E, 25×). (B) Mild epidermal hyperplasia (long arrows), hyperkeratosis (short arrows), hyperplastic sebaceous glands (asterisks) and minimal infiltrates of inflammatory cells within the papillary dermis, male rat exposed to 75 mg/kg pyrogallol for two years (H&E, 25×). (C) Mild epidermal hyperplasia (arrows) and minimal hyperkeratosis. Note hyperplastic sebaceous gland (asterisk) and brown pigment within the dermis. Skin, site of application, male mouse exposed to 20 mg/kg pyrogallol (H&E, 16×). (D) Moderate epidermal hyperplasia (long arrows) and hyperkeratosis (short arrows). Note inflammatory cells within the dermis. Skin, site of application, male mouse exposed to 20 mg/kg pyrogallol (H&E, 12×).
Increased incidences of fibrosis and pigmentation were observed at 20 mg/kg and 75 mg/kg in male and female mice. Fibrosis was characterized by an increased presence of bands of pale, plump fibroblasts in the dermis. Pigmentation consisted of increased numbers of cells in the dermis containing abundant dark brown, granular, intracytoplasmic pigment considered to be melanin (Figure 1C). Increased incidences of sebaceous gland hyperplasia and ulcers were observed at 75 mg/kg in males and females. Ulcer was characterized by full-thickness loss of epidermis and was invariably accompanied by necrosis and inflammation of the underlying dermis (Figure 2, A and B).
Figure 2.
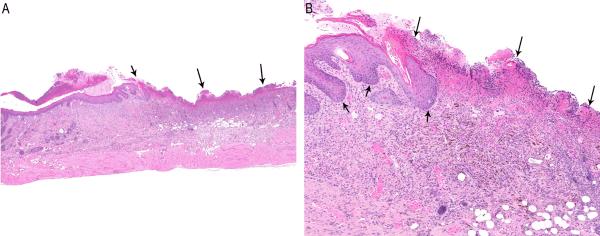
(A) Ulcer (arrows) at the SOA in a male mouse exposed to 75 mg/kg pyrogallol (H&E, 2.5×). (B) Higher magnification of Figure 2A. Note complete loss of the epidermis (long arrows) with superficial necrosis, diffuse inflammation and pigment within the dermis. The adjacent intact epidermis is hyperplastic (short arrows) (H&E, 10×).
Squamous cell carcinomas at the SOA were observed in four female mice exposed to 75 mg/kg pyrogallol; none were observed in controls (Table 4). Microscopically, squamous cell carcinomas were poorly demarcated, unencapsulated, invasive masses arising from the epidermis and extending into the underlying dermis and subcutis (Figure 3); the affected epidermis was ulcerated in some cases. Squamous cell carcinomas were composed of pleomorphic, disorganized proliferations of neoplastic squamous epithelial cells forming irregular cords and islands (Figure 3). The neoplastic epithelial cells often surrounded concentrically arranged aggregates of keratin (keratin pearls) and were surrounded by fibrous connective tissue infiltrated by neutrophils and other inflammatory cells.
Table 4.
Incidence of neoplastic lesions at the site of application in B6C3F1/N mice treated dermally for 2 years with pyrogallol.
| Dose (mg/kg) | ||||
|---|---|---|---|---|
| 0 | 5 | 20 | 75 | |
|
| ||||
| B6C3F1/N Males | ||||
| Squamouscell papilloma | 0† | 0 | 0 | 2 |
| Historical Controls | ||||
| Dermal, ethanol vehicle | 0/200 | |||
| All routes & vehicles | 1/1150 | |||
|
| ||||
| B6C3F1/N Females | ||||
| Squamous cell carcinoma | 0††† | 0 | 0 | 4* |
| Historical Controls | ||||
| Dermal, ethanol vehicle | 0/200 | |||
| All routes and vehicles | 0/1198 | |||
N = 50. Significant positive trend per Poly-3 test:
p = 0.037,
p < 0.001.
Significant pairwise difference from concurrent control by Poly-3 test:
p = 0.033
Figure 3.
