Abstract
Rationale
Assays of schedule-controlled responding can be used to characterize the pharmacology of benzodiazepines and other GABAA receptor modulators, and are sensitive to changes in drug effects that are related to physical dependence.
Objective
The present study used this approach to investigate the role of GABAA receptor subtypes in mediating dependence-like effects following benzodiazepine administration.
Methods
Squirrel monkeys (n=6) were trained on a fixed-ratio schedule of food reinforcement. Initially, the response rate-decreasing effects of chlordiazepoxide (0.1–10 mg/kg; nonselective GABAA receptor agonist), zolpidem (0.032–1.0 mg/kg; α1 subunit-containing GABAA subtype-preferring agonist) and HZ-166 (0.1–10 mg/kg; functionally selective α2 and α3 subunit-containing GABAA receptor agonist) were assessed. Next, acute dependence-like effects following single injections of chlordiazepoxide, zolpidem and HZ-166 were assessed with flumazenil (0.1–3.2 mg/kg; nonselective GABAA receptor antagonist). Finally, acute dependence-like effects following zolpidem administration were assessed with βCCt and 3-PBC (0.1–3.2 mg/kg and 0.32–10 mg/kg, respectively; α1 subunit-containing GABAA receptor antagonists).
Results
Chlordiazepoxide, zolpidem and HZ-166 produced dose- and time-dependent decreases in response rates, whereas flumazenil, βCCt and 3-PBC were ineffective. After the drug effects waned, flumazenil produced dose-dependent decreases in response rates following administration of 10 mg/kg chlordiazepoxide and 1.0 mg/kg zolpidem, but not following any dose of HZ-166. Further, both βCCt and 3-PBC produced dose-dependent decreases in response rates when administered after 1.0 mg/kg zolpidem.
Conclusions
These data raise the possibility that α1 subunit-containing GABAA receptors play a major role in physical dependence-related behaviors following a single injection of a benzodiazepine.
Keywords: GABAA receptors, benzodiazepine, physical dependence, withdrawal, operant behavior
INTRODUCTION
The γ-aminobutyric acid type A (GABAA) receptors are the primary sites of action for benzodiazepines and related drugs used to treat anxiety and sleep disorders. The therapeutic use of benzodiazepine-type drugs for the treatment of these disorders is constrained, however, by other characteristic effects such as daytime drowsiness, motor impairments and reinforcing effects that are thought to contribute to their abuse (Griffiths and Weerts, 1997; Licata and Rowlett, 2008). In addition, benzodiazepines have also the propensity to produce physical dependence which can be observed following not only chronic treatment but may also manifest following a single drug administration (e.g. Lukas and Griffiths, 1984; Spealman, 1986; Bronson, 1994).
Benzodiazepines bind to a specific site on GABAA receptors where they induce a conformational change, leading to an allosteric enhancement in the ability of GABA to increase chloride conductance. Over the past two decades, research has revealed the existence of multiple subtypes of the GABAA receptor (e.g., Pritchett et al., 1989; Rudolph et al., 2001; Olsen and Sieghart, 2008); and subsequent reports have provided evidence to support the notion that the diverse behavioral effects of benzodiazepine-type drugs may reflect their action at these different subtypes (Rudolph et al., 1999; McKernan et al., 2000; Löw et al., 2000; Rowlett et al., 2005). These observations raise the possibility for a pharmacological dissociation between the clinically advantageous effects and unwanted side-effects of these compounds.
GABAA receptors in the central nervous system are pentamers constituted from structurally distinct proteins, with each protein family consisting of different subunits (for review, see Rudolph et al., 2001). The majority of GABAA receptors are composed of α, β and γ subunits; and benzodiazepines bind to a site on the native GABAA receptor that is located at the interface of the γ2 subunit and one of the α1, α2, α3 or α5 subunits. Studies in rodent models have suggested a differential anatomical distribution among these GABAA subunit-containing receptors. In this regard, GABAA receptors containing α1 subunits (α1GABAA receptors) are ubiquitously located, and have been implicated in the sedative, operant rate-reducing and reinforcing effects of benzodiazepines (Rudolph et al., 1999; Licata et al., 2005; Fischer et al., 2010; Tan et al., 2010). In contrast, GABAA receptors containing α2 and α3 subunits (α2GABAA and α3GABAA receptors, respectively) are anatomically distributed in the cortex, limbic system and spinal cord, and have been associated with the anxiolytic and antihyperalgesic effects of benzodiazepines (McKernan et al., 2000; Löw et al., 2000; Rowlett et al., 2005, Fischer et al., 2010; Knabl et al., 2008). Finally, GABAA receptors containing α5 subunits (α5GABAA receptors) are a relatively minor population expressed primarily within the hippocampus and are thought to play a role in certain memory processes associated with benzodiazepines (Collinson et al., 2002; Crestani et al., 2002; Atack et al., 2006).
The contribution that the different GABAA receptor subtypes have in benzodiazepine-induced physical dependence is less clear. Limited data is available to delineate a role of the GABAA receptor subtypes following repeated administration (e.g. Mirza and Nielsen, 2006); and the role of these receptors in acute physical dependence following a single administration has yet to be addressed. In the former study, the GABAA receptor inverse agonist FG-7142 precipitated withdrawal in mice following chronic administration of a series of conventional benzodiazepines, but failed to do so following chronic administration of the α1GABAA receptor-sparing compounds SL651498 and L-838,417 (Mirza and Nielsen, 2006). Together, these findings raise the possibility that α1GABAA receptors may play a key role in benzodiazepine-induced physical dependence following chronic treatment.
The current study was designed to assess further the physical dependence-like effects that follow benzodiazepine administration, particularly the role that GABAA receptor subtypes play in dependence following a single drug administration. Towards this end, squirrel monkeys were trained in an operant conditioning procedure in which behavior was maintained by the presentation of food pellets. This approach was chosen as it is an established method commonly used to quantify changes in behavior related to dependence (e.g. Holtzman and Villarreal, 1973; McMahon and France, 2002), and is a particularly sensitive measure of acute dependence (e.g. Spealman 1986, Bronson, 1994). Moreover, this approach has been shown to be advantageous in delineating receptor subtype mechanisms in other systems (e.g., opioid receptor subtypes, Adams and Holtzman 1990). Initial studies established a model of physical dependence-like effects as evident from an increased potency of the nonselective GABAA receptor antagonist flumazenil following a single injection of the nonselective benzodiazepine chlordiazepoxide. Subsequent studies assessed the role of the GABAA receptor subtypes that mediate this effect by administering the α1GABAA-preferring agonist zolpidem and the α2GABAA/α3GABAA agonist HZ-166 as pretreatment. Finally, the role of α1GABAA receptors was examined further by assessing the rate-decreasing effects of the α1GABAA-preferring antagonists βCCT and 3-PBC following zolpidem pretreatment.
MATERIALS AND METHODS
Subjects
Six adult squirrel monkeys (Saimiri sciureus) were maintained on a 12-hr lights-on/12-hr lights-off cycle (lights on at 7:00 AM). Water was available continuously in the home cage and food was available in the home cage after the session. Monkeys were prepared with a chronic indwelling polyvinyl chloride catheter according to previously described procedures (Platt et al., 2011). In brief, under isoflurane anesthesia and aseptic conditions, one end of a catheter was passed to the level of the right atrium by way of a femoral or jugular vein. The distal end of the catheter was passed subcutaneously and exited the skin in the midscapular region. Catheters were flushed daily with heparinized 0.9% saline solution and sealed with stainless-steel obturators when not in use. Monkeys wore nylon-mesh jackets (Lomir Biomedical, Toronto, Canada) at all times to protect the catheter.
Squirrel monkeys weighed between 0.7 and 1.2 kg throughout the study. Two of the six monkeys were experimentally naïve, two monkeys had previous experience discriminating methamphetamine from vehicle, one monkey had previous experience discriminating a benzodiazepine from vehicle and one monkey had previous experience self-administrating intravenous cocaine. Animals in this study were maintained in accordance with the guidelines of the Committee on Animals of Harvard Medical School and the Guide for Care and Use of Laboratory Animals (8th edition, 2011). Research protocols were approved by the Harvard Medical School Institutional Animal Care and Use Committee.
Operant conditioning behavior
Rates of responding were assessed in an experimental operant chamber during daily sessions, typically 5 days each week (Monday through Friday). Monkeys sat in a Plexiglas chair facing a panel equipped with stimulus lights, a response lever and a food pellet receptacle (MED Associates, St. Albans, VT). The operant conditioning chamber was controlled by a MED-PC interface and an IBM-compatible computer programmed with MED Associates software (MED Associates).
Monkeys were trained under a multiple-cycle procedure consisting of a 7-min pretreatment period followed by a 3-min response period. During the pretreatment period, stimulus lights were not illuminated and responding had no scheduled consequences. During the response period, the stimulus lights were illuminated and monkeys could obtain up to 10 food pellets (Bioserve, Frenchtown, NJ) under a fixed-ratio 10 schedule of food presentation. If all 10 reinforcers were earned before 3-min had elapsed, the light was turned off and responding had no scheduled consequences for the remainder of the response period. Training sessions consisted of five consecutive cycles, and testing began once response rates were stable throughout the session.
Test sessions were conducted in lieu of training sessions once or twice per week if responding was stable throughout the five preceding training sessions, defined as the average rates of responding for each cycle not varying by more than 20%, with no upward or downward trends. Test sessions were identical to training sessions, except that cumulative doses of drug were administered i.v. 2-min into the pretreatment period of each cycle (i.e., 5-min pretreatment time; 10-min inter-injection interval), increasing in one-half log unit increments. The first series of tests assessed the cumulative dose-effect curves and time-course of chlordiazepoxide, zolpidem or HZ-166 alone. Next, doses of chlordiazepoxide, zolpidem or HZ-166 were given as a pre-session pretreatment, followed by dose-effect curves for flumazenil, βCCT and 3-PBC via cumulative dosing. During test sessions in which a pretreatment was administered, the pretreatment time was based upon the duration of action of each compound.