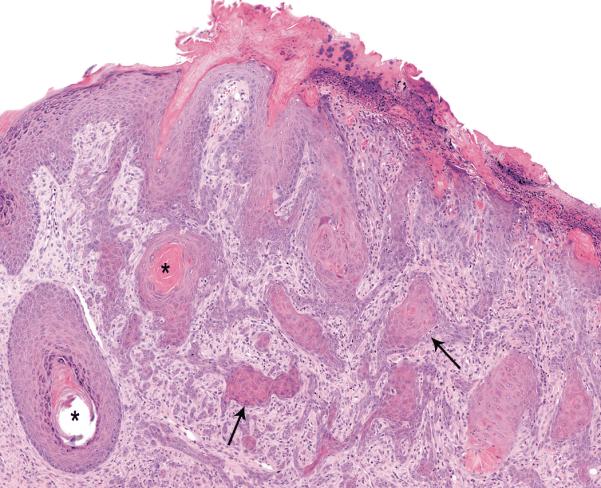
Squamous cell carcinoma (arrows) has effaced the epidermis and invaded into the dermis and subcutis. Note cords and islands of well-differentiated but dysplastic squamous epithelium (arrows) some of which surround keratin pearls (asterisk). Skin, SOA from a female mouse exposed to 75 mg/kg pyrogallol for two years (H&E, 12×).
Two 75 mg/kg male mice had grossly visible masses at the SOA that microscopically were identified as squamous cell papillomas (Table 4); only one squamous cell papilloma has been observed in NTP historical control male mice in dermal application studies (Table 4). One of the mice also had moderate inflammation at the SOA. The squamous cell papillomas were well-circumscribed, exophytic growths composed of an inner connective tissue core forming a stalk with superficial branching fronds that were covered by an outer layer of hyperplastic and hyperkeratotic squamous epithelium (Figure 4).
Figure 4.
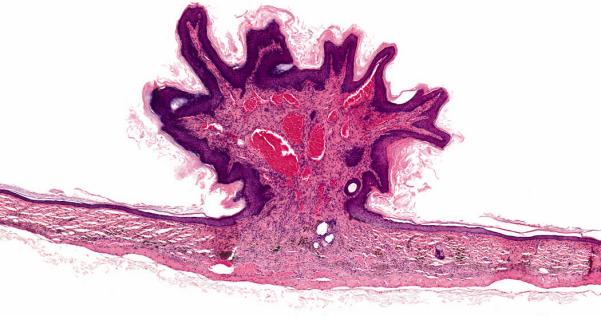
Squamous papilloma occurs as an exophytic growth composed of an inner connective tissue core with branching fronds covered by an outer layer of hyperplastic squamous epithelium. Note dilated blood vessels within the core. Skin, SOA from a male rat exposed to 75 mg/kg Pyrogallol for 2 years (H&E, 12 ×).
Although skin lesions were consistently found at the SOA, a few dosed mice also had morphologically similar lesions in the skin of the neck and back immediately adjacent to the SOA. The incidences of hyperplasia, hyperkeratosis, ulcer, inflammation, and fibrosis at these sites were significantly increased in 75 mg/kg male and female mice (Table 5). The incidences of sebaceous gland hyperplasia were also increased in 75 mg/kg mice, and the increase in females was significant. These lesions were considered related to the test material spreading to or beyond the margins of the clipped skin after application. One 75 mg/kg female had a squamous cell carcinoma of the skin of the right forelimb.
Table 5.
Incidence and severity of non-neoplastic lesions outside the site of application in B6C3F1/N mice treated dermally for 2 years with pyrogallol.