Drugs
Chlordiazepoxide hydrochloride and flumazenil were purchased from Sigma-Aldrich (St. Louis, MO) and zolpidem L-tartaric acid salt was a gift from Dr. K. Fang (Sepracor, Inc.; Marlborough, MA) and dissolved in 50% propylene glycol, 50% sterile water. HZ-166 (8-ethynyl-6-(2'-pyridine)-4H-2,5,10b-triaza-benzo[e]azulene-3-carboxylic acid ethyl ester), βCCT (β-carboline-3-carboxylate-tert-butyl ester) and 3-PBC (3-propoxy-β-carboline hydrochloride) were synthesized at the Department of Chemistry and Biochemistry, University of Wisconsin-Milwaukee and dissolved in 20% ethanol, 60% propylene glycol and 20% sterile water. Pretreatment doses of chlordiazepoxide (1.0–10 mg/kg), zolpidem (0.1–1.0 mg/kg) and HZ-166 (1.0–10 mg/kg) were chosen based upon the preliminary dose-effect curves as determined in the present or previous studies (Spealman, 1986; McMahon and France 2002; Fischer et al., 2010).
Data analysis
The number of responses on the lever per second were recorded and expressed as a percentage of control responding using the average rate of responding from the previous day as the control value (average of five cycles) for individual animals. Data are expressed graphically as the mean (± the standard error of the mean, SEM) percent control response rate from the group. Rates of responding during time-course experiments were considered to have returned to baseline if they were within 10% of the control baseline response rates (i.e. a minimum of 90% control). To compare changes in the behavioral effects following antagonist (flumazenil, βCCT and 3-PBC) administration, a two way analysis of variance (ANOVA) was conducted. Additional Bonferroni t-tests were also conducted to compare individual doses versus the antagonist alone control. For all tests, the level of statistical significance was set at p < 0.05. If significant antagonist-induced rate decreasing effects were detected, potency values (dose reducing responding to 75% of control, ED75) were calculated by log-linear regression from the descending limb of the group dose-effect curve.
RESULTS
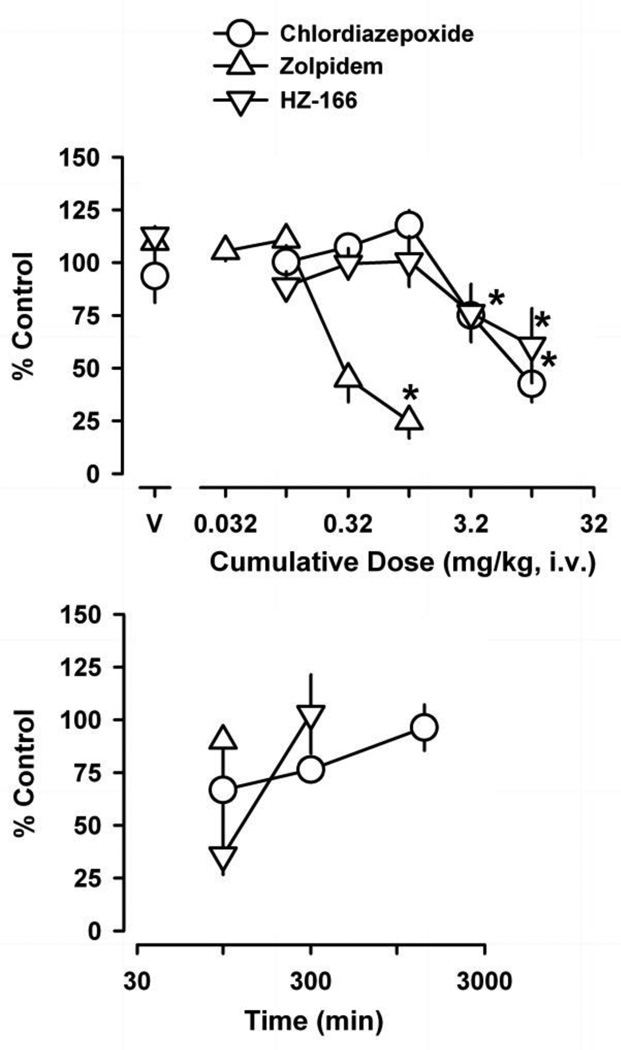
Figure 1 (top) shows the rate-decreasing effects of chlordiazepoxide, zolpidem and HZ-166. Chlordiazepoxide, zolpidem and HZ-166 produced statistically significant decreases in schedule-controlled responding across the dose ranges studied, resulting in ED75 values (mg/kg, i.v.) of 3.5 (±0.42 SEM), 0.21 (±0.044 SEM) and 3.2 (±1.5 SEM), respectively. When assessed over time (Figure 1, bottom), operant responding returned to baseline values (i.e., ≥ 90% control) at 100 min following administration of zolpidem, 300 min following administration of HZ-166 and 1440 min following administration of chlordiazepoxide, and these values were used as pretreatment times during subsequent experiments.