| Dose (mg/kg) | Hyperplasia | Hyperkerat osis | Inflammation | Fibrosis | Sebaceous gland hyperplasia | Ulcer |
|---|---|---|---|---|---|---|
| Males | ||||||
| 0 | 1 (2.0) | 1 (2.0) | 1 (4.0) | 1 (2.0) | 1 (1.0) | 0 |
| 5 | 1 (3.0) | 1 (2.0) | 1 (4.0) | 1 (4.0) | 1 (1.0) | 1 (2.0) |
| 20 | 3 (3.7) | 3 (2.7) | 3 (4.0) | 3 (4.0) | 0 | 3 (4.0) |
| 75 | 10** (3.3) | 10** (3.1) | 10** (3.5) | 10** (3.6) | 5 (1.8) | 10** (3.7) |
| Females | ||||||
| 0 | 1 (4.0) | 1 (4.0) | 1 (3.0) | 1 (3.0) | 1 (2.0) | 1 (3.0) |
| 5 | 2 (3.0) | 2 (3.0) | 0 | 2 (3.5) | 0 | 1 (2.0) |
| 20 | 1 (4.0) | 1 (4.0) | 0 | 1 (4.0) | 1 (1.0) | 1 (4.0) |
| 75 | 9** (3.9) | 9** (3.3) | 9** (3.7) | 9** (4.0) | 7* (1.3) | 9** (3.4) |
N = 50. Average severity grades indicated in parenthesis: 1=minimal, 2=mild, 3=moderate, 4=marked.
Significant differences from vehicle control group:
p ≤ 0.05 by Williams' test;
p ≤ 0.01 by Fisher exact test.
Discussion
The present studies are the first to fully characterize the subchronic and chronic dermal toxicity (i.e. after application to naïve skin) of pyrogallol in rodents. Skin at the SOA was the primary site of toxicity for pyrogallol for both 3-months and 2-year dosing paradigms. In the 3-month studies, the incidences of most lesions at the SOA were similar in all dosed groups and in both species (i.e., 100% incidence was the maximum response), however the severity of the lesions was dose-dependent. While dose selection for the 3-month studies was limited by the maximum solubility of pyrogallol in the ethanol vehicle and the fixed dosing volumes, dermal toxicity observed in the 3-month studies precluded the use of doses greater than 75 mg/kg for the 2-year studies. In the two-year studies, mice were more sensitive to the effects of pyrogallol than rats and female mice were more sensitive than males; treated mice groups showed significant increases in hyperkeratosis and inflammation at lower doses than rats. Mice treated for 2 years had additional increases in fibrosis and ulcers at the SOA not seen in the rats or in the three-month mice study. Non-neoplastic dermal toxicity also led to decreased survival of female mice.
Previous mouse studies in the literature did not report dermal toxicity from pyrogallol [19, 20], however they used lower doses and different formulations as compared to the current studies. Burnett et. al. [21] reported skin irritation in mice at a dose of 8 mg/kg, which is similar to the lowest dose in the current subchronic study that produced chronic active inflammation in rats.
Two types of neoplastic skin lesions of concern occurred at the SOA in mice: squamous cell papilloma in males and squamous cell carcinoma in females. The incidence of squamous cell papilloma at the SOA in 75 mg/kg male mice (2/50, 4%) was not statistically different from that in the vehicle control group (0/50); however, it exceeded the historical control ranges for 2-year ethanol dermal studies (0/200) and for all routes (1/1150). The incidence of squamous cell carcinoma of the skin at the SOA was significantly increased in 75 mg/kg female mice (4/50, 8%) when compared to the vehicle controls (0/50). In fact, no squamous cell carcinomas have been observed in NTP historical control female mice in dermal studies regardless of route or vehicle (0/1198).
Only one other report in the literature shows carcinogenic effects of pyrogallol. Van Duuren and Goldschmidt reported that pyrogallol more than doubled the number of gross papillomas produced by benzo[a]pyrene when the chemicals were applied together to mouse skin in a 63-week assay [27]; however pyrogallol alone produced no gross papillomas; skin was not examined microscopically. The mice studies reported here were of longer duration (105 weeks) and only a few animals developed papillomas or carcinomas, most of them observable only by microscopic histopathological analysis. Pyrogallol is known to produce reactive oxygen species (ROS) [28, 29, 30], and in the present study produced chronic inflammation; two processes that are interrelated (inflammation produces endogenous ROS), and are known mechanisms of cancer promotion [31, 32]. Taken together, the available experimental data suggest pyrogallol acts as a tumor promoter.
There were dose-related, significantly increased incidences of squamous epithelial hyperplasia at the site of application in all dose groups of male and female rats and mice. Squamous epithelial hyperplasia is considered a pre-neoplastic lesion in rats and mice. However, relatively few squamous epithelial neoplastic lesions were observed in the skin. This is not an uncommon finding in NTP studies as a review of the NTP database shows several studies in which this phenomenon has occurred.