Figure 1.
Dose-effect curves (top panel) and time-course (bottom panel) of the positive GABAA receptor modulators chlordiazepoxide, zolpidem and HZ-166 on schedule-controlled responding. Abscissae, dose of drug in milligrams per kilogram or time after drug administration. Ordinate, mean (±SEM) rate of responding as percentage of control. Points above “V’ indicate average rates of responding (±SEM) following vehicle administration. Asterisks represent significant differences relative to vehicle (Bonferroni t-tests, p<0.05). Time-course data were determined following the highest dose tested for each drug (10 mg/kg chlordiazepoxide, 1 mg/kg zolpidem and 10 mg/kg HZ-166).
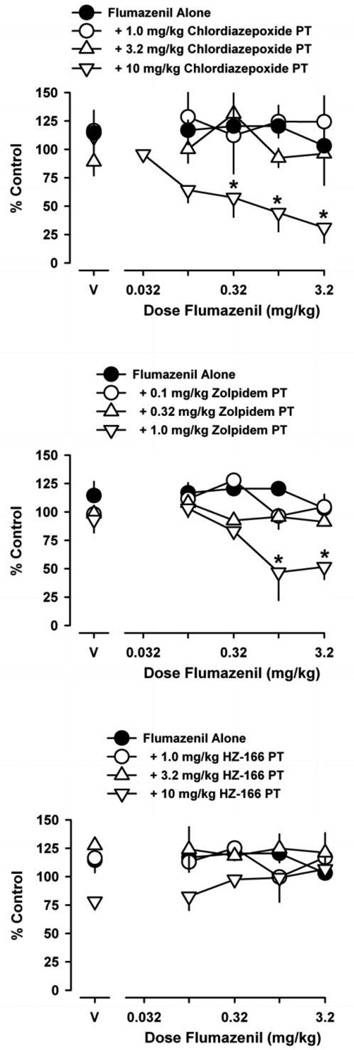
Figure 2 (top) shows the rate-decreasing effects of flumazenil alone and following the 1440 min pretreatment with chlordiazepoxide. Flumazenil was without effect across the dose range tested. Flumazenil did, however, suppress schedule-controlled responding in a dose-dependent manner following chlordiazepoxide pretreatment [F(3,36) = 17; p < 0.001]. In this regard, flumazenil did not decrease rates of responding following pretreatment doses of 1.0 mg/kg chlordiazepoxide and 3.2 mg/kg chlordiazepoxide, whereas flumazenil did decrease rates of responding following a pretreatment dose of 10 mg/kg chlordiazepoxide, resulting in a flumazenil ED75 value of 0.074 (± 0.057 SEM). Bonferroni t-tests revealed that flumazenil doses of 0.32–3.2 mg/kg suppressed response rates following pretreatment of 10 mg/kg chlordiazepoxide.
Figure 2.
Effects of flumazenil on schedule-controlled responding, either alone or following pretreatment with chlordiazepoxide (top), zolpidem (middle) or HZ-166 (bottom). Pretreatment times for each GABAA receptor agonist were 1440 min, 100 min or 300 min, respectively. Abscissa, dose of flumazenil in milligrams per kilogram. Ordinate, mean (±SEM) response rate as percentage of control. Points above “V” indicate mean (±SEM) rates of responding following vehicle administration, either alone or following GABAA receptor agonist pretreatment. Asterisks represent significant differences relative to vehicle (Bonferroni t-tests, p<0.05).
Figure 2 (middle) also shows the rate-decreasing effects of flumazenil alone and following pretreatment with zolpidem (100 min pretreatment). Flumazenil suppressed schedule-controlled responding in a dose-dependent manner following zolpidem pretreatment [F(3,28) = 12; p < 0.001]. Here, flumazenil did not decrease rates of responding following 0.1 mg/kg and 0.32 mg/kg zolpidem, but significant decreases in response rates were observed following 1.0 mg/kg zolpidem, resulting in an ED75 value of 0.34 (±0.12 SEM). Bonferroni t-tests revealed that flumazenil doses of 1.0 mg/kg and 3.2 mg/kg decreased response rates following this dose of zolpidem. Also shown in Figure 2 (bottom) are the effects of flumazenil following 300-min pretreatment with HZ-166 on operant behavior. In contrast to zolpidem, pretreatment with HZ-166 failed to affect the flumazenil dose response curve across all doses tested, and an ED75 value could not be calculated.
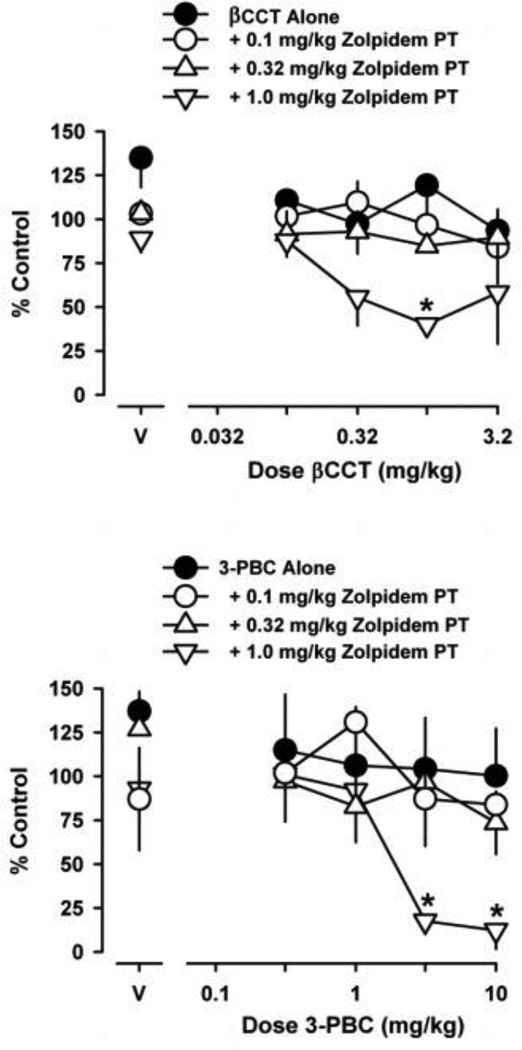
Figure 3 shows the rate-decreasing effects of βCCT (top) and 3-PBC (bottom) alone and following pretreatment with zolpidem. βCCT and 3-PBC were ineffective when administered alone. Following zolpidem pretreatment, however, both βCCT [F(3,32) = 5.9; p = 0.002] and 3-PBC [F(3,32) = 6.1; p = 0.002] were effective, depending on the pretreatment dose. In this regard, βCCT and 3-PBC did not decrease rates of responding following pretreatment doses of 0.1 mg/kg and 0.32 mg/kg zolpidem, whereas both GABAA receptor antagonsits did decrease rates of responding following a pretreatment dose of 1.0 mg/kg zolpidem. The resulting ED75 values for βCCT and 3-PBC were 0.16 (±0.060 SEM) and 1.3 (±0.30 SEM), respectively. Bonferroni t-tests revealed that the βCCT dose of 1.0 mg/kg decreased response rates following 1.0 mg/kg zolpidem. Further, Bonferroni t-tests showed that the 3-PBC doses of 3.2 and 10 mg/kg decreased response rates following 1.0 mg/kg zolpidem.
Figure 3.
Effects of βCCT (top panel) and 3-PBC (bottom panel) on schedule-controlled responding, either alone or following pretreatment with zolpidem. The pretreatment time for zolpidem prior to both GABAA receptor antagonists was 100 min. Abscissae, dose of drug in milligrams per kilogram. Ordinate, mean (±SEM) response rate as percentage of control. Points above “V” indicate mean (±SEM) rates of responding following vehicle administration, either alone or following zolpidem pretreatment. Asterisks represent significant differences relative to vehicle (Bonferroni t-tests, p<0.05).
DISCUSSION
Conventional benzodiazepines bind non-selectively across α1GABAA, α2GABAA, α3GABAA and α5GABAA receptors; however, the role of these receptor subtypes in benzodiazepine-induced physical dependence is understood poorly. In the present study, we used an assay of schedule-controlled responding to characterize the role of α1GABAA, α2GABAA and α3GABAA receptors on an endpoint related to acute physical dependence. Initial studies demonstrated that chlordiazepoxide, zolpidem and HZ-166 produced dose-dependent decreases in rates of responding, whereas the GABAA receptor antagonists flumazenil, βCCT and 3-PBC were ineffective over the dose ranges tested. Flumazenil suppressed rates of responding following pretreatment of the non-selective benzodiazepine chlordiazepoxide, as well as pretreatment with the α1GABAA-preferring agonist zolpidem. In contrast, flumazenil did not suppress rates of responding following pretreatment with the α2GABAA/α3GABAA agonist HZ-166. Further, the α1GABAA-preferring antagonists βCCT and 3-PBC decreased response rates following zolpidem pretreatment. Together, these findings raise the possibility that α1GABAA receptors, but not α2GABAA or α3GABAA receptors, play an important role in behaviors that are related to the initiation of physical dependence following conventional benzodiazepine administration. Moreover, these data suggest that the receptor profile that underpins benzodiazepine-induced physical dependence may be different from receptors that mediate other behavioral effects, including antihyperalgesia and anxiolysis.