In conclusion, dermal administration of pyrogallol caused high incidences of nonneoplastic lesions of the skin at the SOA in male and female rats and mice at doses as low as 5 mg/kg/day. Pyrogallol was considered to be carcinogenic in female mice. In male mice, the presence of two rare benign tumors at the SOA in the high dose group was considered an equivocal finding. These studies provide the first comprehensive assessment of pyrogallol toxicity in mammals and can be used to assess possible risks to humans from dermal pyrogallol exposure.
Acknowledgments
We thank Drs. Rajendra S. Chhabra and Barry S. McIntyre for their helpful review of this manuscript. This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of NIEHS, NIH or the United States government.
Footnotes
Declaration of interest
The authors declare no conflicts of interest.
References
- 1.Allen A. Acid Derivatives of Phenols, Aromatic Acids, Resins, and Essential Oils. Third ed P. Blakiston's Son & Co.; Philadelphia: 1907. [Google Scholar]
- 2.Leston G. Polyhydroxybenzenes. In: Arza Seidel., editor. Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons; New York: 2010. Published Online: 4 DEC 2000, DOI: 10.1002/0471238961.1615122512051920.a01. [Google Scholar]
- 3.Tan KH. Humic Matter in Soil and the Environment: Principles and Controversies. Marcel Dekker Inc.; New York: 2003. [Google Scholar]
- 4.Clayton GD, Clayton FE. Patty's Industrial Hygiene and Toxicology. 3rd ed John Wiley and Sons; New York: 1981. [Google Scholar]
- 5.Cosmetic Ingredient Review Expert Panel Final Report on the Safety Assessment of Pyrogallol. J Am Coll Toxicol. 1991;10(1):67–85. [Google Scholar]
- 6.Mazzei JL, da Silva DN, Oliveira V, Hosomi RZ, do Val RR, Pestana CB, et al. Absence of mutagenicity of acid pyrogallol-containing hair gels. Food Chem Toxicol. 2007;45(4):643–8. doi: 10.1016/j.fct.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Meiser H, Hagedorn HW, Schulz R. Pyrogallol concentrations in rumen content, liver and kidney of cows at pasture. Berl Munch Tierarztl Wochenschr. 2000;113(3):108–11. [PubMed] [Google Scholar]
- 8.Reed JD. Nutritional toxicology of tannins and related polyphenols in forage legumes. J Anim Sci. 1995;73(5):1516–28. doi: 10.2527/1995.7351516x. [DOI] [PubMed] [Google Scholar]
- 9.Gosselin RE, Smith RP, Hodge HC, Braddock JE. Clinical toxicology of commercial products. Williams & Wilkins; 1984. [Google Scholar]
- 10.Pewny R. A Fatal Case of Pyrogallic Acid Poisoning. Medizinische Klinik. 1925 Jun 26;21:970. 1925. [Google Scholar]
- 11.Willsteed E, Regan W. Psoriasis, pyrogallol and skin cancer. Australas J Dermatol. 1985;26(3):144–5. doi: 10.1111/j.1440-0960.1985.tb01769.x. [DOI] [PubMed] [Google Scholar]
- 12.Guerra L, Bardazzi F, Tosti A. Contact dermatitis in hairdressers' clients. Contact Dermatitis. 1992;26(2):108–11. doi: 10.1111/j.1600-0536.1992.tb00893.x. [DOI] [PubMed] [Google Scholar]
- 13.Guerra L, Tosti A, Bardazzi F, Pigatto P, Lisi P, Santucci B, et al. Contact dermatitis in hairdressers: the Italian experience. Gruppo Italiano Ricerca Dermatiti da Contatto e Ambientali. Contact Dermatitis. 1992;26(2):101–7. doi: 10.1111/j.1600-0536.1992.tb00892.x. [DOI] [PubMed] [Google Scholar]
- 14.Keil H. Group reactions in contact dermatitis due to resorcinol. Arch Dermatol. 1962;86:212–6. doi: 10.1001/archderm.1962.01590080082010. [DOI] [PubMed] [Google Scholar]