The positive GABAA receptor modulators that decreased responding did so with a potency ranking of zolpidem > chlordiazepoxide = HZ-166, whereas the GABAA receptor antagonists lacked effects. The finding that zolpidem and chlordiazepoxide reduced rates of responding is consistent with a number of studies demonstrating that various positive GABAA modulators with activity at α1GABAA receptors decrease responding maintained under a variety of operant schedules (e.g., Paronis and Bergman 1999; Vanover et al., 1999; Rowlett et al., 2005; Fischer et al., 2010). Further, flumazenil, βCCT and 3-PBC, compounds that do not substantially modify GABA-mediated chloride flux at α1GABAA receptors (Smith et al., 2001; Harvey et al., 2002), failed to alter rates of responding, consistent with previous studies (McMahon and France, 2002). In contrast, the observation that HZ-166 reduced rates of responding, albeit not completely, was unexpected considering the relatively low intrinsic efficacy of HZ-166 at α1GABAA receptors, and contrasts previous observations that this and related compounds do not alter response rates under a similar schedule following i.v. administration in rhesus monkeys (Fischer et al., 2010). The reasons for this discrepancy are unclear. One possibility is that weak activity at α1GABAA receptors is sufficient for rate-reducing effects in squirrel monkeys; however this is unlikely since SH-053-2’F-R-CH3, a compound with a similar pharmacological profile to that of HZ-166 at α1GABAA receptors (Fischer et al., 2010; Savic et al., 2010) does not produce rate decreasing effects in this species across a similar dose range (unpublished). Therefore, the results from the present study suggest that activation of α2GABAA and α3GABAA receptors is sufficient for modest behavioral disruption in squirrel monkeys under certain conditions.
The finding that flumazenil suppressed schedule controlled responding following pretreatment with chlordiazepoxide is consistent with the findings described by Spealman (1986). In this study, flumazenil (i.e. Ro 15-1788) did not produce disruptions in behavior when administered alone, but did so following administration with the benzodiazepines chlordiazepoxide and diazepam, as well as the diazepam metabolite N-desmethyldiazepam on responding maintained under both fixed ratio and fixed interval schedules of reinforcement. This alteration of flumazenil potency has been interpreted as a measure of physical dependence, as the rate-decreasing effects of flumazenil are most likely due to the precipitation of withdrawal (Holtzman and Villarreal, 1973; Lukas and Griffiths, 1982; Gerak and France, 1997; McMahon and France, 2002).
The main purpose of the present study was to test the hypothesis that acute benzodiazepine-induced physical dependence-like effects may be mediated by specific α subunit-containing GABAA receptors. In order to explore GABAA receptor mechanisms in this effect, initial studies examined the rate-decreasing effects of flumazenil following pretreatment with zolpidem and HZ-166. Here, flumazenil decreased response rates following pretreatment with zolpidem in a similar manner to that seen after chlordiazepoxide pretreatment. This observation raises the possibility that α1GABAA receptors may play a substantial role in benzodiazepine-induced physical dependence. In contrast, flumazenil did not decrease response rates following pretreatment with doses as high as 10 mg/kg HZ-166. It is important to note that the doses of HZ-166 administered as pretreatment were behaviorally effective in the initial dose- and time-effect determinations. Therefore, the lack of effect from this drug in inducing a dependence-like state is likely not due to insufficient modulation of GABAA-mediated chloride influx at α2GABAA and/or α3GABAA receptors.
The finding that flumazenil suppressed schedule-controlled responding following zolpidem pretreatment supports further the hypothesis that α1GABAA receptors mediate acute dependence-like effects. However, although zolpidem has preferential (~10-fold) selectivity for α1GABAA receptors relative to α2GABAA and α3GABAA receptors, it does activate the latter subtypes at higher doses. Therefore, to explore further the role of α1GABAA receptors on this endpoint, we sought to characterize the enhanced effectiveness of two α1GABAA-preferring antagonists, βCCT and 3-PBC, following zolpidem administration. βCCT and 3-PBC have an approximately 10 to 100-fold greater affinity for α1GABAA receptors relative to α2GABAA and α3GABAA and α5GABAA receptors (Harvey et al., 2002). Although βCCT and 3-PBC were ineffective when administered alone, both drugs decreased rates of operant responding following zolpidem pretreatment. Considering the affinity of these compounds for α1GABAA receptors, this observation provides additional support for a role of this receptor subtype in zolpidem-induced physical dependence, and in turn provides additional evidence to suggest that α1GABAA receptors may play an important role in benzodiazepine-induced physical dependence.
In consideration of evidence suggesting that physical dependence is an α1GABAA receptor-mediated phenomenon, it is perhaps surprising that initial reports following the introduction of zolpidem suggested that it had a reduced propensity to produce dependence. These initial reports demonstrated a lack of withdrawal-like behaviors following long-term administration and drug discontinuation (Perrault et al., 1992; Elliot and White 2000). Subsequently, these reports were used to suggest that zolpidem was a safer compound relative to conventional benzodiazepines (e.g. Holm and Goa, 2000). However, these reports have been contrasted by other studies in which zolpidem-induced physical dependence was apparent (e.g. Griffiths et al., 1992; Weerts and Griffiths, 1998; Kliethermes et al., 2004), and is in concordance with numerous clinical accounts of zolpidem dependence (cf. Victorri-Vigneau et al., 2007). The extent to which these differences across studies reflect variables such as species and/or methodology used to demonstrate dependence remains to be determined. Regardless, our findings add to the growing empirical support of the presence of physical dependence-like effects associated with zolpidem administration.
Taken together with previously described experiments, the data from the present study provides additional evidence that the behavioral effects of benzodiazepines are mediated by different α subunit-containing GABAA receptor subtypes. Our findings implicate α1GABAA receptors in benzodiazepine-induced physical dependence-like effects, and suggest further that the receptor mechanisms that underpin physical dependence may be different than those that mediate anxiolysis and antihyperalgesia (α2GABAA and α3GABAA receptor subtypes). Further, our findings suggest that it may be difficult to dissociate α1GABAA receptor-mediated therapeutic effects (e.g. the sedative effects found in sleep aids) from dependence-like effects. These hypotheses should provide an important framework for studying the role of different GABAA receptor subtypes in the abuse-related effects of benzodiazepine-type drugs, which in turn should help guide both the current clinical use of benzodiazepines as well as the development of improved therapeutic agents for treating anxiety- and pain-related disorders.
ACKNOWLEDGMENTS
This work was supported by USPHS grants DA011792, DA033795, RR000168/OD011103 and MH046851.
REFERENCES
- Adams JU, Holtzman SG. Pharmacologic characterization of the sensitization to the rate-decreasing effects of naltrexone induced by acute opioid pretreatment in rats. J Pharmacol Exp Ther. 1990;253:483–489. [PubMed] [Google Scholar]
- Atack JR, Wafford KA, Tye SJ, Cook SM, Sohal B, Pike A, Sur C, Melillo D, Bristow L, Bromidge F, Ragan I, Kerby J, Street L, Carling R, Castro JL, Whiting P, Dawson GR, McKernan RM. TPA023 [7-(1,1-dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine], an agonist selective for alpha2- and alpha3-containing GABAA receptors, is a nonsedating anxiolytic in rodents and primates. J Pharmacol Exp Ther. 2006;316:410–422. doi: 10.1124/jpet.105.089920. [DOI] [PubMed] [Google Scholar]
- Bronson ME. Chlordiazepoxide, but not bretazenil, produces acute dependence, as evidenced by disruptions in schedule-controlled behavior. Pharmacol Biochem Behav. 1994;48:397–401. doi: 10.1016/0091-3057(94)90543-6. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot EE, White JM. Precipitated and spontaneous withdrawal following administration of lorazepam but not zolpidem. Pharmacol Biochem Behav. 2000;66:361–369. doi: 10.1016/s0091-3057(00)00176-3. [DOI] [PubMed] [Google Scholar]
- Fischer BD, Licata SC, Edwankar RV, Wang ZJ, Huang S, He X, Yu J, Zhou H, Johnson EM, Jr, Cook JM, Furtmüller R, Ramerstorfer J, Sieghart W, Roth BL, Majumder S, Rowlett JK. Anxiolytic-like effects of 8-acetylene imidazobenzodiazepines in a rhesus monkey conflict procedure. Neuropharmacology. 2010;59:612–618. doi: 10.1016/j.neuropharm.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, France CP. Repeated administration of flumazenil does not alter its potency in modifying schedule-controlled behavior in chlordiazepoxidetreated rhesus monkeys. Psychopharmacology. 1997;131:64–70. doi: 10.1007/s002130050266. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Weerts EM. Benzodiazepine self-administration in humans and laboratory animals--implications for problems of long-term use and abuse. Psychopharmacology. 1997;134:1–37. doi: 10.1007/s002130050422. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Sannerud CA, Ator NA, Brady JV. Zolpidem behavioral pharmacology in baboons: self-injection, discrimination, tolerance and withdrawal. J Pharmacol Exp Ther. 1992;260:1199–1208. [PubMed] [Google Scholar]
- Harvey SC, Foster KL, McKay PF, Carroll MR, Seyoum R, Woods JE, 2nd, Grey C, Jones CM, McCane S, Cummings R, Mason D, Ma C, Cook JM, June HL. The GABA(A) receptor alpha1 subtype in the ventral pallidum regulates alcohol-seeking behaviors. J Neurosci. 2002;22:3765–3775. doi: 10.1523/JNEUROSCI.22-09-03765.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm KJ, Goa KL. Zolpidem: an update of its pharmacology, therapeutic efficacity and tolerability in the treatment of insomnia. Drugs. 2000;59:865–889. doi: 10.2165/00003495-200059040-00014. [DOI] [PubMed] [Google Scholar]
- Holtzman SG, Villarreal JE. Operant behavior in the morphine-dependent rhesus monkey. J Pharmacol Exp Ther. 1973;184:528–541. [PubMed] [Google Scholar]
- Kliethermes CL, Metten P, Belknap JK, Buck KJ, Crabbe JC. Selection for pentobarbital withdrawal severity: correlated differences in withdrawal from other sedative drugs. Brain Res. 2004;1009:17–25. doi: 10.1016/j.brainres.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Knabl J, Witschi R, Hösl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, Fritschy J-M, Rudolph U, Möhler H, Zeilhofer HU. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- Licata SC, Platt DM, Cook JM, Sarma PV, Griebel G, Rowlett JK. Contribution of GABAA receptor subtypes to the anxiolytic-like, motor, and discriminative stimulus effects of benzodiazepines: studies with the functionally selective ligand SL651498 [6-fluoro-9-methyl-2-phenyl-4-(pyrrolidin-1-yl-carbonyl)-2,9-dihydro-1H-pyridol[3,4-b]indol-1-one] J Pharmacol Exp Ther. 2005;313:1118–1125. doi: 10.1124/jpet.104.081612. [DOI] [PubMed] [Google Scholar]