- 15.Frosch PJ, Burrows D, Camarasa JG, Dooms-Goossens A, Ducombs G, Lahti A, Menné T, Rycroft RJG, Shaw S, White IR, Wilkinson JD. Allergic reactions to a hairdressers' series: Results from 9 European centres. Contact Dermatitis. 1993;28:180–183. doi: 10.1111/j.1600-0536.1993.tb03383.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang MZ, Farmer SA, Richardson DM, Davis MDP. Patch-testing with hairdressing chemicals. Dermatitis. 2011;22:16–26. [PubMed] [Google Scholar]
- 17.Hillen U, Grabbe S, Uter W. Patch test results in patients with scalp dermatitis: Analysis of data of the Information Network of Departments of Dermatology. Contact Dermatitis. 2007;56:87–93. doi: 10.1111/j.1600-0536.2007.01000.x. [DOI] [PubMed] [Google Scholar]
- 18.National Toxicology Program Final Report: Assessment of Contact Hypersensitivity to Pyrogallol in Female BALB/c Mice. 2006 [Google Scholar]
- 19.Jacobs MM, Burnett CM, Penicnak AJ, Herrera JA, Morris WE, Shubik P, et al. Evaluation of the Toxicity and Carcinogenicity of Hair Dyes in Swiss Mice. Drug and Chemical Toxicology. 1984;7(6):573–86. doi: 10.3109/01480548409042820. [DOI] [PubMed] [Google Scholar]
- 20.Stenbäck F, Shubik P. Lack of toxicity and carcinogenicity of some commonly used cutaneous agents. Toxicology and Applied Pharmacology. 1974;30(1):7–13. [Google Scholar]
- 21.Burnett C, Goldenthal EI, Harris SB, Wazeter FX, Strausburg J, Kapp R, et al. Teratology and percutaneous toxicity studies on hair dyes. J Toxicol Environ Health. 1976;1(6):1027–40. doi: 10.1080/15287397609529406. [DOI] [PubMed] [Google Scholar]
- 22.Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc. 1955;50:1096–1121. [Google Scholar]
- 23.Williams DA. A test for differences between treatment means when several dose levels are compared with a zero dose control. Biometrics. 1971;27:103–117. [PubMed] [Google Scholar]
- 24.Williams DA. The comparison of several dose levels with a zero dose control. Biometrics. 1972;28:519–531. [PubMed] [Google Scholar]
- 25.Cox DR. Regression models and life-tables. J. R. Stat. Soc. 1972;B34:187–220. [Google Scholar]
- 26.Tarone RE. Tests for trend in life table analysis. Biometrika. 1975;62:679–682. [Google Scholar]
- 27.Van Duuren BL, Goldschmidt BM. Cocarcinogenic and Tumor-Promoting Agents in Tobacco Carcinogenesis. J. of the Natl. Cancer Inst. 1976;56(6):1237–1242. doi: 10.1093/jnci/56.6.1237. [DOI] [PubMed] [Google Scholar]
- 28.Marklund S, Marklund G. Involvement of Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 29.Lin JK, Lee SF. Enhancement of the Mutagenicity of Polyphenols by Chlorination and Nitrosation in Salmonella typhimurium. Mutat. Research. 1992;269:217–224. doi: 10.1016/0027-5107(92)90202-d. [DOI] [PubMed] [Google Scholar]
- 30.Lee SF, Lin JK. Generation of Hydrogen Peroxide, Superoxide Anion and the Hydroxyl Free Radical From Polyphenols and Active Benzene Metabolites: Their Possible Role in Mutagenesis. J. Biomed. Sci. 1994;1:125–130. doi: 10.1007/BF02257986. [DOI] [PubMed] [Google Scholar]
- 31.Mueller MM. Inflammation in Epithelial Skin Tumors: Old Stories and New Ideas. Cancer and Inflammation. 2006;42(6):735–744. doi: 10.1016/j.ejca.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Klaunig JE, Wang Z, Pu X, Shaoyu Z. Oxidative Stress and Oxidative Damage in Chemical Carcinogenesis. Tox. App. Phar. 2011;254(2):86–99. doi: 10.1016/j.taap.2009.11.028. [DOI] [PubMed] [Google Scholar]