- Licata SC, Rowlett JK. Abuse and dependence liability of benzodiazepine-type drugs: GABA(A) receptor modulation and beyond. Pharmacol Biochem Behav. 2008;90:74–89. doi: 10.1016/j.pbb.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löw K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Griffiths RR. Precipitated withdrawal by a benzodiazepine receptor antagonist (Ro 15-1788) after 7 days of diazepam. Science. 1982;217:1161–1163. doi: 10.1126/science.6287579. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Griffiths RR. Precipitated diazepam withdrawal in baboons: effects of dose and duration of diazepam exposure. Eur J Pharmacol. 1984;100:163–171. doi: 10.1016/0014-2999(84)90218-8. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- McMahon LR, France CP. Daily treatment with diazepam differentially modifies sensitivity to the effects of gamma-aminobutyric acid(A) modulators on schedule-controlled responding in rhesus monkeys. J Pharmacol Exp Ther. 2002;300:1017–1025. doi: 10.1124/jpet.300.3.1017. [DOI] [PubMed] [Google Scholar]
- Mirza NR, Nielsen EØ. Do subtype-selective gamma-aminobutyric acid A receptor modulators have a reduced propensity to induce physical dependence in mice? J Pharmacol Exp Ther. 2006;316:1378–1385. doi: 10.1124/jpet.105.094474. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paronis CA, Bergman J. Apparent pA2 values of benzodiazepine antagonists and partial agonists in monkeys. J Pharmacol Exp Ther. 1999;290:1222–1229. [PubMed] [Google Scholar]
- Perrault G, Morel E, Sanger DJ, Zivkovic B. Lack of tolerance and physical dependence upon repeated treatment with the novel hypnotic zolpidem. J Pharmacol Exp Ther. 1992;263:298–303. [PubMed] [Google Scholar]
- Platt DM, Carey G, Spealman RD. Models of neurological disease (substance abuse): self-administration in monkeys. Chapter 10. Curr Protoc Pharmacol. 2011 doi: 10.1002/0471141755.ph1005s56. Unit10.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett DB, Lüddens H, Seeburg PH. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci U S A. 2005;102:915–920. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Möhler H. GABA(A) receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22:188–194. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- Savić MM, Majumder S, Huang S, Edwankar RV, Furtmüller R, Joksimović S, Clayton T, Sr, Ramerstorfer J, Milinković MM, Roth BL, Sieghart W, Cook JM. Novel positive allosteric modulators of GABAA receptors: do subtle differences in activity at alpha1 plus alpha5 versus alpha2 plus alpha3 subunits account for dissimilarities in behavioral effects in rats? Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:376–386. doi: 10.1016/j.pnpbp.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Alder L, Silk J, Adkins C, Fletcher AE, Scales T, Kerby J, Marshall G, Wafford KA, McKernan RM, Atack JR. Effect of α subunit on allosteric modulation of ion channel function in stably expressed human recombinant aminobutyric acidA receptors determined using 36Cl ion flux. Mol Pharmacol. 2001;59:1108–1118. doi: 10.1124/mol.59.5.1108. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Disruption of schedule-controlled behavior by Ro 15-1788 one day after acute treatment with benzodiazepines. Psychopharmacology. 1986;88:398–400. doi: 10.1007/BF00180845. [DOI] [PubMed] [Google Scholar]
- Tan KR, Brown M, Labouèbe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Lüscher C. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanover KE, Robledo S, Huber M, Carter RB. Pharmacological evaluation of a modified conflict procedure: punished drinking in non-water-deprived rats. Psychopharmacology (Berl) 1999;145:333–341. doi: 10.1007/s002130051066. [DOI] [PubMed] [Google Scholar]
- Victorri-Vigneau C, Dailly E, Veyrac G, Jolliet P. Evidence of zolpidem abuse and dependence: results of the French Centre for Evaluation and Information on Pharmacodependence (CEIP) network survey. Br J Clin Pharmacol. 2007;64:198–209. doi: 10.1111/j.1365-2125.2007.02861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weerts EM, Griffiths RR. Zolpidem self-injection with concurrent physical dependence under conditions of long-term continuous availability in baboons. Behav Pharmacol. 1998;9:285–297. [PubMed] [Google Scholar]